Abstract
The pathogenic yeast Candida albicans can grow in multiple morphological states including budded, pseudohyphal and true hyphal forms. The ability to interconvert between budded and hyphal forms, herein termed the budded-to-hyphal transition (BHT), is important for C. albicans virulence, and is regulated by multiple environmental and cellular signals. To identify small-molecule inhibitors of known cellular processes that can also block the BHT, a microplate-based morphological assay was used to screen the BIOMOL–Institute of Chemistry and Cell Biology (ICCB) Known Bioactives collection from the ICCB-Longwood Screening Facility (Harvard Medical School, Boston, MA, USA). Of 480 molecules tested, 53 were cytotoxic to C. albicans and 16 were able to block the BHT without inhibiting budded growth. These 16 BHT inhibitors affected protein kinases, protein phosphatases, Ras signalling pathways, G protein-coupled receptors, calcium homeostasis, nitric oxide and guanylate cyclase signalling, and apoptosis in mammalian cells. Several of these molecules were also able to inhibit filamentous growth in other Candida species, as well as the pathogenic filamentous fungus Aspergillus fumigatus, suggesting a broad fungal host range for these inhibitory molecules. Results from secondary assays, including hyphal-specific transcription and septin localization analysis, were consistent with the inhibitors affecting known BHT signalling pathways in C. albicans. Therefore, these molecules will not only be invaluable in deciphering the signalling pathways regulating the BHT, but also may serve as starting points for potential new antifungal therapeutics.
INTRODUCTION
Candida albicans is a major opportunistic pathogen of immunocompromised hosts (Schmidt-Westhausen et al., 1991; Warnock, 2007), as well as a leading cause of nosocomial bloodstream infections, especially in patients with indwelling medical devices (Kojic & Darouiche, 2004; Lynch & Robertson, 2008). C. albicans can grow as either budded (yeast-like) or filamentous cells, the latter comprising pseudohyphae and true hyphae (Sudbery et al., 2004). Both budded and hyphal cells are found at sites of infection, and the ability to undergo the budded-to-hyphal transition (BHT) is important for virulence (Saville et al., 2003). C. albicans mutants that have defects in the BHT have a reduced ability to become internalized and to cause endothelial cell injury in vitro (Phan et al., 2000), and small-molecule inhibitors of the BHT can protect endothelial cells from C. albicans-induced cell damage (Toenjes et al., 2005). These data are indicative that the BHT can modulate the ability of C. albicans to cause endothelial cell injury and suggest that the BHT is critical for systemic candidiasis.
The BHT occurs in response to a variety of environmental signals, including temperature above 35 °C, pH above 6.5, nutrient starvation and growth in serum (Ernst, 2000). Therefore, it is not surprising that multiple signalling pathways regulate the BHT (Biswas et al., 2007; Brown, 2002). Genetic analysis of these signalling pathways has been hampered by the inability to isolate recessive loss-of-function mutants in the diploid C. albicans. For this reason, a ‘forward genetic’ approach using small-molecule inhibitors to study the BHT was initiated (Toenjes et al., 2005).
The use of small organic molecules in deciphering complex biological processes has been very informative (Lokey, 2003; Nehil et al., 2007; Ward et al., 2002). Previously, we developed a microplate-based morphological assay that identified seven novel small molecules that inhibited the BHT without affecting budded growth (Toenjes et al., 2005). To augment this forward genetic approach, the BIOMOL–Institute of Chemistry and Cell Biology (ICCB) Known Bioactives collection, which contains 480 molecules that inhibit known cellular targets or processes, was screened for cytotoxic molecules and BHT inhibitors. We identified 53 molecules that were cytotoxic to C. albicans and 16 molecules that inhibited the BHT without affecting budded growth. Several of these molecules also inhibited filamentous growth in the pathogenic filamentous fungus Aspergillus fumigatus. This raises the possibility that these molecules or their bioactive derivatives may be good starting points for the development of new antifungal therapeutics.
METHODS
C. albicans strains and media.
Protocols for the growth and maintenance of C. albicans strains have been described previously (Toenjes et al., 2005). Growth in nutrient-limiting Spider medium (Liu et al., 1994) was used to induce hyphal growth at 37 °C, as described previously (Toenjes et al., 2005). The following C. albicans strains were used in this study: SC5314 (wild-type clinical isolate); YAW2 (pADH-CDC10-GFP ura3 : : λimm434/ura3 : : λimm434 his1 : : hisG/his1 : : hisG arg4 : : hisG/arg4 : : hisG, kindly provided by James Konopka, Stony Brook University, Stony Brook, NY, USA (Warenda & Konopka, 2002; Warenda et al., 2003); and KTCa1 (URA3-pHWP1-GFP ura3 : : limm434/ura3 : : limm434; Toenjes et al., 2005), which has the green fluorescent protein (GFP) encoding gene expressed under the control of the hyphal-induced HWP1 promoter (HWP1 pr–GFP). Induction of HWP1 pr–GFP expression was observed upon growth in Spider medium at 37 °C.
Microplate-based morphological assay.
The microplate-based morphological assay utilized in these studies has been described previously (Toenjes et al., 2005). In the initial screen, C. albicans SC5314 cells from a single colony were grown in YNB medium (0.67 % yeast nitrogen base, 2 % glucose and required amino acids, pH 7) overnight at 30 °C with shaking. The culture was diluted 1 : 25 000 into hyphal-inducing Spider medium and 100 μl was placed into each well of a 384-well microplate containing small molecules from the BIOMOL–ICCB Known Bioactives collection (BIOMOL International, LP, Plymouth Meeting, PA, USA; see http://iccb.med.harvard.edu/screening/compound_libraries/bioactives_biomol_med.htm for a list of the molecules) obtained from the ICCB-Longwood Screening Facility (Harvard Medical School, Boston, MA, USA). Molecules were tested at two concentrations: high concentration was ∼130 μM for most compounds or ∼13 μM for ‘potent’ compounds (see http://iccb.med.harvard.edu for more details), whereas medium concentration was ∼30 μM for most compounds or ∼3 μM for ‘potent’ compounds. Plates were incubated at 37 °C for 4 h, at which time the cells in each well were fixed by adding glutaraldehyde (electron microscopy grade; Acros Organics/Fisher Scientific) to a final concentration of 5 % (v/v) for light microscopy.
Quantification of BHT inhibition was accomplished by counting the number of individual budded cells versus the number of hyphae in the population. More than 100 cells were counted for each well and all assays were repeated at least twice. Those molecules that showed more than 95 % budded cells after 4 h were examined further. The IC100 value for selected BHT inhibitory molecules (i.e. lowest concentration of molecule used at which 100 % budded cells were observed) was determined using serial dilutions from 200 to 1 μM of the individual molecules.
To test for cytotoxicity, C. albicans cells were incubated with the molecule in Spider medium for 24 h at 37 °C, after which time the wells were checked visually for turbidity and the cells examined morphologically. If there was no difference between the 4 and 24 h time points, the molecules were deemed either cytotoxic or cytostatic. The BHT inhibitors displayed significant budded growth over the 24 h time period. To verify further that the BHT inhibitors were not cytotoxic, the well contents from the 24 h incubations were resuspended by pipetting and 5 μl plus serial dilutions were incubated on YPD+uridine plates at 37 °C to test for growth.
The reversibility assay was performed as described previously (Toenjes et al., 2005). Briefly, the growth medium+molecule was removed after 4 h of incubation at 37 °C, and the cells were washed once with Spider medium, resuspended in 100 μl of fresh Spider medium without the molecule and incubated for 8 h at 37 °C before fixation.
A. fumigatus conidial germination.
A. fumigatus clinical isolate strain Af293, from which the Aspergillus genomic sequence has been determined (Nierman et al., 2005), was kindly provided by Dr Kieren Marr, University of Washington, Seattle, WA, USA. Approximately 1×105 conidia from strain Af293 were incubated in either YNB medium (germination negative control) or Spider medium (germination positive control) with 100 μM selected BHT inhibitor for 8 h at 37 °C and then fixed for microscopic examination. The reversibility of these molecules was tested by incubation of the A. fumigatus with the molecules for 8 h followed by removal of the medium, washing with Spider medium and reincubation with fresh Spider medium without the molecules for 8 h. Inhibition of hyphal elongation was tested by incubating A. fumigatus conidia in Spider medium without the molecule for 7 h at 37 °C to allow hyphae to form, followed by removal of the medium, replacement with Spider medium containing the molecule and incubation for an additional 5 h at 37 °C.
Microscopy techniques.
For light microscopy, C. albicans cells in 384-well microplates with an optical plastic bottom (BD Falcon) were routinely viewed on a Nikon TE300 or TE200 microscope with differential interference contrast/Hoffman optics and a 20× objective. Images of each well were obtained either using a SpotRT monochrome camera (TE300) or driven by In Vivo software (QED Imaging). For fluorescent microscopy, C. albicans cells in 384-well microplates with an optical-glass bottom (BD Falcon) were viewed on a Nikon TE300 microscope using Nikon filter sets. HWP1 pr–GFP expression was observed using KTCa1 cells in the assay as described previously (Toenjes et al., 2005), using a GFP HYQ filter set and a 20× objective. Localization of the Cdc10p septin protein in YAW2 cells was as described previously (Toenjes et al., 2005), using a Nikon TE200 inverted microscope, a GFP HYQ filter set and a 60× objective.
RESULTS AND DISCUSSION
Current antifungal therapies are limited to drugs such as amphotericins, azoles and echinocandins that inhibit the growth of C. albicans cells rather than specific virulence processes. Unfortunately, these drugs can have inhibitory effects on human cells, leading to serious side effects for the host. Therefore, new approaches to discovering possible antifungal therapeutics that target specific virulence factors and processes are needed. One such approach could be based on the ability of small molecules to inhibit the BHT without affecting the growth of C. albicans cells. Data from a number of laboratories have been indicative that the BHT is required for C. albicans virulence (Saville et al., 2003).
Identification of cytotoxic molecules
A microplate-based morphogenesis assay (Toenjes et al., 2005) was used to screen the BIOMOL–ICCB Known Bioactives collection, which contains 480 small molecules whose mammalian cellular targets and/or biological activities have been well characterized. The initial assay was for molecules that inhibited C. albicans hyphal formation in Spider medium, i.e. only budded cells were observed in the wells. This inhibition could be the result of either cytotoxicity of the molecule or inhibition of the BHT. Initially, two concentrations of molecules were assayed. The medium concentration (∼30 μM concentration) screen yielded 21 molecules that inhibited hyphae formation, whereas the high concentration (∼130 μM concentration) screen yielded 74 molecules. Subsequent retesting of the high-concentration collection eliminated five molecules from the screen. Cytotoxicity assays of the remaining 69 molecules indicated that 53 molecules were cytotoxic after incubation for 24 h (Table 1), whereas 16 molecules did not inhibit budded growth but did inhibit the BHT (Table 2).
Table 1.
Cytotoxic molecules
Unless indicated otherwise, the molecules were tested at high concentrations of ∼130 μM against SC5314 cells in Spider medium for 4 h at 37 °C.
| Molecule | Mechanism of action | Reference |
|---|---|---|
| Kinases and phosphatases | ||
| 5-Iodotubercidin | Adenosine kinase inhibitor, ERK2 inhibitor | Massillon et al. (1994) |
| AG213 (tyrphostin 47) | EGF receptor kinase inhibitor | Yaish et al. (1988) |
| BAY 11-7082* | Inhibits TNF-α-inducible phosphorylation | Pierce et al. (1997) |
| Chelerythrine* | Protein kinase C inhibitor | Herbert et al. (1990) |
| Edelfosine (Et-18-OCH3) | Protein kinase C inhibitor, Na+/K+ ATPase inhibitor | Zheng et al. (1990) |
| AMG-PC (Et-16-OCH3) | Protein kinase C and diacylglycerol kinase inhibitor | Grosman (1991) |
| N,N-Dimethylsphingosine† | Protein kinase C and sphingosine kinase inhibitor | Yatomi et al. (1996) |
| Geldanamycin | Binds HSP90, tyrosine kinase inhibitor | Whitesell et al. (1994) |
| K252A† | Protein kinase inhibitor, broad spectrum | Kase et al. (1987) |
| ML-7 | Myosin light chain kinase inhibitor | Saitoh et al. (1987) |
| ML-9 | Myosin light chain kinase inhibitor | Saitoh et al. (1987) |
| Rapamycin | Known antifungal, FK506-binding protein inhibitor | Cruz et al. (2001) |
| Ascomycin (FK520) | FK506-binding protein binder, inhibits calcineurin phosphatase | Wallace et al. (1994) |
| Ro 31-8220 | Protein kinase C inhibitor | McKenna & Hanson (1993) |
| Staurosporine* | Protein kinase inhibitor, broad spectrum | Tamaoki et al. (1986) |
| Tamoxifen* | Known antifungal, protein kinase C inhibitor | Beggs (1993) |
| Calyculin A† | Protein phosphatase 1 and 2A inhibitor | Ishihara et al. (1989) |
| RK-682* | Protein tyrosine phosphatase inhibitor | Hamaguchi et al. (1995) |
| Anisomycin* | Bacterial translation inhibitor, activates JNK/SAPKs | Zinck et al. (1995) |
| Receptor antagonists or agonists | ||
| Enantio-PAF C16† | Platelet-activating factor receptor antagonist | Wykle et al. (1981) |
| Mastoparan* | G protein activator | Higashijima et al. (1988) |
| Ion homeostasis | ||
| Calcimycin (A-23187) | Ca2+ ionophore | Gietzen et al. (1982) |
| Bepridil* | Ca2+ channel blocker | Zeller & Spinler (1987) |
| Dichlorobenzamil | Ca2+ channel blocker | Xu et al. (1999) |
| FCCP* | Mitochondrial uncoupling agent | Collins et al. (2000) |
| Hinokitiol* | Fe2+ chelator, induces apoptosis | Tanaka et al. (1999) |
| Penitrem A | Ca2+-activated K+ (maxi-K) channel blocker | Knaus et al. (1994) |
| Phenamil | Amiloride-sensitive Na+ channel inhibitor | Garvin et al. (1985) |
| SK&F-96365 | Receptor-mediated Ca2+ entry inhibitor | Merritt et al. (1990) |
| Tetrandrine | Ca2+ and K+ channel blocker | Wang & Lemos (1992) |
| TPEN | Zn2+ chelator, induces apoptosis | McCabe et al. (1993) |
| Trifluoperazine* | Calmodulin antagonist, anti-schizophrenic drug | Rao (1987) |
| Valinomycin* | K+ ionophore | Pressman (1976) |
| NO and guanylate cyclase | ||
| Diphenyleneiodonium chloride | NO synthase inhibitor | Stuehr et al. (1991) |
| Thiocitrulline* | NO synthase inhibitor | Frey et al. (1994) |
| Furoxan | NO donor, guanylate cyclase activator | Medana et al. (1994) |
| LY-83583 | Guanylate cyclase inhibitor | Mülsch et al. (1989) |
| Signalling pathways | ||
| Cerulenin | Known antifungal, protein prenylation inhibitor | Hoberg et al. (1983) |
| Gliotoxin* | Fungal toxin, farnesyltransferase and geranylgeranyltransferase inhibitor | Vigushin et al. (2004) |
| Manumycin A | Farnesyltransferase inhibitor | Hara et al. (1993) |
| U73122* | Phospholipase C activation inhibitor | Smith et al. (1990) |
| Vinpocetine | Phosphodiesterase I inhibitor | Hagiwara et al. (1984) |
| Wiskostatin* | N-WASP inhibitor, inhibits actin filament assembly | Peterson & Mitchison (2002) |
| Caffeic acid phenethylester | NF-κB inhibitor | Natarajan et al. (1996) |
| Lipooxygenase inhibitors | ||
| 5,8,11-Eicosatriynoic acid† | 5-, 12- and 15-lipooxygenase inhibitor | Salari et al. (1984) |
| Cinnamyl-3,4-dihydroxycyanocinnamate | 12-Lipooxygenase inhibitor | Cho et al. (1991) |
| Ebselen* | Lipooxygenase and protein kinase C inhibitor | Cotgreave et al. (1989) |
| Assorted cellular functions | ||
| 3,4-Dichloroisocoumarin | Serine protease inhibitor | Powers et al. (1989) |
| β-Lapachone | Known antifungal, inhibits DNA topoisomerase I | Guiraud et al. (1994) |
| Brefeldin A* | Known antifungal, endoplasmic reticulum-to-Golgi transport inhibitor | Klausner et al. (1992) |
| Lycorine | Protein synthesis inhibitor, apoptosis inducer | Vrijsen et al. (1986) |
| Splitomycin | Sir2p histone deacetylase inhibitor | Bedalov et al. (2001) |
| Tunicamycin* | Inhibits N-linked glycosylation | Duksin & Mahoney (1982) |
EGF, Epidermal growth factor; FCCP, carbonylcyanide-4-(trifluoromethoxy)-phenylhydrazone; TPEN, N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine.
*These molecules were tested at medium concentrations of ∼28.5 μM.
†These were high-potency molecules that were tested at concentrations of ∼13 μM; cytotoxicity was determined by a lack of cell growth after 24 h.
Table 2.
BHT inhibitors
Molecules were tested against SC5314 cells in Spider medium for 4 h at 37 °C. Inhibition of the BHT was determined by a lack of hyphal cells after 4 h incubation.
| Molecule (IC100) | Mechanism of action | Reference |
|---|---|---|
| Kinases, phosphatases, Ras signalling pathways | ||
| A-3 (60 μM) | PKA inhibitor, broad spectrum | Inagaki et al. (1986) |
| W7 (40 μM) | Protein kinase C inhibitor, calmodulin antagonist | Kawamoto & Hidaka (1984) |
| Tyrphostin AG1478 (80 μM) | EGF receptor tyrosine kinase inhibitor | Fan et al. (1995) |
| Tyrphostin 9 (80 μM) | EGF and platelet-derived growth factor receptor tyrosine kinase inhibitor | Levitzki & Gilon (1991) |
| GW 5074 (60 μM) | cRaf-1 kinase inhibitor | Lackey et al. (2000) |
| FK506 (60 μM) | Known antifungal, calcineurin phosphatase inhibitor | Schreiber & Crabtree (1992) |
| L-744,832 (130 μM) | Farnesyltransferase inhibitor | Barrington et al. (1998) |
| Receptor antagonists or agonists | ||
| Clozapine (50 μM) | Dopamine GPCR antagonist | Ereshefsky et al. (1989) |
| Fluspirilene (40 μM) | Dopamine receptor antagonist | Sah & Bean (1994) |
| GW 9662 (130 μM) | Peroxisome proliferator-activated receptor antagonist | Leesnitzer et al. (2002) |
| ETYA (12 μM) | Lipoxygenase, cyclooxygenase inhibitor | Salari et al. (1984) |
| Ion homeostasis | ||
| CGP-37157 (40 μM) | Mitochondrial Na+/Ca2+ exchanger inhibitor | Cox et al. (1993) |
| TMB-8 (40 μM) | Intracellular Ca2+ antagonist, inhibits Ca2+ release from endoplasmic reticulum | Chiou & Malagodi (1975) |
| Nigericin (130 μM) | Known weak antifungal, K+/H+ ionophore | Vercesi et al. (1993) |
| NO and guanylate cyclase | ||
| YC-1 (60 μM) | Guanylate cyclase activator | Mülsch et al. (1997) |
| Apoptosis signalling pathways | ||
| HA14-1 (20 μM) | Bcl-2 inhibitor | Degterev et al. (2001) |
Among the cytotoxic molecules were several molecules that have known antifungal activity, such as tamoxifen (Beggs, 1993), β-lapachone (Guiraud et al., 1994), brefeldin A (Arioka et al., 1991), rapamycin (Cruz et al., 2001) and cerulenin (Hoberg et al., 1983). In addition to the protein kinase C inhibitor tamoxifen, 17 other known protein kinase or protein phosphatase inhibitors were identified as cytotoxic (Table 1). The calcineurin phosphatase inhibitor tacrolimus (FK506) was not cytotoxic to C. albicans, which has been determined by Steinbach et al. (2007), but it did inhibit the BHT (Table 2). Interestingly, ascomycin (FK520), another calcineurin phosphatase inhibitor, along with the phosphatase inhibitor calyculin A were cytotoxic, similar to rapamycin, an FK506-binding protein inhibitor, whereas other calcineurin or phosphatase inhibitors in the collection, such as cyclosporine A, cypermethrin and okadaic acid, had no effect (data not shown). These observations indicated that inhibitors of calcineurin or its regulators could have variable effects on C. albicans growth and morphogenesis.
Inhibitors of a variety of other mammalian signalling pathways were cytotoxic to C. albicans, including receptor antagonists such as enantio-PAF C16, and molecules that affected calcium and other ion homeostasis such as bepridil and valinomycin. As with the calcineurin inhibitors described above, other molecules in the Known Bioactives collection that inhibited these cellular processes had variable effects on C. albicans. Overall, these data reinforce the notion that inhibiting cellular signalling pathways can have a detrimental effect on cell growth, and raise the possibility that one or more of these molecules may have potent antifungal activity that can be exploited in the future.
Of particular interest was the identification of inhibitors of lipooxygenases and cyclooxygenases within the screen. Cyclooxygenases and lipooxygenases convert arachidonic acid to eicosanoids, specifically prostaglandins and leukotrienes, respectively. C. albicans cells produce a variety of eicosanoids, which may regulate cell growth, morphogenesis, biofilm formation and virulence (Alem & Douglas, 2005; Noverr et al., 2003). However, no cyclooxygenases or lipooxygenases have been identified within the annotated C. albicans genome database. In our assay, addition of arachidonic acid, as well as 40 other eicosanoids or bioactive lipids, such as prostaglandins B1, B2, D2, E1, E2, F2, F1a and I2, and linoleic acid, had no effect on cell viability or the BHT. However, the lipooxygenase inhibitors 5,8,11-eicosatriynoic acid, cinnamyl-3,4-dihydroxycyanocinnamate and ebselen were cytotoxic to C. albicans (Table 1), whereas the lipooxygenase inhibitor 5,8,11,14-eicosatetraynoic acid (ETYA; IC100 12 μM) inhibited the BHT (Table 2), and the lipooxygenase/cyclooxygenase inhibitors 5(S)-HETE, BW-B 70C, AA-861, NS-398, REV-5901, indomethacin, nimesulide, piroxicam and resveratrol had no effect (data not shown). Indomethacin, nimesulide and piroxicam were previously shown to have modest effects on C. albicans hyphal formation and biofilm formation (Alem & Douglas, 2004). Interestingly, aspirin, one of the most commonly used cyclooxygenase inhibitors, has been shown to be cytotoxic and to inhibit C. albicans biofilm formation (Alem & Douglas, 2004). As C. albicans does not have any recognizable cyclooxygenases or lipooxygenease, it remains to be determined whether ETYA is inhibiting the BHT by inhibiting lipooxygenases and eicosanoid synthesis. Taken together, these data reinforce the notion that eicosanoid synthesis is important for cell viability and regulating the BHT in C. albicans, and that different inhibitors of the same cellular enzymes can have differential effects in vivo.
Interestingly, the nitric oxide (NO) synthase inhibitors diphenyleneiodonium chloride and thiocitrulline, along with the guanylate cyclase inhibitor LY-83583 and activator furoxan, were cytotoxic to C. albicans (Table 1), whereas the guanylate cyclase activator YC-1 (IC100 60 μM) inhibited the BHT (Table 2). NO is an activator of soluble guanylate cyclases in mammalian cells and has been shown to have potent candidacidal activity independent of its guanylate cyclase activation when produced by macrophages within the host (Ullmann et al., 2004). As was seen with other molecules within the collection, certain NO synthase inhibitors (1400W, 3-bromo-7-nitroindazole), guanylate cyclases activators (LY-83583, minoxidil) and cyclic GMP phosphodiesterase inhibitors (MY-5445, MBCQ, 8-bromo-cGMP) had no effect in the assay (data not shown). Surprisingly, there is no recognizable guanylate cyclase or NO synthase encoded in the C. albicans genome, so the mechanism of action of the BHT inhibitor YC-1 and the other cytotoxic molecules is not obvious.
Clearly, the 53 cytotoxic molecules identified in this study could have powerful roles in antifungal therapeutics, and thus may be excellent starting points for the development of new antifungal drugs. It is important to note that there were many other molecules in the Known Bioactives collection that were predicted to inhibit the same cellular processes as these 53 cytotoxic molecules, yet these molecules had no effect on C. albicans growth. This disparity could be partially explained by the inability of some of the inactive molecules to enter the C. albicans cell through its cell wall and membrane, although this explanation is unlikely to suffice for all inactive molecules. The fact that different molecules predicted to affect the same cellular process or protein can have differential effects is more likely to be the result of dosage effects or different interactions between the molecules and their cellular targets. Therefore, identifying and characterizing the cellular targets for the BHT inhibitors is a high priority for future studies.
Identification of BHT inhibitors
The 16 BHT inhibitors identified in this study (Table 2; Supplementary Table S1 available with the online journal) were not fungicidal, suggesting that these molecules were not inhibiting essential proteins and processes needed for growth in C. albicans. These inhibitors affected a variety of signalling pathway components, including kinases and phosphatases. Among the kinase inhibitors, molecule A-3 (IC100 60 μM) has a broad inhibitory spectrum against protein kinases, but seems to have better efficacy against protein kinase A (PKA) (Inagaki et al., 1986), whereas its structural homologue W7 (IC100 40 μM) is a more effective protein kinase C inhibitor (Kawamoto & Hidaka, 1984; Tanaka et al., 1983). Identification of these kinase inhibitors as BHT inhibitors was particularly encouraging, as the role of PKA in regulating the BHT is well established (Sonneborn et al., 2000). The two tyrphostin molecules AG1478 (IC100 80 μM) and tyrphostin 9 (IC100 80 μM) are effective against mammalian tyrosine kinases (Fan et al., 1995), whereas GW 5074 (IC100 60 μM) inhibits the cRaf-1 kinase (Lackey et al., 2000), which functions downstream of the Ras GTPase. Identification of L-744,832, a known farnesyltransferase and Ras inhibitor (Barrington et al., 1998), as a BHT inhibitor, together with GW 5074, reinforces the central role that the Ras signalling pathway plays in regulating the BHT (Leberer et al., 2001).
Identification of the dopamine G protein-coupled receptor (GPCR) antagonists clozapine (IC100 50 μM) and fluspirilene (IC100 40 μM) as BHT inhibitors was very interesting, especially given that clozapine and a number of its bioactive derivatives are FDA approved for the treatment of atypical schizophrenia (Ereshefsky et al., 1989). There are only three annotated GPCRs in C. albicans, with two being the STE2 and STE3 pheromone receptors. The remaining GPCR is Gpr1p, which has been implicated in a nutrient-regulated BHT signalling pathway upstream of PKA (Maidan et al., 2005; Miwa et al., 2004). It remains to be determined whether clozapine and fluspirilene function through the Gpr1p GPCR.
The BHT inhibitors CGP-37157 (IC100 40 μM), TMB-8 (IC100 40 μM), FK506 (IC100 60 μM), W7 (IC100 40 μM), HA14-1 (IC100 20 μM) and nigericin (IC100 130 μM) are known to affect calcium homeostasis in the cell, by affecting calcium/calmodulin-dependent protein kinases (W7; Kawamoto & Hidaka, 1984), the calcineurin phosphatase (FK506; Schreiber & Crabtree, 1992) or intracellular calcium levels (CGP-37157, Cox et al., 1993; TMB-8, Chiou & Malagodi, 1975; nigericin, Vercesi et al., 1993; and HA14-1, Degterev et al., 2001). Although FK506 does not inhibit the growth of C. albicans cells, it has been shown to display synergistic effects with the known azole antifungal drug fluconazole (Uppuluri et al., 2008). Cyclosporine A also showed this synergy, but did not inhibit the BHT on its own (data not shown), as FK506 did.
Interesting, HA14-1 has also been shown to be an inducer of apoptosis in mammalian cells (Degterev et al., 2001). However, several other inducers of apoptosis in the collection (C8 ceramine, 5d-prostaglandin J2, lysophosphatidic acid and Y-27632) were not cytotoxic and did not inhibit the BHT (data not shown). Apoptosis is regulated in mammalian cells by the Bcl-2 family proteins Bcl-2, Bcl-lx, Bak and Bax (Danial & Korsmeyer, 2004). HA14-1 induces apoptosis by specifically binding to the Bcl-2 homology 3 domain of Bcl-2, thereby preventing the antagonistic interaction between Bcl-2 and the Bak/Bax proteins. Unfortunately, there are no recognizable Bcl-2 family proteins encoded in fungal genomes (Fedorova et al., 2005; Madeo et al., 2002); therefore, the target of HA14-1 and its mechanism of action in BHT inhibition remain to be elucidated.
BHT inhibitors block conidial germination and hyphal formation in A. fumigatus and other Candida species
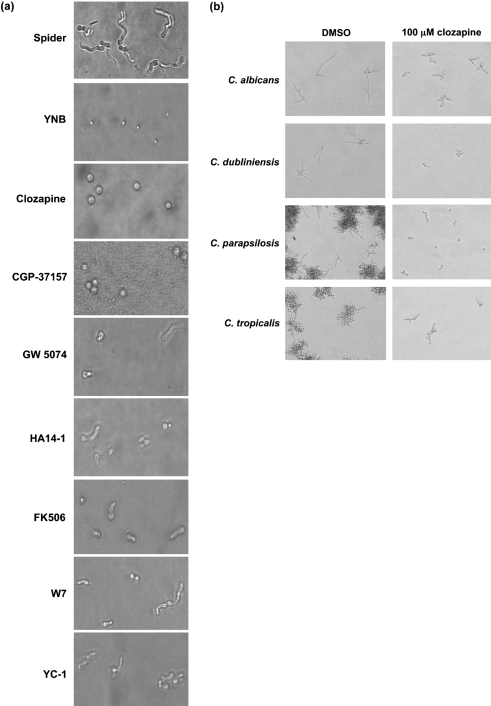
The effects of 11 of the 16 BHT inhibitors (excluding tyrphostin 9, L-744,832, fluspirilene, ETYA and nigericin) on conidial germination and hyphal elongation in the pathogenic filamentous fungus A. fumigatus were also examined. Aspergillus species are second only to Candida species in causing systemic human fungal infections, with A. fumigatus being the most prevalent cause of human aspergillosis (Warnock, 2007). We observed that addition of clozapine and CGP-37157 blocked conidia germination and hyphae formation, whilst addition of GW 5074, HA14-1, FK506, W7 and YC-1 had partial effects after an 8 h incubation (Fig. 1a). Incubation with HA14-1, FK506, W7 and YC-1 for an additional 6 h showed significant conidial germination and hyphal elongation, suggesting that these molecules were not effective inhibitors (data not shown). The effects of clozapine and CGP-37157 were reversible, as they are in C. albicans, and both molecules could also inhibit hyphal elongation following conidial germination (data not shown).
Fig. 1.
(a) Effects of BHT inhibitors on conidial germination in A. fumigatus. The indicated small molecules (100 μM final concentration) were incubated with ∼105 A. fumigatus conidia in Spider medium for 8 h at 37 °C. (b) Effects of clozapine on hyphal formation in Candida species. Clozapine (100 μM final concentration) was incubated with the indicated Candida species in Spider medium for 4 h at 37 °C. Hyphal growth of cells with the addition of DMSO was used as a control.
The same 11 molecules were examined for their effects on morphogenesis in other Candida species. Only three molecules, clozapine (Fig. 1b), HA14-1 and ETYA (data not shown), inhibited filamentous growth in Candida dubliniensis, Candida parapsilosis and Candida tropicalis. Taken together, these results suggested that a subset of the BHT inhibitors, including clozapine, may have a broad host range among yeast and filamentous fungi, and hence may be better starting candidates for antifungal therapeutics.
Effects of BHT inhibitors on hyphae-associated cellular processes
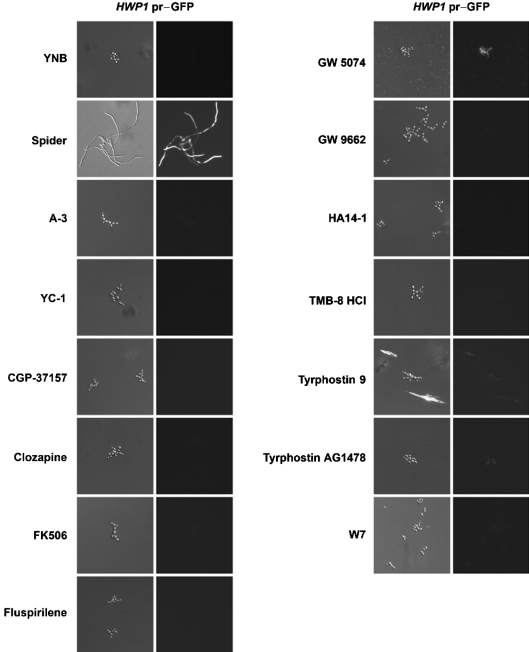
The effects of 13 of the 16 BHT inhibitors (excluding L-744,832, ETYA and nigericin) on the expression of the hyphal-specific HWP1 pr–GFP reporter gene were determined. Previously, we showed that expression of the HWP1 pr–GFP reporter gene was strongly induced under hypha-inducing conditions, such as growth in Spider medium, and that small-molecule BHT inhibitors could have differential effects on HWP1 pr–GFP expression (Toenjes et al., 2005). Of the 13 BHT inhibitors tested, only GW 5074-treated cells showed appreciable fluorescence (Fig. 2). This result suggested that the remaining 12 molecules tested inhibited the expression of HWP1 pr–GFP, possibly by inhibiting a component of the EFG1, NRG1 or TUP1 transcriptional pathway that regulates HWP1 expression. GW 5074 inhibits mammalian c-Raf kinase, which functions downstream of the Ras GTPase. In C. albicans, Ras is believed to signal through the EFG1 transcription factor pathway (Biswas et al., 2007), so it is not clear why HWP1 pr–GFP expression was observed in GW 5074-treated cells; an alternative target for GW 5074 is a likely possibility.
Fig. 2.
Effects of BHT inhibitors on HWP1 pr–GFP expression. The indicated small molecules (100 μM final concentration) were incubated with KTCa1 cells in Spider medium for 4 h at 37 °C.
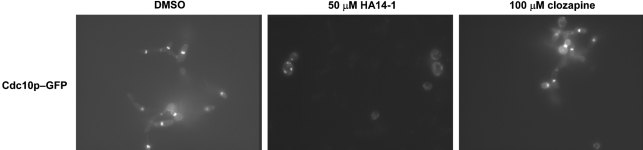
The effects of the bioactive molecules on septin ring assembly were also examined. Septins play an important role in cellular morphogenesis and polarized growth in C. albicans and most other eukaryotes (Warenda et al., 2003). YAW2 cells expressing a septin Cdc10p–GFP fusion protein were used to examine septin organization. Of the nine BHT inhibitors tested (YC-1, tyrphostin 9, HA14-1, GW 5074, FK506, GW 9662, fluspiriline, clozapine and TMB-8), only HA14-1 showed a defect in the localization of the Cdc10p–GFP fusion protein (Fig. 3). It remains to be determined whether this abnormal septin localization is a cause of, or the consequence of, BHT inhibition.
Fig. 3.
Effects of BHT inhibitors on septin localization. HA14-1 (50 μM final concentration) and clozapine (100 μM final concentration) were incubated with YAW2 cells in Spider medium for 4 h at 37 °C. Addition of HA14-1 resulted in abnormal septin localization, whereas addition of clozapine had no effect. Addition of DMSO was used as a control.
The molecules identified in this study will hopefully prove to be a useful and informative resource, not only for new antifungal therapeutics but also as tools to decipher the signal-transduction pathways that regulate cell growth and the BHT in C. albicans. The fact that several of these molecules also affected morphogenetic processes in other Candida species and in the filamentous fungus A. fumigatus raises the exciting possibility of a broad fungal host range for these molecules, as well as conservation of function for the affected signalling pathways and the targets of the molecules in both budded and filamentous fungal species.
Supplementary Material
Acknowledgments
We thank Gary Ward, James Konopka and Kieren Marr for valuable reagents, the ICCB-Longwood Screening Facility for the BIOMOL–ICCB Known Bioactives collection, Ted Wilson and Dan Reichert for technical support, and Gary Ward, David K. Butler and members of the Johnson and Toenjes laboratories for valuable discussions and critical comments on this manuscript. This research was supported in part by USDA-Hatch grant VT-H01308 from the Vermont Experiment Station (D. I. J.), and NIH grant P20 RR16455-04 from the INBRE Program of the National Center for Research Resources (K. A. T.).
Abbreviations
BHT, budded-to-hyphal transition
ETYA, 5,8,11,14-eicosatetraynoic acid
GFP, green fluorescent protein
GPCR, G protein-coupled receptor
ICCB, Institute of Chemistry and Cell Biology
NO, nitric oxide
PKA, protein kinase A
Footnotes
A table of the structures of the BHT inhibitors is available as supplementary material with the online version of this paper.
References
- Alem, M. A. S. & Douglas, L. J. (2004). Effects of aspirin and other nonsteroidal anti-inflammatory drugs on biofilms and planktonic cells of Candida albicans. Antimicrob Agents Chemother 48, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alem, M. A. S. & Douglas, L. J. (2005). Prostaglandin production during growth of Candida albicans biofilms. J Med Microbiol 54, 1001–1005. [DOI] [PubMed] [Google Scholar]
- Arioka, M., Hirata, A., Takatsuki, A. & Yamasaki, M. (1991). Brefeldin A blocks an early stage of protein transport in Candida albicans. J Gen Microbiol 137, 1253–1262. [DOI] [PubMed] [Google Scholar]
- Barrington, R. E., Subler, M. A., Rands, E., Omer, C. A., Miller, P. J., Hundley, J. E., Koester, S. K., Troyer, D. A., Bearss, D. J. & other authors (1998). A farnesyltransferase inhibitor induces tumor regression in transgenic mice harboring multiple oncogenic mutations by mediating alterations in both cell cycle control and apoptosis. Mol Cell Biol 18, 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedalov, A., Gatbonton, T., Irvine, W. P., Gottschling, D. E. & Simon, J. A. (2001). Identification of a small molecule inhibitor of Sir2p. Proc Natl Acad Sci U S A 98, 15113–15118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs, W. H. (1993). Anti-Candida activity of the anti-cancer drug tamoxifen. Res Commun Chem Pathol Pharmacol 80, 125–128. [PubMed] [Google Scholar]
- Biswas, S., Dijck, P. V. & Datta, A. (2007). Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev 71, 348–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, A. J. P. (2002). Morphogenetic signaling pathways in Candida albicans. In Candida and Candidiasis, pp. 95–106. Edited by R. A. Calderone. Washington, DC: American Society for Microbiology.
- Chiou, C. Y. & Malagodi, M. H. (1975). Studies on the mechanism of action of a new Ca2+ antagonist, 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride in smooth and skeletal muscles. Br J Pharmacol 53, 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, H., Ueda, M., Tamaoka, M., Hamaguchi, M., Aisaka, K., Kiso, Y., Inoue, T., Ogino, R., Tatsuoka, T. & other authors (1991). Novel caffeic acid derivatives: extremely potent inhibitors of 12-lipoxygenase. J Med Chem 34, 1503–1505. [DOI] [PubMed] [Google Scholar]
- Collins, T. J., Lipp, P., Berridge, M. J., Li, W. & Bootman, M. D. (2000). Inositol 1,4,5-trisphosphate-induced Ca2+ release is inhibited by mitochondrial depolarization. Biochem J 347, 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotgreave, I. A., Duddy, S. K., Kass, G. E. N., Thompson, D. & Moldeus, P. (1989). Studies on the anti-inflammatory activity of ebselen: ebselen interferes with granulocyte oxidative burst by dual inhibition of NADPH oxidase and protein kinase C. Biochem Pharmacol 38, 649–656. [DOI] [PubMed] [Google Scholar]
- Cox, D. A., Conforti, L., Sperelakis, N. & Matlib, M. A. (1993). Selectivity of inhibition on Na+–Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J Cardiovasc Pharmacol 21, 595–599. [DOI] [PubMed] [Google Scholar]
- Cruz, M. C., Goldstein, A. L., Blankenship, J., Del Poeta, M., Perfect, J. R., McCusker, J. H., Bennani, Y. L., Cardenas, M. E. & Heitman, J. (2001). Rapamycin and less immunosuppressive analogs are toxic to Candida albicans and Cryptococcus neoformans via FKBP12-dependent inhibition of TOR. Antimicrob Agents Chemother 45, 3162–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial, N. N. & Korsmeyer, S. J. (2004). Cell death: critical control points. Cell 116, 205–219. [DOI] [PubMed] [Google Scholar]
- Degterev, A., Lugovsky, A., Cardone, M., Mulley, B., Wagner, G., Mitchison, T. & Yuan, J. (2001). Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-XL. Nat Cell Biol 3, 173–182. [DOI] [PubMed] [Google Scholar]
- Duksin, D. & Mahoney, W. C. (1982). Relationship of the structure and biological activity of the natural homologues of tunicamycin. J Biol Chem 257, 3105–3109. [PubMed] [Google Scholar]
- Ereshefsky, L., Watanabe, M. D. & Tran-Johnson, T. K. (1989). Clozapine: an atypical antipsychotic agent. Clin Pharm 8, 691–709. [PubMed] [Google Scholar]
- Ernst, J. F. (2000). Transcription factors in Candida albicans – environmental control of morphogenesis. Microbiology 146, 1763–1774. [DOI] [PubMed] [Google Scholar]
- Fan, Z., Lu, Y., Wu, X., DeBlasio, A., Koff, A. & Mendelsohn, J. (1995). Prolonged induction of p21Cip1/WAF1/CDK2/PCNA complex by epidermal growth factor receptor activation mediates ligand-induced A431 cell growth inhibition. J Cell Biol 131, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova, N. D., Badger, J. H., Robson, G. D., Wortman, J. R. & Nierman, W. C. (2005). Comparative analysis of programmed cell death pathways in filamentous fungi. BMC Genomics 6, 177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey, C., Narayanan, K., McMillan, K., Spack, L., Gross, S. S., Masters, B. S. & Griffith, O. W. (1994). l-Thiocitrulline. A stereospecific, heme-binding inhibitor of nitric-oxide synthases. J Biol Chem 269, 26083–26091. [PubMed] [Google Scholar]
- Garvin, J. L., Simon, S. A., Cragoe, E. J. J. & Mandel, L. J. (1985). Phenamil: an irreversible inhibitor of sodium channels in the toad urinary bladder. J Membr Biol 87, 45–54. [DOI] [PubMed] [Google Scholar]
- Gietzen, K., Sadorf, I. & Bader, H. (1982). A model for the regulation of the calmodulin-dependent enzymes erythrocyte Ca2+-transport ATPase and brain phosphodiesterase by activators and inhibitors. Biochem J 207, 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosman, N. (1991). Effects of the ether phospholipid AMG-PC on mast cells are similar to that of the ether lipid AMG but different from that of the analogue hexadecylphosphocholine. Immunopharmacology 22, 39–47. [DOI] [PubMed] [Google Scholar]
- Guiraud, P., Steiman, R., Campos-Takaki, G. M., Seigle-Murandi, F. & Simeon de Buochberg, M. (1994). Comparison of antibacterial and antifungal activities of lapachol and β-lapachone. Planta Med 60, 373–374. [DOI] [PubMed] [Google Scholar]
- Hagiwara, M., Endo, T. & Hidaka, H. (1984). Effects of vinpocetine on cyclic nucleotide metabolism in vascular smooth muscle. Biochem Pharmacol 33, 453–457. [DOI] [PubMed] [Google Scholar]
- Hamaguchi, T., Sudo, T. & Osada, H. (1995). RK-682, a potent inhibitor of tyrosine phosphatase, arrested the mammalian cell cycle progression at G1 phase. FEBS Lett 372, 54–58. [DOI] [PubMed] [Google Scholar]
- Hara, M., Akasaka, K., Akinaga, S., Okabe, M., Nakano, H., Gomez, R., Wood, D., Uh, M. & Tamanoi, F. (1993). Identification of Ras farnesyltransferase inhibitors by microbial screening. Proc Natl Acad Sci U S A 90, 2281–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert, J. M., Augereau, J. M., Gleye, J. & Maffrand, J. P. (1990). Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 172, 993–999. [DOI] [PubMed] [Google Scholar]
- Higashijima, T., Uzu, S., Nakajima, T. & Ross, E. M. (1988). Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J Biol Chem 263, 6491–6494. [PubMed] [Google Scholar]
- Hoberg, K. A., Cihlar, R. L. & Calderone, R. A. (1983). Inhibitory effect of cerulenin and sodium butyrate on germination of Candida albicans. Antimicrob Agents Chemother 24, 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki, M., Kawamoto, S., Itoh, H., Saitoh, M., Hagiwara, M., Takahashi, J. & Hidaka, H. (1986). Naphthalenesulfonamides as calmodulin antagonists and protein kinase inhibitors. Mol Pharmacol 29, 577–581. [PubMed] [Google Scholar]
- Ishihara, H., Martin, B. L., Brautigan, D. L., Karaki, H., Ozaki, H., Kato, Y., Fusetani, N., Watabe, S., Hashimoto, K. & other authors (1989). Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun 159, 871–877. [DOI] [PubMed] [Google Scholar]
- Kase, H., Iwahashi, K., Nakanishi, S., Matsuda, Y., Yamada, K., Takahashi, M., Murakata, C., Sato, A. & Kaneko, M. (1987). K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun 142, 436–440. [DOI] [PubMed] [Google Scholar]
- Kawamoto, S. & Hidaka, H. (1984). 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Commun 125, 258–264. [DOI] [PubMed] [Google Scholar]
- Klausner, R. D., Donaldson, J. G. & Lippincott-Schwartz, J. (1992). Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol 116, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus, H. G., McManus, O. B., Lee, S. H., Schmalhofer, W. A., Garcia-Calvo, M., Helms, L. M., Sanchez, M., Giangiacomo, K., Reuben, J. P. & other authors (1994). Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 33, 5819–5828. [DOI] [PubMed] [Google Scholar]
- Kojic, E. M. & Darouiche, R. O. (2004). Candida infections of medical devices. Clin Microbiol Rev 17, 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey, K., Cory, M., Davis, R., Frye, S. V., Harris, P. A., Hunter, R. N., Jung, D. K., McDonald, O. B., McNutt, R. W. & other authors (2000). The discovery of potent cRaf1 kinase inhibitors. Bioorg Med Chem Lett 10, 223–226. [DOI] [PubMed] [Google Scholar]
- Leberer, E., Harcus, D., Dignard, D., Johnson, L., Ushinsky, S., Thomas, D. Y. & Schroppel, K. (2001). Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol Microbiol 42, 673–687. [DOI] [PubMed] [Google Scholar]
- Leesnitzer, L. M., Parks, D. J., Bledsoe, R. K., Cobb, J. E., Collins, J. L., Consler, T. G., Davis, R. G., Hull-Ryde, E. A., Lenhard, J. M. & other authors (2002). Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 41, 6640–6650. [DOI] [PubMed] [Google Scholar]
- Levitzki, A. & Gilon, C. (1991). Tyrphostins as molecular tools and potential antiproliferative drugs. Trends Pharmacol Sci 12, 171–174. [DOI] [PubMed] [Google Scholar]
- Liu, H., Köhler, J. & Fink, G. R. (1994). Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266, 1723–1726. [DOI] [PubMed] [Google Scholar]
- Lokey, R. S. (2003). Forward chemical genetics: progress and obstacles on the path to a new pharmacopoeia. Curr Opin Chem Biol 7, 91–96. [DOI] [PubMed] [Google Scholar]
- Lynch, A. S. & Robertson, G. T. (2008). Bacterial and fungal biofilm infections. Annu Rev Med 59, 415–428. [DOI] [PubMed] [Google Scholar]
- Madeo, F., Herker, E., Maldener, C., Wissing, S., Lächelt, S., Herlan, M., Fehr, M., Lauber, K., Sigrist, S. J. & other authors (2002). A caspase-related protease regulates apoptosis in yeast. Mol Cell 9, 911–917. [DOI] [PubMed] [Google Scholar]
- Maidan, M. M., De Rop, L., Serneels, J., Exler, S., Rupp, S., Tournu, H., Thevelein, J. M. & Van Dijck, P. (2005). The G protein-coupled receptor Gpr1 and the Gα protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol Biol Cell 16, 1971–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massillon, D., Stalmans, W., van de Werve, G. & Bollen, M. (1994). Identification of the glycogenic compound 5-iodotubercidin as a general protein kinase inhibitor. Biochem J 299, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe, M. J. J., Jiang, S. A. & Orrenius, S. (1993). Chelation of intracellular zinc triggers apoptosis in mature thymocytes. Lab Invest 69, 101–110. [PubMed] [Google Scholar]
- McKenna, J. P. & Hanson, P. J. (1993). Inhibition by Ro 31-8220 of acid secretory activity induced by carbachol indicates a stimulatory role for protein kinase C in the action of muscarinic agonists on isolated rat parietal cells. Biochem Pharmacol 46, 583–588. [DOI] [PubMed] [Google Scholar]
- Medana, C., Ermondi, G., Fruttero, R., Di Stilo, A., Ferretti, C. & Gasco, A. (1994). Furoxans as nitric oxide donors. 4-Phenyl-3-furoxancarbonitrile: thiol-mediated nitric oxide release and biological evaluation. J Med Chem 37, 4412–4416. [DOI] [PubMed] [Google Scholar]
- Merritt, J. E., Armstrong, W. P., Benham, C. D., Hallam, T. J., Jacob, R., Jaxa-Chamiec, A., Leigh, B. K., McCarthy, S. A., Moores, K. E. & Rink, T. J. (1990). SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J 271, 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa, T., Takagi, Y., Shinozaki, M., Yun, C.-W., Schell, W. A., Perfect, J. R., Kumagai, H. & Tamaki, H. (2004). Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans. Eukaryot Cell 3, 919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch, A., Lückhoff, A., Pohl, U., Busse, R. & Bassenge, E. (1989). LY 83583 (6-anilino-5,8-quinolinedione) blocks nitrovasodilator-induced cyclic GMP increases and inhibition of platelet activation. Naunyn Schmiedebergs Arch Pharmacol 340, 119–125. [DOI] [PubMed] [Google Scholar]
- Mülsch, A., Bauersachs, J., Schäfer, A., Stasch, J. P., Kast, R. & Busse, R. (1997). Effect of YC-1, an NO-independent, superoxide-sensitive stimulator of soluble guanylyl cyclase, on smooth muscle responsiveness to nitrovasodilators. Br J Pharmacol 120, 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan, K., Singh, S., Burke, T. R., Grunberger, D. & Aggarwal, B. B. (1996). Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci U S A 93, 9090–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehil, M. T., Tamble, C. M., Combs, D. J., Kellogg, D. R. & Lokey, R. S. (2007). Uncovering genetic relationships using small molecules that selectively target yeast cell cycle mutants. Chem Biol Drug Des 69, 258–264. [DOI] [PubMed] [Google Scholar]
- Nierman, W. C., Pain, A., Anderson, M. J., Wortman, J. R., Kim, H. S., Arroyo, J., Berriman, M., Abe, K., Archer, D. B. & other authors (2005). Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438, 1151–1156. [DOI] [PubMed] [Google Scholar]
- Noverr, M. C., Erb-Downward, J. R. & Huffnagle, G. B. (2003). Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin Microbiol Rev 16, 517–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, J. R. & Mitchison, T. J. (2002). Small molecules, big impact: a history of chemical inhibitors and the cytoskeleton. Chem Biol 9, 1275–1285. [DOI] [PubMed] [Google Scholar]
- Phan, Q. T., Belanger, P. H. & Filler, S. G. (2000). Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect Immun 68, 3485–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, J. W., Schoenleber, R., Jesmok, G., Best, J., Moore, S. A., Collins, T. & Gerritsen, M. E. (1997). Novel inhibitors of cytokine-induced IkBa phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem 272, 21096–21103. [DOI] [PubMed] [Google Scholar]
- Powers, J. C., Kam, C. M., Narasimhan, L., Oleksyszyn, J., Hernandez, M. A. & Ueda, T. (1989). Mechanism-based isocoumarin inhibitors for serine proteases: use of active site structure and substrate specificity in inhibitor design. J Cell Biochem 39, 33–46. [DOI] [PubMed] [Google Scholar]
- Pressman, B. C. (1976). Biological applications of ionophores. Annu Rev Biochem 45, 501–530. [DOI] [PubMed] [Google Scholar]
- Rao, G. H. (1987). Influence of calmodulin antagonist (stelazine) on agonist-induced calcium mobilization and platelet activation. Biochem Biophys Res Commun 148, 768–775. [DOI] [PubMed] [Google Scholar]
- Sah, D. W. & Bean, B. P. (1994). Inhibition of P-type and N-type calcium channels by dopamine receptor antagonists. Mol Pharmacol 45, 84–92. [PubMed] [Google Scholar]
- Saitoh, M., Ishikawa, T., Matsushima, S., Naka, M. & Hidaka, H. (1987). Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem 262, 7796–7801. [PubMed] [Google Scholar]
- Salari, H., Braquet, P. & Borgeat, P. (1984). Comparative effects of indomethacin, acetylenic acids, 15-HETE, nordihydroguaiaretic acid and BW755C on the metabolism of arachidonic acid in human leukocytes and platelets. Prostaglandins Leukot Med 13, 53–60. [DOI] [PubMed] [Google Scholar]
- Saville, S. P., Lazzell, A. L., Monteagudo, C. & Lopez-Ribot, J. L. (2003). Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2, 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Westhausen, A., Schiller, R. A., Pohle, H. D. & Reichart, P. A. (1991). Oral Candida and Enterobacteriaceae in HIV-1 infection: correlation with clinical candidiasis and antimycotic therapy. J Oral Pathol Med 20, 467–472. [DOI] [PubMed] [Google Scholar]
- Schreiber, S. L. & Crabtree, G. R. (1992). The mechanism of action of cyclosporin A and FK506. Immunol Today 13, 136–142. [DOI] [PubMed] [Google Scholar]
- Smith, R. J., Sam, L. M., Justen, J. M., Bundy, G. L., Bala, G. A. & Bleasdale, J. E. (1990). Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther 253, 688–697. [PubMed] [Google Scholar]
- Sonneborn, A., Bockmuhl, D. P., Gerads, M., Kurpanek, K., Sanglard, D. & Ernst, J. F. (2000). Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol Microbiol 35, 386–396. [DOI] [PubMed] [Google Scholar]
- Steinbach, W. J., Reedy, J. L., Cramer, R. A., Jr, Perfect, J. R. & Heitman, J. (2007). Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol 5, 418–430. [DOI] [PubMed] [Google Scholar]
- Stuehr, D. J., Fasehun, O. A., Kwon, N. S., Gross, S. S., Gonzalez, J. A., Levi, R. & Nathan, C. F. (1991). Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J 5, 98–103. [DOI] [PubMed] [Google Scholar]
- Sudbery, P., Gow, N. & Berman, J. (2004). The distinct morphogenetic states of Candida albicans. Trends Microbiol 12, 317–324. [DOI] [PubMed] [Google Scholar]
- Tamaoki, T., Nomoto, H., Takahashi, I., Kato, Y., Morimoto, M. & Tomita, F. (1986). Staurosporine, a potent inhibitor of phospholipid/Ca++ dependent protein kinase. Biochem Biophys Res Commun 135, 397–402. [DOI] [PubMed] [Google Scholar]
- Tanaka, T., Ohmura, T. & Hidaka, H. (1983). Calmodulin antagonists' binding sites on calmodulin. Pharmacology 26, 249–257. [DOI] [PubMed] [Google Scholar]
- Tanaka, T., Satoh, T., Onozawa, Y., Kohroki, J., Itoh, N., Ishidate, M. J., Muto, N. & Tanaka, K. (1999). Apoptosis during iron chelator-induced differentiation in F9 embryonal carcinoma cells. Cell Biol Int 23, 541–550. [DOI] [PubMed] [Google Scholar]
- Toenjes, K. A., Munsee, S. M., Ibrahim, A. S., Jeffrey, R., Edwards, J. E., Jr & Johnson, D. I. (2005). Small-molecule inhibitors of the budded-to-hyphal-form transition in the pathogenic yeast Candida albicans. Antimicrob Agents Chemother 49, 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann, B. D., Myers, H., Chiranand, W., Lazzell, A. L., Zhao, Q., Vega, L. A., Lopez-Ribot, J. L., Gardner, P. R. & Gustin, M. C. (2004). Inducible defense mechanism against nitric oxide in Candida albicans. Eukaryot Cell 3, 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppuluri, P., Nett, J., Heitman, J. & Andes, D. (2008). Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob Agents Chemother 52, 1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercesi, A. E., Moreno, S. N. J., Bernardes, C. F., Meinicke, A. R., Fernandes, E. C. & Docampo, R. (1993). Thapsigargin causes Ca2+ release and collapse of the membrane potential of Trypanosoma brucei mitochondria in situ and of isolated rat liver mitochondria. J Biol Chem 268, 8564–8568. [PubMed] [Google Scholar]
- Vigushin, D. M., Mirsaidi, N., Brooke, G., Sun, C., Pace, P., Inman, L., Moody, C. J. & Coombes, R. C. (2004). Gliotoxin is a dual inhibitor of farnesyltransferase and geranylgeranyltransferase I with antitumor activity against breast cancer in vivo. Med Oncol 21, 21–30. [DOI] [PubMed] [Google Scholar]
- Vrijsen, R., Berghe, D. A. V., Vlietinck, A. J. & Boeye, A. (1986). Lycorine: a eukaryotic termination inhibitor? J Biol Chem 261, 505–507. [PubMed] [Google Scholar]
- Wallace, K. K., Reynolds, K. A., Koch, K., McArthur, H. A. I., Brown, M. S., Wax, R. G. & Moore, B. S. (1994). Biosynthetic studies of ascomycin (FK520): formation of the (1R,3R,4R)-3,4-dihydroxycyclohexanecarboxylic acid-derived moiety. J Am Chem Soc 116, 11600–11601. [Google Scholar]
- Wang, G. & Lemos, J. R. (1992). Tetrandrine blocks a slow, large-conductance, Ca2+-activated potassium channel besides inhibiting a non-inactivating Ca2+ current in isolated nerve terminals of the rat neurohypophysis. Pflugers Arch 421, 558–565. [DOI] [PubMed] [Google Scholar]
- Ward, G. E., Carey, K. L. & Westwood, N. J. (2002). Using small molecules to study big questions in cellular microbiology. Cell Microbiol 4, 471–482. [DOI] [PubMed] [Google Scholar]
- Warenda, A. J. & Konopka, J. B. (2002). Septin function in Candida albicans morphogenesis. Mol Biol Cell 13, 2732–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warenda, A. J., Kauffman, S., Sherrill, T. P., Becker, J. A. & Konopka, J. B. (2003). Candida albicans septin mutants are defective for invasive growth and virulence. Infect Immun 71, 4045–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock, D. W. (2007). Trends in the epidemiology of invasive fungal infections. Nippon Ishinkin Gakkai Zasshi 48, 1–12. [DOI] [PubMed] [Google Scholar]
- Whitesell, L., Mimnaugh, E. G., De Costa, B., Myers, C. E. & Neckers, L. M. (1994). Inhibition of heat shock protein HSP90–pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A 91, 8324–8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykle, R. L., Miller, C. H., Lewis, J. C., Schmitt, J. D., Smith, J. A., Surles, J. R., Piantadosi, C. & O'Flaherty, J. T. (1981). Stereospecific activity of 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine and comparison of analogs in the degranulation of platelets and neutrophils. Biochem Biophys Res Commun 100, 1651–1658. [DOI] [PubMed] [Google Scholar]
- Xu, W., Leung, S., Wright, J. & Guggino, S. E. (1999). Expression of cyclic nucleotide-gated cation channels in airway epithelial cells. J Membr Biol 171, 117–126. [DOI] [PubMed] [Google Scholar]
- Yaish, P., Gazit, A., Gilon, C. & Levitzki, A. (1988). Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science 242, 933–935. [DOI] [PubMed] [Google Scholar]
- Yatomi, Y., Ruan, F., Megidish, T., Toyokuni, T., Hakomori, S. & Igarashi, Y. (1996). N,N-dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry 35, 626–633. [DOI] [PubMed] [Google Scholar]
- Zeller, F. P. & Spinler, S. A. (1987). Bepridil: a new long-acting calcium channel blocking agent. Drug Intell Clin Pharm 21, 487–492. [DOI] [PubMed] [Google Scholar]
- Zheng, B., Oishi, K., Shoji, M., Eibl, H., Berdel, W. E., Hajdu, J., Vogler, W. R. & Kuo, J. F. (1990). Inhibition of protein kinase C, (sodium plus potassium)-activated adenosine triphosphate, and sodium pump by synthetic phospholipid analogues. Cancer Res 50, 3025–3031. [PubMed] [Google Scholar]
- Zinck, R., Cahill, M. A., Kracht, M., Sachsenmaier, C., Hipskind, R. A. & Nordheim, A. (1995). Protein synthesis inhibitors reveal differential regulation of mitogen-activated protein kinase and stress-activated protein kinase pathways that converge on Elk-1. Mol Cell Biol 15, 4930–4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.