Abstract
Purpose of review
Cancer cachexia is associated with marked alterations in skeletal muscle protein metabolism that lead to muscle wasting and, in some cases, death. The inflammatory response elicited by cancer is a likely, if not primary, mediator of these alterations. This review focuses on the possible relationship between inflammatory signaling and altered amino acid metabolism in cancer.
Recent findings
Loss of skeletal muscle in cancer patients can potentially be due to anorexia and early satiety, reduced muscle protein synthesis, and/or increased muscle protein breakdown. Inflammation has been associated with each of these mechanisms. Effects on appetite appear to be mediated by the melanocortin system in the hypothalamus. Studies in animal models of cachexia suggest that modulation of orexigenic and anorexigenic pathways in this system may improve nutrient consumption. Inflammatory cytokines such as IL-6 and TNFα likely contribute to the effects of inflammation on muscle protein metabolism through several pathways.
Summary
Limited studies in humans suggest that targeted anti-inflammatory and nutritional interventions may ameliorate the net catabolic effect on skeletal muscle protein metabolism. Future studies of the precise mechanism of muscle protein loss, as well as novel or combination therapies to inhibit inflammation and promote anabolism, are warranted.
Keywords: amino acid, ubiquitin proteasome pathway
Introduction
Cachexia is a multifaceted syndrome describing the loss of body mass as a result of both accelerated catabolism of fat and skeletal muscle and anorexia. Cachexia does not simply refer to a loss of body weight [1•] and can be differentiated from sarcopenia (age-induced loss of skeletal muscle) and starvation (in which body mass loss is caused by nutrient deficiency and preferential loss of adipose tissue occurs) [2]. In cancer cachexia, profound inflammation is both a diagnostic characteristic [3] as well as a possible mechanism for the accompanying anorexia and wasting. In the present review, we focus on the relationship between cancer-induced inflammation and muscle wasting.
Characteristics of cancer cachexia
Although descriptions vary, weight loss, anorexia, and inflammation are the cardinal characteristics of cancer cachexia and are correlated with adverse outcomes [3]. Although muscle weakness and fatigue are often present (e.g. 70–100% of cancer patients experience fatigue [4]) in cancer patients and significantly impact quality of life, they are also common in noncachectic cancer patients and thus may not be predictive of cancer cachexia [3]. Elevated resting energy expenditure has also been associated with cachexia and is thought to be at least partially due to the acute-phase inflammatory response (as indicated, for example, by increased circulating levels of C-reactive protein, fibrinogen, and white blood cells) present in these patients [5•].
Although weight loss occurs in roughly half of cancer patients [5•], in cachexia, the weight loss represents a marked loss of skeletal muscle and fat. Strictly speaking, cachexia describes the manifestations (e.g. excessive loss of body weight, lean body mass, and adipose tissue) of a process that was initiated at some point in the past. Accordingly, patients who exhibit characteristics (e.g. systemic inflammation, anorexia) that are associated with the state of cachexia as well as 5% or greater weight loss may be considered ‘precachectic’ [1•]. When the loss of body weight reaches 15% or greater the patient is in a frank cachectic state [1•,6]. In cachectic patients, a loss of 25% of body weight represents an approximately 75% reduction in skeletal muscle protein [7]. The excessive weight loss of cachexia is an ominous sign, with roughly 20% of cancer deaths attributed to cachexia [8]. Thus, early identification of the factors predisposing an individual to cachexia is important for designing potential therapies to reverse and/or prevent the catabolic process.
Possible causal relationships between inflammation and cancer cachexia
The idea that cancer and inflammation are linked has been around since the hypothesis of Virchow in the 19th Century [9]. Apart from a role in cancer development and progression, cancer-associated inflammation may contribute to the development of cachexia. As inflammation is implicated in the elevated rates of muscle protein breakdown, reduced rates of muscle protein synthesis, and reduced intake of amino acids due to cancer-induced anorexia (Fig. 1 Fig. 1), it has been suggested to play a dominant mechanistic role in the development of cachexia-induced loss of skeletal muscle [3,9,10••].
Figure 1.
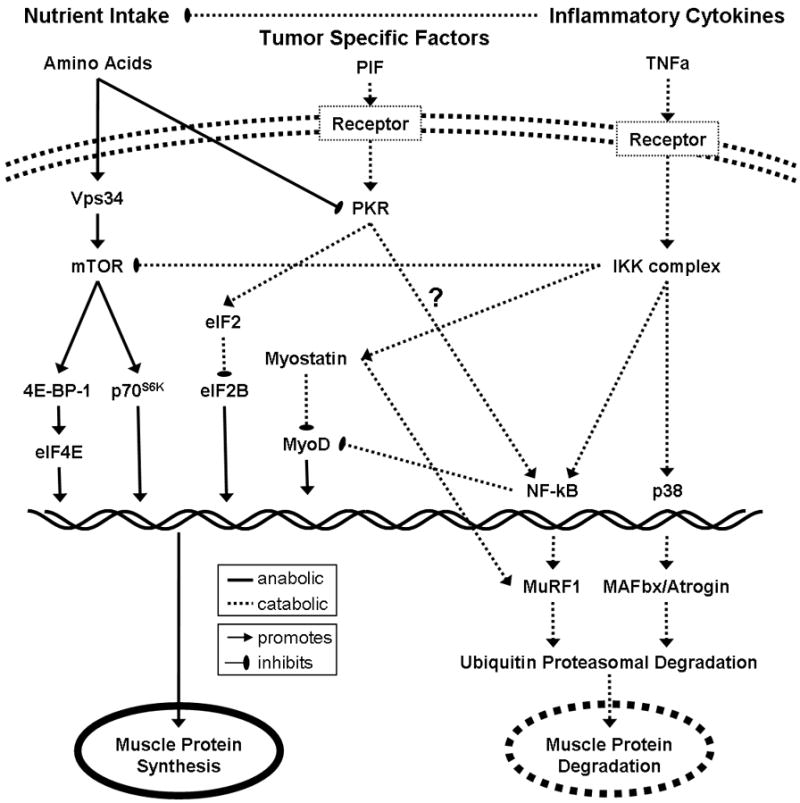
Key signaling pathways between amino acids, inflammatory cytokines, and tumor-specific factors gr1
Amino acids stimulate muscle protein synthesis through the mTOR pathway. Inflammatory cytokines and tumor specific factors inhibit muscle protein synthesis and promote muscle protein degradation.
Anorexia
Inflammatory cytokines released as part of the cancer-induced inflammatory response act upon the hypothalamus to limit food intake and thus the supply of amino acids to the muscle [2,11••]. This effect is thought to occur as a result of increased stimulation of melanocortin receptors in the hypothalamus [11••]. Reduced release of the orexigenic molecule neuropeptide Y may also contribute to this response [2]. Interestingly, circulating levels of the orexigenic hormone ghrelin are paradoxically elevated in cancer patients [12–14], suggesting possible inflammation-induced resistance to its actions.
Impaired muscle protein synthesis
Inflammatory-mediated signaling may limit muscle protein synthesis by several mechanisms. Inflammatory cytokines such as TNF-α and IL-6 are likely important in this regard. TNF-α can activate the transcription factor NF-κB, which inhibits the synthesis of the muscle-specific transcription factor MyoD, thereby inhibiting differentiation [10••]. As discussed below, TNF-α also influences the anabolic mTOR signaling pathway. The potential role of IL-6 in human cachexia has not received much attention; however, a recent study found that skeletal muscle protein synthesis was dramatically reduced by relatively low-dose IL-6 infusion in humans [15••].
Myostatin is a negative regulator of muscle mass, inhibiting myogenic proliferation and differentiation [10••,16••]. Animals and humans that lack myostatin exhibit markedly increased muscle mass [16••]. The study of the role of myostatin in cancer cachexia is still in its infancy; however, preliminary reports have described marked elevations in myostatin in cachectic cancer patients [10••]. Animal models suggest that the inflammatory cytokine TNF-α is at least partially responsible for this increase [17•].
As touched on above, inflammation initiates central anorexic pathways that limit dietary consumption of nutrients. As amino acids, and perhaps leucine in particular, are required for muscle protein synthesis [18•,19•,20••], the reduced availability of amino acids represents a major contributor to the inflammation-induced loss of skeletal muscle mass in cachectic patients. In addition to the reduced supply of amino acids, the anorexic response also reduces the exposure to insulin, a potent stimulator of muscle protein synthesis [21].
The mTOR signaling pathway is a major mediator of anabolic responses in skeletal muscle. Specifically, mTOR activation by insulin or amino acids activates the downstream targets eukaryotic initiation factor binding protein 4E and ribosomal S6 kinase 1, which promote translation initiation, and eukaryotic elongation factor, which stimulates elongation [18•]. Although more research is needed, limited human [22] and animal [20••] studies suggest that mTOR signaling is inhibited in cancer cachexia. Multiple mechanisms may contribute, including anorexia-induced reductions in amino acid intake as well as inflammatory mediators such as TNF-α, which potentially oppose mTOR signaling by several means [19•].
Recent work has identified dsRNA-dependent protein kinase (PKR), which is activated by the tumor-secreted proteolysis-inducing factor (PIF), as a potential inhibitor of skeletal muscle protein synthesis in cachexia [23••]. This effect is apparently because of phosphorylation of eukaryotic initiation factor (eIF) 2, which in turn interferes with the activity of eIF2B [23••] to promote translation initiation.
Stimulated muscle protein breakdown
Inflammatory stimulation activates pathways associated with muscle protein breakdown [10••]. In cancer cachexia, the ubiquitin proteasome pathway is greatly influenced [10••]. Many of these effects are thought to occur through activation of NF-κB by upstream factors such as TNF-α [10••] and PIF (acting through its stimulatory effect on PKR) [20••,23••]. NF-κB stimulates transcription of the ubiquitin E3 ligase muscle ring finger (MuRF)-1, which is known to positively regulate activity of the ubiquitin proteasome pathway [10••]. Members of the Foxo family of transcription factors may also stimulate expression of MuRF-1 and atrogin-1 [10••,24•,25]. Myostatin has recently been shown to stimulate expression of ubiquitin proteasome pathway elements in an NF-κB-independent manner that may involve regulation of Foxo [16••].
Although IL-6 has been considered as a procachectic inflammatory cytokine, it may reduce skeletal muscle protein breakdown [15••]. However, as its effect to reduce protein synthesis (see above) is greater than its effect on breakdown, the net response to IL-6 appears to be a loss of muscle protein [15••]. Interestingly, IL-6 infusion increases whole body protein turnover and reduces circulating amino acid concentrations, suggesting that profound effects on nonmuscle tissues may influence the response in skeletal muscle [15••].
Notwithstanding evidence for upregulation of elements of the ubiquitin proteasome pathway in cancer cachexia, we are not aware of studies in humans in which elevated rates of muscle protein breakdown have been reported. On the contrary, some studies suggest that muscle protein breakdown is unaltered by cancer in humans [26••,27]. In the study by Dillon et al. [26••], this was demonstrated despite increased skeletal muscle IL-6 and NF-κB and systemic elevations of hs-CRP. Indirect measures of muscle protein breakdown in animal models suggest activation of skeletal muscle protein breakdown during cachexia [10••], although exceptions exist [28]. One possibility is that in animal models, in which the relative tumor mass is greater than in humans, the tumor represents a large enough ‘sink’ for amino acids that it effectively competes with skeletal muscle [29] and stimulates breakdown. Another possibility is that humans with cancer spend longer periods throughout the day in negative protein balance during the postabsorptive and postprandial periods than their healthy counterparts. Whether this effect is more pronounced as a result of a diminished synthetic response to a meal or enhanced protein breakdown due to the upregulation of elements of the ubiquitin proteasome pathway is not known. Clearly, this is an area in which further studies are warranted.
Therapeutic approaches
The inflammatory burden of cancer, with potential influences on appetite, muscle protein synthesis, and muscle protein breakdown, presents a challenge to the development of anabolic therapies. Cachectic patients often exhibit reduced appetite and early satiety, hampering their ability to ingest amino acid-dense foods such as meats to stimulate muscle protein synthesis. Further, cancer-induced inflammation appears to reduce the sensitivity of skeletal muscle protein synthesis to amino acid supplementation [26••] and may increase muscle protein breakdown [10••]. This tug-of-war kinetic scenario suggests that normal intake of food containing amino acids, as well as supplemental amino acids for preventing or reversing muscle loss could preferentially stimulate tumor growth [29] by stealing key nutrients away from muscle tissue. Accordingly, there exist several options for therapeutic intervention, namely anti-inflammatory treatments, appetite stimulation, anabolic agents to stimulate synthesis, and various approaches to reduce proteolysis. These approaches could in theory work in a synergistic manner to significantly ameliorate loss of skeletal muscle.
Anti-inflammatory treatments
If inflammatory signaling pathways are responsible for cachexia, then anti-inflammatory agents should ameliorate the condition. Accordingly, a number of anti-inflammatory agents have been tested for their potential to reduce the symptoms of cachexia. Eicosapentaenoic acid (EPA), a naturally occurring fatty acid in fish oils, has been shown to inhibit inflammatory responses. Animal studies using EPA have been promising, suggesting significant benefit on maintenance of body weight and lean body mass [10••]. Consistent with these findings, human studies have found beneficial effects of EPA on body weight, lean body mass, appetite, and quality of life [30••,31]. However, other studies have found marginal or no benefit [32]. Although a consensus on the use of EPA still does not exist, the weight of current evidence suggests marginal benefit [32,33].
Pharmacological inhibition of the inflammatory response has been moderately successful. Cyclooxygenase inhibition in cancer patients has been associated with improved survival and preservation of adipose tissue [34,35]; however, loss of lean body mass was not attenuated. Given the overlapping nature of inflammatory signaling, it seems likely that combination treatments may be necessary to see positive effects on skeletal muscle mass.
Appetite stimulation
The inflammation-induced anorexia promotes a catabolic state by limiting the supply of amino acids, limiting insulin exposure, and promoting an energetic state that does not favor stimulation of mTOR signaling. Although human trials are lacking, promising results have been obtained in animal models of cachexia through the use of melanocortin receptor antagonists [11••]. In these models, melanocortin inhibition has been shown to reduce both the anorexia and hypermetabolism induced by implanted tumors, while preserving lean body mass [11••]. Ghrelin, the only known circulating orexigenic factor, stimulates orexigenic signaling by the melanocortin system [11••]. In an animal model of cancer cachexia, ghrelin has shown promising effects on lean body mass and appetite, apparently through both orexigenic and anti-inflammatory effects [36•].
Stimulation of muscle protein synthesis
Essential amino acids and leucine in particular are potent stimulators of muscle protein synthesis in healthy individuals [36•]. Until recently, however, it was not known whether this response is also present in cachectic skeletal muscle, in which inflammatory mediators and impaired energetic status may limit the response to amino acids. Fortunately, recent studies suggest that this response is also present in cachexia.
Dillon et al. [26••] tested the effectiveness of a 40 g balanced amino acid supplement in patients with ovarian cancer. Notably, these patients exhibited evidence of marked inflammation, with significantly increased levels of circulating C-reactive protein as well as elevations in skeletal muscle TNF-α and IL-6. Despite this state of inflammation, as well as ongoing treatment, the amino acid supplement improved skeletal muscle protein net balance. This effect was achieved through stimulation of muscle protein synthesis, with no effect on muscle protein breakdown. Notably, however, the anabolic response in these patients was tempered relative to an age-matched control group.
These findings are complemented by a recent study in cachectic mice that also demonstrated improved preservation of skeletal muscle mass in response to supplementation with branched chain amino acids [20••]. This effect was apparently due to improved muscle protein synthesis and a possible reduction in muscle protein breakdown [20••]. mTOR signaling was enhanced, which should favor increased translation initiation. In addition, phosphorylation of eukaryotic elongation factor 2 was reduced, consistent with a stimulation of translation elongation. This study also suggests that PKR activity is modulated by branched chain amino acids though induction of increased expression of protein phosphatase 1.
Biolo et al. [37] investigated the acute effects of BCAA on muscle protein metabolism in the setting of the combined stresses of cancer and recovery from surgery and radiation therapy. In this study, provision of a balanced mixture of amino acids had no effect on muscle protein synthesis and breakdown. However, when an isonitrogenous supplement that was enriched in essential amino acids was given, muscle protein synthesis was stimulated [37].
As insulin is anabolic to skeletal muscle and inhibits lipolysis, it is an attractive candidate for treatment of cachexia. Accordingly, Lundholm et al. [38••] studied the response to chronic insulin treatment (0.11 U/kg per day) in a large group of patients with assorted malignancies. Notably, both the control group and the insulin-treated group of patients in this study received anti-inflammatory treatment with indomethacin [38••]. Despite the concomitant indomethacin treatment, insulin did not affect lean body mass, although it did significantly increase body fat stores and survival [38••].
Biolo et al. [39••] also investigated the response to insulin. This study differed from the Lundholm et al. study in that it examined the acute response to insulin given to maintain euglycemia on the day after abdominal surgery in female cancer patients. In addition, the circulating insulin concentrations of both the control (hyperglycemic) and insulin (euglycemic) groups were higher than in the cancer patients studied by Lundholm et al. [38••]. Insulin significantly stimulated muscle protein synthesis and did not affect muscle protein breakdown; thus, the net balance between synthesis and breakdown was improved by insulin under these conditions.
Finally, testosterone is anabolic to muscle protein through stimulation of the IGF-1 pathway [40]. Recent evidence suggests that testosterone also has anti-inflammatory properties, making this hormone a therapeutic candidate for use in cachectic patients [41–43].
Inhibition of muscle protein breakdown
As noted above, there may be a discrepancy between indicators of ubiquitin proteasome pathway activation and actual rates of muscle protein breakdown. Nevertheless, whether muscle protein breakdown is actually stimulated by cancer-induced inflammation or not, inhibition of breakdown represents a viable strategy for improving the net balance between muscle protein synthesis and breakdown.
One agent that may reduce muscle protein breakdown is insulin [44••]. However, in the study by Biolo et al. [39••] cited above, skeletal muscle protein breakdown was not reduced by insulin treatment. In the Lundholm et al. study [38••], muscle protein breakdown in response to insulin therapy was not measured; however, the lack of effect on lean body mass suggests that insulin did not affect muscle protein breakdown [38••].
Testosterone is a hormone with well known anabolic effects. It apparently increases muscle mass by reducing the loss of skeletal muscle amino acids, either by stimulating the reutilization of amino acids released from proteolysis for protein synthesis or by reducing the rate of muscle protein breakdown [45]. Thus, testosterone is an attractive candidate for reducing loss of muscle mass in cachectic patients. However, to date, testosterone treatment of cancer patients has not been reported.
Conclusion
Although the inflammatory burden of cancer exerts profound effects on skeletal muscle protein metabolism, recent studies suggest that appropriate interventions may ameliorate these responses.
Acknowledgments
M.S.-M. gratefully acknowledges the following research support: NCI R01 CA 127971-01.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest.
•• of outstanding interest.
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1 •.Tan HL, Fearon KCH. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care. 2008;11:400–407. doi: 10.1097/MCO.0b013e328300ecc1. The study places cancer cachexia in its context within the broad category of cachexia. [DOI] [PubMed] [Google Scholar]
- 2.Morley JE, Thomas DR, Wilson M-MG. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;84:735–743. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 3.Fearon KC, Voss AC, Hustead DS CB for the Cancer Cachexia Study Group. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345–1350. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
- 4.Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362:640–650. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- 5•.Skipworth RJE, Stewart GD, Dejong CHC, et al. Pathophysiology of cancer cachexia: much more than host-tumor interaction? Clin Nutr. 2007;26:667–676. doi: 10.1016/j.clnu.2007.03.011. Provides good description and discussion of the acute-phase response elicited in cancer cachexia. [DOI] [PubMed] [Google Scholar]
- 6.Stewart GD, Skipworth RJE, Fearon KCH. Cancer cachexia and fatigue. Clin Med. 2006;6:140–143. doi: 10.7861/clinmedicine.6-2-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fearon KC. The Sir David Cuthbertson Medal Lecture 1991: the mechanisms and treatment of weight loss in cancer. Proc Nutr Soc. 1992;51:251–265. doi: 10.1079/pns19920036. [DOI] [PubMed] [Google Scholar]
- 8.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 9.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 10••.Acharyya S, Guttridge DC. Cancer signaling pathways continue to emerge yet much still points to the proteasome. Clin Cancer Res. 2007;13:1356–1361. doi: 10.1158/1078-0432.CCR-06-2307. Discussion of recent advances in understanding of the signaling pathways implicated in cancer cachexia, particularly evidence for a role of the ubiquitin proteasome pathway. [DOI] [PubMed] [Google Scholar]
- 11••.DeBoer MD. Melanocortin interventions in cachexia: how soon from bench to bedside? Curr Opin Clin Nutr Metab Care. 2007;10:457–462. doi: 10.1097/MCO.0b013e328108f441. Excellent review of the role of the melanocortin system in cachexia. Includes discussion of therapeutic approaches. [DOI] [PubMed] [Google Scholar]
- 12.Garcia JM, Garcia-Touza M, Hijazi RA, et al. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab. 2005;90:2920–2926. doi: 10.1210/jc.2004-1788. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu Y, Nagaya N, Isobe T, et al. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res. 2003;9:774–778. [PubMed] [Google Scholar]
- 14.Wolf I, Sadetzki S, Kanety H, et al. Adiponectin, ghrelin, and leptin in cancer cachexia in breast and colon cancer patients. Cancer. 2006;106:966–973. doi: 10.1002/cncr.21690. [DOI] [PubMed] [Google Scholar]
- 15••.van Hall G, Steensburg A, Fischer C, et al. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab. 2008;93:2851–2858. doi: 10.1210/jc.2007-2223. Human study suggesting that IL-6 reduces muscle protein synthesis. Notably, IL-6 reduced muscle protein breakdown but had profound effect on protein turnover in nonmuscle tissues. [DOI] [PubMed] [Google Scholar]
- 16••.McFarlane C, Sharma M, Kambadur R. Myostatin is a procachectic growth factor during postnatal myogenesis. Curr Opin Clin Nutr Metab Care. 2008;11:422–427. doi: 10.1097/MCO.0b013e32830007e2. Discussion of the possible role of myostatin in muscle protein breakdown, adding a new dimension to our understanding of how this protein regulates muscle mass. [DOI] [PubMed] [Google Scholar]
- 17•.Costelli P, Muscaritoli M, Bonetto A, et al. Muscle myostatin signaling is enhanced in experimental cancer cachexia. Eur J Clin Invest. 2008;38:531–538. doi: 10.1111/j.1365-2362.2008.01970.x. Evidence of a role for myostatin signaling, possibly initiated by tumor necrosis factor α, in cancer cachexia. [DOI] [PubMed] [Google Scholar]
- 18•.Proud CG. Signaling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–234. doi: 10.1042/BJ20070024. Comprehensive review of the regulation of translation. [DOI] [PubMed] [Google Scholar]
- 19•.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459. doi: 10.1152/ajpendo.00204.2007. Concise review including discussion of the effects of inflammatory signaling on translation initiation. [DOI] [PubMed] [Google Scholar]
- 20••.Eley HL, Russell ST, Tisdale MJ. Effect of branched-chain amino acids on muscle atrophy in cancer cachexia. Biochem J. 2007;407:113–120. doi: 10.1042/BJ20070651. Shows efficacy of branched chain amino acids in the face of inflammation in a model of cancer cachexia. Effects on PKR suggest novel mechanism for responses to branched chain amino acids in cancer cachexia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest. 1995;95:811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt TL, Martignoni ME, Bachmann J, et al. Activity of the Akt-dependent anabolic and catabolic pathways in muscle and liver samples in cancer-related cachexia. J Mol Med. 2007;85:647–654. doi: 10.1007/s00109-007-0177-2. [DOI] [PubMed] [Google Scholar]
- 23••.Eley HL, Tisdale MJ. Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J Biol Chem. 2007;282:7087–7097. doi: 10.1074/jbc.M610378200. Provides a novel mechanistic link between accelerated protein breakdown and inhibited protein synthesis involving PKR. [DOI] [PubMed] [Google Scholar]
- 24•.Wu A-L, Kim J-H, Zhang C, et al. Forkhead box protein 01 negatively regulates skeletal myocyte differentiation through degradation of mammalian target of rapamycin pathway components. Endocrinology. 2008;149:1407–1414. doi: 10.1210/en.2007-1470. Describes a new role for FOXO signaling in skeletal muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stitt TN, Drujan D, Clarke BA, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 26••.Dillon EL, Volpi E, Wolfe RR, et al. Amino acid metabolism and inflammatory burden in ovarian cancer patients undergoing intense oncological therapy. Clin Nutr. 2007;26:736–743. doi: 10.1016/j.clnu.2007.07.004. Demonstrates efficacy of amino acid supplementation in cancer patients with evidence of marked inflammation. Also suggests that muscle protein breakdown is not elevated in these patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundholm K, Bennegard K, Eden E, et al. Efflux of 3-methylhistidine from the leg in cancer patients who experience weight loss. Cancer Res. 1982;42:4807–4811. [PubMed] [Google Scholar]
- 28.Svaninger G, Bennegard K, Ekman L, et al. Lack of evidence for elevated breakdown rate of skeletal muscles in weight-losing, tumor-bearing mice. J Natl Cancer Inst. 1983;71:341–346. [PubMed] [Google Scholar]
- 29.McNurlan MA, Heys SD, Park KG, et al. Tumour and host tissue responses to branched-chain amino acid supplementation of patients with cancer. Clin Sci. 1994;86:339–345. doi: 10.1042/cs0860339. [DOI] [PubMed] [Google Scholar]
- 30••.Skipworth RJE, Fearon KCH. The scientific rationale for optimizing nutritional support in cancer. Eur J Gastroenterol Hepatol. 2007;19:371–377. doi: 10.1097/MEG.0b013e3280bdbf87. Discussion of importance of nutrition in cancer treatment. Also provides partial explanation for divergent results in animal models and humans with cancer. [DOI] [PubMed] [Google Scholar]
- 31.Colomer R, Moreno-Nogueira JM, Garcia-Luna PP, et al. n-3 fatty acids, cancer and cachexia: a systemic review of the literature. Br J Nutr. 2007;97:823–831. doi: 10.1017/S000711450765795X. [DOI] [PubMed] [Google Scholar]
- 32.Fearon KCH, Barber MD, Moses AG, et al. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J Clin Oncol. 2006;24:3401–3407. doi: 10.1200/JCO.2005.04.5724. [DOI] [PubMed] [Google Scholar]
- 33.Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care. 2005;8:265–269. doi: 10.1097/01.mco.0000165004.93707.88. [DOI] [PubMed] [Google Scholar]
- 34.Lundholm K, Daneryd P, Korner U, et al. Evidence that long-term COX-treatment improves energy homeostasis and body composition in cancer patients with progressive cachexia. Int J Oncol. 2004;24:505–512. [PubMed] [Google Scholar]
- 35.Lundholm K, Gelin J, Hyltander A, et al. Anti-inflammatory treatment may prolong survival in undernourished patients with metastatic solid tumors. Cancer Res. 1994;54:5602–5606. [PubMed] [Google Scholar]
- 36•.Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signaling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care. 2008;11:222–226. doi: 10.1097/MCO.0b013e3282fa17fb. Discussion of the role of leucine in skeletal muscle anabolic signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biolo GD, De Cicco M, Dal Mas V, et al. Response of muscle protein and glutamine kinetics to branched-chain enriched amino acids in intensive care patients after radical cancer surgery. Nutrition. 2006;22:475–482. doi: 10.1016/j.nut.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 38••.Lundholm K, Korner U, Gunnebo L, et al. Insulin treatment in cancer cachexia: effects on survival, metabolism, and physical functioning. Clin Cancer Res. 2007;13:2699–2706. doi: 10.1158/1078-0432.CCR-06-2720. Reports that low-dose insulin, when given as part of a combination treatment including anti-inflammatories, improves survival. This insulin treatment regimen does not, however, appear to prevent loss of skeletal muscle. [DOI] [PubMed] [Google Scholar]
- 39••.Biolo G, De Cicco M, Lorenzon S, et al. Treating hyperglycemia improves skeletal muscle protein metabolism in cancer patients after major surgery. Crit Care Med. 2008;36:1768–1775. doi: 10.1097/CCM.0b013e318174de32. Reports that insulin treatment, given to reduce hyperglycemia, helps preserve muscle in cancer patients during the immediate postsurgery period. [DOI] [PubMed] [Google Scholar]
- 40.Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 41.Khosla S, Atkinson EJ, Dunstan CR, O’Fallon WM. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J Clin Endocrinol Metab. 2002;87:1550–1554. doi: 10.1210/jcem.87.4.8397. [DOI] [PubMed] [Google Scholar]
- 42.Malkin CJ, Pugh PJ, Jones RD, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 43.Yesilova Z, Ozata M, Kocar IH, et al. The effects of gonadotropin treatment on the immunological features of male patients with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2000;85:66–70. doi: 10.1210/jcem.85.1.6226. [DOI] [PubMed] [Google Scholar]
- 44••.Greenhaff PL, Karagounis LG, Peirce N, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin-ligases and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. Suggests that pathways putatively involved in the regulation of skeletal muscle protein metabolism do not always respond to nutritional or hormonal stimuli in accordance with observed responses of muscle protein synthesis and breakdown. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrando AA, Tipton KD, Doyle D, et al. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998;275:E864–871. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]


