Abstract
Objectives
To determine if there were any differences in antiretroviral treatment (ART) use across the three eastern states of Australia, New South Wales, Victoria and Queensland, during the period 1997 to 2006.
Methods
We used data from a clinic-based cohort, the Australian HIV Observational Database (AHOD), to determine the proportion of HIV-infected patients on ART in selected clinics in each state and the proportion of treated patients with an undetectable viral load. Data from the national Highly Specialised Drugs program and AHOD was used to estimate total numbers of individuals on ART and the proportion of individuals living with HIV on ART nationally and by state. Data from the HIV Futures Survey and the Gay Community Periodic Survey (GCPS) were used to determine the proportion of community-based men who have sex with men (MSM) on ART. The proportion of patients with primary HIV infection (PHI) who commenced ART within one year of diagnosis was obtained from the Acute Infection and Early Disease Research Program (AIEDRP) CORE01 protocol and Primary HIV and Early Disease Research: Australian cohort (PHAEDRA) cohorts.
Results
We estimated that the numbers of individuals on ART increased from 3,181 to 4,553 in NSW, 1,309 to 1,926 in Victoria and 809 to 1615 in Queensland between 2000 and 2006. However, these numbers may reflect a lower proportion of individuals living with HIV on ART in NSW compared to the other states (37% compared to 49 and 55% in 2000). We found similar proportions of HIV-positive MSM participants were on ART in all three states over the study period in the clinic-based AHOD cohort (81-92%) and two large, community based surveys in Australia (69-85% and 49-83%) . Similar proportions of treated patients had an undetectable viral load across the three states, with a consistently increasing trend over time observed in all states. We found that more PHI patients commenced treatment in the first year following HIV diagnosis in NSW compared to Victoria; however, the sample size was very small.
Conclusions
For the most part, patterns of ART use were similar across NSW, Victoria and Queensland using a range of available data from cohort studies, community surveys and national prescription databases in Australia. However, there may be a lower proportion of individuals living with HIV on ART in NSW compared to the other states, and there is some indication of a more aggressive treatment approach with PHI patients in NSW compared to Victoria.
Introduction
HIV viral load has been shown to be the key predictor of HIV-1 transmission among heterosexual sero-discordant couples (1). HIV viral load is significantly reduced, often to levels below those detectable on available assays, in the majority of people who receive combination antiretroviral treatment (cART) (2-4). For this reason, it is plausible that patterns of antiretroviral treatment (ART) use are one factor that could influence HIV transmission in populations.
Over the past decade, differential rates of HIV notifications have been observed between New South Wales, Victoria and Queensland, the three most populated states of Australia (5, 6). The extent to which these differences may be attributed to differences in ART use between the three states remains largely unexplored. Recently, Marrone and colleagues (7) discounted their hypothesis that the states with higher notification rates would have lower proportions of ART use among their affected populations using estimates of person years of effective ART based on data from the Australian Government Highly Specialised Drugs (s100) Program and national HIV notification data. While this suggests that treatment patterns are not associated with notification rates in the three eastern states of Australia, there are a range of other data sources and methods that may shed further light on this issue, and we have utilised these in the present study.
The objective of this paper is to assess if there were any differences in ART use among people living with HIV/AIDS (PLWHA), by state in Australia between 1997 and 2006. Since the vast majority of HIV infections in Australia to date have been in people reporting male homosexual contact as their HIV exposure (5, 6), this sub-population is the primary focus in this paper.
Methods
We used a variety of data sources, including data from observational cohort studies of patients with primary or chronic HIV infection, community based, repeat cross-sectional behavioural surveys, and a national prescription dataset, to investigate ART trends across the states and territories of Australia between 1997 and 2006. All analyses were limited to the states of New South Wales (NSW), Victoria and Queensland due to small numbers of patients from the other states and territories in the existing datasets.
Australian HIV Observational Database
The Australian HIV Observational Database (AHOD) is a longitudinal, observational cohort study of patients with HIV infection in Australia, whose methods have previously been described in detail (8). Briefly, data is collected from 27 clinical sites throughout Australia, including hospitals, sexual health clinics and general medical practices. Prospective data collection for the cohort commenced in 1999, with retrospective data provided where available. All participants provided written, informed consent on enrolment to the cohort. Data are transferred electronically to the National Centre in HIV Epidemiology and Clinical Research (NCHECR) every six months. The core data collected in AHOD include: date of birth, sex, date of first positive HIV test result, date of most recent clinic visit, HIV exposure category, hepatitis B virus (HBV) and hepatitis C virus (HCV) status, CD4 and CD8 T-lymphocyte counts, HIV viral load, antiretroviral and prophylactic treatment histories (including start and stop dates), AIDS defining illness history and cause of death.
Data collected to March 31, 2007 were included in this analysis for the 20 sites that submitted complete data by this date (N=2066). To allow direct comparison with data from the Gay Community Periodic Surveys (GCPS) and HIV Futures Surveys (see below), the sample was restricted to the 75% of all patients recruited to AHOD who were male and reported male homosexual contact, with or without injecting drug use, as their HIV exposure (N=1543). Analyses were repeated on the unrestricted AHOD sample with similar results (data not shown). In this paper, we report on the proportion of patients on ART in the states of NSW, Victoria and Queensland. We also report on the proportion of patients with an undetectable viral load by treatment status, state and year. We defined an undetectable viral load as <400 copies/mL because more sensitive assays were not uniformly available throughout the study period. For the purpose of this analysis, patients were classified as being on ART if they had a treatment regimen that was longer than 14 days during a calendar year period. For these patients, the median viral load of all viral load measures recorded during the period of their longest treatment regimen in a particular calendar year was calculated. For patients classified as being off ART during a calendar year period, the median viral load of all viral load measures recorded during the same calendar year was calculated. The proportion of patients in the off ART group who had previously received ART was also calculated. Patients were then classified as having an undetectable or detectable viral load during the calendar year by this median viral load summary statistic. Patients without at least one viral load measure during the period of interest were excluded from this analysis.
Confidence intervals (95%) were calculated using binomial proportions.
Highly Specialised Drugs Program (s100) data
Data on the number of patients who were dispensed antiretroviral (ARV) drugs per state per financial year quarter reported in the Public Hospital Dispensed National Patient Report from the Australian Government's Highly Specialised Drugs (HSD) (s100) program were analysed together with data on ART use from the AHOD sample to estimate total numbers of patients on ART by state and nationally, by year. At this time, all ARV drugs in Australia are publicly funded under Section 100 (s100) of the National Health Act 1953, through the HSD program. Since patients with HIV infection generally receive three or more ARV drugs in combination, and because the s100 program only collects data on individual ARV drugs, it is not possible to enumerate directly the number of individuals receiving ART from the s100 data.
One of the commonly used ARV drugs for treatment of HIV infection is lamivudine; however, it is also used for the treatment of hepatitis B infection. As the PBS code is not included in the Public Hospital Dispensed National Patient Report, it is not possible to separate the number of patients who were dispensed lamivudine treatment for HIV from those receiving lamivudine for HBV. Therefore, we estimated the number of person years of lamivudine (100mg tablets) for HBV treatment from the Public Hospital Dispensed National Pack Number Report, which includes the PBS code, dosage and the total numbers of packs dispensed for each drug per financial quarter. To estimate the total number of individuals dispensed lamivudine for HIV treatment, we deducted the total number of person years of lamivudine treatment for HBV each year from the total number of individuals dispensed lamivudine for HIV and HBV treatment. This method is based on the assumption that the majority of individuals received a complete year of treatment during any calendar year period.
To estimate the number of individuals receiving ART, we combined data on the proportion of patients receiving certain mutually exclusive ARVs in AHOD with data from the s100 program on the total number of individuals receiving the same ARVs. For example, lamivudine and emtricitabine are a common component of combination ART regimens in Australia, but should not be prescribed in combination. We calculated the proportion of all treated patients in AHOD who received lamivudine or emtricitabine as part of an ART regimen by year and state, with 95% confidence intervals. We also estimated the total number of individuals dispensed lamivudine or emtricitabine for HIV infection each year through the s100 program by calculating the average number of individuals prescribed each drug from the corresponding four financial year quarters. An estimate of the total number of individuals receiving any ART was then obtained by dividing the total number of individuals receiving lamivudine or emtricitabine through the s100 program by the proportion of treated patients in AHOD receiving the same ARV drugs. As a sensitivity analysis, we repeated this calculation for other commonly mutually exclusive drugs, including: 1) efavirenz and nevirapine; 2) Kaletra (lopinavir and ritonavir) and ritonavir; and 3) stavudine and zidovudine containing ARVs.
Finally, we estimated the proportion of people living with diagnosed HIV or AIDS (PLWDHA) receiving ART by state and nationally as the estimated number of individuals on ART (described above) divided by the estimated number of PLWDHA. The number of PLWDHA was estimated from the National HIV Registry and National AIDS Registry which are aggregated at the National Centre in HIV Epidemiology and Clinical Research (NCHECR) on behalf of state and territory health authorities. We estimated the number of PLWDHA by state and nationally by subtracting the cumulative number of deaths following HIV or AIDS diagnosis from the cumulative number of HIV diagnoses at the 31st December each year.
Linear regression methods were used to estimate the annual change in s100 expenditure with 95% confidence intervals. P values less than 0.05 were considered significant.
Primary HIV infection cohorts
The sample of patients with primary HIV infection (PHI) included in this analysis includes patients recruited to two PHI cohorts: 1) The Acute Infection and Early Disease Research Program CORE 01 protocol, established by the National Institutes of Health (NIH), Bethesda, Maryland, USA (9); and 2) The Primary HIV and Early Disease Research: Australian Cohort (PHAEDRA), established by the National Centre in HIV Epidemiology and Clinical Research. These prospective observational cohorts were established to support basic and clinical research aiming to define the virologic, immunologic and host factors that are at play in the determination of the long-term outcome of HIV-1 infection, as well as identifying new therapeutic strategies. Patients with newly acquired HIV were recruited from six clinical sites in Sydney, NSW, and five in Melbourne, Victoria, between 2002 and 2006. Acute primary infection was defined as a negative or indeterminate serology with positive plasma viremia, and early PHI was defined as HIV seroconversion within the previous 6-12 month period (defined by either proven negative serology within that period or a positive result on a detuned ELISA). The protocols provided for rigorous laboratory-based inclusion and exclusion criteria (9). Following inclusion to the study, patients were followed over time with 6-7 study visits in the first year, quarterly visits in the second year and then 2-4 visits per year in subsequent years. Blood samples were drawn at each study visit to assess virological and immunological status, ART history and AIDS related events were recorded on specifically designed case report forms. A total of 238 patients were recruited to the CORE01 and PHAEDRA cohorts, with 186 recruited in NSW and 52 in Victoria.
HIV Futures Surveys
We report on data from the HIV Futures surveys, five Australian nationwide studies of multiple aspects of the lives of PLWHA, both clinical and social, conducted in 1997 (N=925), 1999 (N=921), 2001 (N=894) 2003 (N=1059) and 2005 (N=973). The study methods have been described elsewhere in detail (10-12). Briefly, respondents completed an anonymous, self-administered, mail-back questionnaire that included sections on demographics, accommodation, health and treatments, services and community, sex and relationships, employment, recreational drug use and finances. The sample participants were recruited through multiple clinical and community sites and many participants would have obtained multiple copies of the survey. As many questionnaires were distributed through third parties, it was not possible to calculate a response rate for the survey. Confidence intervals (95%) were calculated using binomial proportions.
Gay Community Periodic Surveys
We used data from the GCPS, cross-sectional surveys that recruit participants from a range of sites in Sydney (NSW) and Melbourne (Victoria), and in several locations in Queensland (Brisbane, Cairns and the Sunshine Cost). More detailed information on the study methodology has previously been published in study reports (http://nchsr.arts.unsw.edu.au/publications_gay.html). The GCPS have been conducted twice a year in NSW since their inception in 1996, and annually in Victoria and Queensland since 1998, with the exception of 1999 in Victoria. The GCPS participation rates have ranged between 70% and 85% over the years. A brief self-administered questionnaire was used to collect information about the HIV sero-status, risky and safer sexual practices, HIV/STI testing history, recreational drug use and socio-demographic details of participants. Since the survey commenced in 1996, the following question has been asked of all HIV positive participants: “Are you on combination antiretroviral therapy (ART)?” with a closed response of “yes” or “no” required. In this paper, we present the proportion of all HIV positive men who self-reported being on cART, by state and year. Confidence intervals (95%) were calculated using binomial proportions.
Results
AHOD
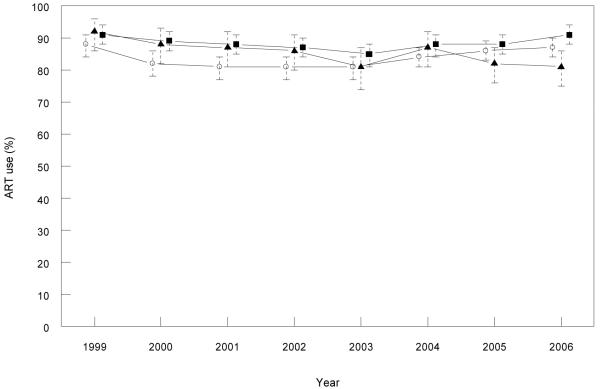
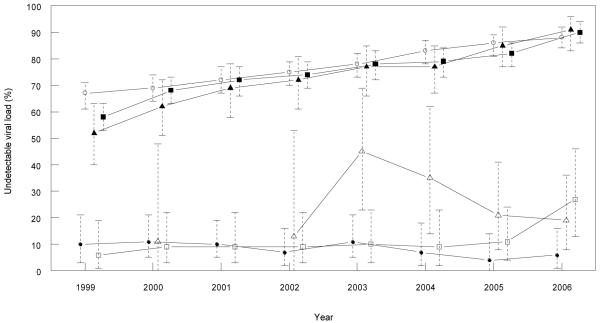
We found a similar proportion of MSM patients from the AHOD cohort were on any ART between 1999 and 2006 in each of the three eastern states (Figure 1). We observed a gradual increase in the proportion of treated patients with an undetectable viral load between 1999 and 2006 in all three eastern states, ranging from 67-87% in NSW, 39-88% in Victoria and 51-90% in Queensland (Figure 2). In the national sample, the proportion of patients with an undetectable viral load in the off ART group ranged from 10-12% between 1999 and 2002, and 15-19% between 2003 and 2006. The proportion of patients with an undetectable viral load in the off ART group in NSW and Victoria were consistently below 12% between 1999 and 2005, with an increase to 27% in Victoria in 2006 (Figure 2). There was more variation in the proportion with an undetectable viral load in off ART group in Queensland, ranging from 11% to 45% between 1999 and 2006, although the 95% confidence intervals mostly overlap with NSW and Victorian estimates. The proportion of patients in the off ART group who had received previous ART ranged from 67-98% in NSW, 67-97% in Queensland, and 77-99% in Victoria over the study period.
FIGURE 1. Antiretroviral treatment (ART) use (%) among patients under follow up in AHOD* by year and state.
*Only patients with male homosexual contact reported as their HIV exposure.
Legend: Open circles = NSW, closed squares = Victoria and closed triangles = Queensland.
FIGURE 2. Undetectable viral load (%) among patients in AHOD* by antiretroviral treatment (ART) status, year and state.
*Only patients with male homosexual contact reported as their HIV exposure.
Legend: Open circles = NSW patients on ART, closed circles = NSW patients off ART, closed squares = Victorian patients on ART, open squares = Victorian patients off ART, closed triangles = Queensland patients on ART and open triangles = Queensland patients off ART.
Highly Specialised Drugs Program (s100) data
The estimated total number of individuals on ART nationally and by state is presented in Table 1. Using the lamivudine and emtricitabine model, we found that the estimated number of individuals on ART nationally increased from 5,757 in 2000 to 9,463 in 2006. The same pattern was observed in each of the three eastern states, with increases from 3,181 to 4,553, 1,309 to 2,334 and 809 to 1,615 between 2000 and 2006 in NSW, Victoria and Queensland, respectively. A similar pattern was observed using the efavirenz and nevirapine model, although the estimated total numbers of individuals on ART were lower between 2003 and 2006 compared to the lamivudine and emtricitabine model, particularly in NSW and nationally. A gradual increase in the estimated number of individuals on ART between 2002 and 2006 was observed using the Kaletra and ritonavir model; however, it generated the lowest estimates in all years compared to the other three models, with the exception of 2006. There was variation in estimated patient numbers on ART using the stavudine and zidovudine containing ARVs model; however, there was no distinct trend over time. Consistent with our estimates of numbers of individuals receiving ART, the HSD reported increasing public hospital expenditure on HIV ARVs via the s100 program, from 66 million Australian dollars (AUD) in 2001 to 111 million AUD in 2006, with a decrease of approximately 2.5 million AUD between 2000 and 2001 (Table 2). This increase in expenditure was highly statistically significant across all three states.
Table 1.
Estimates of the total number of individuals on antiretroviral treatment annually in Australia and by state1
| Year | |||||||
|---|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | |
| Model 1 (lamivudine & emtricitabine) | |||||||
| NSW | 3,181 | 3,337 | 3,416 | 3,712 | 3,810 | 4,209 | 4,553 |
| Victoria | 1,309 | 1,388 | 1,605 | 1,809 | 1,909 | 1,926 | 2,334 |
| Queensland | 809 | 856 | 900 | 972 | 1,095 | 1,340 | 1,615 |
| Australia | 5,757 | 6,113 | 6,440 | 7,173 | 7,598 | 8,453 | 9,463 |
| Model 2 (efavirenz & nevirapine) | |||||||
| New South Wales | 3,251 | 3,297 | 3,456 | 3,421 | 3,615 | 3,684 | 3,909 |
| Victoria | 1,319 | 1,433 | 1,491 | 1,587 | 1,792 | 2,436 | 2,225 |
| Queensland | 675 | 785 | 877 | 933 | 1,072 | 1,206 | 1,345 |
| Australia | 5,946 | 6,266 | 6,683 | 6,778 | 7,263 | 8,350 | 8,669 |
| Model 3 (Kaletra & ritonavir) | |||||||
| New South Wales | 2,283 | 1,428 | 2,383 | 2,601 | 2,710 | 2,951 | 3,371 |
| Victoria | 673 | 620 | 993 | 1,090 | 1,318 | 1,390 | 1,676 |
| Queensland | 745 | 488 | 779 | 936 | 935 | 1,148 | 1,197 |
| Australia | 4,354 | 3,142 | 4,920 | 5,521 | 5,997 | 6,530 | 7,364 |
| Model 4 (stavudine & zidovudine) | |||||||
| New South Wales | 3,210 | 3,337 | 3,564 | 3,682 | 4,027 | 3,649 | 3,891 |
| Victoria | 1,501 | 1,707 | 1,595 | 1,777 | 1,943 | 1,849 | 1,742 |
| Queensland | 835 | 980 | 982 | 1,049 | 1,174 | 1,269 | 1,277 |
| Australia | 6,107 | 6,737 | 6,635 | 7,034 | 7,539 | 7,274 | 7,165 |
Data sources used in the models include s100 data from the Australian Government Highly Specialised Drugs Program and the Australian HIV Observational Database.
Table 2.
Annual expenditure ($'000sa) on HIV antiretroviral drugs dispensed via public hospitals (s100 program) in Australia and by state
| Year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | Annual increase | 95% CI | p-value | |
| NSW | 34,431 | 32,044 | 34,566 | 38,711 | 40,649 | 45,585 | 49,635 | 3912 | 1715, 3912 | 0.001 |
| Victoria | 16,204 | 16,052 | 17,586 | 19,583 | 21,472 | 25,500 | 28,623 | 2846 | 1443, 2846 | 0.001 |
| Queensland | 8,704 | 8,864 | 9,857 | 11,214 | 11,577 | 14,326 | 16,958 | 1832 | 840, 1832 | 0.001 |
| Australia | 68,483 | 65,945 | 71,470 | 79,654 | 85,293 | 98,485 | 110,512 | 7321 | 4689, 9953 | 0.001 |
Australian dollars (AUD)
Source of annual expenditure data: Australian Government Department of Health and Ageing, Highly Specialised Drugs (s100) program
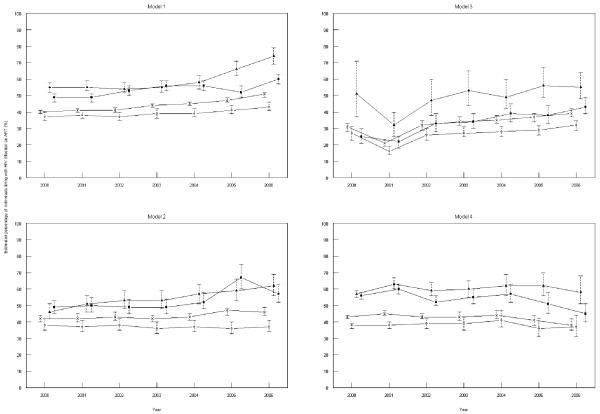
The estimated number of PLWDHA in Australia increased from 14260 in 2000 to 18735 in 2006, with a similar increasing trend observed across all states. We observed an increase over time in the estimated proportion of PLWDHA on ART in all states and nationally using the estimated total number of individuals on ART from the lamivudine and emtricitabine model, while there was more variation over time among the other models (Figure 3). The state of NSW had the lowest estimated proportion of PLWDHA on ART using estimated numbers of individuals on ART from all models. There was an increase in the estimated proportion of PLWDHA on ART in Victoria and Queensland over time using the efavirenz and nevirapine model, while the estimates remained relatively stable in NSW using the same model.
Figure 3. Estimated percentage of individuals living with HIV on antiretroviral treatment (ART) annually in Australia and by state.
Legend: Open circles = NSW, closed squares = Victoria and closed triangles = Queensland, crosses = Australia.
PHI cohorts
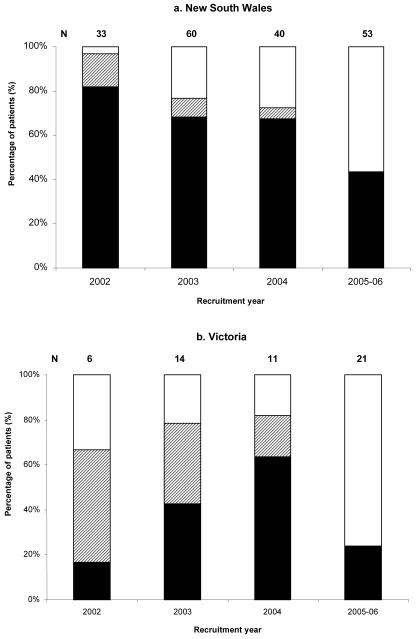
Analysis of the total number of patients who commenced treatment from 2002-2006 in the PHI cohorts shows that in NSW, 91% (118/130) were treated in the first year of diagnosis in NSW compared to 51% (15/29) in Victoria (Figure 4). There was a substantial decrease in the proportion of PHI patients in NSW who commenced treatment since cohort recruitment from 82% in 2002 (N=33) to 43% in 2005-2006 (N=53); however, the time since HIV diagnosis and cohort recruitment, and therefore the period of follow up, is less among those recruited to the cohort in 2005-2006 compared to 2002. In Victoria, there was an increase in the proportion of PHI patients who commenced treatment since cohort recruitment from 17% in 2002 (N=6) to 64% in 2004 (N=11), followed by a decrease to 24% in 2005-2006 (N=21).
Figure 4. Treatment status of patients recruited to the primary HIV infection cohorts.
Legend: Black represents patients who commenced ART within one year of their first positive ELISA test; diagonal lines represent patients who commenced ART more than one year after their first positive ELISA test; and white represents patients who have not commenced ART.
HIV Futures Surveys
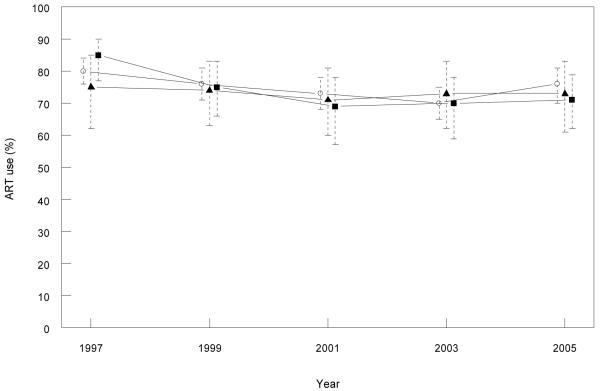
The largest numbers of participants recruited to the HIV Futures Surveys were in NSW, ranging between 288 and 362 across the survey years, followed by Victoria (85 to 123) and Queensland (59 to 84). A similar proportion of participants self-reported any ART use across the three states over the survey years (Figure 5).
Figure 5. Self reported current antiretroviral treatment (ART) use (%) among participants in the HIV Futures Survey* by state and year.
*Only patients reporting male homosexual contact.
Legend: Open circles = NSW, closed squares = Victoria and closed triangles = Queensland.
Gay Community Periodic Surveys
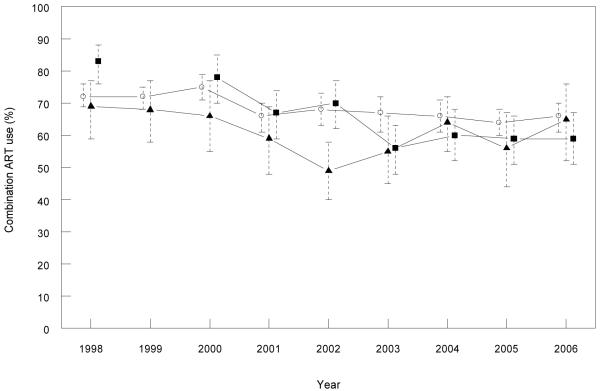
The number of HIV positive participants ranged between 330 and 606, 138 and 177, and 68 and 122 across the survey years in NSW, Victoria and Queensland, respectively. Overall, there was little difference in the proportion on cART across the three states. A decrease in the proportion of MSM self-reporting cART use in the GCPSs was observed in both Victoria (from 83% to 59%) and NSW (from 72% to 66%) across the survey years (p<0.001 in both states) (Figure 6). cART decreased in Queensland from 1998 to 2002, then increased through to 2006. While there was variation in the proportion of participants recruited via clinics over time, their exclusion from this analysis did not appreciably alter results (data not shown).
Figure 6. Self reported combination antiretroviral treatment (ART) use (%) among participants in the Gay Community Periodic Survey by state and year.
*Only patients reporting male homosexual contact.
Legend: Open circles = NSW, closed squares = Victoria and closed triangles = Queensland.
Discussion
Our estimates show an increasing numbers of individuals on ART between 2000 and 2006 in all three states, as well as nationally, in line with increases in government expenditure on ART over the same time period. While we found no difference in ART use between NSW, Victoria and Queensland among patients in the AHOD cohort, or survey participants in the HIV Futures Survey or GCPS, and consistent trends in the proportion of treated patients in AHOD with viral suppression in all three states, our model estimates indicate that there may be a smaller proportion of individuals living with HIV on ART in NSW compared to the other two states. Another difference in ART use emerged from analysis of the PHI research cohort data, with more patients commencing ART in the first year following HIV diagnosis in NSW compared to Victoria in that study.
Our finding of a consistent increase in estimated numbers of individuals on ART over time in all three states and nationally is in contrast to recently reported estimates of effective person years of ART in another Australian study (7). Marrone and colleagues reported a decreasing trend in effective person years of ART in NSW and Queensland between 1999 and 2005 and no trend over time in national estimates. Differences in methodology are likely to account for much of the difference in findings between the two studies. The estimates generated in the Marrone et al study were based entirely on the s100 data source, including data on the number of tablets, capsules and volume of suspensions of each ARV drug dispensed by year and state, and assumptions regarding average treatment combinations. Our estimates were based on numbers of patients prescribed certain mutually exclusive drugs using AHOD to estimate the proportion of patients receiving those drugs. While there are weaknesses in both analyses, it is notable that the increase in estimated numbers of individuals on ART over time in each state in our study correlates well with the increase in total drug costs by state in the s100 program.
In our analyses of the s100 data, three of the four models used to estimate total numbers of individuals on ART showed increasing estimated numbers over the study period, with the exception of a decrease from 2004-2006 using the stavudine/zidovudine model. This is likely to be related to new developments in ARVs in recent years and the subsequent shift away from zidovudine and stavudine based regimens. Furthermore, AHOD, which is based on a very treatment experienced cohort, may not have accurately captured these population level trends in ART use. Interestingly, the decrease in estimated total patient numbers on ART from 2000 to 2001 using the Kaletra/ritonavir model corresponds temporally to the decrease of approximately 2.5 million AUD in public hospital dispensed HIV ARV expenditure, while none of the other models produced a decrease in patient numbers during the same time period. However, overall our analyses of the s100 data suggest a consistent pattern of increasing numbers of individuals on ART across all three states. Of note is the lower estimated proportion of individuals living with HIV on ART in NSW compared to Victoria and Queensland, which is consistent with estimates from a recent ecological study by Marrone and colleagues (7). This may be explained by the challenges in accurately estimating the number of PLWDHA in each state, including duplicate reporting, under ascertainment of deaths, and no allowance for migration between states. These factors could all lead to overestimates of the numbers of PLWDHA in NSW, with underestimates in Victoria and particularly Queensland. In particular, due to difficulties in accurately tracing individuals in national surveillance registries in Australia individuals remain attributed to the state of their first diagnosis, and are not reclassified on migration. This overestimation of PLWDHA in NSW, and underestimation in Victoria and Queensland, could have contributed at least in part to the larger estimates of the proportions of PLWDHA receiving ARVs in the latter two states, because s100 estimates of people receiving ARVs would be unbiased by migration. It is also possible that differential rates of new HIV diagnoses across the three states may have contributed to the observed differences in estimated proportions of individuals living with HIV on ART over the study period.
One of the limitations of the public hospital dispensed patient number report from the s100 program was the lack of distinction between the numbers of patients who were dispensed lamivudine for the treatment of HIV compared to HBV. To overcome this, we estimated the number of person years of lamivudine for HBV treatment from the public hospital dispensed national pack number report, and subsequently deducted this figure from the overall number of patients who were dispensed lamivudine for HIV and HBV. This operates on the assumption that the majority of patients received a complete year of treatment during any calendar year period. We conclude that in the absence of data on the distribution of treatment duration for HBV, this method produces a reasonably accurate estimate of the total number of patients who were dispensed lamivudine for HIV. Further, when conducting sensitivity analyses, this method produced a similar estimate of person years of treatment using adefovir compared to the numbers of patients who were reportedly dispensed adefovir via the s100 program.
There was no observed difference in ART use between the three states over time in the AHOD study, HIV Futures Survey or the GCPS, with the exception of a lower proportion on ART in Queensland in 2002 in the GCPS. We found a higher proportion of MSM patients on any ART in the AHOD cohort compared to the HIV Futures Survey. This is probably explained by differences in the study samples. AHOD is entirely comprised of people attending clinics for the management of HIV disease and is a highly treatment experienced cohort at this point in time. In comparison, the HIV Futures Survey and GCPS are both primarily community based samples of MSM, and so may include men not attending clinics who are not on treatment. While the proportion of patients on ART over time was relatively consistent and similar across all three states in the HIV Futures Survey, there was more variation in the proportion on cART in the GPCS over time. The reason for this is uncertain but again may reflect differences in survey sampling methods.
There were a similar and increasing proportion of treated patients with viral suppression between 1999 and 2006 in AHOD in NSW, Victoria and Queensland. This may reflect that consistent and increasingly effective treatment strategies were being employed in clinical settings in all three states over the study period, but also may be related to the majority of AHOD patients being recruited between 1999 and 2002 and therefore representing a very highly treated subgroup of all patients receiving ARVs. The small proportion of patients with an undetectable viral load in the off ART group can be explained by the fact that the vast majority of patients who were off ART in any calendar year period had previously used ART. Further, the proportion that had previously used ART increased over time in each state, reflecting the ageing nature and treatment experience of the cohort. Given the AHOD study has not historically collected data on the reasons why patients cease ART or change their treatment regimen, we are unable to provide any further analysis of why patients in the off ART group ceased treatment. However, in light of the previous treatment experience of many patients in the off ART group and the popularity of structured treatment interruptions as a treatment strategy at the time of data collection, it is plausible that some of these patients stopped ART because they achieved virological suppression and decided to take a break from treatment or for a reason other than virological failure.
There is little published data to date on treatment patterns in Australia among PHI patients. Data from the PHI cohorts in this paper suggest that there may have been a more aggressive approach to commencing ART in NSW compared to Victoria over the study period, which may have impacted on the number of new cases of HIV infection in each state. However, the results should be interpreted with caution since the number of patients recruited in Victoria was low, and no patients were recruited from other states in Australia.
In conclusion, for the most part the patterns of ART use was similar across the states of NSW, Victoria and Queensland using a range of available data from cohort studies, community surveys and national prescription databases in Australia. However, our estimates suggest there may be a lower estimated proportion of individuals living with HIV on ART in NSW compared to the other states over the study period as well as some indication of a more aggressive treatment approach with PHI patients in NSW compared to Victoria. While challenging to collect, data on migration of individuals with HIV infection between states would allow more firm conclusions to be drawn regarding reasons why there are differing estimated proportions on ART across the three states. Further, the possibility to link ARV drugs taken by individuals in the prescription dataset would allow us to determine the total number of patients dispensed ART in Australia and each state in any calendar year, and consequently, considerably improve estimates of the proportion of PLWDHA on ART in Australia and each state. Finally, further investigation of treatment trends across Australia in patients with primary infection are needed to establish if indeed there is a difference in early treatment strategies between the states.
Acknowledgements
The Australian HIV Observational Database is funded as part of the Asia Pacific HIV Observational Database, a program of The Foundation for AIDS Research, amfAR, and is support in part by a grant from the U.S. National Institutes of Health's National Institute of Allergy and Infectious Diseases (Grant No. U01-AI069907). The authors would like to thank participating AHOD sites and steering committee members (Appendix 1) and all patients who participated in this study. We acknowledge the Australian Government Highly Specialised Drugs program for provision of the s100 data on antiretroviral treatment dispensed in Australia. The contribution of the National Blood Borne Virus and Sexually Transmissible Infections Surveillance Committee to monitoring new diagnoses of HIV/AIDS and its outcome in Australia is gratefully acknowledged. The National Institute of Allergy and Infectious Diseases Division of AIDS through the Acute HIV Infection and Early Disease Research Program (AIEDRP) funded the study entitled “Pathogenesis and Treatment of Acute HIV Infection” (Grant No. 5 UOI A152403-04) which provided the data for the primary HIV infection analysis. The authors would like to thank the members of the Primary Infection Advisory Committee, including Don Smith, Pat Grey, Tim Ramacciotti, Rob Finlayson, Mark Bloch, Rob McFarland, Nick Medland, Norm Roth, Cassy Workman, Andrew Carr, David Cooper, John Kaldor, John Murray and Anthony Kelleher, and the participating clinics: Taylor Square Private Clinic, 407 Doctors, AIDS Research Initiative, Holdsworth House, St. Vincent's Hospital, Sydney Sexual Health Clinic, The Burwood Street Practice, The Centre Clinic, Prahran Market Clinic, Carlton Clinic, The Alfred Hospital, and the Melbourne Sexual Health Centre. They also express their gratitude to the participants of this study. The HIV Futures Survey was funded by the Australian Government Department of Health and Ageing. The authors of this paper acknowledge the contribution of co-investigators and coauthors on the five HIV Futures studies. The Gay Community Periodic Survey in NSW was funded by the New South Wales Health Department, in Victoria by Victoria Department of Human Services, and in Queensland by Queensland Health. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales.
Appendix 1: The Australian HIV Observational Database
Asterisks indicate steering committee members in 2006-2007.
New South Wales: D Ellis, General Medical Practice, Coffs Harbour; M Bloch, T Franic, S Agrawal, Holdsworth House General Practice, Darlinghurst; D Allen, Holden Street Clinic, Gosford; D Smith, C Mincham, Lismore Sexual Health & AIDS Services, Lismore; D Baker*, R Vale, 407 Doctors, Surry Hills; C O'Connor; Royal Prince Alfred Hospital Sexual Health, Camperdown; E Jackson, D Hunter, K McCallum, Blue Mountains Sexual Health and HIV Clinic, Katoomba; M Gotowski, S Taylor, L Stuart-Hill, Bligh Street Clinic, Tamworth; D Cooper, A Carr, M Lacey, K Hesse, St Vincent's Hospital, Darlinghurst; R Finlayson, I Prone, Taylor Square Private Clinic, Darlinghurst; MT Liang, Nepean Sexual Health and HIV Clinic, Penrith; K Brown, N Skobalj, Illawarra Sexual Health Clinic, Warrawong; L Wray, H Lu, Sydney Sexual Health Centre, Sydney; Dubbo Sexual Health Centre, Dubbo; P Canavan*, National Association of People living with HIV/AIDS; C Lawrence*, National Aboriginal Community Controlled Health Organisation; I Zablotska*, National Centre in HIV Social Research, University of NSW; B Mulhall*, School of Public Health, University of Sydney; M Law*, K Petoumenos*, K Falster, National Centre in HIV Epidemiology and Clinical Research, University of NSW.
Northern Territory: A Kulatunga, P Knibbs, Communicable Disease Centre, Royal Darwin Hospital, Darwin.
Queensland: J Chuah*, D Lester, W Fankhauser, B Dickson, Gold Coast Sexual Health Clinic, Miami; D Russell, J Leamy, C Remington, Cairns Sexual Health Service, Cairns; D Sowden, A Walker*, Clinic 87, Sunshine Coast & Cooloola HIV Sexual Health Service, Nambour; D Orth; D Youds, Gladstone Road Medical Centre, Highgate Hill; M Kelly, P Negus, H Magon, AIDS Medical Unit, Brisbane.
South Australia: W Donohue, A Lohmeyer, The Care and Prevention Programme, Adelaide University, Adelaide.
Victoria: J Anderson, P Cortissos, The Carlton Clinic, Carlton; NJ Roth*, J Nicholson, Prahran Market Clinic, South Yarra; T Read, J Silvers, Melbourne Sexual Health Centre, Melbourne; A Mijch, J Hoy, K Watson*, M Bryant, The Alfred Hospital, Melbourne; I Woolley, Monash Medical Centre, Clayton.
Western Australia: S Mallal, C Forsdyke, S Bulgannawar, Department of Clinical Immunology, Royal Perth Hospital, Perth.
Table A1.
Proportion of patients in the Australian HIV Observational Database on one of a mutually exclusive set of antiretroviral therapies (ARV) annually, nationally and by state
| Mutually exclusive ARVs | Year | |||||||
|---|---|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | ||
| Lamivudine or emtricitabine containing ARVs | NSW | 0.69 | 0.69 | 0.68 | 0.68 | 0.73 | 0.76 | 0.79 |
| Victoria | 0.80 | 0.80 | 0.76 | 0.75 | 0.76 | 0.77 | 0.78 | |
| Queensland | 0.81 | 0.85 | 0.84 | 0.80 | 0.78 | 0.78 | 0.76 | |
| National | 0.78 | 0.79 | 0.77 | 0.75 | 0.77 | 0.78 | 0.79 | |
| Efavirenz or nevirapine | NSW | 0.52 | 0.53 | 0.52 | 0.54 | 0.54 | 0.54 | 0.53 |
| Victoria | 0.62 | 0.60 | 0.55 | 0.57 | 0.56 | 0.57 | 0.55 | |
| Queensland | 0.59 | 0.64 | 0.59 | 0.58 | 0.60 | 0.56 | 0.55 | |
| National | 0.55 | 0.56 | 0.53 | 0.55 | 0.56 | 0.55 | 0.53 | |
| Kaletra or ritonavir | NSW | 0.27 | 0.39 | 0.4 | 0.45 | 0.49 | 0.5 | 0.52 |
| Victoria | 0.25 | 0.28 | 0.32 | 0.36 | 0.37 | 0.42 | 0.43 | |
| Queensland | 0.14 | 0.19 | 0.24 | 0.27 | 0.35 | 0.38 | 0.43 | |
| National | 0.23 | 0.3 | 0.34 | 0.38 | 0.41 | 0.44 | 0.46 | |
| Stavudine or zidovudine containing ARVs | NSW | 0.81 | 0.71 | 0.61 | 0.49 | 0.42 | 0.35 | 0.24 |
| Victoria | 0.90 | 0.83 | 0.76 | 0.66 | 0.56 | 0.50 | 0.41 | |
| Queensland | 0.90 | 0.83 | 0.76 | 0.67 | 0.57 | 0.53 | 0.43 | |
| National | 0.88 | 0.79 | 0.72 | 0.61 | 0.53 | 0.46 | 0.35 | |
Table A2.
Number of individuals in New South Wales dispensed antiretroviral treatment (ART) through the Highly Specialised Drugs Program by year and antiretroviral agent
| Year ARV drugs dispensed1 | |||||||
|---|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | |
| NRTIs | |||||||
| Abacavir | 636 | 825 | 822 | 831 | 872 | 856 | 448 |
| Didanosine | 726 | 660 | 772 | 712 | 673 | 483 | 320 |
| Emtricitabine | n/a | n/a | n/a | n/a | n/a | 145 | 90 |
| Lamivudine2 | 1,469 | 1,441 | 1,363 | 1,554 | 1,803 | 2,028 | 1,191 |
| Stavudine | 1,675 | 1,338 | 1,076 | 711 | 476 | 290 | 154 |
| Tenofovir | n/a | n/a | 436 | 909 | 1,253 | 1,630 | 1,237 |
| Zalcitabine | 52 | 44 | 34 | 22 | 13 | 7 | 3 |
| Zidovudine | 200 | 171 | 138 | 123 | 237 | 107 | 88 |
| Lamivudine & Zidovudine | 726 | 813 | 772 | 728 | 754 | 700 | 530 |
| Abacavir & Lamivudine | n/a | n/a | n/a | n/a | n/a | 145 | 728 |
| Abacavir & Lamivudine & Zidovudine | n/a | 49 | 188 | 243 | 225 | 181 | 162 |
| Tenofovir & Emtricitabine | n/a | n/a | n/a | n/a | n/a | n/a | 897 |
| NNRTIs | |||||||
| Delavirdine | 37 | 43 | 26 | 18 | 13 | 8 | 5 |
| Efavirenz | 544 | 572 | 648 | 748 | 872 | 976 | 1,065 |
| Nevirapine | 1,147 | 1,176 | 1,150 | 1,100 | 1,080 | 1,013 | 1,007 |
| PIs | |||||||
| Amprenavir | n/a | n/a | 74 | 70 | 48 | 21 | 9 |
| Atazanavir | n/a | n/a | n/a | n/a | 323 | 642 | 857 |
| Indinavir | 645 | 511 | 385 | 239 | 160 | 101 | 66 |
| Fosamprenavir | n/a | n/a | n/a | n/a | n/a | 66 | 109 |
| Kaletra (Lopinavir & ritonavir) | n/a | n/a | 478 | 774 | 837 | 800 | 838 |
| Nelfinavir | 584 | 435 | 323 | 227 | 168 | 103 | 64 |
| Ritonavir | 617 | 557 | 476 | 397 | 491 | 676 | 916 |
| Saquinavir | 501 | 426 | 361 | 277 | 229 | 169 | 128 |
| FI | |||||||
| Enfuvirtide | n/a | n/a | n/a | n/a | 21 | 75 | 81 |
We have estimated the number of people dispensed each antiretroviral drug during a calendar year period by calculating the average of the total number of patients dispensed each drug during the corresponding finanical year quarters
The number of patients on lamivudine per calendar year is estimated by deducting the number of patient years of lamivudine for HBV treatment (calculated from the national pack numbers report) from the total number of patients dispensed lamivudine for the purpose of HIV & HBV treatment; n/a indicates that the data or the ARV drug were not available.
FI, fusion inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; PI, protease inhibitors.
Source: Australian Government Highly Specialised Drugs (S100) Program.
Table A3.
Number of individuals in Victoria dispensed antiretroviral treatment (ART) through the Highly Specialised Drugs Program by year and antiretroviral agent
| Year ARV drugs dispensed1 | |||||||
|---|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | |
| NRTIs | |||||||
| Abacavir | 198 | 316 | 273 | 304 | 331 | 344 | 181 |
| Didanosine | 291 | 288 | 289 | 231 | 259 | 194 | 142 |
| Emtricitabine | n/a | n/a | n/a | n/a | n/a | 37 | 19 |
| Lamivudine2 | 681 | 623 | 563 | 592 | 694 | 734 | 442 |
| Stavudine | 764 | 629 | 471 | 336 | 265 | 156 | 99 |
| Tenofovir | n/a | n/a | 210 | 392 | 480 | 668 | 609 |
| Zalcitabine | 35 | 28 | 17 | 8 | 6 | 5 | 2 |
| Zidovudine | 220 | 301 | 84 | 72 | 66 | 57 | 51 |
| Lamivudine & Zidovudine | 367 | 451 | 482 | 536 | 558 | 548 | 436 |
| Abacavir & Lamivudine | n/a | n/a | n/a | n/a | n/a | n/a | 456 |
| Abacavir & Lamivudine & Zidovudine | n/a | 37 | 176 | 229 | 199 | 164 | 129 |
| Tenofovir & Emtricitabine | n/a | n/a | n/a | n/a | n/a | n/a | 339 |
| NNRTIs | |||||||
| Delavirdine | 4 | 11 | 10 | 11 | 13 | 9 | 10 |
| Efavirenz | 235 | 243 | 217 | 250 | 297 | 363 | 489 |
| Nevirapine | 583 | 616 | 603 | 655 | 706 | 1,025 | 735 |
| PIs | |||||||
| Amprenavir | n/a | n/a | 37 | 43 | 30 | 8 | 3 |
| Atazanavir | n/a | n/a | n/a | n/a | 110 | 253 | 407 |
| Indinavir | 259 | 226 | 164 | 106 | 86 | 51 | 36 |
| Fosamprenavir | n/a | n/a | n/a | n/a | 2 | 37 | 56 |
| Kaletra (Lopinavir & ritonavir) | n/a | n/a | 184 | 271 | 330 | 330 | 329 |
| Nelfinavir | 196 | 165 | 119 | 98 | 82 | 56 | 33 |
| Ritonavir | 168 | 174 | 134 | 122 | 158 | 254 | 392 |
| Saquinavir | 173 | 143 | 91 | 66 | 53 | 39 | 37 |
| FI | |||||||
| Enfuvirtide | n/a | n/a | n/a | n/a | 19 | 47 | 53 |
We have estimated the number of people dispensed each antiretroviral drug during a calendar year period by calculating the average of the total number of patients dispensed each drug during the corresponding finanical year quarters
The number of patients on lamivudine per calendar year is estimated by deducting the number of patient years of lamivudine for HBV treatment (calculated from the national pack numbers report) from the total number of patients dispensed lamivudine for the purpose of HIV & HBV treatment; n/a indicates that the data or the ARV drug were not available.
FI, fusion inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; PI, protease inhibitors.
Source: Australian Government Highly Specialised Drugs (S100) Program.
Table A4.
Number of individuals in Queensland dispensed antiretroviral treatment (ART) through the Highly Specialised Drugs Program by year and antiretroviral agent
| Year ARV drugs dispensed1 | |||||||
|---|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | |
| NRTIs | |||||||
| Abacavir | 122 | 162 | 145 | 140 | 156 | 164 | 97 |
| Didanosine | 147 | 165 | 168 | 202 | 177 | 120 | 94 |
| Emtricitabine | n/a | n/a | n/a | n/a | n/a | 26 | 28 |
| Lamivudine2 | 376 | 364 | 344 | 320 | 363 | 424 | 292 |
| Stavudine | 418 | 383 | 274 | 191 | 128 | 87 | 59 |
| Tenofovir | n/a | n/a | 146 | 236 | 324 | 463 | 396 |
| Zalcitabine | 19 | 16 | 11 | 3 | 1 | 1 | n/a |
| Zidovudine | 54 | 67 | 60 | 54 | 50 | 50 | 48 |
| Lamivudine & Zidovudine | 280 | 331 | 309 | 341 | 392 | 438 | 364 |
| Abacavir & Lamivudine | n/a | n/a | n/a | n/a | n/a | 59 | 187 |
| Abacavir & Lamivudine & Zidovudine | n/a | 33 | 104 | 117 | 99 | 99 | 79 |
| Tenofovir & Emtricitabine | n/a | n/a | n/a | n/a | n/a | n/a | 277 |
| NNRTIs | |||||||
| Delavirdine | 16 | 14 | 11 | 7 | 4 | 2 | 1 |
| Efavirenz | 109 | 143 | 163 | 204 | 258 | 289 | 355 |
| Nevirapine | 290 | 360 | 354 | 338 | 385 | 387 | 384 |
| PIs | |||||||
| Amprenavir | n/a | n/a | 9 | 10 | 6 | 3 | 1 |
| Atazanavir | n/a | n/a | n/a | n/a | 131 | 231 | 287 |
| Indinavir | 151 | 119 | 84 | 57 | 34 | 28 | 21 |
| Fosamprenavir | n/a | n/a | n/a | n/a | n/a | 5 | 9 |
| Kaletra (Lopinavir & ritonavir) | n/a | n/a | 118 | 159 | 180 | 218 | 234 |
| Nelfinavir | 175 | 152 | 109 | 90 | 64 | 48 | 26 |
| Ritonavir | 104 | 93 | 69 | 94 | 148 | 219 | 281 |
| Saquinavir | 124 | 94 | 80 | 68 | 65 | 48 | 35 |
| FI | |||||||
| Enfuvirtide | n/a | n/a | n/a | n/a | 9 | 25 | 32 |
We have estimated the number of people dispensed each antiretroviral drug during a calendar year period by calculating the average of the total number of patients dispensed each drug during the corresponding finanical year quarters
The number of patients on lamivudine per calendar year is estimated by deducting the number of patient years of lamivudine for HBV treatment (calculated from the national pack numbers report) from the total number of patients dispensed lamivudine for the purpose of HIV & HBV treatment; n/a indicates that the data or the ARV drug were not available.
FI, fusion inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; PI, protease inhibitors.
Source: Australian Government Highly Specialised Drugs (S100) Program.
Footnotes
Potential conflicts of interest ML has received consultancies and travel grants from CSL, GlaxoSmithKline, Janssen-Cilag, Johnson & Johnson Research and Roche. JC has served on advisory boards for Gilead, Pfizer, MSD & Jannsen-Cilag and has previously received unconditional educational grants to attend conferences from the following: GSK, BMS, Boehringer Ingelheim, Roche, MSD & Abbott. In the past 12 months, JA has received consultancies, speaker fees and/or been an advisory board member for the Australian Department of Immigration and Citizenship, Gilead, MSD, Tibotec and Pfizer.
References
- 1.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 2.Amin J, Moore A, Carr A, et al. Combined analysis of two-year follow-up from two open-label randomized trials comparing efficacy of three nucleoside reverse transcriptase inhibitor backbones for previously untreated HIV-1 infection: OzCombo 1 and 2. HIV Clin Trials. 2003;4:252–61. doi: 10.1310/K2U9-QC2V-1Y3V-5DYF. [DOI] [PubMed] [Google Scholar]
- 3.Carr A, Chuah J, Hudson J, et al. A randomised, open-label comparison of three highly active antiretroviral therapy regimens including two nucleoside analogues and indinavir for previously untreated HIV-1 infection: the OzCombo1 study. AIDS. 2000;14:1171–80. doi: 10.1097/00002030-200006160-00014. [DOI] [PubMed] [Google Scholar]
- 4.Garcia F, Romeu J, Grau I, et al. A randomized study comparing triple versus double antiretroviral therapy or no treatment in HIV-1-infected patients in very early stage disease: the Spanish Earth-1 study. AIDS. 1999;13:2377–88. doi: 10.1097/00002030-199912030-00009. [DOI] [PubMed] [Google Scholar]
- 5.Guy RJ, McDonald A, Bartlett M, et al. HIV diagnoses in Australia: diverging epidemics within a low-prevalence country. Med J Aust. 2007;187:1–4. doi: 10.5694/j.1326-5377.2007.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 6.National Centre in HIV Epidemiology and Clinical Research . HIV/AIDS, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2006. National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, NSW; Australian Institute of Health and Welfare, Canberra, ACT; 2006. [Google Scholar]
- 7.Marrone J, Fairley CK, Chen M, Hocking JS. Comparisons of trends in antiretroviral use and HIV notification rates between three Australian states. Aust N Z J Public Health. 2007;31:131–4. doi: 10.1111/j.1753-6405.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- 8.Australian HIV Observational Database Rates of combination antiretroviral treatment change in Australia, 1997-2000. HIV Med. 2002;3:28–36. doi: 10.1046/j.1464-2662.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- 9.Hect FM, Wang L, Collier A, et al. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis. 2006;194:725–33. doi: 10.1086/506616. [DOI] [PubMed] [Google Scholar]
- 10.Grierson JW, de Visser R, Bartos M. More cautious, more optimistic: Australian people living with HIV/AIDS, 1997-1999. Int J STD AIDS. 2001;12:670–6. doi: 10.1258/0956462011923903. [DOI] [PubMed] [Google Scholar]
- 11.Grierson JW, Pitts MK, Misson S. Health and wellbeing of HIV-positive Australians: findings from the third national HIV Futures Survey. Int J STD AIDS. 2005;16:802–6. doi: 10.1258/095646205774988190. [DOI] [PubMed] [Google Scholar]
- 12.Grierson JW, Pitts MK, Thorpe RD. State of the (positive) nation: findings from the fourth national Australian HIV futures survey. Int J STD AIDS. 2007;18:622–5. doi: 10.1258/095646207781568510. [DOI] [PubMed] [Google Scholar]