Abstract
Background
No medication is currently approved for the treatment of cocaine dependence, but several preclinical and clinical reports suggest agonist-like medications, e.g. amphetamine analogues, may be a productive strategy for medication development.
Objective
This current proof-of-concept study sought to evaluate the safety, tolerability, and effectiveness of methamphetamine as a candidate treatment for cocaine dependence.
Methods
A randomized, double-blind, placebo-controlled study served to evaluate three treatment conditions in 82 cocaine-dependent individuals: (1) placebo (0 mg, 6×/day; n = 27), (2) immediate release (IR) methamphetamine (5 mg, 6×/day; n = 30), (3) sustained release (SR) methamphetamine (30 mg first pill, 1×/day; 0 mg 5×/day; n = 25). The study employed a sequential, two-phase design (i.e., 4 weeks of medication and counseling followed by 4 weeks of medication/counseling plus a contingency management procedure).
Results
Both preparation forms of methamphetamine were well tolerated, with similar retention to placebo (0 mg, 33%; 30 mg IR, 30%, 30 mg SR, 32%). Methamphetamine SR was associated with decreased sleep and increased weight loss. Medication adherence rates were high for the first dose of the day (95%), while adherence for subsequent capsules was lower. Those in the SR condition exhibited consistently lower rates of cocaine-positive urine samples (0 mg, 60%; 30 mg IR, 66%, 30 mg SR, 29%), p<0.0001, and reported the greatest reduction in craving for cocaine, p<0.05.
Conclusions
SR methamphetamine significantly reduced cocaine use and craving. Additional research is warranted to develop and evaluate agonist-like medications that may effectively treat cocaine dependence.
Keywords: cocaine, methamphetamine, dextroamphetamine, agonist-like treatment
1. Introduction
Of the numerous medications evaluated for treatment of cocaine dependence, few have meaningfully reduced cocaine use. The quest for effective pharmacotherapies has been challenged by the complex acute and long-term effects of cocaine use on the central nervous system. Among medication treatment strategies, agonist-like interventions that enhance dopaminergic functioning in the central nervous system have shown the most success. Of these, some inhibit dopamine reuptake or metabolism (e.g., bupropion, disulfiram, methylphenidate Carroll et al., 2004a; Grabowski et al., 1997; Margolin et al., 1995; Petrakis et al., 2000; Poling et al., 2006), or replenish dopamine stores (e.g., levodopa, Mooney et al., 2007; Schmitz et al., 2008; Shoptaw et al., 2005; Wolfsohn et al., 1993). Additionally, researchers have widely investigated medications that indirectly enhance dopaminergic functioning via reversal of the dopamine transporter, most notably dextroamphetamine (Grabowski et al., 2001; Grabowski et al., 2004; Shearer et al., 2003). In humans, double-blind clinical studies have demonstrated as much as a 50% reduction in cocaine use following dextroamphetamine treatment in cocaine-dependent individuals (Grabowski et al., 2001; Grabowski et al., 2004; Shearer et al., 2003).
Given the significant, but not complete, reduction in cocaine use achieved with dextroamphetamine, the potential of achieving greater treatment response with other amphetamine analogues is of considerable interest. A kindred amphetamine, methamphetamine, has shown some promise as an agonist-like therapy for cocaine dependence in pre-clinical experiments. This replacement strategy is supported by animal studies showing that methamphetamine substitutes for cocaine in the drug discrimination paradigm (Johanson and Barrett, 1993; Negus et al., 2007). Additionally, pretreatment with methamphetamine produces large reductions in cocaine, but not food self-administration (Negus et al., 2007), and some data suggest that methamphetamine is less reinforcing than cocaine (Newman and Carroll, 2006).
There is abundant evidence of methamphetamine's abuse liability in humans, and chronic, high-dose, non-therapeutic use of methamphetamine has serious health consequences (Anglin et al., 2000; Barr et al., 2006; Lineberry and Bostwick, 2006; Villemagne et al., 1998). However, immediate release (IR) and sustained release (SR) preparations of oral methamphetamine are approved by the FDA for short-term treatment of refractory attention-deficit/hyperactivity disorder (Desoxyn®), and simple obesity (Obetrol®) (Micromedex® Healthcare Series., n.d.). Under close clinical supervision, numerous studies indicate that oral methamphetamine is safe and well-tolerated in humans, with minimal abuse liability (e.g., 10 – 20 mg/day from 6 – 23 days, Comer et al., 2001; Hart et al., 2001; Hart et al., 2003; Perez-Reyes et al., 1991).
This proof-of-concept study is the first medication trial to evaluate the safety, tolerability, and efficacy of methamphetamine as an agonist-like therapy for cocaine dependence. We chose to compare the effects of placebo (0 mg/day, 6×/day; n = 27) to immediate-release (IR; 30mg/day; 5 mg, 6×/day; n = 30) and sustained-release (SR) methamphetamine (30 mg/day; 30 mg 1×/day, 0 mg, 5×/day; n = 25) on cocaine use, under close medical supervision. This 8-week study involved a sequential treatment design. During phase 1 (weeks 1-4), participants received medications and cognitive-behavioral therapy. During phase 2 (weeks 5-8), a contingency management (CM) procedure was added to study medication effects under a high-intensity behavioral therapy platform designed to reduce ongoing cocaine use (Dutra et al., 2008). Compared to placebo, we hypothesized that the greatest reduction in cocaine use would occur in individuals receiving sustained-release methamphetamine followed by the immediate release condition, and that this advantage would be found across phases of treatment. Safety was determined via monitoring of self-reported side effects and vital signs, and tolerability was operationalized in terms of treatment retention and medication adherence. Efficacy outcomes included cocaine use, cocaine craving, and mood.
2. Methods
2.1 Participants
The study and all related materials were approved by University of Texas-Houston Committee for the Protection of Human Subjects. Participants were recruited through advertisements in local media sources, and underwent a telephone interview to establish initial eligibility. To be included in the study, participants had to be: (a) English-speakers; (b) between the ages of 18 and 55; and (c) cocaine-dependent at time of intake by Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria (American Psychiatric Association, 1994); (d) reporting recent use of cocaine (confirmed by qualitative urine benzoylecgonine testing during the intake procedures); and (e) in generally good psychiatric and medical health with normal electrocardiogram and no history of heart disease. Exclusion criteria included: (a) pregnancy or nursing; (b) current dependence on substances other than nicotine; (c) current psychotic, affective, or anxiety disorders; (d) serious medical conditions precluding study participation and; (e) legal status that might prevent study completion. Study enrollment and attrition data are presented in Figure 1.
Figure 1.
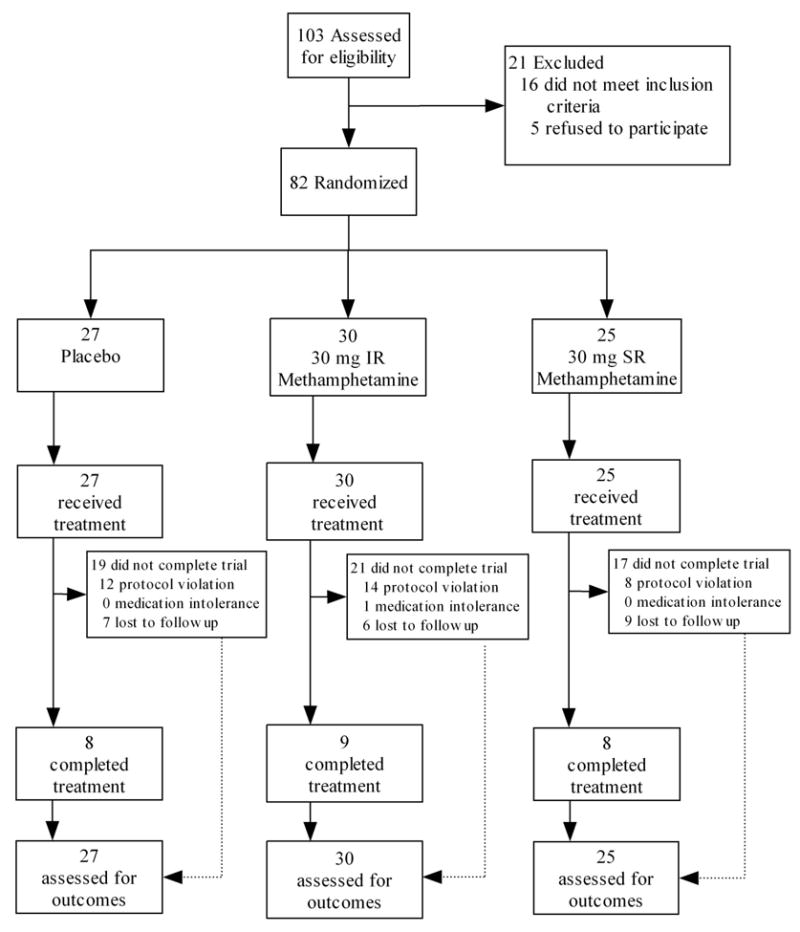
Participant enrollment and retention figure. Protocol violations indicate high absenteeism or failure to provide self-report or biological data.
2.2 Procedures
The research was conducted at the Treatment Research Clinic (TRC, Grabowski et al., 1997) of the Substance Abuse Research Center, a component of the Department of Psychiatry and Behavioral Sciences at the University of Texas at Houston. The four study phases were: (1) intake, (2) stabilization, (3) treatment, and (4) run-down.
2.2.1 Intake
Callers meeting initial telephone screen criteria received an appointment for the consent process and a pre-treatment evaluation (3-10 days duration), which included a medical history and complete physical examination. This assessment involved laboratory evaluation of liver, kidney, and thyroid functioning, cardiac functioning (i.e., 12-lead electrocardiogram), heart rate and blood pressures, and weight. In addition, tests were conducted for pregnancy (serum), drug toxicology (e.g., urinalysis for over 90 illicit and prescription drugs), tuberculosis, and HIV. Diagnostic interviews were conducted to assess psychiatric history (i.e., Structured Clinical Interview for DSM-IV Axis I Disorders [SCID], First et al., 1995), as well as substance abuse and psychosocial functioning (Addiction Severity Index, [ASI], McLellan et al., 1992). A benzoylecgonine (BE; cocaine metabolite) positive urine sample was required during intake. Study entry followed completion of the pre-treatment evaluation.
2.2.2. Stabilization
Following completion of intake procedures, participants were randomly assigned to a treatment condition, and medication administration was initiated during a stabilization phase (5-7 days duration), with daily clinic attendance. Subjects received an initial dose of 5 mg with 5 mg/day increments to 20 mg, then a 10 mg dose increase, with the potential for 2 days flexibility in run-up.
2.2.3. Treatment
After stabilization, subjects began the 8-week intervention phase of the study, during which thrice weekly attendance was required. In this two-phase sequential design, three medication treatment conditions were compared: (1) placebo, (2) 30-mg immediate release (IR) methamphetamine, or (3) 30-mg sustained release (SR) methamphetamine. At week 5, an abstinence-based contingency management procedure was introduced in each group (see below).
2.2.4. Run-down
Following the treatment phase, participants completed a 1-week rundown.
2.2.5. Exclusion from the Study
Given the potentially significant health risks associated with this study, we applied exceptionally stringent criteria for continuing participation in the trial. For any two-week period in the trial, participants had to provide at least 75% of the requested data. Failing to attend two visits in a given week would in most cases result in the participant being discontinued from the study.
2.3 Interventions
2.3.1. Medication
A procedure with multiple daily capsules for all groups was necessary to use the IR preparation. A simple color coding procedure was established for the first versus subsequent 5 doses each day. For all groups, the first dose of the day was in a yellow capsule and the remaining five doses were in blue capsules. The placebo group received six inactive capsules each day. For the IR condition there were six active doses of 5 mg each, while for the SR condition, there was one active 30 mg dose, (yellow capsule), and five inactive doses (blue capsules). Subjects were instructed to take the yellow capsule within two hours of awakening, and the remaining 5 capsules at intervals no less than two hours. On 3 days each week (Monday, Wednesday, Friday), ingestion of the first dose of the day was observed at the clinic.
Several strategies were utilized to ascertain medication compliance. First, capsules were distributed in Medication Event Monitoring Systems (MEMS, Aprex Corp.) pill bottles with a cap that recorded the time and date of each bottle opening. A separate MEMS bottle was used for each day and contained only that day's capsules. Thus, on Monday and Wednesday subjects received two medication bottles, and they received three bottles on Friday. The bottles were returned at each clinic visit, a pill count was obtained, and MEMS data were transferred and stored to a computer for later analysis. Compliance was also monitored by amending capsules with supradietary levels of riboflavin, followed by detection (see below) in urine samples (Del Boca et al., 1996). Riboflavin (100 mg/day) was evenly distributed across each capsule (16.67 mg/capsule).
2.3.2. Therapy
Manual-based, cognitive-behavioral therapy was provided for one hour each week by master's-level therapists. The cognitive-behavioral therapy emphasized relapse prevention and coping skills (for a full description see Schmitz et al., 2001).
2.3.3. Contingency management
Contingency management was implemented in weeks 5-8, based on CM's ability to reduce cocaine use and potentially improve response to pharmacotherapies (Carroll et al., 2004b; Carroll and Rounsaville, 2007; Higgins et al., 1991; Petry and Martin, 2002; Petry et al., 2004; Preston et al., 2001; Silverman et al., 1996; Stitzer and Vandrey, 2008). A simple fixed-ratio schedule was employed where each BE-negative urine sample was reinforced with a US$20 payment. A total of 12 payments were possible (3/week × 4 weeks). This was added to the baseline platform of medication plus cognitive behavioral therapy.
2.4. Measures
At each visit, subjects provided urine samples for analysis of BE, methamphetamine, and riboflavin. BE was assessed qualitatively and semi-quantitatively through analyses in our onsite analytical neurochemistry lab using the Syva EMIT system and the Abbott Toxi-Lab thin layer chromatographic system. Creatinine-adjusted samples were classified as positive with BE concentrations equaling or exceeding 300 ng/mL (Wilkins, 1997). Methamphetamine was also assessed semi-quantitatively. Semi-quantitative riboflavin levels were obtained using a Model 4-8202 Aminco-Bowman spectrophotofluorometer (American Instrument Co., Silver Springs, Maryland). Riboflavin levels range from 0 to 99 fluorescence units, with levels at or below 35 units considered to reflect non-compliance with medication administration (Mooney et al., 2007). On a weekly basis, patients completed measures of cocaine craving (Halikas et al., 1997). Mood was assessed with the Beck Depression Inventory (BDI, Beck et al., 1961). Medication side effects were determined with a questionnaire used in our other clinic studies plus additional questions regarding commonly reported methamphetamine side effects (i.e., changes in sleep, mood, and appetite). Nursing staff reviewed side effects and assessed each participant's weight, blood pressure, and heart rate each week. EKGs were conducted biweekly.
2.5. Statistical Analyses
2.5.1. Randomization and Assumptions
This study employed an adaptive enrollment strategy in which subject enrollment continued until 8 subjects in each condition completed the 8-week trial. The target sample size of 8 subjects was based on available study resources, and requirements of competing protocols. However data from all enrolled subjects were analyzed before a supplemental analysis of treatment completers. All analyses were conducted using the Statistical Analysis System, Version 9.1.3. (SAS Institute Inc., 2008). Values of p<.05 were considered statistically significant for main effects and interactions. Type I error rate in all post-hoc comparisons was controlled using Tukey-Kramer adjustments. Due to participant attrition and frequent missing data, the number of subjects or data points available for statistical analysis varied, with a maximum of 82 participants (57.3% of observations missing) and a minimum of 25 participants (25% observations missing). Missing data were treated as missing, and no imputation was used. For cocaine use and medication adherence, in a given week, the proportion was expressed as the number of events or numerator (e.g., cocaine positive tests, medication positive tests) to the number of tests or denominator (e.g., cocaine urine tests, urine riboflavin fluorescence tests).
2.5.2. Techniques
Comparability of study groups across baseline demographic and substance-use variables was evaluated using ANOVA for continuous variables and chi-square tests for categorical variables. Kaplan-Meier survival analysis with right censoring was used to test for differences in the duration of treatment as a function of condition. In the case of repeated measures analyses, we employed multilevel models with between-subjects effects of treatment, within-subjects effects of time, and the interaction of treatment and time.
2.5.3. Models
In repeated measures models, each model included tests for effects of Medication (i.e., 0 = placebo, 1 = methamphetamine, IR, 2 = methamphetamine, SR), Time (i.e., 1 – 8 weeks), and their interaction. In addition, to account for differences between Phase 1 and Phase 2, when the CM procedure was introduced, a bent-line parameterization was used, including a Knot (0 = weeks 1 – 4, else Knot = Time), and Knot × Medication interaction (e.g., Poling et al., 2006). In instances of non-significant Knot or Knot × Medication effects, these terms were eliminated from models before final interpretation. The value of the dependent measure during the intake phase was used as a covariate. One exception was cocaine use analyses in which self-reported cocaine use in the 30 days preceding treatment was employed as the covariate (Carroll et al., 2004a; McLellan et al., 1992).
3. Results
3.1. Sample Description
Sample characteristics including demographics, substance use variables, and psychosocial functioning are presented in Table 1. No differences were observed across conditions.
Table 1. Sample Characteristics.
| Condition | 0 mg (n = 27) |
30 mg IR (n = 30) |
30 mg SR (n = 25) |
|---|---|---|---|
| Demographic | |||
| Age (Years) | 36.8 (6.3) | 35.9 (5.1) | 36.5 (6.5) |
| %Female | 22.2 (11) | 10.0 (9) | 12.0 (8) |
| Education (Years) | 12.8 (1.7) | 13.3 (2.8) | 12.1 (2.2) |
| %Race | |||
| White | 25.9 (7) | 23.3 (7) | 36.0 (9) |
| Black | 70.4 (19) | 60.0 (18) | 48.0 (12) |
| Hispanic | 3.7 (1) | 16.7 (5) | 16.0 (4) |
| %Married | 14.8 (10) | 20.0 (12) | 16.0 (9) |
| %Employed | 70.4 (13) | 73.3 (13) | 60.0 (13) |
| Drug Use | |||
| %Intake Cocainea | 73.1 (20) | 69.0 (17) | 70.8 (21) |
| %Crack Cocaineb | 70.4 (19) | 70.0 (21) | 72.0 (18) |
| Cocaine Use (30 days) | 11.7 (9.1) | 12.3 (9.0) | 11.0 (5.8) |
| Cocaine (Years) | 10.5 (6.2) | 10.1 (6) | 9.6 (5) |
| Alcohol (Years) | 12.1 (9.3) | 14.6 (10.7) | 15.3 (8.9) |
| Marijuana (Years) | 12.6 (8.9) | 9.8 (7.3) | 8.5 (9) |
| ASI Composite Scores | |||
| Medical | 0.09 (0.19) | 0.02 (0.08) | 0.02 (0.07) |
| Employment | 0.56 (0.29) | 0.47 (0.29) | 0.56 (0.27) |
| Alcohol | 0.12 (0.16) | 0.21 (0.20) | 0.15 (0.15) |
| Drug | 0.23 (0.07) | 0.24 (0.09) | 0.23 (0.06) |
| Legal | 0.02 (0.06) | 0.06 (0.12) | 0.06 (0.16) |
| Family/Social | 0.17 (0.23) | 0.10 (0.18) | 0.19 (0.21) |
| Psychiatric | 0.03 (0.08) | 0.07 (0.13) | 0.08 (0.12) |
Note. No significant differences were observed among conditions on any variable. For continuous variables, mean values are reported with standard deviations in parentheses while for categorical variables, percentages are reported with subsample sizes in parentheses.
% positive for cocaine use at intake based on urine benzoylecgonine test.
% of individuals who were primarily crack cocaine users.
3.2. Retention
Survival analysis indicated no difference in dropout rates across the three treatment groups (Log Rank Statistic, χ2(2) = .40, p = .82), with 32% of participants randomized to treatment completing treatment (0 mg, 33%; 30 mg IR, 30%, 30 mg SR, 32%). The three groups did not differ in the number of weeks completed (M = 4.1, SD = 3.6). However, 49% (n = 40) of participants dropped from the study in the stabilization phase (52% [n = 20] lost to follow up; 2% [n = 1] due medication intolerance; and 48% [n = 19] did not adhere to the protocol, e.g., sporadic attendance). Inspection of retention rates in the non-randomized sample of 42 participants who entered the formal 8-week treatment phase revealed substantially higher completion rates, (0 mg, 53%; 30 mg IR, 64%, 30 mg SR, 67%).
3.3. Adverse Events
Side effect rates (i.e., reporting the side effect at least once in the 8-week treatment phase) are presented in Table 2. Those in the SR group reported higher rates of sleeping less and feeling less anxious. Only one subject, in the IR group, discontinued treatment due to intolerance of study medication (see Figure 1).
Table 2. Rates of 33 Self-Reported Medication Side Effects in the Treatment Phase.
| Side Effect | 0 mg (n = 16) |
30 mg IR (n = 12) |
30 mg SR (n = 10) |
|
|---|---|---|---|---|
| 1. | Change in appetite | 50.0 | 66.7 | 80.0 |
| 2. | Sleeping More | 50.0 | 58.3 | 50.0 |
| 3. | Sleeping Less | 18.8 | 33.3 | 70.0* |
| 4. | More Anxious | 18.8 | 33.3 | 50.0 |
| 5. | Less Anxious | 6.3 | 33.3 | 50.0* |
| 6. | More Unhappy | 12.5 | 33.3 | 20.0 |
| 7. | Happier | 75.0 | 83.3 | 90.0 |
| 8. | Coughing | 62.5 | 66.7 | 70.0 |
| 9. | Trouble Concentrating | 31.3 | 25.0 | 40.0 |
| 10. | Weight Changing | 37.5 | 66.7 | 60.0 |
| 11. | More Angry | 25.0 | 25.0 | 50.0 |
| 12. | Hands Shaking | 0.0 | 0.0 | 20.0 |
| 13. | Diarrhea | 12.5 | 8.3 | 40.0 |
| 14. | Constipation | 18.8 | 8.3 | 30.0 |
| 15. | Nausea | 12.5 | 16.7 | 20.0 |
| 16. | More Energy | 50.0 | 58.3 | 60.0 |
| 17. | Less Energy | 18.8 | 8.3 | 30.0 |
| 18. | Felt High | 0.0 | 16.7 | 10.0 |
| 19. | Chills | 0.0 | 16.7 | 10.0 |
| 20. | Fever | 0.0 | 16.7 | 10.0 |
| 21. | Heart Beating Slower | 6.3 | 16.7 | 20.0 |
| 22. | Heart Beating Faster | 12.5 | 33.3 | 40.0 |
| 23. | Breathe Faster | 12.5 | 50.0 | 40.0 |
| 24. | Dry Mouth | 25.0 | 33.3 | 70.0 |
| 25. | Runny Nose | 31.3 | 25.0 | 40.0 |
| 26. | Trouble with Eyes | 37.5 | 33.3 | 60.0 |
| 27. | Increased Urination | 50.0 | 50.0 | 60.0 |
| 28. | Drowsy | 18.8 | 16.7 | 60.0 |
| 29. | Muscles/Bones Ache | 12.5 | 33.3 | 50.0 |
| 30. | Felt Dizzy | 6.3 | 8.3 | 30.0 |
| 31. | Sleeping Better | 43.8 | 66.7 | 70.0 |
| 32. | Medication Too High | 0.0 | 0.0 | 10.0 |
| 33. | Medication Too Low | 18.8 | 33.3 | 50.0 |
Note. A total of 42 subjects entered the 8-week treatment phase, but 4 subjects did not provide side effects ratings before being dropped from the study in weeks 1 (n = 2) and 2 (n = 2) of treatment for protocol violations. Accordingly side effect ratings are available for 38 subjects. The rates of ever experiencing a side effect in the 8-week treatment periods are presented, collapsing across time due to low weekly endorsement rates.
p<. 05.
3.4. Vital Signs
3.4.1. Weight
A significant effect of medication group, F(2, 62.7) = 8.37, p = 0.0006, on body weight were observed. Body weights differed between the three treatment groups, (placebo, M = 186.4 lbs., SE = 0.84, MWeight Change = 2.60, SEWeight Change = 1.1; IR, M = 183.5 lbs., SE = 0.84, MWeight Change = 0.70, SEWeight Change = 1.14; SR, M = 181.3 lbs., SE = 0.92, MWeight Change = -2.30, SEWeight Change = 1.24). Post-hoc analysis revealed that the SR, t(63.3) = 4.05, p = 0.0004, lost more weight than the placebo group, and IR groups, t(63.4) = 2.46, p = 0.0436, gained less weight than the placebo group (Figure 2A).
Figure 2.
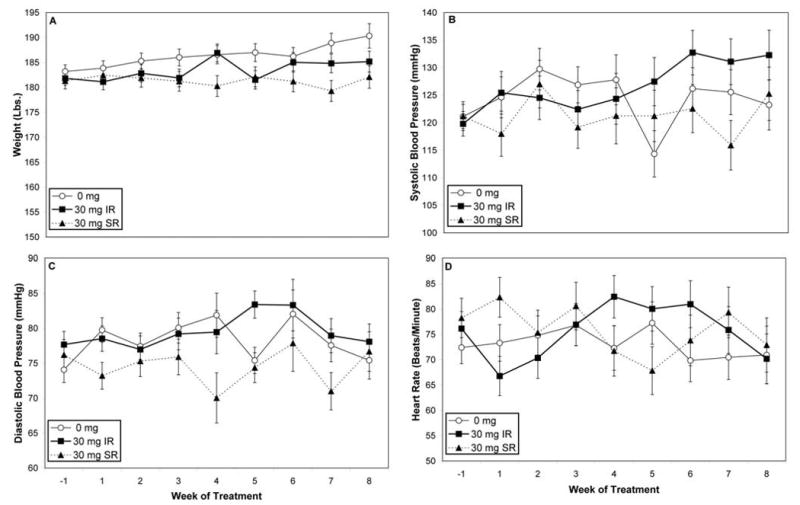
2A. Bodyweight over the 8-week treatment period. Group differences by weight were observed, where the SR condition lost weight while the placebo (0 mg) group gained weight. 2B. Systolic blood pressure tended to change over time, and as a functioning of medication group and time. 2C. Diastolic blood pressure did not vary across group, time, or their interaction. 2D. Heart rate did not vary across group, time, or their interaction.
3.4.2. Blood pressure and heart rate
Systolic blood pressure tended to change over time, F(8, 16.8) = 2.94, p = 0.0299, and as a function of medication group and time, F(16, 17.1) = 3.11, p = 0.0127, (placebo, M = 124.4, SE = 2.6; IR, M = 126.7, SE = 2.7; SR, M = 121.3, SE = 2.9) (see Figure 2B). No effects of medication group, time, or their interaction were observed for diastolic blood pressure (placebo, M = 78.2, SE = 1.3; IR, M = 79.5, SE = 1.4; SR, M = 74.5, SE = 1.5), (see Figure 2D) or heart rate, F(14, 157) = 1.83, p = 0.0387, (placebo, M = 73.1, SE = 2.2; IR, M = 75.5, SE = 2.3; SR, M = 75.8, SE = 2.6), (see Figure 2D).
3.5. Medication Adherence
3.5.1. Pill counts
During the treatment period, pill counts of the first medication dose each day (yellow pills; 0 mg, 5 mg IR, or 30 mg SR) revealed high compliance (95%) and no differences across treatment groups, although rates declined over time, F(1,1099) = 5.82, p = 0.0160. The proportion of first doses taken declined from 98% at week 1 to 87% at end of treatment. In the case of the subsequent medication doses (blue pills), those in the placebo group tended to take more pills (M = 2.35 pills/day) than those in the active treatment conditions, F(1,1092) = 2.82, p = 0.0534, (IR, M = 1.67 pills/day; and SR, M = 1.82 pills/day). As with the first (yellow) dose, the number of pills taken declined over the treatment period, F(1,1092) = 8.07, p = 0.0046. The number of blue pills taken declined from 2.4 pills/day (SE = .16) at week 1 to 1.4 pills/day (SE = .18) at end of treatment.
3.5.2. MEMS
On days with clinic appointments, 5 bottle openings were expected, since the yellow pill was administered during the visit, while 6 bottle openings were expected for intervening days. Groups did not differ in bottle openings (M = 2.21/day). Consistent with pill count data, the number of openings declined over the treatment period, F(1,1109) = 4.07, p = 0.0439. The number of bottle openings taken declined from 2.3 pills/day (SE = .13) at week 1 to 1.8 pills/day (SE = .15) at end of treatment.
3.5.3. Riboflavin
Adherence rates based on riboflavin did not differ by group, time, or their interaction, with an overall rate of 56%.
3.5.4. Methamphetamine exposure
Rates of positive urine tests for methamphetamine differed significantly across groups, F(2, 629) = 5.31, p = 0.0052, with all groups different from each other (ps<.0001; placebo, 0%; IR, 63.4%; SR, 94.4%).
3.6. Cocaine Use
3.6.1. Intention-to-treat sample
Analysis of cocaine use rates revealed a medication effect, F(2, 344) = 14.7, p< .0001, and effect of time, F(9, 344) = 2.49, p = .0091 (see Figure 3A). Post-hoc comparisons indicated that individuals in the SR condition exhibited a decreased proportion of cocaine-positive urine samples (29.0% positive) compared to either placebo (60.0%) or IR condition (66.0%).
Figure 3.
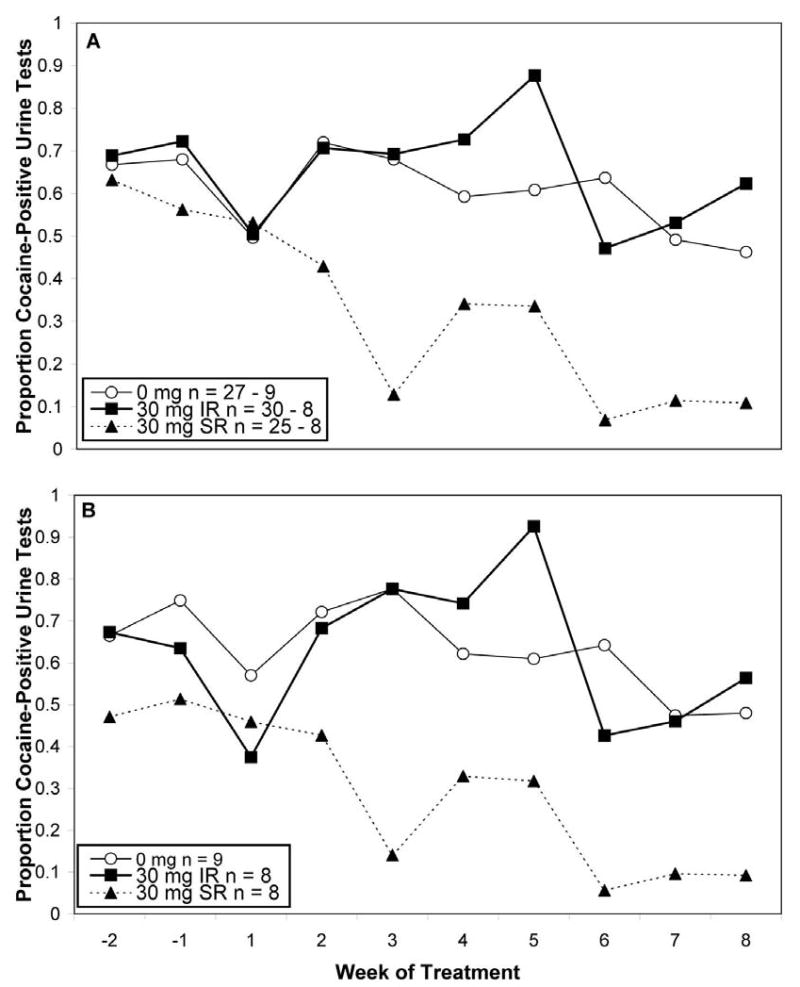
3A. Cocaine-use proportion in the intention-to-treat sample. Beginning (week 1 of treatment) and ending (week 8 of treatment) sample sizes are shown in the legend. The SR group had significantly fewer BE-positive urine tests than the placebo (0 mg) or IR conditions. 3B. Cocaine-use proportion in those completing treatment. Samples sizes are shown in the legend. The SR group had significantly fewer BE-positive urine tests than the placebo (0 mg) or IR conditions.
3.6.2. Completers sample
To evaluate the effects of completed treatment, we conducted an analysis focused on the 25 subjects finishing the 8-week trial (see Figure 3B). Prior to analysis, we compared completion status (completer versus non-completer), treatment condition, and their interaction on baseline variables in Table 1. No interactions between completion status and treatment condition were observed. Completers were employed at a higher rate than non-completers (84.0% vs. 61.4%), χ2(1) = 4.10, p = 0.0429. In addition, completers were somewhat more likely to have a urine sample negative for cocaine at their first visit (60.0% versus 76.0%), although this was not statistically significant. Accordingly, we included employment status and cocaine use at first visits as covariates. Analysis of this reduced sample again revealed a medication effect, F(2, 225) = 10.63, p< .0001. In this restricted subsample, post-hoc comparisons showed that the SR condition had a significantly lower rate of cocaine use (18% positive) than both placebo (64% positive), t(225) = 4.19, p = 0.0001, and IR groups (65% positive), t(225) = 39.972, p =0.0003.
3.7 Cocaine Craving
As assessed on a 10-cm visual analogue scale of craving for cocaine, a significant medication group effect was detected, F(2, 35.1) = 3.50, p = 0.0410, (placebo, M = 3.4, SE = 0.60; IR, M = 3.1, SE = 0.60; SR, M = 1.0, SE = 0.73). Post-hoc analysis revealed that the SR group reported less craving than the placebo group, t(36.3) = 2.49, p = 0.0451.
3.8. Mood
An effect of time was noted for the BDI with scores tending to decline over the course of the study, F(8, 200) = 2.21, p<0.0078. At the beginning of medication induction, BDI levels were in the non-depressed range (M = 9.1, SE = 1.07).
3.9. Contingency management
During the second, 4-week segment of the trial, there were non-significant but potentially meaningful differences in earnings, F(2, 25) = 3.01, p = 0.0675, (placebo, M = US$94.0, SE = 24.8; IR, M = US$108.0, SE = 36.3; SR, M = US$190.0, SE = 18.1).
All of the foregoing models (i.e., safety, tolerability, efficacy, and subjective measures) were also run including a Knot and a Knot×Medication terms. In all cases, these terms were not statistically significant, indicating that medication effects on study outcomes did not change following the introduction of the CM procedures at week 5 of treatment.
4. Discussion
In this double-blind, randomized, placebo-controlled trial, we investigated the safety, tolerability, and efficacy of methamphetamine in cocaine-dependent humans seeking to quit cocaine. Methamphetamine was generally safe and well-tolerated under the restricted conditions of this study. Adherence for the initial daily dose was high, but decreased for subsequent doses. Interestingly, in the IR group, the majority of doses were not taken, but instead returned to the clinic. The SR preparation of methamphetamine was superior to placebo and to the IR preparation in reducing cocaine use and craving. The IR preparation did not differ from placebo on these outcomes. However, it must be noted that compliance in the IR condition was so low as to prevent achievement of therapeutic drug levels, thus making it little different from the placebo condition. Addition of a contingency management procedure in the second half of the treatment period had little discernible effect on outcome measures.
Major concerns in using methamphetamine treatment in a stimulant dependent population include safety and tolerability. No differences in treatment retention were observed between groups, and only one patient, in the IR group, discontinued treatment due to medication intolerance. Those in the SR group were more likely to report sleeping less than the IR or placebo groups. Those in the SR group lost about 2 pounds, but generally showed no changes in appetite. Methamphetamine is a sympathomimetic, and thus we monitored cardiovascular functioning rigorously. As in our previous reports with dextroamphetamine (Grabowski et al. 2001; Grabowski et al. 2004), we observed few differences between groups, with average blood pressures and heart rates falling in the normal range. In the context of this relatively short trial, methamphetamine was generally safe and well tolerated, consistent with other recent studies (Comer et al., 2001; Hart et al., 2001; Hart et al., 2003).
The mechanisms of action of agonist-like treatments for cocaine dependence are not completely understood. Preclinical and clinical studies suggest that chronic cocaine use results in deficits in the dopamine (DA) and serotonin (5-HT) systems, and alterations in noradrenergic (NE) functioning (McDougle et al., 1994; Rothman et al., 2002). Many studies have utilized medications to selectively enhance DA, 5-HT, or NE systems, with mostly negative results in the absence of robust behavioral treatments (Gorelick et al., 2004). In contrast, mounting data suggest promise with medications possessing a more broad action at monoamine systems. Withdrawal from chronic cocaine use is associated with deficits in DA and 5-HT functioning which are thought to underlie negative effects, including cocaine withdrawal and craving as well as anhedonia, impulsivity, and depression (Rothman et al., 2006; Rothman et al., 2000). Rothman and Baumann have proposed a dual-deficit model of stimulant dependence, in which deficits in DA and 5-HT functioning are the focus of pharmacotherapeutic intervention (Rothman et al., 2006; Rothman et al., 2005). Three widely-studied DA/5-HT releasers with respect to cocaine dependence include phentermine plus fenfluramine (Phen-Fen), PAL-287, and methamphetamine. In pre-clinical models, all three have been shown to substitute for cocaine in drug discrimination tasks (Negus et al., 2007; Schechter and McBurney, 1996). Additionally, pretreatment with these medications produces large reductions in cocaine self administration, with lesser alteration on food reinforcement (Glowa et al., 1997; Negus et al., 2007), and are, by themselves, self-administered at lower rates than cocaine (Griffiths et al., 1978; Newman and Carroll, 2006; Rothman et al., 2005). Collectively, previous data as well as the current proof-of-concept study support a pharmacotherapy approach that broadly targets multiple monoaminergic systems (Gorelick et al., 2004).
Considerable research has focused on the relative merits of IR versus SR preparations of stimulants for ADHD. IR formulation provides pulsatile exposure to medication, and this rapid, intermittent exposure could potentially provide more rapid relief of craving. On the other hand, this pattern might actually potentiate abuse (Parasrampuria et al., 2007). However, treatment adherence is negatively associated with number of daily doses, with sharp drops in compliance after 3 daily doses (Claxton et al., 2001). In contrast, SR formulation allows for steady, continuous release of medication, minimizing fluctuations in drug levels and reducing side effects. Since SR formulations require one or two doses each day, compliance is more readily achieved. We were surprised by the poor compliance in the IR condition, expecting that the reinforcing effects of methamphetamine would surmount the challenges of taking 6 daily doses. Whatever advantages IR formulations confer, the adherence burden of the present regime argues against their use. However, advances in drug formulations systems now permit for hybrid formulations that combine an IR component with a longer acting SR component (Markowitz et al., 2003).
Given the preliminary nature of this trial, we were interested in evaluating medication effects alone, before adding a potent CM intervention. No CM advantage or potentiation of medication effects was found. It is possible that the robust ability of methamphetamine SR to reduce cocaine use left little room for further improvement with CM. In choosing a behavioral therapy platform, CM is recommended when evaluating pharmacotherapies having partial or weak effects (Carroll et al., 2004). It is surprising, however, that the CM used here, which included a moderately high magnitude reward schedule (FR US$20) did not significantly alter cocaine use in the non-responding treatment groups (placebo, IR). Use of an escalating schedule of reinforcement, contingent on continuous abstinence, may have yielded a treatment response (e.g., Preston et al., 2001). The lack of typical CM benefit may be explained by the short duration of the intervention. Cocaine-using participants who remained in treatment weeks 5-8 may have lacked the motivation and the time needed to detoxify from cocaine needed to come into contact with CM incentives. Thus, the lack of CM effects likely owes to methodological limitations in our design, and stand in contrast to the robust literature showing the efficacy of CM procedures to reduce cocaine use (Higgins et al., 1991; Petry and Martin, 2002; Petry et al., 2004; Preston et al., 2001; Silverman et al., 1996; Stitzer and Vandrey, 2008).
This study has some limitations. As described above, the lack of effect for CM should be interpreted with caution. Methamphetamine and cocaine levels were assessed semi-quantitatively; however in other studies by our group, quantitative and semi-quantitative assessments have yielded similar results (Grabowski et al., 2001; Grabowski et al., 2004). Furthermore, despite our best efforts to maximize retention, overall rates of treatment completion were low, with fewer than half of subjects receiving the full course of treatment. Finally, though substantial effort was made to have equal numbers of males and females in this study, the current disparity limits the opportunity to explore potential gender differences in response to treatment.
In summary, this current proof-of-concept trial demonstrated that an SR formulation of methamphetamine can substantially reduce cocaine use over an 8-week interval with close supervision and psychological support. While methamphetamine cannot be advocated as standard treatment, further evaluation of mixed monoaminergic medications for the treatment of cocaine dependence appears warranted.
Contributor Information
Marc E. Mooney, Department of Psychiatry, University of Minnesota, Minneapolis
David V. Herin, Department of Psychiatry, University of Minnesota, Minneapolis
Joy M. Schmitz, Department of Psychiatry and Behavioral Sciences, University of Texas Houston
Nidal Moukaddam, Department of Psychiatry and Behavioral Sciences, University of Texas Houston
Charles Green, Department of Psychiatry and Behavioral Sciences, University of Texas Houston
John Grabowski, Research Division SMDC, Duluth MN/Department of Behavioral Science/Department of Psychiatry University of Minnesota
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S. History of the methamphetamine problem. J Psychoactive Drugs. 2000;32:137–141. doi: 10.1080/02791072.2000.10400221. [DOI] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Beck A, Ward C, Mendelson M, Mack J, Erbaugh J. An inventory of measuring depression. Archives of General Psychiatry. 1961;49:599–608. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004a;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kosten TR, Rounsaville BJ. Choosing a behavioral therapy platform for pharmacotherapy of substance users. Drug Alcohol Depend. 2004b;75:123–134. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ. A vision of the next generation of behavioral therapies research in the addictions. Addiction. 2007;102:850–862. doi: 10.1111/j.1360-0443.2007.01798.x. discussion 863-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Effects of repeated oral methamphetamine administration in humans. Psychopharmacology (Berl) 2001;155:397–404. doi: 10.1007/s002130100727. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-VI Axis I Disorders- Patient Edition (SCID -I / P, Version 2.0) Biometric Research Department; NY: 1995. [Google Scholar]
- Glowa JR, Rice KC, Matecka D, Rothman RB. Phentermine/fenfluramine decreases cocaine self-administration in rhesus monkeys. Neuroreport. 1997;8:1347–1351. doi: 10.1097/00001756-199704140-00006. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Roache JD, Schmitz JM, Rhoades H, Creson D, Korszun A. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol. 1997;17:485–488. doi: 10.1097/00004714-199712000-00008. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Brady JV, Snell JD. Progressive-ratio performance maintained by drug infusions: comparison of cocaine, diethylpropion, chlorphentermine, and fenfluramine. Psychopharmacology (Berl) 1978;56:5–13. doi: 10.1007/BF00571401. [DOI] [PubMed] [Google Scholar]
- Halikas JA, Crosby RD, Pearson VL, Graves NM. A randomized double-blind study of carbamazepine in the treatment of cocaine abuse. Clin Pharmacol Ther. 1997;62:89–105. doi: 10.1016/S0009-9236(97)90155-7. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Methamphetamine self-administration by humans. Psychopharmacology (Berl) 2001;157:75–81. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Nasser J, Foltin RW. Methamphetamine attenuates disruptions in performance and mood during simulated night-shift work. Psychopharmacology (Berl) 2003;169:42–51. doi: 10.1007/s00213-003-1464-4. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Barrett JE. The discriminative stimulus effects of cocaine in pigeons. J Pharmacol Exp Ther. 1993;267:1–8. [PubMed] [Google Scholar]
- Lineberry TW, Bostwick JM. Methamphetamine abuse: a perfect storm of complications. Mayo Clin Proc. 2006;81:77–84. doi: 10.4065/81.1.77. [DOI] [PubMed] [Google Scholar]
- Margolin A, Kosten TR, Avants SK, Wilkins J, Ling W, Beckson M, Arndt IO, Cornish J, Ascher JA, Li SH, et al. A multicenter trial of bupropion for cocaine dependence in methadone- maintained patients. Drug Alcohol Depend. 1995;40:125–131. doi: 10.1016/0376-8716(95)01198-6. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Straughn AB, Patrick KS. Advances in the pharmacotherapy of attention-deficit-hyperactivity disorder: Focus on methylphenidate formulations. Pharmacotherapy. 2003;23:1281–1299. doi: 10.1592/phco.23.12.1281.32697. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Black JE, Malison RT, Zimmermann RC, Kosten TR, Heninger GR, Price LH. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Arch Gen Psychiatry. 1994;51:713–719. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Greenwood Village, CO: Thompson Healthcare; Micromedex® Healthcare Series. n.d. Retrieved June 9, 2008 http://www.thomsonhc.com. [Google Scholar]
- Mooney ME, Schmitz JM, Moeller FG, Grabowski J. Safety, tolerability and efficacy of levodopa-carbidopa treatment for cocaine dependence: Two double-blind, randomized, clinical trials. Drug Alcohol Depend. 2007;88:214–223. doi: 10.1016/j.drugalcdep.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Newman JL, Carroll ME. Reinforcing effects of smoked methamphetamine in rhesus monkeys. Psychopharmacology (Berl) 2006;188:193–200. doi: 10.1007/s00213-006-0479-z. [DOI] [PubMed] [Google Scholar]
- Parasrampuria DA, Schoedel KA, Schuller R, Silber SA, Ciccone PE, Gu J, Sellers EM. Do formulation differences alter abuse liability of methylphenidate? A placebo-controlled, randomized, double-blind, crossover study in recreational drug users. J Clin Psychopharmacol. 2007;27:459–467. doi: 10.1097/jcp.0b013e3181515205. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, White WR, McDonald SA, Hicks RE, Jeffcoat AR, Hill JM, Cook CE. Clinical effects of daily methamphetamine administration. Clin Neuropharmacol. 1991;14:352–358. doi: 10.1097/00002826-199108000-00007. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95:219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J Consult Clin Psychol. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine users: how low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, Martell B, Kosten TR. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Wong CJ, Epstein DH. Shaping cocaine abstinence by successive approximation. J Consult Clin Psychol. 2001;69:643–654. doi: 10.1037//0022-006x.69.4.643. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Appetite suppressants as agonist substitution therapies for stimulant dependence. Ann N Y Acad Sci. 2002;965:109–126. doi: 10.1111/j.1749-6632.2002.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine-5-HT releasers: potential treatment agents for cocaine addiction. Trends Pharmacol Sci. 2006;27:612–618. doi: 10.1016/j.tips.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, Roth BL, Baumann MH. Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther. 2005;313:1361–1369. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Partilla JS, Dersch CM, Carroll FI, Rice KC, Baumann MH. Methamphetamine dependence: medication development efforts based on the dual deficit model of stimulant addiction. Ann N Y Acad Sci. 2000;914:71–81. doi: 10.1111/j.1749-6632.2000.tb05185.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. The SAS System for Windows. SAS Institute Inc.; Cary, NC: 2008. [Google Scholar]
- Schechter MD, McBurney D. Phentermine+fenfluramine produce cocaine-like discriminative cues. Life Sci. 1996;59:PL303–308. doi: 10.1016/s0024-3205(96)00513-9. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Mooney ME, Moeller FG, Stotts AL, Green C, Grabowski J. Levodopa pharmacotherapy for cocaine dependence: Choosing the optimal behavioral therapy platform. Drug Alcohol Depend. 2008;94:142–150. doi: 10.1016/j.drugalcdep.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26:167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98:1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Watson DW, Reiber C, Rawson RA, Montgomery MA, Majewska MD, Ling W. Randomized controlled pilot trial of cabergoline, hydergine and levodopa/carbidopa: Los Angeles Cocaine Rapid Efficacy Screening Trial (CREST) Addiction. 2005;100 1:78–90. doi: 10.1111/j.1360-0443.2005.00991.x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, Preston KL. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry. 1996;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Vandrey R. Contingency management: utility in the treatment of drug abuse disorders. Clin Pharmacol Ther. 2008;83:644–647. doi: 10.1038/sj.clpt.6100508. [DOI] [PubMed] [Google Scholar]
- Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci. 1998;18:419–427. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins JN. NIDA Res Monogr. Vol. 175. 1997. Quantitative urine levels of cocaine and other substances of abuse; pp. 235–252. [PubMed] [Google Scholar]
- Wolfsohn R, Sanfilipo M, Angrist B. A placebo-controlled trial of L-dopa/carbidopa in early cocaine abstinence. Neuropsychopharmacology. 1993;9:49–53. doi: 10.1038/npp.1993.42. [DOI] [PubMed] [Google Scholar]


