Abstract
A panel of retinoids and carotenoids was screened as potential inducers of CYP3A4 through the RXR/VDR-mediated signaling pathway. Transient transfection assays revealed that 3 out of 12 retinoids screened transactivated RXRα/VDR and induced CYP3A4 reporter activity. These three retinoids are the active metabolites of retinoids, 9-cis-retinal, 9-cis-retinoic acid (9-cis-RA), and all-trans-retinoic acid (all-trans-RA). 9-cis-RA and all-trans-RA, preferentially transactivated the RXR/VDR heterodimers and RXR homodimers. Retinoids and VDR agonist 1α, 25-dihydroxyvitamin D3, but not PXR or CAR activator, could induce Cyp3a11 mRNA level in hepatocytes derived from PXR/CAR double null mouse. Moreover, retinoids induced CYP3A4 enzyme activity in HepG2 human hepatoma and Caco-2 human colorectal adenocarcinoma cells. A direct role of retinoid-mediated CYP3A4 induction through RXRα/VDR was proved by the results that 9-cis-retinal, 9-cis-RA, and all-trans-RA recruited RXRα and VDR to CYP3A4 regulatory region pER6 (proximal everted repeat with a 6-nucleotide spacer) and dXREM (distal xenobiotic-responsive enhancer module). Thus, using various approaches, we have unequivocally demonstrated that retinoids transactivate RXR/VDR heterodimers and RXR homodimers and induce CYP3A expression at mRNA as well as enzyme activity levels in both liver and intestinal cells. It is possible that retinoids might alter endobiotic metabolism through CYP3A4 induction in vivo.
1. Introduction
Retinoids play a key role in differentiation, proliferation, and apoptosis and their potential clinical applications in treating cancer and skin diseases are well documented. Retinoids comprise a family of polyisoprenoid lipids including vitamin A (retinol) and its natural and synthetic analogs. Retinol comes from the diet as retinyl esters, mostly from animal products such as liver, eggs, and milk, or as carotenoid precursors in plant products, particularly green leafy vegetables. Retinyl esters (primarily retinyl palmitates), as well as β-carotene, are hydrolyzed to retinol in the intestine. Once taken up in the enterocytes, retinol can be esterified and stored as retinyl esters mainly in liver stellate cells. On the other hand, retinol may enter the circulation bound to its specific transporter, retinol-binding protein (RBP). Retinal (from β-carotene) is converted to retinol for membrane transport then converted back to retinyl esters for cellular storage. In the target cells, retinol undergoes bio-activation to form retinal, retinoic acids (RAs), or hydroxyretroretinol [1]. Alcohol dehydrogenase/reductases (ADHs) are the key enzymes responsible for inter-conversion between retinol and retinal. Aldehyde dehydrogenases (ALHDs) and several cytochrome P450 (CYP) enzymes, such as CYP1A1/2, CYP1B1, CYP2C19, and CYP3A4 participate in the irreversible conversion of retinal to RA [2].
Retinol and RAs are metabolized through oxidation on the β-ionone ring and subsequent conjugation to form water-soluble retinoyl glucuronides in the liver. Several CYP enzymes, including CYP3A4, are known to contribute to the conversion of all-trans-RA to more polar metabolites through all-trans-RA 4-hydroxylation. Using a range of expressed human cytochrome P450 enzymes and human liver microsomes, studies have revealed the precise contributions of various CYPs isoforms to 4-hydroxylation of all-trans-RA. Among them, CYP2C8 and CYP3A4 are two enzymes that make major contributions in this process; they together account for 50 to 60 % of 4-hydroxylation of all-trans-RA in human liver. CYP2C9 contributes about 5 to 10 % to the total 4-hydroxylation of all-trans-RA. CYP26, which expresses in very low levels in a number of tissues including cell lines, also plays a role in the process [1, 3].
Retinoids exert their biological actions through binding and transactivation of retinoid × receptor (RXRα, β, and γ) and retinoic acid receptors (RARα, β, and γ). RXRα is activated by 9-cis-RA, whereas RARα can be activated by both all-trans-RA and 9-cis-RA. RXRs form heterodimers with various nuclear receptors, such as the estrogen receptor (ER), vitamin D receptor (VDR), thyroid hormone receptor (TR), peroxisome proliferator-activated receptor (PPAR), farnesoid X receptor (FXR), liver X receptor (LXR), constitutive androstane receptor (CAR), and pregnane X receptor (PXR). These diverse interactions may constitute the molecular basis for the diversity of biological actions of retinoids.
CYP3A4, the principal cytochrome P450 in the human liver and small intestine, catalyzes the synthesis and metabolic conversion of steroid hormones, cholesterol, and other lipids that have important physiological roles in intracellular signaling pathways. It also participates in the metabolism of xenobiotics and bio-activation of environmental pro-carcinogens. Several members of the nuclear receptor superfamily including CAR [4], PXR [5–8], VDR [9, 10], and the glucocorticoid receptor (GR) [11] have been shown to be responsible for endobiotic- and xenobiotic-mediated CYP3A induction. Human PXR (hPXR) or steroids and xenobiotic receptor (SXR) [12, 13], CAR [4], and VDR [9, 10] control CYP3A4 expression by targeting the same cis-acting elements located in the regulatory region of target genes. One of the shared motifs is pER6, an everted repeat separated by 6 nucleotides, in the proximal region of the CYP3A4 promoter [7]. The second is dXREM, a distal xenobiotic-responsive enhancer module [4]. The cross-talk among these intracellular signaling pathways in regulating CYP3A4 gene expression is a key defensive mechanism in response to a diversity of harmful xenobiotic challenges.
We have recently reported that certain retinoids and carotenoids transactivate the RXR/hPXR-mediated pathway and up-regulate CYP3A4 gene expression in a human hepatoma cell line [14]. Given that both hPXR and VDR control the transcription of CYP3A4, we reasoned that retinoids and carotenoids may activate the RXR/VDR-mediated pathway and up-regulate CYP3A4 gene expression by targeting the shared specific response elements in the CYP3A4 promoter. Therefore, a panel of retinoids and carotinoids was screened as potential activators for RXRs/VDR. The data demonstrate that RAs (9-cis-RA and all-trans-RA) and retinyl aldehyde (9-cis-retinal) are efficacious activators of RXR/VDR to induce CYP3A4 gene expression.
2. Materials and methods
2.1. Reagents
All-trans-RA, 9-cis-retinal, 13-cis-retinol, 9-cis-RA, all-trans-retinol palmitate (all-trans-RP), β-carotene, lycopene, 4-(E-2-[5,6,7,8-tet-rahydro-5,5,8,8-tetramethyl-2-naphthalenyl]-1-propenyl) benzoic acid (TTNBP), fenretinide, sterile dimethyl sulfoxide (DMSO), pregnenalone 16α-carbonitrile (PCN), 1,4-bis[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP), 1α, 25-dihydroxyvitamin D3 (1α, 25-(OH)2D3 or D3), rifampin, and 6-(4-chlorophenyl) imidazo[2,1-b][1,3] thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl) oxime (CITCO) were purchased from Sigma-Aldrich. Retinol acetate and 13-cis-retinal were obtained from Toronto Research Chem. Lutein was purchased from US Biological, and 13-cis-RA was obtained from BIOMOL Research Laboratories Inc.
2.2. Transient transfection and luciferase reporter activity assay
HepG2 and Caco-2 cells were cultured in Dulbecco’s Modification of Eagle’s Medium (Mediatech). The media was supplemented with 10% charcoal-stripped fetal bovine serum (FBS) (Biomeda) for HepG2 cells, and 20% FBS for Caco-2 cells. Approximately 2.5 × 105 cells (HepG2 or Caco-2 cells) per well were plated onto 24-well plates and cultured at 37°C in 5% CO2 with a relative humidity of 95%. The plated cells were cultured overnight and then co-transfected with expression plasmids using FuGENE 6 (Roche Diagnostics) for HepG2 cells according to the manufacturers’ instructions. The expression plasmid of mouse mRXRαY402A was kindly provided by Dr. Hinrich Gronemeyer (Institut de Genetique et de Biologie Moleculaire et Cellulaire, France). Human RXRα, mouse RXRα, β, and γ expression plasmids were generously provided by Dr. Ronald Evans (Howard Hughes Medical Institute, The Salk Institute for Biological Studies, USA). The tk-(3A4)3-Luc reporter vector, which contains three copies of ER6, and the human VDR expression plasmid [15] were used for transfection. For each transfection, herpes simplex virus thymidine kinase promoter-driven Renilla reniformis luciferase was used as an internal control for normalization. After transfection, cells were treated with retinoids, carotinoids (10 μM each) or DMSO (0.1%). Fresh medium with retinoids or carotinoids were provided every 24 h. Forty-eight hours after treatment, cells were harvested and firefly and renilla luciferase activities were determined using a single tube TD20/20 luminometer.
2.3. Isolation of mouse hepatocytes
Hepatocytes were isolated from 4-month-old male mice, using in situ two-step collagenase perfusion method via the portal vein as described in literature [16]. The mice were housed at 22°C with a 12/12-h light/dark cycle and provided food and water ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Kansas University Medical Center Institutional Animal Care and Use Committee. Cell viability, assessed by Trypan blue stain (0.4%) (Sigma-Aldrich) exclusion, was greater than 80%. Hepatocytes were seeded in 24-well type I collagen-coated plates at 37°C in 5% CO2 with a relative humidity of 95%. Cells were cultured in William’s E culture medium (Sigma-Aldrich) supplemented with HEPES buffer (10 mM, pH 7.4), FBS (10%) (Biomeda), L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml) (Invitrogen), and insulin (5 μg/ml) (Sigma-Aldrich) for 4 h. After attachment, hepatocytes were cultured in serum-free media for 48 h then treated with retinoids, carotinoids (10 μM each), or DMSO (0.1%) for 48 h. Fresh medium with retinoids or carotinoids was provided every 24 h.
2.4. RNA preparation
Hepatocytes were washed with ice-cold PBS and total RNA was isolated using TRIzol reagent (Invitrogen). The reverse transcription (RT) reaction was performed using total RNA (1 μg) and random primers (Invitrogen).
2.5. Primers and probes
Taqman real-time PCR primers and fluorescent probe sequences were designed using PrimerExpress software (Applied Biosystems). To avoid amplification of genomic DNA, primer sets or probe were designed to span introns. All primers and probes were submitted to the National Center for Biotechnological Information (NCBI) for nucleotide comparison to ensure specificity. Probes and primers were synthesized by Sigma-Aldrich and Integrated DNA Technologies. Probes were labeled with the reporter FAM (6-carboxyfluorescein) and quencher BQH1 (black hole quencher 1) at the 5′ and 3′ ends, respectively. The primer sets and probe used were as follows: Cyp3a11, F-5′-TCACAGACCCAGAGACGATTAAGA-3′, R-5′-CCCGCCGGTTTGTGAAG-3′, 5′-6FAM-TGTGCTAGTGAAGGAATGTTTTTCT-BHQ1-3′; and mouse glyceraldehyde-3-phosphate dehydrogenase (Gapdh) F-5′-TGTGTCCGTCGTGGATCTGA-3′, R-5′-CCTGCTTCACCACCTTCTTGA-3′, 5′-6FAM-CCGCCTGGAGAAACCTGCCA-BHQ1-3′. Primers (900 nM) and probe (250 nM) were used for Taqman real-time PCR amplification on ABI Prism 7900 Sequence Detection System (Applied Biosystems). Fold inductions were calculated using the ΔΔCt method according to the manufacturer’s instructions.
2.6. CYP3A4 enzyme activity measurement
HepG2 and Caco-2 cells were plated in 24-well plates and treated with retinoids for 72 h. CYP3A4 enzyme activity was measured using P450-Glo CYP3A4 enzyme activity kit (Promega) according to the protocol provided by the manufacturer.
2.7. Chromatin immunoprecipitation (ChIP) assay
HepG2 cells were cultured in 6-well plates to about 80% confluence and transfected with human RXRα and VDR expression plasmids. The cells were treated with retinoids for 48 h. ChIP assays were performed using a ChIP Assay kit (Upstate Biotechnology) according to the manufacturer’s protocol. Briefly, cells were treated with 1% formaldehyde for 10 min, followed by sonication with a Model 500 Sonic Dismembrator (Fisher Scientific). Ten percent of the cell lysates were used as “input”. The cross-linked DNA-protein complexes were precipitated by incubating the cell lysates with rabbit anti-RXRα, rabbit anti-VDR antibodies, or non-immune IgG (Santa Cruz Biotechnology) at 4°C overnight, followed by incubating with protein A-agarose beads. The beads were washed and samples eluted. The cross-links were reversed by heating samples at 65°C overnight and DNA was purified using a Qiaquick Spin column (Qiagen). The DNA fragments containing either the pER6 or dXREM were amplified using primers flanking the pER6 or dXREM motifs. The PCR primers used for pER6 region (-281 to -80) were: F-5′GGCGATTTAATAGATTT TATGC-3′ and R-5′-TGCTCTGCCTGCAGTTGGAA-3′; and for the dXREM region (dDR3/ER6, -7,771 to −7,562) were: F-5′-CCCAATTAA AGGTCATAAA-3′ and R-5′-CAGAAGTTCAGCTTGTGATTC-3′.
3. Results
3.1. Retinoids transactivate the RXRα/VDR-mediated pathway
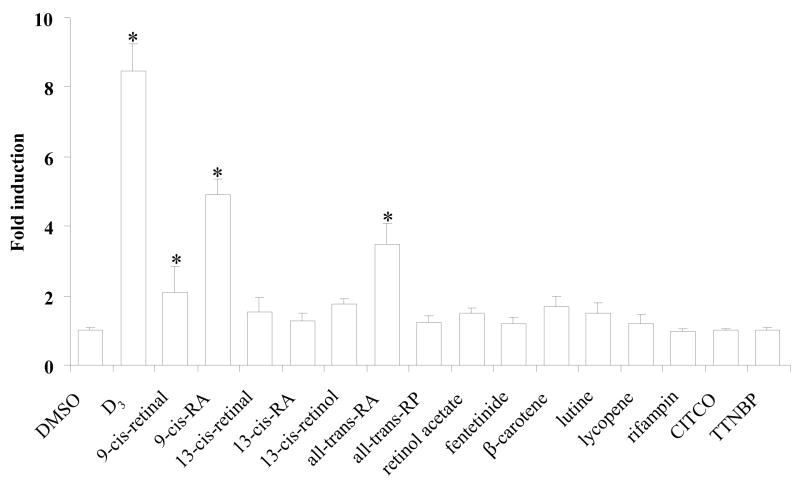
A transient transfection assay was employed to determine if retinoids and carotenoids can transactivate the RXRα/VDR-mediated pathway. The human hepatoma cell line, HepG2 cells, which expresses undetectable mRNA levels of VDR and RXRγ by Taqman real-time PCR, but high basal levels of RXRα and medium levels of RXRβ (our unpublished data), were transfected with the human VDR (hVDR) and human RXRα (hRXRα) expression plasmids. The tk-(3A4)3-Luc reporter construct, which contains 3-copies of ER6, served as a reporter. The cells were treated with retinoids (10 μM) or 1α, 25-dihydroxyvitamin D3 (1α, 25-(OH)2D3 or D3) (0.01 μM or 0.1 μM), the most active metabolite of vitamin D3. Luciferase activity was measured after 48 h treatment. As shown in figure 1, D3 induced reporter activity about 9-fold. Among the 12 retinoids and carotenoids screened, 9-cis-retinal (2.1-fold), 9-cis-RA (4.9-fold), and all-trans-RA (3.5-fold) were effective activators of RXRα/VDR heterodimers (Fig. 1). Additionally, a lower dose (1 μM) of 9-cis-RA was capable of transactivating RXRα/VDR heterodimers and inducing reporter luciferase activity by 2.2-fold (data not shown). No significant induction of reporter activity was observed by 13-cis-retinal, 13-cis-RA, 13-cis-retinol, all-trans-retinol palmitate (all-trans-RP), retinol acetate, fenretinide, β-carotene, lutine, and lycopene (Fig. 1). The specific activator for hPXR (rifampin), CAR (CITCO), and RAR (TTNBP) showed no induction of reporter activity (Fig. 1). When compared on equal molar basis, D3 is more effective than retinoids in transactivating the reporter activity through RXRα/VDR-mediated pathway.
Fig. 1.
Retinoids transactivate the RXRα/VDR-mediated pathway. HepG2 cells were transiently transfected with human RXRα (hRXRα) and human VDR (hVDR) expression plasmids (50 ng each), the tk-(3A4)3-Luc reporter construct (300 ng), and the renilla luciferase expression vector (10 ng). The transfected cells were treated with 1α, 25-dihydroxyvitamin D3 (D3) (0.1 μM), rifampin (10 μM), CITCO (10 μM), TTNBP (10 μM), the indicated retinoids (10 μM), or DMSO (0.1%) for 48 h and then assayed for luciferase activity. The results are expressed as relative fold changes of luciferase activity to DMSO control. Each value represents the mean ± SD of three independent experiments. *p < 0.05, compared to DMSO treated cells.
3.2. 9-cis-RA and D3 co-treatment displayed an additive effect on RXRα/VDR-mediated pathway
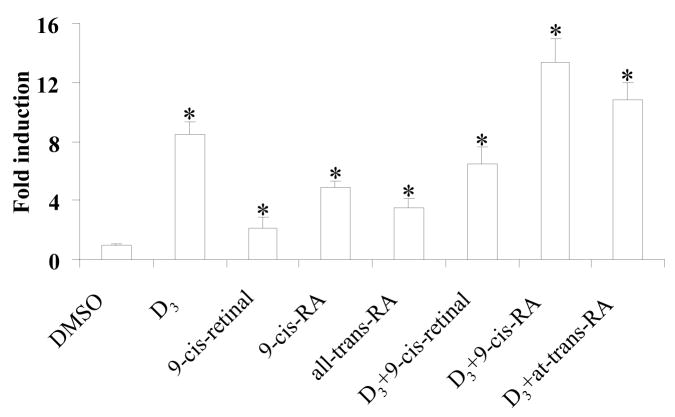
To test the interaction between D3 and retinoids, 9-cis-retinal, 9-cis-RA, and all-trans-RA were dosed in combination with D3. HepG2 cells were transiently transfected with the hRXRα and hVDR expression plasmids, then treated with D3, retinoid alone or retinoid plus D3 for 48 h. Concomitant 9-cis-RA and D3 showed an additive effect on transactivation of tk-(3A4) 3-Luc activity (Fig. 2). However, neither additive nor synergistic effect on activating reporter activity was observed by 9-cis-retinal or all-trans-RA plus D3 co-treatment (Fig. 2).
Fig. 2.
9-cis-RA plus D3 treatment has an additive effect on RXRα/VDR-mediated activation of tk-(3A4)3-Luc. HepG2 cells were transiently transfected with the hRXRα and hVDR expression plasmids (50 ng each), the tk-(3A4)3-Luc reporter construct (300 ng), and the renilla luciferase expression vector (10 ng). Cells were treated with D3 (0.1 μM), retinoid (10 μM), D3 (0.1 μM) plus retinoid (10 μM), or DMSO for 48 h then cells were harvested for luciferase assays. The results are expressed as relative fold changes of luciferase activity to DMSO control. Each value represents the mean ± SD of three independent experiments. *p < 0.05, compared to DMSO treated cells.
3.3. Redundant role of RXRs in mediating 9-cis-RA-induced activation of tk-(3A4)3-Luc
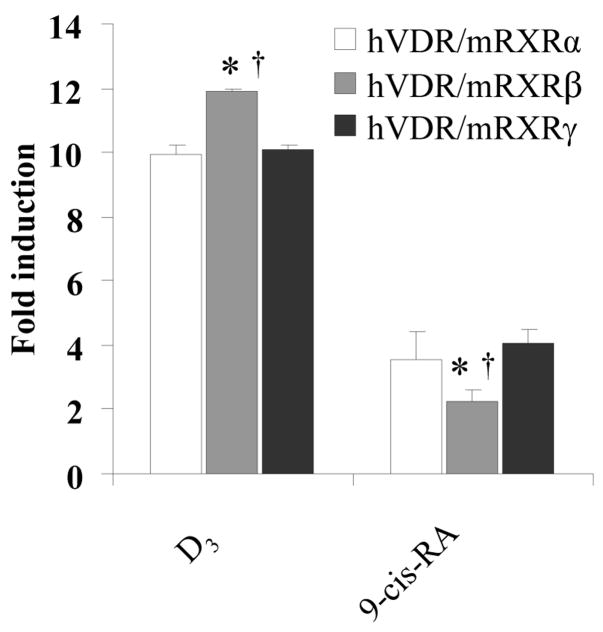
To study the differential roles of RXR isoforms in transactivation of tk-(3A4)3-Luc reporter, HepG2 cells were transfected with the expression plasmids encoding mouse RXRα, β, or γ and hVDR followed by treatment with DMSO, D3, or 9-cis-RA for 48 h. The results revealed that over-expressed mouse RXRs and VDR in HepG2 cells displayed redundant effects in transactivating reporter activity in response to D3 or 9-cis-RA (Fig. 3). D3 preferentially activated mouse RXRβ/hVDR heterodimers. In contrast, 9-cis-RA preferentially activated mouse RXRα/hVDR and mouse RXRγ/VDR heterodimers. Thus, dependent upon the ligand, VDR preferentially dimerizes with different mRXR isoforms and leads to differential transactivation of tk-(3A4)3-Luc reporter activity though the difference is minor.
Fig. 3.
9-cis-RA differentially transactivates the RXR isoforms/VDR heterodimers. HepG2 cells were transiently transfected with the hVDR expression plasmids (50 ng), mRXRα, β, or γ (50 ng each), the tk-(3A4)3-Luc reporter construct (300 ng), and the renilla luciferase expression vector (10 ng). Cells were treated with the indicated retinoids (10 μM), or DMSO (0.1%) for 48 h and then assayed for luciferase activity. The results are expressed as relative fold changes of luciferase activity to DMSO control. Each value represents the mean ± SD of three independent experiments. Comparison was performed between each group: *p < 0.05, compared to hVDR/mRXRα group, †p < 0.05, compared to hVDR/mRXRγ group.
3.4. Retinoids differentially transactivate the homodimers and RXRα/VDR heterodimers
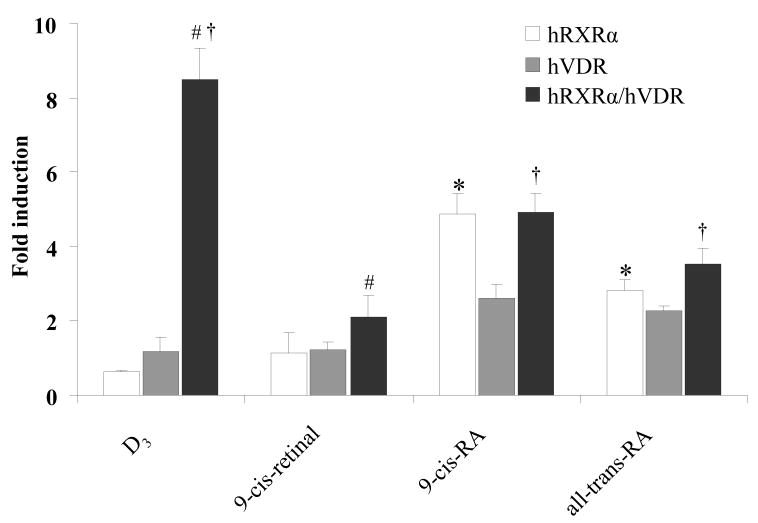
To further distinguish the possible role of homodimers and heterodimers (RXRα/VDR) in transactivation of tk-(3A4)3-Luc reporter activity, hRXRα or hVDR expression plasmid alone or a combination of both were transfected into HepG2 cells. The cells were treated with retinoids or D3 for 48 h followed by luciferase activity assay. The results revealed that neither D3 nor 9-cis-retinal was capable of transactivating tk-(3A4)3-Luc when only hRXRα or hVDR were over-expressed (Fig. 4). In contrast, reporter activity was elevated by 9-cis-RA (4.8- and 2.6-fold) and all-trans-RA (2.8- and 2.3-fold) respectively (Fig. 4) when hRXRα or hVDR were included in the transfection. The highest induction of reporter activity was observed when both hRXRα and hVDR were transfected, and D3 displayed the highest induction fold (8.7-fold), followed by 9-cis-RA (4.9-fold) and all-trans-RA (3.5-fold) (Fig. 4).
Fig. 4.
Redundant role of RXRs in mediating 9-cis-RA-induced activation of tk-(3A4)3-Luc. HepG2 cells were transiently transfected with the hVDR expression plasmids (50 ng), hRXRα (50 ng), the tk-(3A4)3-Luc reporter construct (300 ng), and the renilla luciferase expression vector (10 ng). Cells were treated with the indicated retinoids (10 μM) or DMSO (0.1%) for 48 h and then assayed for luciferase activity. The results are expressed as relative fold changes of luciferase activity to DMSO control. Each value represents the mean ± SD of three independent experiments. Comparison was performed between each group: *p < 0.05, RXRα compared to VDR, #p < 0.05, RXRα compared to RXRα/VDR, † p < 0.05, VDR compared to RXRα/VDR.
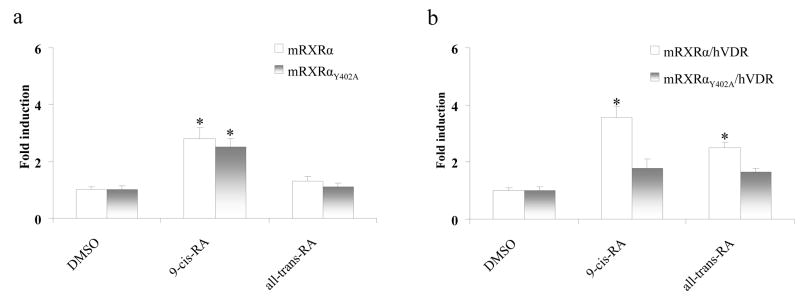
mRXRαY402A plasmid encoding mouse RXRα, which is impaired in RXRα/VDR heterodimer formation and is incapable of mediating the transactivation by D3, but does form homodimers and mediates RA effects [17], was employed for transient transfection to discern if retinoids activate the reporter activity through mouse RXRα homodimers. Both homodimers of mouse wild-type RXRα and mutant mRXRαY402A yielded about 3-fold induction of the reporter activity upon 9-cis-RA treatment. However, all-trans-RA was not able to transactivate the homodimers (Fig. 5a). On the contrary, transfection of mRXRαY402A and VDR expression plasmids remarkably aborted induction of reporter activity by 9-cis-RA (3.6-fold vs. 1.8-fold, wild-type RXRα vs. mRXRαY402A) and all-trans-RA (2.5-fold v.s 1.7-fold, wild-type RXRα vs. mRXRαY402A) (Fig. 5b).
Fig. 5.
RXRα homodimers and RXRα/VDR heterodomers differentially mediate retinoid-induced tk-(3A4)3-Luc activity. HepG2 cells were transiently transfected with the wild-type mouse RXRα (mRXRα) (a) or mutated mouse RXRαY402A (mRXRαY402A) (b) and hVDR expression plasmids (50 ng), the tk-(3A4)3-Luc reporter construct (300 ng), and the renilla luciferase expression vector (10 ng). Cells were treated with indicated RA (10 μM) or DMSO (0.1%) for 48 h, then cells were harvested for luciferase assays. The results are expressed as relative fold changes of luciferase activity to DMSO control. Each value represents the mean ± SD of three independent experiments. *p < 0.05, compared to DMSO treated cells.
3.5. Retinoids induce Cyp3a11 mRNA and increase CYP3A4 enzyme activity
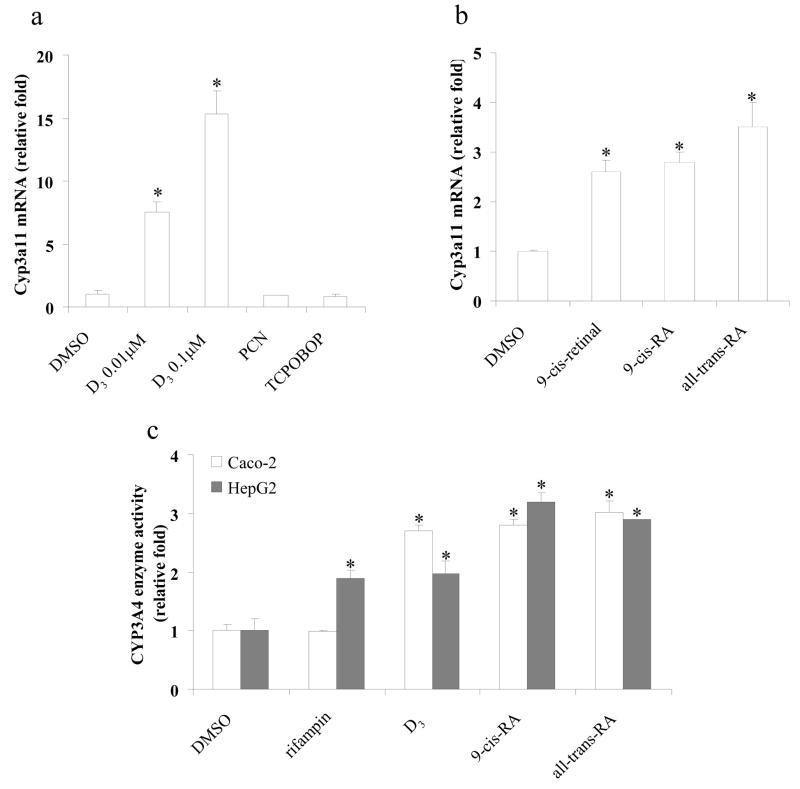
It has been well characterized that CYP3A4 gene is regulated by PXR [5–8], CAR [4], and VDR [9, 10]. Induction of Cyp3a11 (homologous of human CYP3A4) mRNA by retinoids in hepatocytes which are deficient in both PXR and CAR would strongly suggest the role of VDR in regulation of Cyp3a11. The PXR/CAR double-knockout mice were created by cross-breeding PXR-null [12] and CAR-null [18] mice. The absence of both PXR and CAR was confirmed by treating primary hepatocytes with pregnenalone 16α-carbonitrile (PCN), 1,4-bis[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP), and D3 for 48 h. The Cyp3a11 mRNA level was monitored using Taqman real-time PCR after the treatment. As expected, Cyp3a11 mRNA expression was induced by D3, but not by PCN or TCPOBOP (Fig. 6a). The induction fold was 7.5-fold at 0.01 μM and 15.3-fold at 0.1 μM of D3 (Fig. 6a). Basal Cyp3a11 mRNA was detectable in primary hepatocytes derived from the PXR and CAR double knockout mice, suggesting that basal Cyp3a11 gene expression is maintained in the absence of both PXR and CAR. Retinoids were then tested for Cyp3a11 mRNA induction in the double-null mouse hepatocytes. The results indicated that 9-cis-retinal, 9-cis-RA, and all-trans-RA induced Cyp3a11 mRNA levels by 2.5-, 2.6-, and 3.5- fold, respectively (Fig. 6b). The data demonstrated that RA (9-cis-RA and all-trans-RA) and aldehyde (9-cis-retinal) are capable of inducing Cyp3a11 gene expression in mouse primary hepatocytes, which is consistent with the transfection data. Moreover, these three retinoids induced Cyp3a11 gene expression through signaling pathways which are independent of PXR and CAR.
Fig. 6.
Retinoids induce Cyp3a11 mRNA and increase CYP3A4 enzyme activity. Forty-eight hours after plating, primary hepatocytes derived from PXR/CAR double-knockout mice hepatocytes were treated with (a) D3 (0.01 μM, or 0.1 μM), pregnenalone 16α-carbonitrile (PCN) (10 μM), 1,4-bis[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP) (10 μM), or DMSO (0.1%); (b) retinoids (10 μM), or DMSO (0.1%) for 48 h. Total RNA was extracted, Cyp3a11 and Gapdh mRNA levels were quantified by Taqman real-time PCR. (c) Caco-2 or HepG2 cells were treated with rifampin (10 μM), D3 (0.1 μM for Caco-2 cells, or 0.25 μM for HepG2 cells), RA (10 μM), or DMSO (0.1%) for 48 h. CYP3A4 enzyme activity was measured using P450-Glo assay kit. The results are expressed as relative fold changes of activity to DMSO-treated control. Each value represents the mean ± SD of three independent experiments. *p < 0.05, compared to DMSO treated cells.
The human colorectal adenocarcinoma cell line, Caco-2, spontaneously undergoes enterocytic differentiation in culture, acquiring morphological as well as biochemical features of small intestinal enterocytes, and expresses high levels of VDR. Previous work has revealed that D3 functions as a transcriptional inducer of CYP3A4 in Caco-2 cell lines [19]. To further demonstrate that retinoids can transactivate the endogenous CYP3A4 gene, CYP3A enzyme activity assay was performed in both Caco-2 and HepG2 cells. Rifampin, an hPXR activator, did not induce CYP3A4 activity in Caco-2 cells; about 2.0-fold induction of CYP3A4 activity was observed in HepG2 cells (Fig. 6c). However, D3 induced CYP3A4 activity about 2.5-fold in Caco-2 cells at the concentration of 0.1 μM (Fig. 6c), but less than 2.0-fold in HepG2 cells even at the concentration of 0.25 μM (Fig. 6c). Our data is in accordance with previous findings that the induction of CYP3A4 by D3 is cell line-specific and that the differential induction fold in two cell lines may be due to the relative abundance of the related nuclear receptors and co-factors (18). RA such as 9-cis-RA and all-trans-RA induced CYP3A4 activity by 2.6- to 3.3-fold in both Caco-2 and HepG2 cells (Fig. 6c).
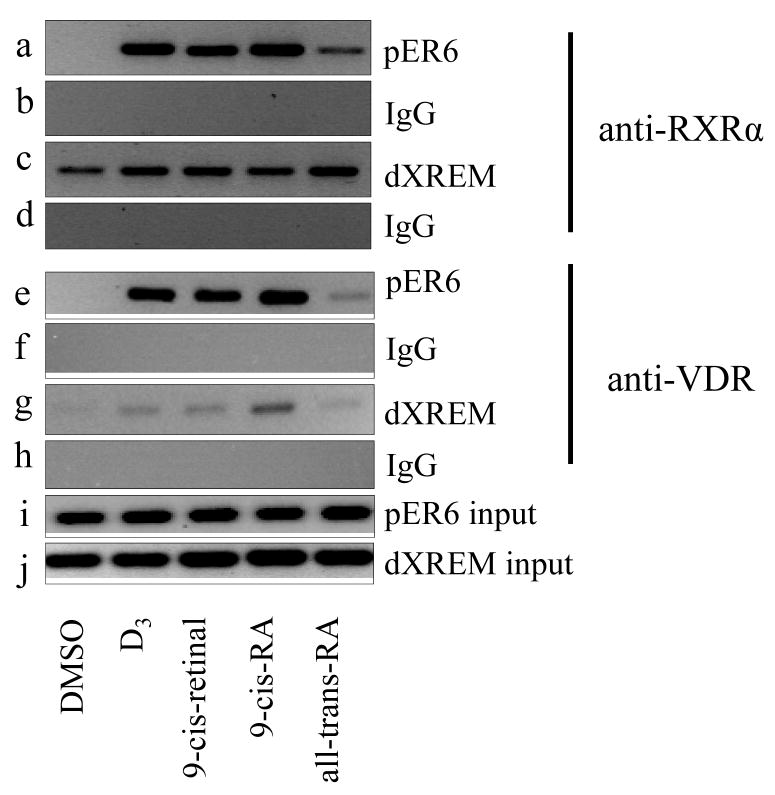
3.6. Retinoids recruit RXRα and VDR to the promoter region of CYP3A4 chromatin
To study the effect of retinoids on recruitment of RXRα and VDR to the CYP3A4 chromatin, which contains pER6 and dXREM binding sites, ChIP assays were performed. Antibodies against RXRα or VDR were used to immunoprecipitate the cross-linked DNA-protein complexes in HepG2 cells treated with retinoids. PCR primer sets were designed to amplify the DNA fragments containing either the pER6 or the dXREM response elements. The chromosomal occupancy of RXRα and VDR was specific because PCR signals were at the background level when the nonimmune IgG was used (Fig. 7b, d, f, and h). HepG2 cells were treated with D3 or retinoids for 48 h and harvested for ChIP assay. The results revealed that anti-RXRα and anti-VDR antibodies precipitated markedly higher amounts of the CYP3A4 promoter fragments than that observed from DMSO-treated cells (Fig. 7a, c, e, and g). D3, 9-cis-retinal, and 9-cis-RA recruited both RXRα and VDR to pER6 with similar binding signals, but a weaker signal was noted in all-trans-RA treated cells (Fig. 7a and e). For the dXREM binding site, slightly enhanced RXRα binding signals were observed by D3 or retinoids treatment (Fig. 7c), compared with DMSO treated control. The most enhanced VDR binding signal to dXREM was observed upon 9-cis-RA treatment. D3 and 9-cis-retinal exerted weak signals, and all-trans-RA showed no VDR binding signal at dXREM binding site (Fig. 7g).
Fig. 7.
Retinoids recruit RXRα and VDR to the promoter region of the CYP3A4 chromatin. HepG2 cells were treated with D3 (0.1 μM), retinoids (10 μM) or DMSO (0.1%) for 48 h. ChIP assays were performed as described under Materials and Methods. Anti-RXRα antibody (a, c) or anti-VDR antibody (e, g) was used to precipitate the DNA-protein complexes. DNA fragments containing pER6 or dXREM were PCR-amplified and analyzed on a 2% agarose gel. Normal IgG was used as nonimmune control (b, d, f, h); Total cell lysate (10%) was used as input (i, j).
4. Discussion
CYP3A4 is the predominant CYP450 isozyme expressed in both human liver and small intestine contributing to the biotransformation of approximately 50% of drugs on the current market [20, 21]. In addition, CYP3A4 is involved in the oxidation of a variety of endogenous substrates such as steroids, lipids, bile acids, and retinoids [22]. Expression of the CYP3A4 gene can be induced by an array of structurally diverse compounds. Previous work has demonstrated the marked correlation between retinoid treatment and induction of CYP450s enzymes in both liver and intestine [23, 24]. However, the molecular basis for the induction of the CYP3A4 gene by retinoids is not fully understood. We have recently reported that retinoids activate the RXRα/hPXR-mediated pathway and induce endogenous CYP3A4 activity in Huh7 human hepatoma cells [14]. Given that hPXR and VDR share 64% amino acid identity in their ligand binding domain [7], and the networks of hPXR, VDR, and CAR control CYP3A4 gene expression, we anticipated that retinoids may also regulate CYP3A4 gene expression through the RXR/VDR signaling pathway. Using transient transfection assays, real-time PCR for quantification of mRNA levels, enzyme activity assays, gene knockout models, and ChIP assays, we have unequivocally demonstrated that retinoid-activated RXRs/VDR heterodimers and RXRα homodimers, are responsible for the induction of CYP3A4.
As a promiscuous nuclear partner, RXRα can exert its effect in three different ways. First, RXRα acts as a permissive partner of FXR, LXRs, and PPARs, displaying more than an additive effect upon both ligands treatment [25, 26]. RXRα is characterized as a conditional permissive partner in the RXRα/RAR heterodimer, as full response to retinoids occurs only in the existence of an RAR agonist [27]. RXRα/VDR and RXRα/TR heterodimers have been suggested to be non-permissive because they neither bind nor show activation by RXR ligands [28]. However, RXRα has been reported as a non-silent partner in the context of TR heterodimers [29]. Conflicting results have been documented regarding the role of RXR in ligand binding and transactivation as a heterodimeric partner of VDR. It has been demonstrated that RXR is silent in the RXR/VDR heterodimers on binding to the DR3 (direct repeats spaced by 3 nucleotides) response element [25]. However, additive or synergistic transcriptional activation has been observed when RXR/VDR heterodimers bind to particular response elements (osteopontin vitamin D response element and DR3) in different cells (human breast cancer cell line MCF-7 and Drosophila SL-3 cells) [30], suggesting that the role of RXR in VDR-mediated transactivation is gene- and cell line-specific. It has been demonstrated that RXR is an essential partner of VDR and actively participates in VDR-dependent gene expression [31]. Similar observations that RXR is a significant contributor to VDR-mediated gene expression have been reported elsewhere [32]. Notably, our transfection data demonstrated that retinoid alone can activate RXRα/VDR heterodimers on the ER6 response element of CYP3A4 gene. In addition, the specific agonist for hPXR (rifampin), CAR (CITCO), and RAR (TTNBP) is incapable of increasing the reporter activity in the same experimental settings, demonstrating that endogenous hPXR, CAR, and RAR is not involved in retinoid-mediated reporter induction in HepG2 cells (Fig. 1). These findings suggest that upon retinoid treatment, RXRα actively participates in RXRα/VDR-mediated activation of tk-(3A4)3-Luc reporter activity.
To further support the idea that retinoids actively contribute to transactivation of the tk-(3A4)3-Luc reporter through the RXR/VDR-mediated pathway, transient transfection assays were performed using wild-type mouse RXRα (mRXRα), mutant mouse RXRα (mRXRαY402A) expression plasmid, or both. mRXRαY402A is impaired in RXRα/hVDR heterodimer formation and blocks transactivation by D3 [17]. As expected, 9-cis-RA and all-trans-RA activate mRXRα/hVDR heterodimers and yield a higher reporter activity induction than that of mRXRαY402A/hVDR heterodimers. 9-cis-RA moderately but significantly activates both wild-type and mutant mRXRα homodimers and induces tk-(3A4)3-Luc reporter activity. The data demonstrate that both RXR/VDR and RXR/RXR signaling pathways can be activated by RA, thus RXR/VDR and RXR/RXR may be the pathways for retinoid-mediated activation of the CYP3A4 gene.
Although VDR levels in liver might be low, it has been revealed recently that VDR is present in human liver [9, 33]; as well as fetal, neonatal, and adult rat liver [34]. Furthermore, the level of VDR is much higher (>20-fold) in mouse than in human or rat hepatocytes [35]. Using VDR knockout mice might provide a direct evidence for the role of VDR in regulating Cyp3a11 gene expression. However, the liver expresses high levels of PXR and CAR, and these two nuclear receptors play the dominant roles in regulating Cyp3a11 gene expression [4, 12]. Thus, using VDR knockout mice might not demonstrate an obvious reduction of retinoid-mediated induction of Cyp3a11. However, PXR/CAR-double null mice will be a good model for investigating VDR in induction of Cyp3a11. Neither PXR (PCN) nor CAR (TCPOBOP) agonist could induce Cyp3a11 mRNA in double-null mice hepatocytes; in contrast, D3 induced Cyp3a11 mRNA in PXR/CAR double null mice. Therefore, our data showing the induction of Cyp3a11 mRNA by retinoids in PXR/CAR double knockout mice strongly suggests the in vivo role of VDR in mediating the induction of Cyp3a11. Although we exclude PXR and CAR mediated pathways in the up-regulation of Cyp3a11 gene expression by using PXR/CAR double knockout mice, we could not rule out the possibility that the elevated Cyp3a11 mRNA is mediated through RXRα homodimers or other unknown signaling pathways. PXR/CAR/VDR-triple knockout mouse model is required to definitely characterize the role of VDR in retinoid-mediated up-regulation of the Cyp3a11 gene. Moreover, a direct role of retinoid-mediated CYP3A induction through RXRα/VDR is evidenced by the result that 9-cis-retinal, 9-cis-RA, and all-trans-RA treatment is associated with the recruitment of RXRα and VDR to the response motifs (pER6 and dXREM) of the CYP3A4 gene as demonstrated by ChIP assays. These data strengthen the conclusion that the direct interaction of retinoids with the chromatin bound by RXRα and VDR is essential for the CYP3A4 induction.
Retinoids are promising in cancer therapy and chemoprevention, nevertheless, retinoid resistance in clinical treatment is frequent and therefore may be limited in clinical applications. Endogenous concentrations of retinoids in human plasma vary between nanomolar to micromolar range, thus the effects at 10 μM of RA used in the culture medium are not likely to be physiologically relevant. However, in HepG2 cells, the lowest dose that transactivated the RXRα/VDR heterodimer was 1 μM. In clinical treatment, plasma concentrations of all-trans-RA may be as high as 10 μM [36] and the concentrations within liver may exceed this level. It is likely that at pharmacological or toxicological doses, high levels of RA may be achieved and be sufficient to activate the RXR/VDR signaling pathway and up-regulate CYP3A4 gene expression. CYP3A4 enzyme plays dual roles in the biological fate of RA, converting retinal to biological active RA and deactivating RA through the oxidation pathway [2, 3]. The induced CYP3A4 may alter the synthesis and metabolism of retinoids, the metabolism of xenobiotics as well as endogenous compounds, which may be the basis for retinoid resistance and drug-drug interactions. Moreover, CYP3A4 is particularly sensitive to dietary constituents due to its high expression levels in the intestine as well as its broad substrate specificity. Many daily dietary components, such as egg yolk, milk, fish oil, liver and plants that contain large amounts of natural vitamin A and carotenoids, are enriched with retinoids. Furthermore, under normal cellular conditions in vivo, both retinal and RA may be present. Consequently, it is possible that inter-individual differences in dietary habits may partly account for inter-individual variations in CYP3A4 expression and related metabolic processes.
Taken together, this work suggests that certain retinoids such as 9-cis-retinal and RAs can induce CYP3A4 through the RXR/VDR- and RXR/RXR-mediated signaling pathway. It is likely that RXR/VDR and RXR/hPXR serve as a primary pathway in intestine and liver, respectively, to mediate retinoids regulated CYP3A4 expression.
Acknowledgments
The authors thank Dr. Hinrich Gronemeyer for providing the mRXRαY402A expression plasmid, Dr. Ronald Evans for RXRα, β, and γ expression plasmids. The authors thank Mr. Matthew Wortham for editing the manuscript.
The financial support from NIH grants CA53596, AA14147, and COBRE P20 RR021940 as well as the Molecular Biology Core under COBRE are greatly appreciated.
Abbreviations
- CYP
cytochrome P450
- RA
retinoic acid
- all-trans-RP
all-trans-retinol palmitate
- RBP
retinol-binding protein
- ADHs
alcohol dehydrogenase/reductases
- ALHDs
aldehyde dehydrogenase
- ER
everted repeat (prefixes “p” and “d” indicate proximal and distal, respectively)
- DR
direct repeat
- NR
nuclear receptor
- dXREM
distal xenobiotic-responsive enhancer module
- D3 or 1α25-(OH)2D3
1α, 25-dihydroxyvitamin D3
- VDRE
vitamin D-responsive element
- RT
reverse transcription
- Gadph
glyceraldehyde-3-phosphate dehydrogenase
- PCN
pregnenalone 16α-carbonitrile
- TCPOBOP
1,4-bis[2-(3,5-dichloropyridyloxy)]benzene
- CITCO
6-(4-Chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime
- TTNBP
4-(E-2-[5,6,7,8-tet-rahydro-5,5,8,8-tetramethyl-2-naphthalenyl]-1-propenyl) benzoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Njar VC, Gediya L, Purushottamachar P, Chopra P, Vasaitis TS, Khandelwal A, et al. Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases. Bioorg Med Chem. 2006;14:4323–40. doi: 10.1016/j.bmc.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 2.Zhang QY, Dunbar D, Kaminsky L. Human cytochrome P-450 metabolism of retinals to retinoic acids. Drug Metab Dispos. 2000;28:292–7. [PubMed] [Google Scholar]
- 3.McSorley LC, Daly AK. Identification of human cytochrome P450 isoforms that contribute to all-trans-retinoic acid 4-hydroxylation. Biochem Pharmacol. 2000;60:517–26. doi: 10.1016/s0006-2952(00)00356-7. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol. 1999;56:1329–39. doi: 10.1124/mol.56.6.1329. [DOI] [PubMed] [Google Scholar]
- 5.Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, et al. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci U S A. 1998;95:12208–13. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–23. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem. 2002;277:25125–32. doi: 10.1074/jbc.M201323200. [DOI] [PubMed] [Google Scholar]
- 10.Thummel KE, Brimer C, Yasuda K, Thottassery J, Senn T, Lin Y, et al. Transcriptional control of intestinal cytochrome P-4503A by 1alpha,25-dihydroxy vitamin D3. Mol Pharmacol. 2001;60:1399–406. doi: 10.1124/mol.60.6.1399. [DOI] [PubMed] [Google Scholar]
- 11.Pascussi JM, Drocourt L, Gerbal-Chaloin S, Fabre JM, Maurel P, Vilarem MJ. Dual effect of dexamethasone on CYP3A4 gene expression in human hepatocytes. Sequential role of glucocorticoid receptor and pregnane X receptor. Eur J Biochem. 2001;268:6346–58. doi: 10.1046/j.0014-2956.2001.02540.x. [DOI] [PubMed] [Google Scholar]
- 12.Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–9. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- 13.Xie W, Barwick JL, Simon CM, Pierce AM, Safe S, Blumberg B, et al. Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR. Genes Dev. 2000;14:3014–23. doi: 10.1101/gad.846800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang K, Mendy AJ, Dai G, Luo HR, He L, Wan YJ. Retinoids activate the RXR/SXR-mediated pathway and induce the endogenous CYP3A4 activity in Huh7 human hepatoma cells. Toxicol Sci. 2006;92:51–60. doi: 10.1093/toxsci/kfj207. [DOI] [PubMed] [Google Scholar]
- 15.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–6. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 16.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 17.Vivat-Hannah V, Bourguet W, Gottardis M, Gronemeyer H. Separation of retinoid X receptor homo- and heterodimerization functions. Mol Cell Biol. 2003;23:7678–88. doi: 10.1128/MCB.23.21.7678-7688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–3. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 19.Schmiedlin-Ren P, Thummel KE, Fisher JM, Paine MF, Watkins PB. Induction of CYP3A4 by 1 alpha,25-dihydroxyvitamin D3 is human cell line-specific and is unlikely to involve pregnane X receptor. Drug Metab Dispos. 2001;29:1446–53. [PubMed] [Google Scholar]
- 20.de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet. 1999;37:485–505. doi: 10.2165/00003088-199937060-00004. [DOI] [PubMed] [Google Scholar]
- 21.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–62. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 23.Goerz G, Bolsen K, Kalofoutis A, Tsambaos D. Influence of oral isotretinoin on hepatic and cutaneous P-450-dependent isozyme activities. Arch Dermatol Res. 1994;286:104–6. doi: 10.1007/BF00370735. [DOI] [PubMed] [Google Scholar]
- 24.Howell SR, Shirley MA, Ulm EH. Effects of retinoid treatment of rats on hepatic microsomal metabolism and cytochromes P450. Correlation between retinoic acid receptor/retinoid × receptor selectivity and effects on metabolic enzymes. Drug Metab Dispos. 1998;26:234–9. [PubMed] [Google Scholar]
- 25.Forman BM, Umesono K, Chen J, Evans RM. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–50. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 26.Schulman IG, Li C, Schwabe JW, Evans RM. The phantom ligand effect: allosteric control of transcription by the retinoid X receptor. Genes Dev. 1997;11:299–308. doi: 10.1101/gad.11.3.299. [DOI] [PubMed] [Google Scholar]
- 27.Germain P, Iyer J, Zechel C, Gronemeyer H. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature. 2002;415:187–92. doi: 10.1038/415187a. [DOI] [PubMed] [Google Scholar]
- 28.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 29.Li D, Li T, Wang F, Tian H, Samuels HH. Functional evidence for retinoid X receptor (RXR) as a nonsilent partner in the thyroid hormone receptor/RXR heterodimer. Mol Cell Biol. 2002;22:5782–92. doi: 10.1128/MCB.22.16.5782-5792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlberg C, Bendik I, Wyss A, Meier E, Sturzenbecker LJ, Grippo JF, et al. Two nuclear signalling pathways for vitamin D. Nature. 1993;361:657–60. doi: 10.1038/361657a0. [DOI] [PubMed] [Google Scholar]
- 31.Thompson PD, Remus LS, Hsieh JC, Jurutka PW, Whitfield GK, Galligan MA, et al. Distinct retinoid X receptor activation function-2 residues mediate transactivation in homodimeric and vitamin D receptor heterodimeric contexts. J Mol Endocrinol. 2001;27:211–27. doi: 10.1677/jme.0.0270211. [DOI] [PubMed] [Google Scholar]
- 32.Bettoun DJ, Burris TP, Houck KA, Buck DW, 2nd, Stayrook KR, Khalifa B, et al. Retinoid X receptor is a nonsilent major contributor to vitamin D receptor-mediated transcriptional activation. Mol Endocrinol. 2003;17:2320–8. doi: 10.1210/me.2003-0148. [DOI] [PubMed] [Google Scholar]
- 33.Berger U, Wilson P, McClelland RA, Colston K, Haussler MR, Pike JW, et al. Immunocytochemical detection of 1,25-dihydroxyvitamin D receptors in normal human tissues. J Clin Endocrinol Metab. 1988;67:607–13. doi: 10.1210/jcem-67-3-607. [DOI] [PubMed] [Google Scholar]
- 34.Segura C, Alonso M, Fraga C, Garcia-Caballero T, Dieguez C, Perez-Fernandez R. Vitamin D receptor ontogenesis in rat liver. Histochem Cell Biol. 1999;112:163–7. doi: 10.1007/s004180050403. [DOI] [PubMed] [Google Scholar]
- 35.Gascon-Barre M, Demers C, Mirshahi A, Neron S, Zalzal S, Nanci A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology. 2003;37:1034–42. doi: 10.1053/jhep.2003.50176. [DOI] [PubMed] [Google Scholar]
- 36.Lee JS, Newman RA, Lippman SM, Fossella FV, Calayag M, Raber MN, et al. Phase I evaluation of all-trans retinoic acid with and without ketoconazole in adults with solid tumors. J Clin Oncol. 1995;13:1501–8. doi: 10.1200/JCO.1995.13.6.1501. [DOI] [PubMed] [Google Scholar]