Abstract
Amyotrophic Lateral Sclerosis (ALS) is a fatal neurological disorder characterized by degeneration of motor neurons throughout the central nervous system. Mutations of the free radical scavenging enzyme superoxide dismutase-1 (SOD1) are a cause of familial ALS but it is not known how mutations lead to cell death. Free radicals such as nitric oxide (NO) are thought to play a key pathogenic role. NO is synthesized by NO synthases (NOSs) from arginine, which is a rate-limiting factor for NO production. We found that neuronal NOS (nNOS)-positive motor neurons were depleted while inducible NOS (iNOS)-positive activated glial cells were increased in transgenic mtSOD1 (G93A) ALS mice. iNOS expression was up regulated consistent with the increases of motor neuron loss and glial activation and citrulline and NO levels while nNOS expression was decreased in G93A ALS mice. Administration of L-arginine to G93A mice reduced the severity of motor neuron depletion and glial activation. In treated animals, nNOS expression was preserved while citrulline and NO were reduced, possibly due to reduced activation of glia expressing iNOS. Our findings show that high concentrations of NO correlate with iNOS expression rather than nNOS expression in G93A ALS mice. This suggests that therapy focused on iNOS inhibition might be a fruitful direction for future ALS therapeutic trials.
Keywords: amyotrophic lateral sclerosis (ALS), nitric oxide synthase (NOS), nitric oxide, astrocytes, motor neuron, L-arginine
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by selective degeneration of motor neurons located in the spinal cord, brain stem, and motor cortex, resulting in progressive atrophy and paralysis of limb, bulbar, and respiratory muscles [1–3]. Mutations of the free radical scavenging enzyme superoxide dismutase-1 (SOD1) are a cause of familial ALS. Transgenic mice expressing human mutant superoxide dismutase (mtSOD1) develop age-dependent clinical and pathological features closely mirroring those found in human ALS and thus provide a comprehensive model to study pathogenic mechanisms that may underlie the human disease [4–7]. In ALS, oxidative damage of spinal cord proteins occurs (8) and motor neurons are particularly vulnerable to oxidative stress, a phenomena attributed to low levels of antioxidant enzymes, a high content of easily oxidized substrates, and an inherently high flux of ROS generated during energy metabolism [1–3]. The free radical nitric oxide (NO) has been shown to be cytotoxic in ALS animal models [9–11]. NO is derived from the terminal nitrogen atom of L-arginine through the catalytic activity of NO synthase (NOS). Three major NOSs, neuronal NOS (nNOS/NOS1), inducible NOS (iNOS/NOS2) and endothelial NOS (eNOS/NOS3), are encoded by separate genes. Even though it is widely accepted that NO and nitrotyrosylation of macromolecules trigger the death of motor neurons, it still remains to be determined what type of NOS is specifically linked to the pathogenesis of ALS. As expected, we found that the net amount of NO was increased mtSOD1 (G93A) ALS mice. iNOS gene expression was strongly induced in astrocytes while nNOS gene expression was significantly reduced in motor neurons of mtSOD1 (G93A) ALS mice. The administration of L-arginine to mtSOD1 (G93A) ALS mice reduced neuronal loss, glial activation, iNOS expression and NO concentrations while nNOS levels were preserved at near normal levels.
MATERIALS AND METHODS
Motor neuron culture
The procedure for motor neuron culture was based on previously described methods with several modifications [12–14]. Spinal motor neurons were prepared from mouse embryo (embryonic day 12.5 [E12.5]) spinal cord by a combination of BSA cushion and Opti-prep (Axis Shield PoC, Oslo, Norway) gradient centrifugation. Briefly, ventral spinal cords were dissected in modified F10 medium (without calcium and magnesium, and with sodium pantothenate), treated with 0.05% trypsin for 15 min at 37°C, and dissociated by gentle trituration in 0.1% bovine serum albumin (BSA) and 0.1 mg/ml DNase I in basal culture medium. The single-cell suspension was centrifuged on a 5.7% Opti-prep density gradient for 15 min at 500g. Motor neurons were removed from the interface. Motor neurons were plated at a density of 280 cells/cm2 on 35 mm dishes or at a density of 103 cells/cm2 on glass coverslips precoated with polyornithine-laminin in basal culture medium. Cultures were maintained at 37°C in an atmosphere 5% CO2/95% humidified air.
RNA isolation, reverse transcription, and real time-PCR
Total RNA was extracted from motor neurons or spinal cord tissues using a kit (RNAeasy; Qiagen, Valencia, CA). For each sample, 1 μg of total RNA was processed for cDNA sysnthesis using iScript cDNA Synthesis Kit (Bio-Rad) for 5 min 25 °C, 30 min at 42 °C and 5 min at 85 °C. Real-tim-PCR was performed with Bio-Rad CFX-96 Real-Time PCR Detection System. Fluoresein dye and iQSYBR Green Supremix (Bio-Rad) were included in the PCR mix. PCR cycle consisted of 5 min at 95 °C, then 40 cycles of amplification for 30 sec at 95 °C, 30 sec at 60 °C, and 30 sec at 72 °C. The primer sequences are as follows (gene name, Genbank accession number, forward primer, reverse primer): nNOS, D14552, 5′-CCT GGG GCT CAA ATG GTA TGG, and 5′-GAA TAG GAG GAG ACG CTG TTG; iNOS, M87039, 5′-CAA CAG GAG AAG GGG GAC GAA C, and 5′-TCT CTG CCT ATC CGT CTC GTC ; eNOS, U53142, 5′-GAA GCG TGT GAA GGC AAC CAT, and 5′-TTG TGG CTC GGG TGG ATT TGC; Arginase I, M14502, 5′-AAA GCC CAT AGA GAT TAT CGG AGC G, and 5′-AGA CAA GGT CAA CGG CAC TGC C; glyceraldehydes-3-phosphate dehydrogenase (GAPDH), M32599, 5′-AAG GGC TCA TGA CCA CAG TC and 5′-ACA CAT TGG GGG TAG GAA CA. After standardization to the housekeeping gene GAPDH, relative expression of each gene compared with that in control wild type animals was calculated according to the manufacturer’s instructions.
HPLC analysis of amino acids in spinal cord
The levels of citrulline in the lumbar spinal cord were determined using HPLC methods as previously described [15, 16]. Spinal cord tissue was lysed with 0.2 mL of 1.5 mol/L HClO4, then 0.1 mL of 2 mol/L K2CO3 was added. The neutralized tissue lysates were centrifuged at 10,000 g for 1 min, and an aliquot (0.2 mL) of the supernatant was used for sample cleanup and determination of citrulline. An aliquot of the derivatized mixture (25 μl) was injected into a Supelco C18 column (150 × 4.6 mm I.D.). Amino acids were separated using a solvent gradient consisting of solution A (0.1 M sodium acetate, 2 mM sodium dodecyl sulfate, 0.5% tetrahydrofuran and 9% methanol, pH 7.2) and solution B (100% methanol and 2 mM sodium dodecyl sulfate). Amino acids in samples were quantified on the basis of authentic standards, using Millenium-32 Software (Waters, Milford, MA).
Measurement of NO− level
Spinal cord lysates (50 μl) were collected and incubated with an equal volume of the Griess reagent for 10 min at room temperature [17]. The concentration of NO2 (nitrite), which is formed by the spontaneous oxidation of NO under physiological condition, was determined by measuring the absorbance at 540 nm. NaNO2 was used as a standard. The value was normalized to the protein concentration of lysates.
Immunofluorescence staining and confocal microscopy
Indirect labeling methods were used to determine NOSs, SMI32, GFAP, and ChAT as previously described [6, 14]. Serial-cut lumbar spinal cord tissue-sections (n=10), from L3–L5 spinal cord segments were used for neuronal analysis. Images were taken by a spinning disk confocal microscopy (IX-81 Olympus DSU, Tokyo, Japan). Size of motor neuronal cell body was analyzed by AQI-X-COMBO-CWF program (Media cybernetics Inc., Bethesda, MD) and NIH ImageJ.
Western blot analysis
The Western blot analysis was performed as previously described [6, 14, 18].
Animals
Male transgenic ALS mice of the mSOD1 (G93A) H1 high-expressor strain (Jackson Laboratories, Bar Harbor, Maine) are bred with females with a similar background (B6/SJLF1). Disease onset may be delayed in female G93A mice due to the neuroprotective effects of female sex hormones [8]. For this reason we only used male G93A transgenic ALS mice as in our previous studies [6, 14]. These experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
L-Arginine supplementation
6% L-arginine was dissolved in water made freshly twice a week and administered to groups (n=10) of wild type and G93A according to the previously described method [14–16].
Statistics
The data was expressed as the mean ± standard error of the mean. Values were analyzed by oneway ANOVA followed by Dunn’s multiple-comparison test. All statistics and graphs were performed using Prism 4.0c (GraphPad Software, San Diego, CA).
RESULTS
nNOS expression versus iNOS expression are differentially modulated in the ALS mice
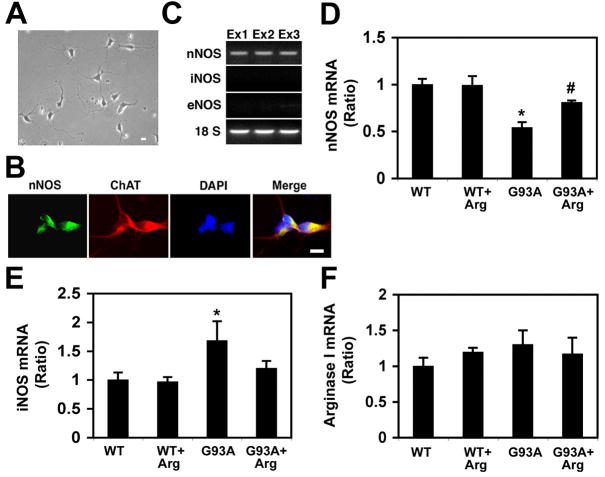
In order to investigate the cell type specific expression of NOS, we established an in vitro primary culture system for mouse motor neurons (Fig. 1A) [14]. The immunoreactivity of nNOS (green) was shown in the cytoplasm of choline acetyltransferase (ChAT)-positive (red) primary motor neurons (3 days in vitro culture) derived from the spinal cord of E12 (Fig. 1B). First, we used a conventional RT-PCR to confirm the expression NOS isoforms in primary motor neurons (Fig. 1C). The only expression of nNOS in primary motor neurons indicated that there are cell type-dependent expressions of NOS isoforms. This data ruled out the possibility that iNOS expression is unlikely occurred in motor neurons and it should be expressed non-neuronal cells in the spinal cord. Then, we evaluated the pattern of NOS expression by quantitative real-time PCR in the spinal cord of WT and mtSOD1 (G93A) ALS mice with or without L-arginine administration (Fig. 1D–F). Interestingly, the expression of nNOS mRNA was lower in the lumbar spinal cord of G93A mice (Fig. 1D). However, in contrast, the level of iNOS mRNA was higher in the lumbar spinal cord of the G93A mice as compared to WT mice (p <0.05) (Fig. 1E). L-arginine administration resulted in an increase of nNOS mRNA in G93A mice in comparison to the untreated control G93A mice (p<0.05) while it decreased the iNOS mRNA level in G93A mice. The mRNA levels of arginase I were measured as a reference enzyme that catalyzes arginine. The mRNA level of arginase I was not markedly induced such as iNOS or nNOS but rather slightly increased in G93A mice (Fig. 1F). There was no significant statistical difference in the mRNA level of arginase I between treated and untreated control mtSOD1 (G93A) mice.
Figure 1. Real-time PCR detection of nNOS, iNOS, and arginase I mRNA from spinal cord from wild type (WT) and mutant SOD1 (G93A) ALS mice with or without arginine supplementation.
A, A photomicrograph of mouse primary motor neurons (3 days in vitro culture) derived from the spinal cord of E12. B, The immunoreactivity of nNOS (green) was shown in the cytoplasm of choline acetyltransferase (ChAT)-positive (red) primary motor neurons. The nuclei (blue) were stained with DAPI (4′,6-diamidino-2-phenylindole). Scale bars: 10 μm. C, The expression of nNOS mRNA, but neither iNOS nor eNOS, is found in primary motor neuron cultures. Each lane (Ex1–3) corresponds to three separate experiments. D, The expression of nNOS mRNA is lower in the lumbar spinal cord of G93A mice. *, Significantly different from WT at p<0.05. #, Significantly increased in comparison to the untreated control G93A mice at p<0.05. E, The expression of iNOS mRNA is higher in the lumbar spinal cord of G93A mice as compared to WT mice (p <0.05). F, The expression of arginase I mRNA was not chnaged in G93A mice compared to WT. The results are shown as the mean (n=5) ± SEM after normalization to GAPDH.
Loss of nNOS-positive motor neurons in the ALS mice and its restoration by L-Arginine
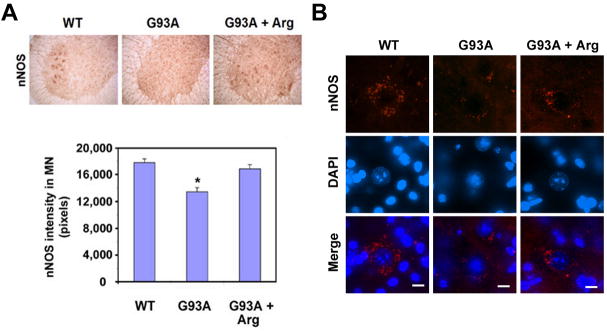
The level of nNOS immunoreactivity was markedly decreased in the lumbar spinal cord of mtSOD1 (G93A) ALS untreated control mice while the L-arginine-treated counterparts showed increased nNOS levels (Fig. 2A). The intensity (pixels) of nNOS immunoreactivity in motor neurons was analyzed with NIH ImageJ image analysis software (Fig. 2A, lower panel). Interestingly, the punctate structures of nNOS particles were found in the cytoplasm of motor neurons (Fig. 2B). Concurrent with the immunohistochemistry data, the spinning disk confocal data in Figure 2B showed that nNOS immunoreactivity is selectively reduced in motor neurons of mtSOD1 (G93A) ALS mice compared to wild type littermate control (Fig. 2B). Considering the decreased level of nNOS immunoeactivity in motor neurons of ALS mice, it seems likely the nitrotyrosine-positive particles might be modulated by NO generated from non-motor neuronal cells such as activated astrocytes and microglia. We further determined whether the level of nitro-tyrosine immunoreactivity is changed in the motor neurons of ALS mice (Supplementary Fig. 1). The punctate structures of nitrotyrosine-positive particles were markedly increased in the nucleus of motor neurons in mtSOD1 (G93A) ALS mice compared to wild type littermate control. L-arginine supplementation reduced the punctate structures of nitro-tyrosine-positive particles (Supplementary Fig. 1).
Figure 2. Loss of nNOS-positive motor neurons in the spinal cord of mutant SOD1 (G93A) ALS mice.
A, nNOS immunoreactivity is markedly decreased in the lumbar spinal cord of G93A mice while arginine (Arg) supplementation preserves nNOS immunoreactivity. The intensity (pixels) of nNOS immunoreactivity in motor neurons was analyzed using NIH ImageJ image analysis software. The results are shown as the mean (n=5) ± SEM *, Significantly different from WT mice at p<0.05. B, Spinning confocal microscopy of nNOS shows the punctate localization in the cytoplasm of motor neurons in the lumbar spinal cord. Scale bars: 10 μm.
iNOS level is increased in the ALS mice
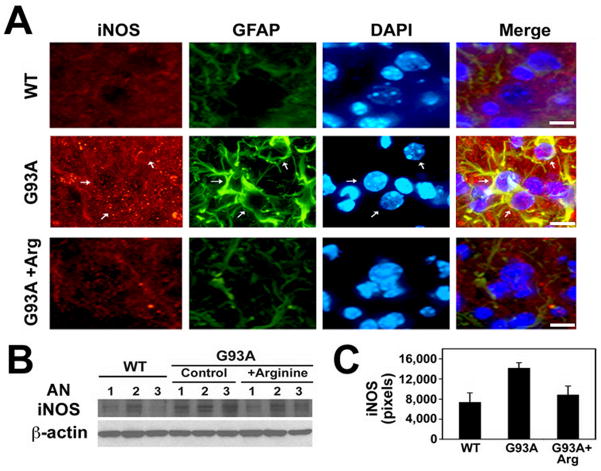
Despite the fact that NO-dependent motor neuronal cytotoxicity has been proposed, it is not clear which NOS subtypes are closely linked to this death pathway. While nNOS levels are markedly decreased in ALS mice, we found that iNOS immunoreactivity is increased in non-motor neuronal cells of the lumbar spinal cord of ALS mice (Fig. 3A). L-arginine reduced the level of iNOS immunoreactivity in the ventral horn of G93A mice. Western blot analysis shows that arginine supplementation reduced the protein level of iNOS in G93A mice (Fig. 3B and C).
Figure 3. Induction of iNOS level the GFAP-positive cells in the spinal cord of mutant SOD1 (G93A) ALS mice.
A, L-Arginine reduces the immunoreactivity of iNOS in the ventral horn of G93A mice lumbar spinal cord. DAPI was used for counter staining of the nucleus. Arrows indicate iNOS- and GFAP-positive astrocytes. Scale bars: 10 μm. B, Western blot shows that L-arginine supplementation reduces the protein level of iNOS in G93A mice. AN, animal number. The numbers refer to individual animals. C, The densitometric analysis of iNOS protein derived from panel B.
The changes of citrulline and NO concentrations in the ALS mice
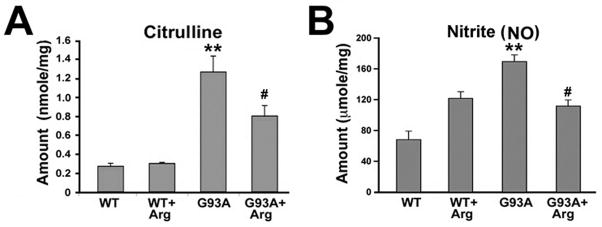
In order to measure the tissue concentrations of citrulline, and NO (measured nitrite concentration by Greiss reagent), spinal cord extracts were prepared as described in the materials and methods. The concentration of citrulline and NO was significantly increased in ALS mice (Fig. 4A and B). L-arginine significantly decreased the level of citrulline and NO in the spinal cord tissue of ALS mice (Fig. 4A and B). Our data suggests that L-arginine down regulates the NO level, presumably by preventing activation of astrocytes and microglia iNOS expression in the spinal cord of ALS mice. We believe that this NO is mainly produced by nNOS residing in motor neurons in wild type mice. As would be expected, we found that nNOS-positive motor neurons are decreased and iNOS-positive activated glial cells are increased in transgenic ALS mice The net amount of NO in the L-arginine treated ALS mice may be reduced compared to untreated ALS mice because of a reduction in inflammation and gliosis with a concomitant reduction in iNOS expression and iNOS-derived NO generation in the absence of nNOS due to motor neuron death.
Figure 4. The changes of citrulline and nitrite (NO) levels in the spinal cord of mutant SOD1 (G93A) ALS mice.
A, The concentration of citrulline in the spinal cord tissue extracts were measured by HPLC. B, The concentration of nitrite (NO) in the spinal cord tissue as measured by Griess reagent as described in Experimental Procedures. WT mice (n=6), WT + Arg mice (n=6), G93A mice (n=6), and G93A + Arg mice (n=6). The results are shown as the mean ± SEM. Significantly different from WT at *, p<0.05 and at **, p<0.01. #, L-arginine supplementation results in a decrease in citrulline and nitrite (NO) in G93A mice in comparison to the untreated control G93A mice at p<0.05.
DISCUSSION
NO has been implicated as a mediator of motor neuron death induced by glutamate [19]. It has been hypothesized that dying motor neurons or other cells release chemotactic factors and cytokines that activate glia and induce iNOS, which is primarily responsible for NO generation and oxidative stress [20]. It has shown that ventral root avulsion in the rat results in the iNOS activation and motor neuronal loss, which is prevented by treatment with NOS inhibitors [21, 22]. In this context and others, the neurodestructive effects of NO are mediated by its diffusion limited interaction with superoxide to form the strong oxidant peroxynitrite [21, 23, 24]. High levels of NO interacting with superoxide form highly toxic peroxynitrite, while low or physiological levels of NO interact with guanylate cyclase to form cGMP which is neuroprotective [24, 25]. The specific intracellular mechanisms that regulate NOS enzyme activity are not well understood but our results suggest that neurotoxicity may depend on changes in the cellular composition of the spinal cord in ALS mice. Our data show that increased tissue levels of NO in the lumbar spinal cord of ALS mice are produced by iNOS located in astrocytes that are activated by cytokines or oxidative stress [26, 27]. Increased NO levels may further exacerbate oxidative stress and trigger motor neuron death, consistent with findings previously reported by Przedborski and colleagues [9]. We recently reported that L-arginine- treatment extends the lifespan of ALS mice and reduces spinal motor neuron degeneration [14]. Interestingly, L-arginine supplementation decreases iNOS levels in glia and improves nNOS and arginase I levels in motor neurons [26, 27]. It therefore seems likely that the down regulation of iNOS associated with L-arginine treatment is due to the suppression of NO-dependent motor neuronal cytotoxicity derived from activated glia [10, 27]. This finding was unexpected given the fact that increased levels of L-arginine, the substrate for NOS, would be expected to result in increased NO concentrations. Indeed, we found a linear relationship between L-arginine concentrations and NO generation in cultured astrocytes [17]. This paradoxical finding suggests that L-arginine’s action in vivo is complicated. It could potentially be acting as an inhibitor of astrogliogenesis or through other free radical-scavenging mechanism [17]. Alternatively, L-arginine may be involved in other of cellular signaling pathways such as polyamine synthesis that could account for its neuroprotective capacity [14]. The relevance of this finding to the striking neuroprotective effects of L-arginine will require further investigation.
In summary, our data provide evidence that differential regulation of neuronal and inducible NOS is linked to degeneration of motor neurons in ALS mice. We further found that neuroprotection by L-arginine may be mediated by a reduction of iNOS derived from glial cells thereby leading to reduced levels of neurotoxic NO in the spinal cord in G93A ALS mice.
Supplementary Material
Acknowledgments
J.L. is an awardee of Les Turner ALS Foundation Grant. NIH P30 AG13846 (J.L.), NIH NS52724 (H.R.), and the Merit Review Grant from the Department of Veterans Affairs (J.L., N.W.K.) supported this work. The WCU Neurocytomics Program Grant (800-20080848) through SNU from KOSEF (H.R.) supported this work, in part.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rowland L, Shneider N. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 2.Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 3.Weiss MD, Weydt P, Carter GT. Current pharmacological management of amyotrophic [corrected] lateral sclerosis and a role for rational polypharmacy. Expert Opin Pharmacother. 2004;5:735–746. doi: 10.1517/14656566.5.4.735. [DOI] [PubMed] [Google Scholar]
- 4.Festoff BW, Suo Z, Citron BA. Prospects for the pharmacotherapy of amyotrophic lateral sclerosis : old strategies and new paradigms for the third millennium. CNS Drugs. 2003;17:699–717. doi: 10.2165/00023210-200317100-00002. [DOI] [PubMed] [Google Scholar]
- 5.Rothstein JD. Of mice and men: reconciling preclinical ALS mouse studies and human clinical trials. Ann Neurol. 2003;53:423–426. doi: 10.1002/ana.10561. [DOI] [PubMed] [Google Scholar]
- 6.Ryu H, Smith K, Camelo SI, Lee J, Iglesias AH, Dangond F, Cormier KA, Cudkowicz ME, Brown RH, Jr, Ferrante RJ. Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J Neurochem. 2005;93:1087–1098. doi: 10.1111/j.1471-4159.2005.03077.x. [DOI] [PubMed] [Google Scholar]
- 7.Zecka JI, Stelmasiak Z, Solski J, Wawrzycki S, Szpetnar M. Plasma amino acids concentration in amyotrophic lateral sclerosis patients. Amino Acids. 2003;25:69–73. doi: 10.1007/s00726-002-0352-2. [DOI] [PubMed] [Google Scholar]
- 8.Russell AS, Ruegg UT. Arginase production by peritoneal macrophages: a new assay. J Immunol Methods. 1980;32:375–382. doi: 10.1016/0022-1759(80)90029-0. [DOI] [PubMed] [Google Scholar]
- 9.Almer G, Vukosavic S, Romero N, Przedborski S. Inducible nitric oxide synthase upregulation in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 1999;72:2415–2425. doi: 10.1046/j.1471-4159.1999.0722415.x. [DOI] [PubMed] [Google Scholar]
- 10.Hensley K, Abdel-Moaty H, Hunter J, Mhatre M, Mou S, Nguyen K, Potapova T, Pye QN, Qi M, Rice H, Stewart C, Stroukoff K, West M. Primary glia expressing the G93A-SOD1 mutation present a neuroinflammatory phenotype and provide a cellular system for studies of glial inflammation. J Neuroinflammation. 2006;3:2. doi: 10.1186/1742-2094-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Chen S, Li L, Wang Q, Le W. Folic acid protects motor neurons against the increased homocysteine, inflammation and apoptosis in SOD1 G93A transgenic mice. Neuropharmacology. 2008;54:1112–1119. doi: 10.1016/j.neuropharm.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363:266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 13.Pennica D, Arce V, Swanson TA, Vejsada R, Pollock RA, Armanini M, Dudley K, Phillips HS, Rosenthal A, Kato AC, Henderson CE. Cardiotrophin-1, a cytokine present in embryonic muscle, supports long-term survival of spinal motoneurons. Neuron. 1996;17:63–74. doi: 10.1016/s0896-6273(00)80281-0. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Ryu H, Kowall NW. Motor neuronal protection by l-arginine prolongs survival of mutant SOD1 (G93A) ALS mice. Biochem Biophys Res Commun. 2009;384:524–529. doi: 10.1016/j.bbrc.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu G, Meininger CJ. Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol. 2008;440:177–189. doi: 10.1016/S0076-6879(07)00810-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Ryu H, Ferrante RJ, Morris SM, Jr, Ratan RR. Translational control of inducible nitric oxide synthase expression by arginine can explain the “arginine paradox”. Proc Natl Acad Sci USA. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Kannagi M, Ferrante RJ, Kowall WN, Ryu H. Activation of Ets-2 by oxidative stress induces Bcl-xL expression and accounts for glial survival in amyotrophic lateral sclerosis. FASEB J. 2009;23:1739–1749. doi: 10.1096/fj.08-121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawson TM, Dawson VL, Snyder SH. Nitric oxide as a mediator of neurotoxicity. NIDA Res Mono. 1993;136:258–271. [PubMed] [Google Scholar]
- 21.Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 22.Wu W, Li L. Inhibition of nitric oxide synthase reduces motoneuron death due to spinal root avulsion. Neurosci Lett. 1993;153:121–124. doi: 10.1016/0304-3940(93)90303-3. [DOI] [PubMed] [Google Scholar]
- 23.Wu W, Han K, Li L, Schinco FP. Implantation of PNS graft inhibits the induction of neuronal nitric oxide synthase and enhances the survival of spinal motoneurons following root avulsion. Exp Neurol. 1994;129:335–339. doi: 10.1006/exnr.1994.1176. [DOI] [PubMed] [Google Scholar]
- 24.Beckman JS. The double-edged role of nitric oxide in brain function and superoxide-mediated injury. J Dev Physiol. 1991;15:53–59. [PubMed] [Google Scholar]
- 25.Beckman JS, Carson M, Smith CD, Koppenol WH. ALS, SOD and peroxynitrite. Nature. 1993;364:584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- 26.Ferri A, Nencini M, Casciati, Cozzolino M, Angelini DF, Longone P, Spalloni A, Rotilio G, Carrì MT. Cell death in amyotrophic lateral sclerosis: interplay between neuronal and glial cells. FASEB J. 2004;18:1261–1263. doi: 10.1096/fj.03-1199fje. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Chen S, Li L, Wang Q, Le W. Folic acid protects motor neurons against the increased homocysteine, inflammation and apoptosis in SOD1 G93A transgenic mice. Neuropharmacology. 2008;54:1112–1119. doi: 10.1016/j.neuropharm.2008.02.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.