Abstract
Aim
To characterize new clinical features in a family with enhanced S-cone syndrome (ESCS) and investigate the pathogenesis of these clinical features in the homozygous Nr2e3rd7rd7 (rd7) mutant mice.
Methods
Four patients from an affected family were included for genotypic and phenotypic study. Eye tissues from rd7 mice were used to detect a possible relationship between macrophages and autofluorescent material by immunohistochemistry (IHC) staining.
Results
Homozygous mutation in R311Q in NR2E3 was detected in this family. Color photographs revealed that white dots do not correlate to hyperautofluorescent spots seen in autofluorescence imaging of the macula. OCT showed rosette-like lesions similar to those found in rd7 mice histology sections. From IHC analysis, we observed that F4/80 (a pan macrophage marker), and autofluorescence were co-localized to the same cells within the retina rosettes.
Conclusions
Retinal structure of a young ESCS patient with homozygous R311Q mutation in the NR2E3 gene is similar to that seen in the rd7 mice. The macrophages were found to contain autofluorescent materials in the retinal rosettes of rd7 mice. Our data are consistent with macrophage infiltration contributing to the hyper-autofluorescent spots found in our patients.
Keywords: photoreceptors, retinal degeneration, macrophages, A2E compound, lipofuscin
INTRODUCTION
Enhanced S-cone syndrome (ESCS; OMIM 268100; http://www.ncbi.nlm.nih.gov/omim/ Online Mendelian Inheritance in Man; NCBI, Bethesda, MD) is a relatively slowly progressive autosomal recessive rod-cone degeneration caused by mutations in the orphan nuclear receptor transcription factor NR2E3 (OMIM: 604485). NR2E3 encodes a retinal nuclear receptor that is a ligand-dependent transcription repressor of cone-specific genes in rod photoreceptors.[1] The NR2E3 protein is part of a large family of nuclear receptor transcription factors that is homologous to an orphan nuclear receptor, Nr2e1 (formerly known as tailless or TLX), which is involved in the development and maintenance of normal function of the central nervous system (CNS) in Drosophila and mammals. NR2E3 is expressed in mitotic progenitor cells during development and its increased expression in cone cells may be due to a prolonged period of cone proliferation, which is followed by a gradual increase in apoptosis. NR2E3 appears to be a dual regulator, suppressing cone cell proliferation in mitotic cells and promoting rod cell genesis in postmitotic cells. NR2E3 mutations were first identified in patients with ESCS, but more recent studies have demonstrated that mutations in NR2E3 are also common causes of clumped pigmentary changes in Goldmann-Favre syndrome, clumped pigmentary retinal degenerations, autosomal recessive retinitis pigmentosa and autosomal dominant retinitis pigmentosa.[2–9]
The spontaneous mutant Nr2e3rd7(rd7) mice have a mutation in NR2E3 gene.[10] This mutant mouse displays increased number and propotion of photoreceptors with S-cone phenotype and abnormal lamination of the outer nuclear layer (ONL).[10, 11]
With confocal scanning-laser ophthalmoscope and spectral domain optical coherence tomography we discovered similar features in a patient. In addition, we demonstrated the origin of these unique clinical features in the homozygous Nr2e3rd7 (rd7) mutant mice.
MATERIALS AND METHODS
Phenotypic Studies
Four patients from an affected family (6, 8, 35, and 46 years of age) were enrolled with the approval of the institutional review board protocol #AAAB6560 at Columbia University. The tenets of the Declaration of Helsinki were followed.
All patients underwent complete ophthalmic evaluation after informed consent was obtained. Fundus photographs, retinal pigment epithelium (RPE) autofluorescence (AF), Fourier-domain optical coherence tomography (OCT) and electroretinography (ERG) were performed in the two children.
Genetic Analyses
The coding region of NR2E3 was sequenced for disease-causing mutations in all patients. DNA was extracted from peripheral blood with the QIAamp DNA Mini Kit (Qiagen Inc., Valencia, CA). Extracted genomic DNA was amplified by polymerase chain reaction (PCR) using primers and cycling temperature as previously described.[12] The PCR products were purified (QIAquick PCR Purification Kit, Qiagen Inc., Valencia, CA) and sequenced.
Autofluorescence Imaging
Autofluorescence images were obtained using a confocal scanning-laser ophthalmoscope (cSLO, Heidelberg Retina Angiograph 2, Heidelberg Engineering, Dossenheim, Germany) by illuminating the fundus with argon laser light (488 nm) and viewing the resultant fluorescence through a band pass filter with a short wavelength cut-off at 495 nm.[13–18]
Optical Coherence Tomography (OCT)
Spectral domain optical coherence tomography (SD-OCT) images were obtained using the Cirrus Spectral Domain OCT from Zeiss (Carl Zeiss Meditec Inc., Dublin, CA, USA). The acquisition protocols included five-line raster scans. Three scans were performed on each eye, and the one with the best signal strength was selected for the final analysis.
Electroretinogram (ERG) Testing
Full-field ERG were performed with silver-impregnated fiber electrodes (DTL; Diagnosys LLC, Littleton, MA) and incorporated the ISCEV Standards.[19] The minimum protocol incorporates the rod-specific and standard bright flash ERGs, both recorded after a minimum of 20 min dark adaptation. Following 10 min of light adaptation, the photopic 30 Hz flicker cone and transient photopic cone ERGs were recorded. S-cone ERGs used a blue stimulus (445 nm, 2–12 cd/m2) on an orange background (620 nm, 100 cd/m2).[12]
Whole Mount Eyecup
To better understand the mechanisms underlying the clinical findings in our patients, we acquired the Nr2e3rd7 mutant mice, a mouse model for ESCS, from Jackson laboratory (stock #004643, Bar Harbor, Maine, United States). Eyes from five week-old Nr2e3rd7 mice and eight week-old C57BL/6J mice (used as a control) were enucleated and placed in 4% paraformaldehyde for one hour at room temperature. The cornea and lens were removed from each eye under a surgical microscope. The whole eyecups were flattened by means of four radial cuts and mounted with mounting medium (VECTASHIELD, Burlingame, CA). Autofluorescence was detected by standard fluorescence microscopy.
Immunohistochemistry (IHC)
Whole eyecups were prepared as described above, and frozen in optimum cutting temperature compound (Tissue-Tek OCT, Miles Laboratories, Elkhart, IN). Frozen eyes were cryosectioned using at a thickness of 10μm. Blocking was performed using 10% donkey serum in PBS with 0.3% Triton X-100 (blocking solution) for 30 min at room temperature (RT). The sections were then incubated with the primary antibodies diluted in 4% donkey serum in PBS with 0.1% Triton X-100 for 2 hours at RT. The samples were subsequently incubated for 40 minutes at RT with the secondary antibodies diluted in PBS with 0.1% Triton. The primary antibody used was directed against F4/80, a pan macrophage marker, (dilution 1:500, eBioscience, San Diego, CA). The secondary antibody used was: Alexa Fluor 488 donkey anti-rat IgG (dilutions 1:1000, Invitrogen, Carlsbad, CA). After washing three times with PBS, the retinas were mounted with mounting medium containing the nuclear dye DAPI (VECTASHIELD, Burlingame, CA) and viewed under a fluorescence microscope (Leica DM 5000 B, Wetzlar, Germany). Images were digitally merged to assess for triple labeling.
RESULTS
Genetic Studies
Direct sequencing of the NR2E3 gene from the proband revealed a homozygous missense mutation 932G→A in exon 6 coding for the R311Q residue (fig 1). His asymptomatic elder brother (8 year old) and consanguineous parents (first cousins) were heterozygous for a mutation at the same DNA site.
Figure 1.
(A). Pedigree of a family in which mutation in the NR2E3 gene were identified. A mutant allele is indicated by (−); a normal allele is indicated by (+). Arrow shows the proband (1, homozygous), and the other members are heterozygous. (B). Direct DNA sequencing from proband’s consanguineous parents and brother showed heterozygous base-substitution at 932G→A in exon 6. (C). Direct sequencing from the proband revealed a homozygous base-substitution at 932G→A with a predicted missense mutation of R311Q (CGG to CAG).
Clinical Examination
Case 1
A 6-year-old boy presented with poor night vision since the age of 2. No history of prematurity or developmental abnormalities was noted, and past ocular history was also unremarkable. The patient is a product of consanguineous mating. Two months prior to presentation, the boy was suspected of having chorioretinitis after failing a standard vision screening at his elementary school. Serum examination was negative for inflammatory and infectious markers, including toxoplasmosis, toxocara, cat scratch disease, Lyme disease and syphilis.
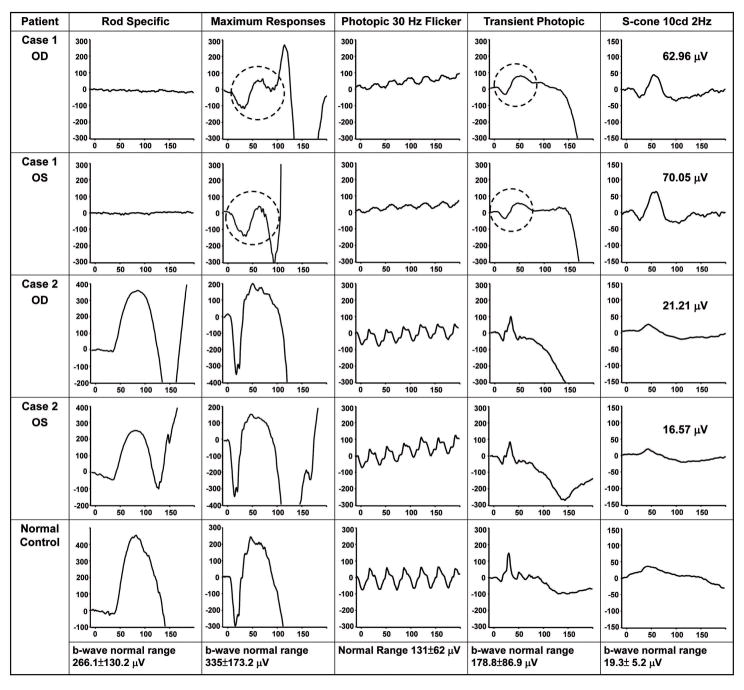
On examination, visual acuity was 20/800 in the right eye and 20/40 in the left eye. The intraocular tension was 13 mmHg in the right eye and 15 mmHg in the left eye. The eye position was orthophoric with full motility. Biomicroscopy was unremarkable except for 1+ vitreous cells in both eyes. Dilated fundus examination of both eyes revealed multiple whitish subretinal deposits and several prominent lesions within the macula (fig 2A). Fundus autofluorescence imaging (FAF) showed hyperautofluorescence in the macula and mid-peripheral retina (fig 3A). Spectral-domain optical coherence tomography (OCT) showed intraretinal cystic changes surrounding the lesion and rosette-like lesions in the mid-peripheral retina, with loss of normal retinal lamination (fig 3B). There was no cystoid macular edema or foveal schisis in the left eye. Fluorescein angiography showed hyperfluorescence that increased with time, suggesting chronic subretinal neovascularization and fibrosis. There was no peripheral vascular leakage. Full-field ERG shows three characteristic findings: 1). no rod responses; 2) the waveforms of scotopic maximal response are very identical to the transient photopic responses except the size (circles); 3) the amplitude of a wave in the transient photopic response is larger than amplitude of photopic 30 Hz flicker. S-cone-specific ERG was of remarkably high amplitude and confirmed the diagnosis of ESCS (fig 4).
Figure 2.
(A). Composite fundus photographs of the right and left eyes of proband (case 1) showing multiple subretinal white deposits and prominent lesions in the macula (red arrows). (B). Composite fundus imaging of the right and left eyes of case 2 exhibited a normal retina without subretinal deposit. There are some artifacts arising from photography (yellow arrows illustrate examples of artifacts from the camera lens, blue arrows show reflections of the nerve fiber layer).
Figure 3.

(A). Fundus autofluorescence exam (FAF) of proband (case 1) showed hyper-autofluorescent spots in the macular area and in the mid-peripheral retina. (B). Optical coherence tomography (OCT) 1, 2, 3, and 4 correspond to scanning sections in fig A. 1 showed cystic changes around the foveal lesion (OD). Note that the rosette-like lesions (arrow head) correspond to hyper-autofluorescent spots and loss of retinal lamination (2, 3 and 4). (C). FAF of case 2 showed several hyper-autofluorescent spots around the disc and arcades. (D). (5,6) OCT sectioning of case 2 (line 5 and 6 from fig C) showed normal retinal lamination without rosette-like lesions. Some shadows casted by retinal vessels are observed.
Figure 4.
Electroretinogram (ERG) from case 1 revealed three characteristic findings: 1). no rod responses; 2) the waveforms of scotopic maximal response are very identical to the transient photopic responses except the size (circles); 3) the amplitude of a wave in the transient photopic response is larger than amplitude of photopic 30 Hz flicker. S-cone-specific ERG was of remarkably high amplitude (60–70 μV) and further confirmed the diagnosis of ESCS. ERG from case 2 was normal.
Case 2
The proband’s older brother was an asymptomatic 8-year-old boy. The best corrected visual acuity was 20/20 (OU) and biomicroscopy was unremarkable. Dilated fundus examination of both eyes revealed a normal retinal appearance without white dots (fig 2B). FAF showed several increased hyperautofluorescence spots around the disc and arcades (fig 3C). OCT showed normal retinal lamination without rosette-like lesions (fig 3D). ERG results were normal (fig 4).
Case 3 & 4
The proband’s parents were first cousins and both were beta-thalassemia carriers. Comprehensive ophthalmic evaluation was unremarkable and there were no fundus white dots or hyper-autofluorescence spots in either parent.
Examination of Nr2e3rd7 mutant mice
Dilated fundus photography from 5-week-old Nr2e3rd7 mutant mice showed diffuse subretinal white dots (fig 5B), and whole mount eyecup from age-matched Nr2e3rd7 mice demonstrated similar hyperautofluorescent spots (fig 5D). Images obtained from 8-week-old wild type C57BL/6J mice showed no white dots in the retina (fig 5A) and no hyperautofluorescent spots (fig 5C). Autofluorescence imaging of retina from Nr2e3rd7 mice has chromophores with a wide spectrum of emission in the visible wavelengths with the highest intensity around 550 nm when excited at 436 nm (fig 5E).
Figure 5.
Color fundus photographs from wild type mouse (A) showed normal retina, whereas image from rd7 mouse (B) showed numerous subretinal white dots. Whole mount eyecup from wild type mouse (C) did not reveal hyperautofluorescence spots whereas image from rd7 mouse (D) showed hyperautofluorescence spots. (E). Spectral fluorescence of hyperautofluorescence spots from rd7 mouse retina demonstrated the wave length of highest fluorescence intensity was 540 nm to 560 nm when excited with light of 436 nm.
DAPI staining of retina from Nr2e3rd7 mice demonstrated rosette and whorl-like structures in the retina and the autofluorescent materials were localized to the region between the outer nuclear layer (ONL) and retinal pigment epithelium (RPE), and mostly within the rosettes (fig 6A). IHC performed with the macrophage marker F4/80 demonstrated that the macrophages were within the rosettes, and the autofluorescent materials were in the cytoplasm of these macrophages (fig 6B).
Figure 6.

(A). Immunohistochemistry of a frozen section from rd7 mouse retina using anti-F4/80 antibodies (macrophage marker) showed one macrophage in the retinal rosette, or whorl lesion. (B). High magnification of white dots lined area from (A) showed images merge from different fluorescence channels. The autofluorescence (red) was co-localized with macrophage marker (green). INL: inner nuclear layer; ONL: outer nuclear layer; AF: autofluorescence; F4/80: macrophage marker; DAP: DAPI staining.
DISCUSSION
Fundus examination
Nummular pigment clumping along the vascular arcades, the classical retinal phenotype seen in adult ESCS patients, is not observed in our proband. Findings from recent reports of young ESCS patients suggest that subtle pigmentary changes with white dots represent an early stage of the disease process,[2, 20] and these white dots progress to characteristic clumped retinopathy in late adulthood.[2] This phenomenon suggested why there were few reports described similar fundus white dots from the ESCS patients. Although a normal fundus appearance without subretinal white dots has been reported in some case series with 6–10 year-old patients,[12, 21] those patients are homozygous for a missense mutation (R104W) that is different from the R311Q mutation seen in our case series. The R311Q substitution, the most common ESCS allele, is found in ~45% of patients.[3] Whether there is genotype-phenotype correlation requires further studies to analyze younger ESCS patients to explain clinical characteristics variability.
OCT
Foveal schisis has been reported in patients with ESCS.[3, 12, 21] In case 1, there is no foveal schisis in the left eye, however, there are intraretinal cysts around the lesion in the macula of the right eye (fig 3B).
Patients with ESCS have histologically dysmorphic retinas in the ONL [4, 22, 23] and our results demonstrate that spectral-domain OCT can detect such rosette formation in the ONL and can be used to monitor photoreceptor loss as the disease progresses.
Autofluorescence imaging
The ring of increased AF in the macula area has been suggested to be associated with fundus white dots and retinal rosette.[12] In case 1, hyperautofluorescent spots are not limited in the macula area but also extended beyond the arcades. The hyperautofluorescent spots outside the macula area correspond to the fundus white dots (fig 3A), whereas the hyperautofluorescent spots within the macula do not correspond to the white dots or pigmentary clumps. In addition, the heterozygous brother (case 2) does not have white dots visible funduscopically, but does have several hyperautofluorescent spots along the arcades. Thus, the hyperautofluorescent spots and fundus white dots may have different origins.
Figure 3C showed several hyperautofluorescence dots around the disc and arcades in case 2. Whether heterozygosity with the mutant NR2E3 allele has any phenotypic manifestation is unknown; the absence of these hyperautofluorescent dots in the asymptomatic consanguineous parents suggests that they may be transient in nature and only detectable in heterozygous children.
Fundus white dots and retinal rosettes of Nr2e3rd7 mutant mice
Subretinal white dots[10] and abnormal lamination of the ONL[10, 11] have been described in Nr2e3rd7 mutant mice. The Nr2e3rd7 mutant mice have numerous white dots over the entire retina at 1 month old that gradually decrease by five months of age, and disappear completely by 16 months.[10] The concordance of the appearance and disappearance of the white spots observed over entire retina with the waves seen under histology suggests that these spots are associated with the retinal rosettes.[10] Milam et al described an approximately 2-fold increase in total cones from a 70-year-old R311Q patient,[4] and Haider et al described a 1.5–2.0 fold increase of S-cones in Nr2e3rd7 mice.[11] Whether the excessive numbers of S-cones contribute to rosette formation is uncertain.
Autofluorescent spots of Nr2e3rd7 mutant mice
In Nr2e3rd7 mice retina, we found that the autofluorescent materials were present in the cytoplasm of macrophages situated within and sometimes adjacent to the retinal rosettes (fig 6). Immunolocalization studies (fig 6) demonstrate that the chromophores are found within macrophages instead of RPE. In addition, the multiple spectral wavelength (fig 5) of the hyperautofluorescent spots from Nr2e3rd7 mutant mice suggests that these spots contain chromophores in addition to A2E, which has an emission maxima of 565 to 570 nm.[24] In a manner analogous to that seen in Nr2e3rd7 mutant mice, we can expect that hyperautofluorescent spots in our case may not from RPE cells, but macrophages. Further in vivo study on ESCS patient with different spectral wavelength can help to determine the origin of these hyperautofluorescent spots.
There are two probable explanations for the presence of macrophages in Nr2e3rd7 mice retina. First, the macrophages are recruited into the retina to remove apoptotic photoreceptors found as early as in P30 Nr2e3rd7 retina.[24] Second, photoreceptor outer segments within the rosettes are inefficiently phagocytosized by RPE due to the abnormal retinal structure, and the accumulation of outer segment debris may in turn trigger macrophage infiltration to remove the waste. However, our IHC studies suggest that these fluorophores within macrophages are derived from the outer segment, rather than apoptotic photoreceptors.
Similar accumulation of outer segment debris and macrophage infiltration has been associated with the development of age-related macular degeneration (AMD).[26] In photoreceptor damage or degeneration, macrophages invade the retina to support the role of RPE in phagocytosing outer segment debris.[27, 28] Thus, infiltration of macrophages can be used as a marker for early phases of various degenerative conditions, and their subsequent disappearance correlates with loss of hyperautofluorescent spots in later stages of retinal degeneration.
In summary, we discovered several novel features (fundus white dots, hyperautofluorescent spots and rosette-like retinal lesions) observed in a child homozygous for the R311Q allele in NR2E3. Findings from the Nr2e3rd7 mutant mice suggest that fundus hyperautofluorescent lesions are likely to be contributed by macrophages.
Acknowledgments
We would like to thank the medical imaging division at the Edward S. Harkness Eye Institute for their excellent work. We greatly appreciate the assistance of the members of the Brown Glaucoma laboratory for sharing ideas, and equipment, especially, Neeco Palmer. NKW is supported by the Taiwan National Science Council NSC-096-2917-I-002-105 and the Chang Gung Memorial Hospital Fellowship CMRPG360571 & 360572.
Funding: Burroughs-Wellcome Program in Biomedical Sciences Fellow, Charles Culpeper Scholarship, Foundation Fighting Blindness, Hirschl Trust, Schneeweiss Stem Cell Fund, Sylvia Wright Retinal Research Trust, Joel Hoffmann Foundation, Jonas Family Fund, Crowley Research Fund, Jahnigen/Hartford/American Geriatrics Society, Eye Surgery Fund, Bernard Becker-Association of University Professors in Ophthalmology-Research to Prevent Blindness (RPB), and EY018213.
Footnotes
Licence for Publication
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted)to be published in BJO editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://bjo.bmj.com/ifora/licence.pdf).”
Competing Interests: No authors have any financial/conflicting interests to disclose.
References
- 1.Chen J, Rattner A, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J Neurosci. 2005;25:118–29. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharon D, Sandberg MA, Caruso RC, Berson EL, Dryja TP. Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch Ophthalmol. 2003;121:1316–23. doi: 10.1001/archopht.121.9.1316. [DOI] [PubMed] [Google Scholar]
- 3.Haider NB, Jacobson SG, Cideciyan AV, et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000;24:127–31. doi: 10.1038/72777. [DOI] [PubMed] [Google Scholar]
- 4.Milam AH, Rose L, Cideciyan AV, et al. The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc Natl Acad Sci U S A. 2002;99:473–8. doi: 10.1073/pnas.022533099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright AF, Reddick AC, Schwartz SB, et al. Mutation analysis of NR2E3 and NRL genes in Enhanced S Cone Syndrome. Hum Mutat. 2004;24:439. doi: 10.1002/humu.9285. [DOI] [PubMed] [Google Scholar]
- 6.Coppieters F, Leroy BP, Beysen D, et al. Recurrent mutation in the first zinc finger of the orphan nuclear receptor NR2E3 causes autosomal dominant retinitis pigmentosa. Am J Hum Genet. 2007;81:147–57. doi: 10.1086/518426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi T, Gekka T, Goto-Omoto S, Takeuchi T, Kubo A, Kitahara K. Novel NR2E3 mutations (R104Q, R334G) associated with a mild form of enhanced S-cone syndrome demonstrate compound heterozygosity. Ophthalmology. 2005;112:2115. doi: 10.1016/j.ophtha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Chavala SH, Sari A, Lewis H, et al. An Arg311Gln NR2E3 mutation in a family with classic Goldmann-Favre syndrome. Br J Ophthalmol. 2005;89:1065–6. doi: 10.1136/bjo.2005.068130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber S, Rozet JM, Takezawa SI, et al. The photoreceptor cell-specific nuclear receptor gene (PNR) accounts for retinitis pigmentosa in the Crypto-Jews from Portugal (Marranos), survivors from the Spanish Inquisition. Hum Genet. 2000;107:276–84. doi: 10.1007/s004390000350. [DOI] [PubMed] [Google Scholar]
- 10.Akhmedov NB, Piriev NI, Chang B, et al. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc Natl Acad Sci U S A. 2000;97:5551–6. doi: 10.1073/pnas.97.10.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haider NB, Naggert JK, Nishina PM. Excess cone cell proliferation due to lack of a functional NR2E3 causes retinal dysplasia and degeneration in rd7/rd7 mice. Hum Mol Genet. 2001;10:1619–26. doi: 10.1093/hmg/10.16.1619. [DOI] [PubMed] [Google Scholar]
- 12.Audo I, Michaelides M, Robson AG, et al. Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci. 2008;49:2082–93. doi: 10.1167/iovs.05-1629. [DOI] [PubMed] [Google Scholar]
- 13.Robson AG, Egan C, Holder GE, Bird AC, Fitzke FW. Comparing rod and cone function with fundus autofluorescence images in retinitis pigmentosa. Adv Exp Med Biol. 2003;533:41–7. doi: 10.1007/978-1-4615-0067-4_6. [DOI] [PubMed] [Google Scholar]
- 14.Robson AG, Saihan Z, Jenkins SA, et al. Functional characterisation and serial imaging of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuity. Br J Ophthalmol. 2006;90:472–9. doi: 10.1136/bjo.2005.082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsang SH, Vaclavik V, Bird AC, Robson AG, Holder GE. Novel phenotypic and genotypic findings in X-linked retinoschisis. Arch Ophthalmol. 2007;125:259–67. doi: 10.1001/archopht.125.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsui I, Casper D, Chou CL, Tsang SH. Electronegative electroretinogram associated with topiramate toxicity and vitelliform maculopathy. Doc Ophthalmol. 2008;116:57–60. doi: 10.1007/s10633-007-9084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsui I, Fuchs BS, Chou CL, Chang S, Tsang SH. Non-vascular vision loss in pseudoxanthoma elasticum. Doc Ophthalmol. 2008;117:65–7. doi: 10.1007/s10633-007-9100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Ruckmann A, Fitzke FW, Bird AC. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br J Ophthalmol. 1995;79:407–12. doi: 10.1136/bjo.79.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holder GE, Brigell MG, Hawlina M, Meigen T, Vaegan, Bach M. ISCEV standard for clinical pattern electroretinography--2007 update. Doc Ophthalmol. 2007;114:111–6. doi: 10.1007/s10633-007-9053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan AO, Aldahmesh M, Meyer B. The enhanced S-cone syndrome in children. Br J Ophthalmol. 2007;91:394–6. doi: 10.1136/bjo.2006.097956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaclavik V, Chakarova C, Bhattacharya SS, et al. Bilateral giant macular schisis in a patient with enhanced S-cone syndrome from a family showing pseudo-dominant inheritance. Br J Ophthalmol. 2008;92:299–300. doi: 10.1136/bjo.2007.120055. [DOI] [PubMed] [Google Scholar]
- 22.Milam AH, Jacobson SG. Photoreceptor rosettes with blue cone opsin immunoreactivity in retinitis pigmentosa. Ophthalmology. 1990;97:1620–31. doi: 10.1016/s0161-6420(90)32358-8. [DOI] [PubMed] [Google Scholar]
- 23.Peyman GA, Fishman GA, Sanders DR, Vlchek J. Histopathology of Goldmann-Favre syndrome obtained by full-thickness eye-wall biopsy. Ann Ophthalmol. 1977;9:479–84. [PubMed] [Google Scholar]
- 24.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci. 1999;40:2988–95. [PubMed] [Google Scholar]
- 25.Haider NB, Demarco P, Nystuen AM, et al. The transcription factor Nr2e3 functions in retinal progenitors to suppress cone cell generation. Vis Neurosci. 2006;23:917–29. doi: 10.1017/S095252380623027X. [DOI] [PubMed] [Google Scholar]
- 26.Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Hoppeler T, Hendrickson P, Dietrich C, Reme C. Morphology and time-course of defined photochemical lesions in the rabbit retina. Curr Eye Res. 1988;7:849–60. doi: 10.3109/02713688808997242. [DOI] [PubMed] [Google Scholar]
- 28.Gordon WC, Casey DM, Lukiw WJ, Bazan NG. DNA damage and repair in light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2002;43:3511–21. [PubMed] [Google Scholar]