Abstract
Skin-stage schistosomula of Schistosoma mansoni were found to secrete molecules that are pro-apoptotic for skin T lymphocytes as measured by annexin V staining, caspase-3 activity, caspase-8 activities, and DNA fragmentation. Caspase-8 activities in lymphocytes peaked ~8 h and caspase-3 activity peaked ~16 h after exposure to the parasite secretions. Subset analysis showed that mainly CD4+ and CD8+ cells (but not B cells) were susceptible to the parasite-induced pro-apoptotic effect. In situ staining confirmed the presence of apoptotic T cells around challenge parasites in the skin of naive or immunized animals. Analysis of T cells to identify the potential molecular pathway of the parasite-induced apoptosis showed increases in the expression of Fas, FasL, and the Fas-associated death domain. Blocking of FasL with a fusion protein reversed the parasite-induced apoptosis, suggesting a role for the Fas/FasL-mediated pathway in the parasite-induced T cell apoptosis. Subsequent analyses of the secretions of skin-stage schistosomula identified the pro-apoptotic activity as being associated with a protein of ~23 kDa. This protein was termed S. mansoni-derived apoptosis-inducing factor.
Schistosomiasis is a chronic debilitating disease that currently affects >200 million people worldwide. Despite enormous effort to control the disease, schistosomiasis continues to be one of the major health hazards in parts of Africa, the Middle East, and Southeast Asia (1). Development of an effective vaccine against this infection remains elusive. Part of the problem may be due to the ability of the parasite to evade host immune mechanisms (2–4). Compelling evidences from recent studies suggest that schistosomes may actually exploit the host immune responses for their own replication and transmission by suppressing host immune responses (5). It is well established that cells obtained from patients with chronic infection fail to respond to antigens derived from Schistosoma mansoni (6–11). Similar to these findings, one of our recent studies showed that lymphocytes isolated from the skin or skin-draining lymph nodes of naive mice or mice immunized previously with radiation-attenuated cercariae of S. mansoni fail to respond to antigens in the excretory secretory (ES)1 products of skin-stage schistosomula (12). Morphologically, these cells appear smaller and show nuclear and cytoplasmic condensation, suggestive of cell death by apoptosis (13). Several lines of evidence suggest that antigens of S. mansoni can induce apoptosis of host T cells (7, 10, 11, 14, 15). In fact, the spontaneous immunoregulation associated with egg-induced granulomatous inflammation (16–20) and the global switch from a Th1-type to a Th2-type cytokine response in schistosomiasis are believed to be due to apoptosis of T cells (10, 11, 18, 20, 21). Lundy et al. (22) recently reported that when spleen cells or lymphocytes isolated from egg granulomas of S. mansoni-infected animals were cultured in the presence of soluble egg antigens (SEAs), a significant proportion of CD4+ T cells in this preparation underwent apoptosis. This pro-apoptotic effect appears to be mediated by B cells that express functional FasL on their surface upon exposure to SEAs (22, 23). In this study, we show that skin-stage schistosomula also similarly secrete antigens that can up-regulate FasL expression on lymphocytes, thus promoting apoptosis of skin T cells. In addition, we have also attempted to analyze the antigens secreted by skin-stage schistosomula to identify the pro-apoptotic molecule.
EXPERIMENTAL PROCEDURES
Animals
Male C57BL/6 mice (6–10 weeks old; Charles River Laboratories, Wilmington, MA) were used in these experiments.
S. mansoni Infection and Immunization
Biomphalaria glabrata snails infected with S. mansoni were obtained from Dr. Fred Lewis (Biomedical Research Institute, Rockville, MD). Cercariae were collected from infected snails as described previously (24), and mice were infected via the abdominal skin with 250 cercariae. For immunization, mice were immunized with 250 γ-radiation-attenuated (20,000 roentgen) live cercariae via the abdominal skin. 2 weeks after immunization, some of the mice were subjected to challenge infection with 250 normal cercariae. For in vitro studies, cercariae were transformed into schistosomula, and ES products were collected as described previously (25).
Cell Preparation and Culture
A single cell suspension of skin-draining (inguinal) lymph nodes was made, and cell viability was determined by trypan blue exclusion. Cell viability was always >99% in all our preparations. In some experiments, cells isolated from skin-draining lymph nodes were separated into different subsets (Thy1.2+, CD4+, CD8+, and B cells) using magnetic beads (Miltenyi Biotech Inc., Auburn, CA, or Dynal, Oslo, Norway) coated with monoclonal antibodies specific for the respective cell subsets. Skin T cells (Thy1.2+) were isolated as described previously (12). The purity of these cells was >95% as confirmed by flow cytometry.
Detection of Annexin V-Binding
1 × 106 cells isolated from the inguinal lymph nodes of infected mice, naive mice or immunized mice were suspended in 200 μl of medium and incubated with 60 μg/ml ES products for 24 h at 37 °C and 5% CO2 in air. Following incubation, cells were washed and stained with fluorescein isothiocyanate-labeled annexin V or propidium iodide (BioSource International, Camarillo, CA). About 500 –1000 cells were counted from each sample under a fluorescent microscope, and the percentage of positive cells were calculated. In some experiments, the percentage of annexin V-positive cells was determined by flow cytometry.
Detection and Time Kinetics of Caspase-3 and Caspase-8 Activities
Approximately 1 × 106 lymphocytes (CD4, CD8, or B cells) isolated from the skin or skin-draining lymph nodes of naive or immunized/challenged mice were suspended in 200 μl of medium and incubated with 100–150 schistosomula or 60 μg/ml ES products of schistosomula for 24 h at 37 °C and 5% CO2 in air. Following incubation, cells were harvested and stained with 50 μl of 10 μM PhiPhiLux substrate solution (Alexis Corp., San Diego, CA) for 45 min at 37 °C according to the manufacturer’s instructions. About 500–1000 cells were counted from each sample, and the percentage of caspase-positive cells was calculated.
Because there was an increase in both caspase-3 and caspase-8 activities after exposure to 60 μg/ml ES products, we performed a time kinetics study (at 0, 8, 16, and 32 h after exposure) to measure differences in caspase-3 and caspase-8 activities in the skin-draining lymph node cells (5 × 106) collected from naive C57BL/6 mice. Specific activities of caspase-3 and caspase-8 were determined by a colorimetric assay using kits purchased from MBL (Nagoya, Japan).
Analysis of DNA Fragmentation
DNA fragmentation was evaluated as described by Kroemer et al. (26). Briefly, a subset of lymphocytes were incubated with ES products (60 μg/ml) or schistosomula (100 –150/1 × 106 cells). 24 h later, the cells were treated with digestion buffer (SDS/EDTA/proteinase K) and subjected to DNA electrophoresis on a 1% agarose gel.
TUNEL Staining for Apoptotic Cells in the Skin
Apoptotic cells around the parasites in the skin of mice were evaluated by TUNEL staining using an in situ apoptosis detection kit (R&D Systems, Minneapolis, MN) following the instructions of the manufacturer. Skin samples collected 24 h after challenge were embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA) and snap-frozen in liquid nitrogen. 8-μm-thick sections were cut using a cryostat and treated with proteinase K. Endogenous peroxidase activity was quenched using 1% hydrogen peroxide in methanol. Sections were then incubated with biotinylated dUTP in the presence of terminal deoxynucleotidyltransferase, which incorporates the biotinylated nucleotides into the 3′-OH ends of fragmented DNA. Finally, the biotinylated nucleotides were detected using streptavidin-conjugated horseradish peroxidase, and color was developed using diaminobenzidine substrate. Methyl green was used as a counterstain. Adjacent sections were stained with a PE-labeled mouse anti-CD3 monoclonal antibody (BD Pharmingen, San Diego, CA), and isotype-matched nonspecific rat monoclonal antibody was used as a control. Some sections were also stained with hematoxylin and eosin.
RNA Isolation and RT-PCR Analysis
Total RNA was extracted from Thy1.2+ cells, skin-draining lymph nodes, or the skin of mice using Trizol (Invitrogen) according to the manufacturer’s recommendations and reverse-transcribed using RETROscript (Ambion Inc., Austin, TX). The cDNA of β-actin in each sample was first PCR-amplified (PerkinElmer Life Sciences) using β-actin-specific primers (27). Band densities of β-actin in different samples were adjusted to approximately the same level. Individual samples were then PCR-amplified for Fas, FasL, and the Fas-associated death domain (FADD) using specific primers. Primer sequences for Fas and FasL were as published previously (28). Primers for FADD were 5′-ATGGAGCTCAAGTTCTTGTGC-3′ and 5′-TCACTCTTGCTCACAGATTCC-3′ (GenBank™ accession number U50406). Primers for β-actin, Fas, FasL, and FADD amplified 550-, 281-, 590-, and 503-bp target fragments, respectively. PCRs were performed as follows: for β-actin, 3 min at 94 °C, 30 s at 57 °C, and 30 s at 72 °C for 30 cycles; for Fas, 3 min at 94 °C, 30 s at 62 °C, and 30 s at 72 °C for 32 cycles; for FasL, 3 min at 94 °C, 30 s at 60 °C, and 30 s at 72 °C for 30 cycles; and for FADD, 3 min at 94 °C, 30 s at 49 °C, and 30 s at 72 °C for 35 cycles. The final elongation was followed by 5 min at 72 °C. The products were resolved on a 1.5% agarose gel and stained with ethidium bromide.
Fas and FasL Detection by Flow Cytometry
1 × 106 skin-draining lymph node cells from naive or immunized mice were incubated with ES products (20, 60, or 80 μg/ml) for 24 –48 h. Following incubation, cells were washed and incubated with rat anti-mouse CD16/CD32 antibody (BD Pharmingen) to block non-antigen-specific binding of immunoglobulins to the Fcγ type III/II receptors. Following this, cells were incubated with fluorescein isothiocyanate-labeled rat anti-mouse CD3 antibody and PE-labeled anti-mouse Fas or anti-mouse FasL monoclonal antibody (BD Pharmingen) for 30 min at 4 °C and analyzed in a flow cytometer (Cytron, Orthodiagnostic Inc., Raritan, NJ). Isotype-matched nonspecific antibodies were used as negative controls.
Effect of FasL Blocking on ES Product-induced T Cell Apoptosis
1 × 106 Thy1.2+ cells isolated from skin-draining lymph nodes of naive mice were cultured with 60 μg/ml ES products or ES products plus 5 μg/ml recombinant Fas-Fc fusion protein (Alexis Corp.) for 24 h and then tested for annexin V binding. Recombinant S. mansoni G-binding factor was used as a control recombinant protein.
Fractionation of ES Products to Identify Pro-apoptotic Activity
Proteins in the ES products of normal schistosomula were size-separated initially into three different fractions by ultrafiltration using Centricon concentrators (Amicon, Inc., Beverly, MA). Fraction 1 contained molecules <3000 Da; fraction 2 contained molecules between 3 and 30 kDa; and fraction 3 contained molecules >30 kDa. Each fraction (60 μg/ml) was then tested for the presence of pro-apoptotic activity. These studies narrowed the pro-apoptotic activity to molecules between 3 and 30 kDa. Subsequently, Sephacryl S-100 high-resolution gel filtration medium (Amersham Biosciences) was used to separate the protein bands in fraction 2. The protein band that showed pro-apoptotic activity was then resolved on a 12% SDS-polyacrylamide gel, transferred onto a polyvinylidene difluoride membrane (Bio-Rad), and sent to the Protein Structure Facility at the University of Michigan for N-terminal amino acid sequencing.
Statistical Analysis
Statistical analysis was performed using a Mann-Whitney U rank sum test using Sigmastat Version 2.0 (Jandel Scientific, San Rafael, CA).
RESULTS
Effect of ES Products on Cell Viability
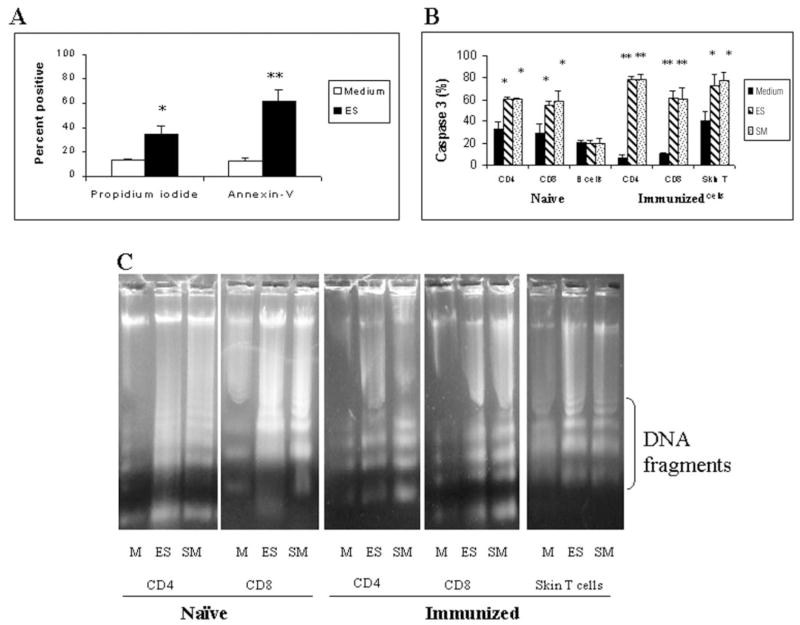
Addition of ES products of normal schistosomula to lymphocytes collected from skin-draining (inguinal) lymph nodes of naive mice resulted in a significant decrease in cell viability as measured by propidium iodide staining. At 24 h after exposure to 60 μg/ml ES products, 34.3 ± 7.5% of cells were positive for propidium iodide. However, when cells were incubated with medium alone, only 13.2 ± 1.3% of cells were positive (p < 0.05) (Fig. 1A). A similar decrease in cell viability was obtained when cells from naive animals were cultured with 100 –150 schistosomula of S. mansoni (data not shown). Under light microscopy, a substantial proportion of cells exposed to the parasites or their ES products appeared smaller with condensed cytoplasm and nuclei (data not shown). Subsequent staining of the cells with annexin V and propidium iodide showed that 62.4 ± 8.9% of cells incubated with ES products (60 μg/ml) were positive for annexin V, whereas only 12.8 ± 2.4% of cells incubated with medium alone were positive for annexin V (p < 0.01) (Fig. 1A). A similar decrease in cell viability was observed when Thy1.2+ cells isolated from the skin of immunized mice were incubated with 100 –150 schistosomula or their ES products (data not shown). These observations suggest that the lymphocytes might undergo apoptosis upon exposure to the parasites or their secretions.
Fig. 1. 1 × 106 cells isolated from the skin or skin-draining lymph nodes of naive or immunized animals were cultured in medium alone (M) or with ES products (60 μg/ml) or 100–150 schistosomula (SM) for 24 h.
Following incubation, apoptosis was measured by annexin V and propidium iodide staining and counting 500 –1000 cells under a fluorescence microscope (A), by measuring caspase-3 activity in various subset of lymphocytes using PhiPhiLux substrate and counting 500 –1000 cells under a fluorescence microscope (B), and by evaluating DNA fragmentation in different subsets after treatment with DNA digestion buffer and separation on a 1% agarose gel (C). Data presented are representative of one of three to five similar experiments using five to seven mice per group in each experiment. A and B show means ± S.D. of the percentage of positive cells. * and **, p < 0.05 and p < 0.01 compared with the medium control, respectively.
Subset of Lymphocytes That Undergo Apoptosis in Response to the Parasites or Their Secretions
In these studies, we monitored caspase-3 activity as a marker of apoptosis in various subsets of lymphocytes (CD4, CD8, and B cells) after incubation with 100 –150 schistosomula or their ES products. Significant increases in caspase-3 activity were observed in both CD4+ and CD8+ subsets of lymphocytes from either naive or immunized mice after exposure to the parasites or their secretions, whereas in B cells, the caspase-3 staining was not significantly different from medium controls (Fig. 1B). Similar results were obtained when Thy1.2+ cells isolated from the skin of immunized and challenged mice were exposed to the parasites or their secretions in vitro (Fig. 1B).
Effect of ES Products on DNA Fragmentation in T Cells
The pro-apoptotic effect of schistosomula or their ES products on CD4 and CD8 subsets of T cells was then further confirmed by analyzing their DNA for fragmentation. These studies showed typical fragmentation of DNA as evidenced by the characteristic ladder formation in both CD4 and CD8 subsets of lymphocytes from both naive and immunized mice that were exposed to schistosomula or their ES products (Fig. 1C). No DNA fragmentation was evident in cells incubated with medium alone (Fig. 1C) or in B cells that were incubated with the parasites or their secretions (data not shown). DNA fragmentation was also evident in Thy1.2+ cells isolated from the skin of immunized and challenged mice following exposure to the parasites or their secretions (Fig. 1C).
Time Kinetics of Caspase-3 and Caspase-8 Activities in Skin-draining Lymph Node Cells
Preliminary studies also showed an increase in caspase-8 activities in T cells exposed to ES products (data not shown). Therefore, we wanted to evaluate the time kinetics of the appearance of caspase-3 and caspase-8 activities in T cells exposed to the ES products. These studies showed that caspase-8 activities appeared first and peaked in the cells ~8 h after incubation (Fig. 2). Thereafter, there was a sharp decline; and by 16 h, caspase-8 activities in the cells were near base-line levels. On the other hand, caspase-3 activity showed a steady increase from 8 h after exposure to ES products and peaked at ~16 h (Fig. 2).
Fig. 2. Time courses of caspase-8 and caspase-3 protease activities.
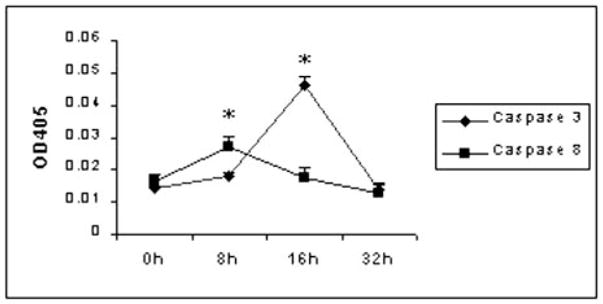
5 × 106 skin-draining lymph node cells from naive C57BL/6 mice were cultured with 60 μg/ml ES products for 0, 8, 16, and 32 h. Cells were harvested at different time points, and caspase-8 or caspase-3 protease activity was examined by a colorimetric protease assay. Data presented are from one of two similar experiments.
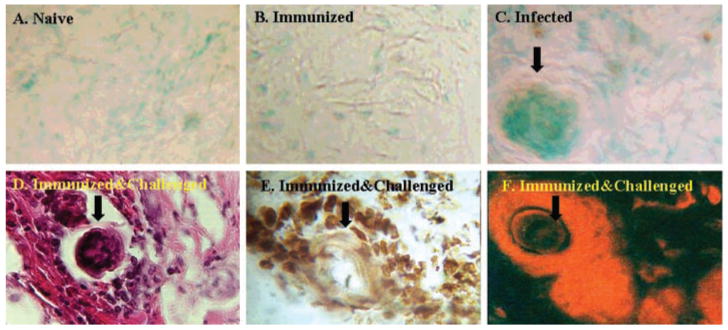
In Situ Analysis of Apoptotic Cells around the Parasites in the Skin
Previous studies showed that mononuclear cells accumulate around challenge parasites in the skin of immunized animals (24). Despite this marked cellular reaction around the parasite, very few normal parasites appear to die in the skin (24). However, if the parasites are attenuated by γ-rays, several of them are retained in the skin; and among these, a few die due to the severe cellular reaction. Because our above results showed that secretions from normal schistosomula can cause apoptosis of T cells, we wanted to evaluate whether T cells that accumulate around normal parasites in the skin undergo apoptosis, thereby helping the parasite to escape. We used TUNEL staining to demonstrate apoptotic cells in the skin. These studies showed the presence of many apoptotic cells around migrating challenge parasites in the skin of immunized and challenged animals (Fig. 3E). Apoptotic cells were also seen around schistosomula in the skin of infected naive mice (Fig. 3C). No apoptotic cells were present in normal skin (Fig. 3A) or in the skin of immunized mice (Fig. 3B). Simultaneous immunohistochemical analyses of adjacent sections with anti-CD3 antibodies showed that the majority of the cells around normal schistosomula (Fig. 3D) in the skin of immunized and challenged animals are T cells (Fig. 3F). There was no PE reactivity in sections incubated with isotype-matched antibody controls (data not shown).
Fig. 3. TUNEL staining for apoptotic cells in the skin.
Skin biopsy samples collected from naive (A), immunized (B), infected naive (C), and immunized and challenged (D–F) mice were processed for cryostat sectioning and stained with biotinylated dUTP (A–C and E), hematoxylin and eosin (D), or PE-labeled anti-CD3 antibody (F). Arrows indicate cut sections of the parasites. The brown-staining cells in C and E are apoptotic cells. Original magnification was ×400. Data presented are from one of three similar experiments using five mice per group.
Fas and FasL Expression in Skin-draining Lymph Node T Cells after Exposure to ES Products
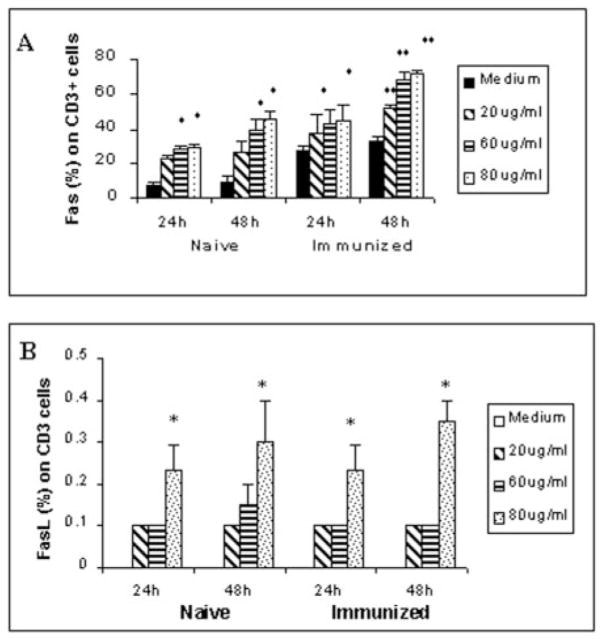
To determine the potential molecular pathway of the parasite-induced apoptosis in T cells, we analyzed the expression of Fas and FasL in skin-draining lymph node T cells from both naive and immunized mice after exposure to ES products. These studies showed a dose- and time-dependent increase in Fas+ CD3+ cells following exposure to ES products (Fig. 4A). Maximum expression was seen when cells were incubated with 60 μg/ml ES products for 48 h. Compared with medium controls, a higher proportion of cells from naive mice expressed Fas on their surface in response to ES products than cells from immunized animals (Fig. 4A). Exposure to ES products also resulted in a significant increase in the number of FasL+ CD3+ cells isolated from both naive and immunized animals (Fig. 4B). However, the levels of FasL detection were low and were significant only at higher concentrations of ES products.
Fig. 4. Fas (A) and FasL (B) expression in CD3+ cells.
Skin-draining lymph node cells isolated from naive or immunized mice were incubated with different concentrations of ES products (20, 60, or 80 μg/ml) for 24 or 48 h. Following incubation, cells were stained with a fluorescein isothiocyanate-labeled anti-CD3+ antibody and a PE-labeled anti-Fas or anti-FasL antibody. The percentage of double-positive cells was evaluated by flow cytometry. Data presented are from one of five similar experiments. * and **, p < 0.05 and p < 0.01 compared with the medium control, respectively.
Expression of Molecules Associated with Apoptosis in T Cells, Skin-draining Lymph Node Cells, and the Skin
RT-PCR analysis showed a relative increase in the message levels for Fas, FasL, and FADD in T cells isolated from the skin-draining lymph nodes of naive mice following incubation with ES products (Fig. 5). Similar semiquantitative increases in the transcript levels of Fas, FasL, and FADD were observed in the skin tissue at the site of infection and in the skin-draining lymph nodes of naive animals or immunized mice following a challenge infection with normal cercariae (Fig. 5). These transcripts were low or absent in the skin and draining lymph nodes of uninfected naive mice (Fig. 5).
Fig. 5. Expression of Fas, FasL, and FADD mRNAs in T cells, skin-draining lymph nodes, and skin.
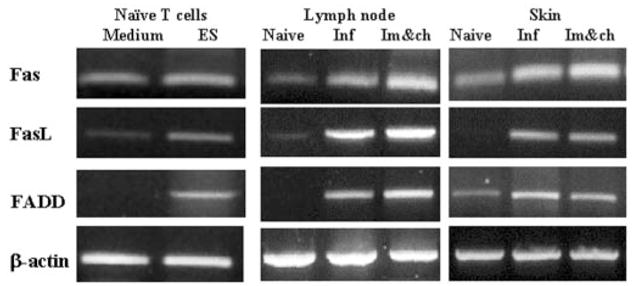
1 × 106 Thy1.2+ cells isolated from skin-draining lymph nodes of naive mice were exposed to 60 μg/ml ES products for 24 h. Tissues (skin-draining lymph nodes and skin) for RT-PCR were collected 24 h after infection with S. mansoni (Inf) or 24 h after challenge infection of mice immunized 2 weeks previously with attenuated parasites (Im&ch). Lymph nodes and skin collected from naive animals served as controls. Gene-specific primers were used in RT-PCR to determine the transcript levels of Fas, FasL, FADD, and β-actin. PCR products were then separated on a 1.5% agarose gel and stained with ethidium bromide. Samples were pooled from three mice, and data presented are representative of one of three similar experiments.
Effect of Blocking FasL on T Cell Apoptosis
Addition of recombinant Fas-Fc fusion protein to T cell cultures incubated with 60 μg/ml ES products resulted in 83.6% reduction in annexin V-positive cells (Fig. 6) compared with control wells that contained ES products, but no blocking recombinant proteins. The percentage of annexin V-positive T cells in Fas-Fc fusion protein-treated wells (27.4 ± 5.7%) was comparable to that in medium controls with no ES products (20.9 ± 3.5%). Addition of a control recombinant protein (S. mansoni G-binding factor) had no effect on the percentage of ES product-induced annexin V-positive cells (data not shown).
Fig. 6. Effect of blocking FasL on parasite-induced T cell apoptosis.
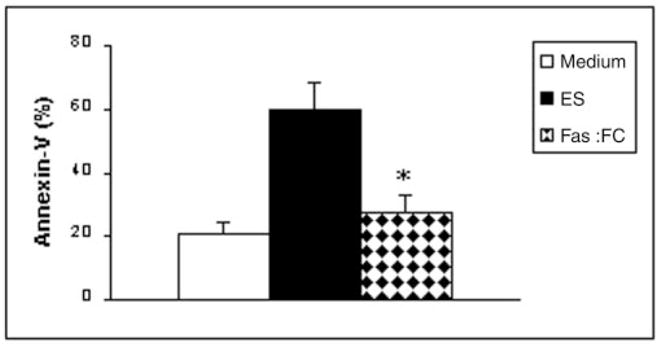
1 × 106 Thy1.2+ cells isolated from skin-draining lymph nodes of naive mice were cultured with 60 μg/ml ES products or ES products plus 5 μg/ml recombinant Fas-Fc fusion protein for 24 h. Apoptosis was evaluated by determining the percentage of annexin V-positive cells. *, p < 0.01 compared with the medium control.
Characterization of the Apoptosis-inducing Activity in the ES Products of Normal Schistosomula
To identify the pro-apoptotic molecule in the ES products of normal schistosomula, we separated the ES products into three fractions and incubated them with T cells isolated from the skin-draining lymph nodes of naive mice. These studies showed that significant pro-apoptotic activity, as measured by annexin V staining, was associated with fraction 2 (3–30 kDa) and fraction 3 (>30 kDa), although the majority of the activity was present in fraction 2 (Fig. 7). Fraction 1 (<3000 Da) did not contain any annexin V-binding activity. Subsequently, bands in fraction 2 were isolated by gel filtration, and each was tested for its ability to induce apoptosis in T cells. These experiments narrowed the pro-apoptotic activity in the ES products of normal schistosomula to a protein of ~23 kDa (Fig. 7).
Fig. 7. Pro-apoptotic activity is associated with a molecule between 3 and 30 kDa in the ES products of normal schistosomula.
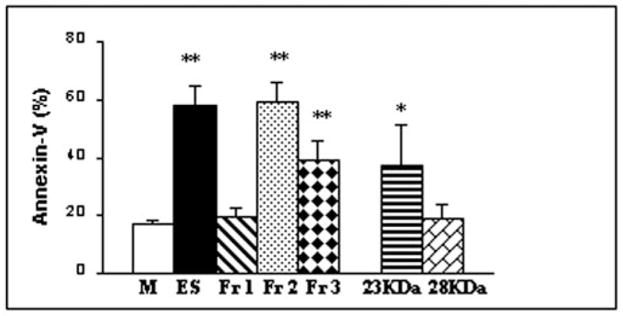
ES products were divided into three fractions, fraction 1 (Fr 1; <3 kDa), fraction 2 (Fr 2; 3–30 kDa), and fraction 3 (Fr 3; >30 kDa). Bands in fraction 2 were again separated by gel filtration. Each peak (at 60 μg/ml) was then tested for its ability to induce apoptosis of T cells (isolated from the skin-draining lymph nodes of naive mice) by staining for annexin V. Only two peaks (23 and 28 kDa) are shown. About 500 cells were counted under a fluorescence microscope. Values are representative of one of three similar experiments and are means ± S.D. of the percentage of positive cells. * and **, p < 0.05 and p < 0.001 compared with the medium control (M), respectively. ES products from normal schistosomula were used as a positive control.
N-terminal Amino Acid Sequence of the 23-kDa Pro-apoptotic Protein
As a preliminary step to identify the molecular structure of the pro-apoptotic molecule, we performed N-terminal amino acid sequencing on the 23-kDa protein. This analysis revealed the sequence as KDMITEDEMFTDSHCPRVVA. We believe that these data will potentially help in cloning the pro-apoptotic gene and may further allow extensive sequence analyses.
DISCUSSION
The results presented in this study show that skin-stage schistosomula of S. mansoni can release molecules in their secretions that can induce apoptosis of T cells in the skin. Subsequent analysis suggested that this pro-apoptotic activity is associated with a molecule of ~23 kDa. Preliminary studies to identify the molecular pathway by which T cells undergo apoptosis in response to the parasite or its secretion suggested a possible role for Fas/FasL mechanisms. Based on this finding, it is suggested that the parasite may use T cell apoptosis as a potential mechanism to subdue initial cellular responses in the skin (24).
Following immunization with radiation-attenuated parasites, there is a significant increase in the numbers of IFN-γ-secreting CD4+ cells in the skin (12) and skin-draining lymph nodes (12, 29, 30). Functional analyses suggest that these IFN-γ-secreting T cells are potentially the effector cells that confer protection against challenge parasites in the lungs (30, 31). However, in one of our earlier studies, when we isolated T cells from the skin or skin-draining lymph nodes of immunized animals and incubated them in vitro with ES antigens from skin-stage schistosomula, they failed to proliferate or secrete IFN-γ (12). Nevertheless, significant amounts of IFN-γ were produced when these cells were stimulated in vitro with mitogen, antigens from radiation-attenuated parasites or with ES antigens from lung-stage schistosomula. This suggests that the normal skin-stage schistosomula might modulate the function of skin or skin-drain lymph node cells to escape host detection in the skin (12). When observed under light microscopy, cells stimulated with ES antigens collected from normal skin-stage schistosomula appear smaller with condensation of cytoplasm and nuclei (13), characteristics that are typical of cells undergoing apoptosis (32, 33).
The ability of schistosomes to induce apoptosis in host cells is well documented in the literature (14). The most striking of the documentation is the studies presented by Carneiro-Santos et al. (7), who suggested an important role for T cell apoptosis in immunoregulation during chronic S. mansoni infection in humans. When peripheral blood CD3+ cells isolated from chronically infected individuals with an asymptomatic intestinal form of the disease were incubated with SEAs of S. mansoni, nearly 30% of these cells became apoptotic (7). In the present study, we have shown that similar to the egg stages, the skin-stage schistosomula also may potentially contain antigens that can induce apoptosis of T cells. However, the implication of this early T cell apoptosis induced by skin-stage schistosomula may be potentially significant and alarming in that the parasite may use apoptosis as a mechanism to eliminate putative effector cells that accumulate around them in the skin and thereby gain more time to adapt in the new host. Previous histological and immunohistochemical analyses showed that mononuclear cells accumulate around challenge parasites in the skin of immunized animals (24). Despite the marked cellular reaction, very few parasites are retained in the skin (24, 31). The results from the present study suggest that this might be because the parasites potentially force the cells that accumulate around them to undergo apoptosis.
Studies by Fallon et al. (11) suggest that the dramatic switch in the cytokine response from the Th1 type to the Th2 type seen in this infection following the onset of egg laying may be due to significant apoptosis of T cells in the spleen. Especially Th1-type cells seem to be more susceptible to the parasite-induced apoptosis than Th2-type cells (20). Although we did not identify the cytokine profile of T cells that undergo apoptosis in our studies, previously, our group (12) and others (30, 31) have demonstrated that the cells isolated from the skin-draining lymph nodes of immunized mice secrete significant amounts of IFN-γ in response to mitogen or antigens from irradiated parasite. However, these cells fail to respond to normal parasites or their antigens. This selective parasite-induced down-regulation of IFN-γ secretion by T cells isolated from the skin and skin-draining lymph nodes of mice (12, 29) may be due to apoptosis of Th1-type cells (21).
Interestingly, not only schistosomes, but other parasites such as Necator americanus (34), Fasciola hepatica (35), Paragonimus westermani (36), Taenia crassiceps (37) Trypanosoma cruzi (38, 39), Leishmania donovani (40), and Cryptosporidium parvum (41) are also known to induce apoptosis of host cells. It has been suggested that the parasites may use host cell apoptosis as a survival strategy to establish infection in their host by creating a site of immune privilege around them (34). Although the mechanism of this parasite-induced apoptosis is not fully understood, it appears that the majority of these parasites induce an increase in caspase-3 activity within the host cell (14). In the present study, we have also shown that caspase-3 activity is increased in T cells following exposure of cells to the parasites or their secretions. Activation of caspase-3 usually occurs when certain specific molecules, collectively called death receptors, are triggered on the surface of lymphocytes (32). Currently, five different death receptors have been described in the literature. The best characterized death receptors are those belonging to the tumor necrosis factor (TNF) receptor family of proteins (42). These include TNF receptor-1, CD95 (Fas/APO-1), TNF receptor-related apoptosis-mediated protein, and TRAIL (TNF-related apoptosis-inducing ligand) receptors (DR-4 and DR-5). The respective ligands for these receptors are TNF, FasL (CD95L), lymphotoxin-α, and TRAIL. The general signaling pathways of apoptosis induced by these receptors appear to be similar (43). Initial attempts to identify the potential death receptors triggered by skin-stage schistosomula showed a significant increase in Fas and FasL on the surface of T cells following exposure to the parasites or their secretions. Both flow cytometry and RT-PCR results confirmed these findings. There was also an increase in the expression of Fas and FasL in the skin and skin-draining lymph nodes after infection, suggesting that the pro-apoptotic effect induced by the skin-stage schistosomula may be mediated via a Fas/FasL pathway. This contention was further validated by in vitro blocking experiments. When Fas binding to FasL was blocked using a Fas-Fc fusion protein, there was a significant reduction in ES product-induced T cell apoptosis, suggesting a central role for Fas/FasL in skin-stage schistosomula-induced T cell apoptosis in the skin. A similar FasL-mediated apoptosis of CD4+ cells may occur in egg-induced granulomatous inflammation in S. mansoni infection as well (18, 19, 22).
Binding of FasL to trimerized Fas on the surface of lymphocytes recruits the intracellular adaptor molecule FADD, which in turn forms a death-inducing signaling complex (42). This complex will activate caspase-8, which is responsible for activating all of the downstream cascade of caspases, including caspase-3 (44). Examination of the different apoptotic signaling molecules within T lymphocytes after activation with ES antigens of schistosomula showed increased expression of FADD, caspase-8, and caspase-3, clearly suggesting the sequence of apoptotic events happening within these cells. A time course study showed that upon exposure to ES antigens from normal skin-stage schistosomula, caspase-8 activity peaked first, followed by caspase-3 activity. These changes started in the cells within hours after exposure to the parasite antigens. Analysis of the skin tissue collected from the site of infection confirmed that similar changes occurred in vivo in the skin of naive or immunized mice following infection.
Activated caspases will, in turn, induce proteolysis of many substrates within the cells, including proteins involved in cell repair, cell cycle control, signal transduction, and/or structural integrity (45). Activation of caspases can also lead to proteolytic cleavage of DNA, resulting in DNA fragmentation, which can be visualized on agar gel. Exposure of lymph node cells to the parasites or their secretions in vitro causes typical DNA fragmentation, further confirming that parasite-induced pro-apoptotic changes occur in T lymphocytes.
Subset analysis of lymphocytes in the skin and skin-draining lymph nodes showed that the skin-stage schistosomula induce apoptosis of CD4+ and CD8+ T cells, but not B cells. Studies by Lundy et al. (22) also showed that >30% of CD4+ cells undergo apoptosis when they are exposed to SEAs of S. mansoni. In these studies, B cells were found not to undergo apoptosis; rather, a subset of B cells (B-1a cells) were shown to impart the death signal to SEA-stimulated CD4+ cells by expressing high levels of FasL (22, 23). Interestingly, in our studies, removal of B cells did not affect ES antigen-induced T cells apoptosis. Furthermore, because a fraction of CD3+ cells also express FasL on their surface in response to ES products, it is possible that a variety of cells are potentially induced by the parasite to express FasL for mediating T cell apoptosis.
Migration of schistosomula through the skin induces a interleukin-10 response in the skin (12). Because interleukin-10 can potentially induce apoptosis of T cells during S. mansoni infection (46), some of the pro-apoptotic activity observed in the skin may be interleukin-10-mediated. Similarly, it is possible that other receptors and adaptors in addition to Fas and FADD could be involved in this parasite-induced pro-apoptotic mechanism. Further studies may help identify whether any other redundant mechanisms are operative in the parasite-induced apoptosis.
Initial attempts to identify the characteristics of the pro-apoptotic molecule in the ES products of normal schistosomula narrowed the activity to a fraction between 3 and 30 kDa. Subsequent fractionation and analysis of the molecules within the 3–30-kDa fraction suggested that the pro-apoptotic activity of skin-stage schistosomula is associated with a protein band at ~23 kDa. Through amino acid analysis, we have identified 20 sequences at the N terminus of the 23-kDa molecule. This will allow us to perform an extensive sequence analysis of the protein and ultimately to clone the gene for the pro-apoptotic molecule. Because this molecule induced significant apoptosis of T cells, we coined the term S. mansoni-derived apoptosis-inducing factor (Smaf) for this molecule(s). Exposure of the parasites to γ-irradiation eliminated Smaf activity, and the pro-apoptotic effect of Smaf was evident only when the cells were incubated with either live parasites or their ES products. Similarly, when schistosomula were heat-inactivated (at 60 °C) and incubated with the skin-draining lymph node cells, there was a significant reduction in the parasite-induced apoptosis.2 This is the first time that the presence of a pro-apoptotic molecule in the secretions of human schistosomes has been demonstrated. We believe that the skin-stage schistosomula release Smaf into their immediate surroundings to potentially eliminate the effector cells that may accumulate around them and thus escape the damaging assaults of the host. Neutralizing the effects of Smaf may help increase development of early immune responses against invading schistosomula in the skin.
Footnotes
This work was supported by National Institutes of Health Grant AI 39066 (to K. R.). Life cycle stages of S. mansoni were obtained from Dr. Fred Lewis through NIAID Contract N01-A1-55270 from the National Institutes of Health.
The abbreviations used are: ES, excretory secretory; SEAs, soluble egg antigens; TUNEL, terminal deoxynucleotidyltransferase-mediated biotinylated UTP nick end labeling; PE, phycoerythrin; RT, reverse transcription; FADD, Fas-associated death domain; IFN-γ, interferon-γ; TNF, tumor necrosis factor; Smaf, S. mansoni-derived apoptosis-inducing factor.
L. Chen and K. Ramaswamy, unpublished data.
References
- 1.World Health Organization Expert Committee on the Control of Schistosomiasis. Bull WHO. 1993;71:657–677. [PMC free article] [PubMed] [Google Scholar]
- 2.Terry RJ, Smithers SR. Symp Soc Exp Biol. 1975:453–465. [PubMed] [Google Scholar]
- 3.Kolata G. Science. 1985;227:285–287. doi: 10.1126/science.3966154. [DOI] [PubMed] [Google Scholar]
- 4.Damian RT. Parasitology. 1997;115:S169–S175. doi: 10.1017/s0031182097002357. [DOI] [PubMed] [Google Scholar]
- 5.McKerrow JH. Parasitology. 1997;115:S107–S112. doi: 10.1017/s0031182097001765. [DOI] [PubMed] [Google Scholar]
- 6.Vieira LQ, Gazzinelli G, Kusel JR, De Souza CP, Colley DG. Parasite Immunol (Oxf) 1986;8:333–343. doi: 10.1111/j.1365-3024.1986.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 7.Carneiro-Santos P, Martins-Filho O, Alves-Oliveira LF, Silveira AM, Coura-Filho P, Viana IR, Wilson RA, Correa-Oliveira R. Parasite Immunol (Oxf) 2000;22:267–277. doi: 10.1046/j.1365-3024.2000.00294.x. [DOI] [PubMed] [Google Scholar]
- 8.Colley DG, Cook JA, Freeman GL, Jr, Bartholomew RK, Jordan P. Int Arch Allergy Appl Immunol. 1977;53:420–433. [PubMed] [Google Scholar]
- 9.Colley DG, Todd CW, Lewis FA, Goodgame RW. J Immunol. 1979;122:1447–1453. [PubMed] [Google Scholar]
- 10.Estaquier J, Marguerite M, Sahuc F, Bessis N, Auriault C, Ameisen JC. Eur Cytokine Netw. 1997;8:153–160. [PubMed] [Google Scholar]
- 11.Fallon PG, Smith P, Dunne DW. Eur J Immunol. 1998;28:1408 –1416. doi: 10.1002/(SICI)1521-4141(199804)28:04<1408::AID-IMMU1408>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 12.Kumar P, Ramaswamy K. Parasitol Int. 1999;48:109–119. doi: 10.1016/s1383-5769(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Ramaswamy K. J Vet Parasitol. 2001;15:1–12. [Google Scholar]
- 14.DosReis GA, Barcinski MA. Adv Parasitol. 2001;49:133–161. doi: 10.1016/s0065-308x(01)49039-7. [DOI] [PubMed] [Google Scholar]
- 15.Remoue F, To Van D, Schacht AM, Picquet M, Garraud O, Vercruysse J, Ly A, Capron A, Riveau G. Clin Exp Immunol. 2001;124:62–68. doi: 10.1046/j.1365-2249.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stadecker MJ, Kamisato JK, Chikunguwo SM. J Immunol. 1990;145:2697–2700. [PubMed] [Google Scholar]
- 17.de Andres B, Mueller AL, Blum A, Weinstock J, Verbeek S, Sandor M, Lynch RG. Blood. 1997;90:1267–1274. [PubMed] [Google Scholar]
- 18.Rumbley CA, Zekavat SA, Sugaya H, Perrin PJ, Ramadan MA, Phillips SM. J Immunol. 1998;161:4129–4137. [PubMed] [Google Scholar]
- 19.Rumbley CA, Sugaya H, Zekavat SA, Perrin PJ, Phillips SM. Am J Trop Med Hyg. 2001;65:442–449. doi: 10.4269/ajtmh.2001.65.442. [DOI] [PubMed] [Google Scholar]
- 20.Fallon PG, Richardson EJ, Smith P, Dunne DW. Eur J Immunol. 2000;30:470–480. doi: 10.1002/1521-4141(200002)30:2<470::AID-IMMU470>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 21.Rumbley CA, Phillips SM. Microbes Infect. 1999;1:499–504. doi: 10.1016/s1286-4579(99)80088-4. [DOI] [PubMed] [Google Scholar]
- 22.Lundy SK, Lerman SP, Boros DL. Infect Immun. 2001;69:271–280. doi: 10.1128/IAI.69.1.271-280.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundy SK, Boros DL. Infect Immun. 2002;70:812–819. doi: 10.1128/iai.70.2.812-819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramaswamy K, He YX, Salafsky B. Exp Parasitol. 1997;86:118–132. doi: 10.1006/expr.1997.4178. [DOI] [PubMed] [Google Scholar]
- 25.Ramaswamy K, Salafsky B, Potluri S, He YX, Li JW, Shibuya T. J Inflamm. 1995;46:13–22. [PubMed] [Google Scholar]
- 26.Kroemer G, Bosca L, Zamzamin N, Marchett P, Hortelano S. In: Immunology Methods Manual. Lefkovits I, editor. Vol. 2. Academic Press Ltd; London: 1997. pp. 1121–1122. [Google Scholar]
- 27.Benavides GR, Hubby B, Grosse WM, McGraw RA, Tarleton RL. J Immunol Methods. 1995;181:145–156. doi: 10.1016/0022-1759(94)00339-x. [DOI] [PubMed] [Google Scholar]
- 28.Chu CY, Tseng J. J Biomed Sci. 2000;7:58–63. doi: 10.1007/BF02255919. [DOI] [PubMed] [Google Scholar]
- 29.Betts CJ, Wilson RA. Immunology. 1998;93:49–54. doi: 10.1046/j.1365-2567.1998.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mountford AP, Coulson PS, Pemberton RM, Smythies LE, Wilson RA. Immunology. 1992;75:250–256. [PMC free article] [PubMed] [Google Scholar]
- 31.Pemberton RM, Smythies LE, Mountford AP, Wilson RA. Immunology. 1991;73:327–333. [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider P, Tschopp J. Pharm Acta Helv. 2000;74:281–286. doi: 10.1016/s0031-6865(99)00038-2. [DOI] [PubMed] [Google Scholar]
- 33.Hengartner MO. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 34.Chow SC, Brown A, Pritchard D. Parasite Immunol (Oxf) 2000;22:21–29. doi: 10.1046/j.1365-3024.2000.00271.x. [DOI] [PubMed] [Google Scholar]
- 35.O’Neill SM, Brady MT, Callanan JJ, Mulcahy G, Joyce P, Mills KH, Dalton JP. Parasite Immunol (Oxf) 2000;22:147–155. doi: 10.1046/j.1365-3024.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- 36.Shin HJ, Cho MS, Kim HI, Lee M, Park S, Sohn S, Im KI. Clin Diagn Lab Immunol. 2000;7:510 –514. doi: 10.1128/cdli.7.3.510-514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Connell KM, Rogan MT. Parasitology. 2000;120:649–655. doi: 10.1017/s0031182099005971. [DOI] [PubMed] [Google Scholar]
- 38.Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 39.Lopes MF, Nunes MP, Henriques-Pons A, Giese N, Morse HC, Davidson WF, Araujo-Jorge TC, DosReis GA. Eur J Immunol. 1999;29:81–89. doi: 10.1002/(SICI)1521-4141(199901)29:01<81::AID-IMMU81>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 40.Das G, Vohra H, Rao K, Saha B, Mishra GC. Scand J Immunol. 1999;49:307–310. doi: 10.1046/j.1365-3083.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 41.Ojcius DM, Perfettini JL, Bonnin A, Laurent F. Microbes Infect. 1999;1:1163–1168. doi: 10.1016/s1286-4579(99)00246-4. [DOI] [PubMed] [Google Scholar]
- 42.Rath PC, Aggarwal BB. J Clin Immunol. 1999;19:350–364. doi: 10.1023/a:1020546615229. [DOI] [PubMed] [Google Scholar]
- 43.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Eur J Biochem. 1998;254:439 –459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 44.Kruidering M, Evan GI. IUBMB Life. 2000;50:85–90. doi: 10.1080/713803693. [DOI] [PubMed] [Google Scholar]
- 45.Rathmell JC, Thompson CB. Annu Rev Immunol. 1999;17:781–828. doi: 10.1146/annurev.immunol.17.1.781. [DOI] [PubMed] [Google Scholar]
- 46.Estaquier J, Ameisen JC. Blood. 1997;90:1618–1625. [PubMed] [Google Scholar]