Abstract
Objective
Pain and depression often occur together. Pain is both a sensation and an affective experience. Similarly, depression is associated frequently with somatic symptoms as well as emotional dysphoria. Existing evidence indicates that major depressive disorder (MDD) may be associated with altered pain processing. However, the extent to which alterations in experimentally controlled heat pain sensations are related to increased affective bias in MDD is unknown. This psychophysical study examined the hypothesis that young adults with MDD would show increased affective bias to painful and non-painful experimental heat stimuli, as evidenced by an increased responsiveness to warm and hot temperatures.
Method
Graded non-noxious and noxious heat stimuli were delivered randomly with a thermode applied to the volar surface of the left arm of 15 unmedicated subjects with current MDD and 15 age- and gender-matched healthy comparison subjects. MDD and non-MDD subjects rated the intensity and unpleasantness of all stimuli.
Results
Two main results were observed. Firstly, MDD relative to non-MDD subjects showed decreased heat pain thresholds. Secondly, a significantly increased affective bias (= unpleasantness/intensity) was observed in MDD subjects, particularly over the range of non-noxious heat stimuli. This bias was independent of the change in sensory pain thresholds.
Conclusion
These findings represent corroborative evidence of abnormal affective heat pain processing in young adults with MDD, and suggest that MDD is associated with “emotional allodynia”, a qualitatively altered negative emotional response to normally non-aversive thermal stimuli.
Keywords: psychophysics, allodynia, MDD, thermode, heat, nociception
Introduction
Pain and depression are common and highly comorbid. Over 75% of patients with a clinical depressive disorder experience chronic or recurring pain, such as headache, stomach pain, neck and back pain, or non-specific generalized pain (1). Conversely, 30–60% of pain patients (i.e., chronic pain, migraine, etc.) report significant depressive symptoms (2). The comorbidity between pain and depression contributes significantly to poorer outcomes and increased cost of treatment (3). The high comorbidity between these conditions potentially suggests a common underlying pathophysiology. Specifically, pain and depression may be related clinical manifestations of altered activity within a common neural network. However, the neurobiological basis of pain processing in major depressive disorder (MDD) is incompletely understood.
Pain is a subjective experience, which can be assessed along two dimensions, i.e., sensory pain intensity, which describes the discrimination of the stimulus intensity, and affective pain unpleasantness, which describes the emotional impact of the stimulus (4, 5). During brief experimental noxious stimuli, these dimensions are highly correlated (6–8), and both of these dimensions are greatly influenced by the cognitive, emotional and physiological state of the individual (9–11). For example, pleasant emotions and cognitions (i.e., expectation of pain relief) can attenuate pain perception, and negative emotions or cognitions, such as catastrophizing (i.e., thinking that pain will never end), can amplify perceived pain intensity and unpleasantness (9). Therefore, increased attention to one’s negative feeling state, which is often observed in depressed patients (12), may fundamentally alter the pain experience and underlying brain circuitry in MDD. This conceptualization is consistent with recent evidence of increased perception and neuronal activation in the brain areas encoding the affective, but not the sensory-discriminative aspect of pain processing in subjects with depressed mood (13, 14) and in patients with comorbid chronic pain and depression (15). In fact, heightened emotional reactivity and emotional biasing of daily self-reports of subjective pain experience is consistently observed in chronic pain patients, especially in those with a history of depression and increased daily depressive moods (16–18).
Several prior studies examined sensitivity to experimental pain in depressed versus non-depressed patients, with discordant results. Decreased pain sensitivity in depressed individuals was observed in several studies, as evidenced by increased pain thresholds (19–24). Other studies reported increased pain sensitivity in depression, as evidenced by decreased pain thresholds (25) and decreased pain tolerance (26–29). Finally, one study reported no difference in pain sensitivity in mild depression (30). Several methodological differences could be responsible for these discrepancies. Firstly, some studies that found decreased or no change in pain perception in depression did not control for the confounding effects of antidepressant use (20, 27, 28) and/or rule out comorbid clinical pain complains (19, 24), which are both known to interfere with pain sensitivity (31, 32). Secondly, other studies looked at a non-homogeneous patient sample including patients with a range of psychiatric disorders as a primary diagnosis (e.g., bipolar, psychosis) that are known to differently influence the perception of pain (reviewed in (33)). Thirdly, the majority of earlier studies on experimental pain did not separately examine the sensory and affective dimensions of the experimental pain experience, which as discussed above is crucial for understanding pain processing in depression (13–15). In the present study, we controlled for all of the above discrepancies in order to identify how MDD affects sensory and affective perception of experimental heat pain.
The aim of the current experiment was to examine whether unmedicated young adults with MDD, but without co-morbid pain conditions or other medical problems, would show altered sensitivity to brief heat stimuli. We hypothesized that MDD relative to non-MDD subjects would demonstrate a bias towards the affective component of brief heat stimuli. Support for this hypothesis would contribute to a more comprehensive understanding of the pathophysiology of pain and depression, as well as suggest a possible mechanism that could explain the emotional bias of daily self-reports of pain in chronic pain patients.
Materials and Methods
Subjects
Fifteen unmedicated (> 30 days) subjects with current MDD (3M, mean age 24.5±5.5) gave written informed consent to participate in this study, which was approved by the UCSD Human Protection Program (IRB protocol # 050511). The data were collected from February 2004 to August 2005. Subjects completed the structured clinical interview for DSM-IV (SCID-P) (34) and met DSM-IV criteria for current MDD (Table 1), based on the diagnosis of a board-certified psychiatrist (S.M). Depressive symptoms severity was measured with the Beck Depression Inventory (BDI-II) (35). Ten of the fifteen subjects were naïve to psychotropic medication. Two female subjects had past PTSD, three subjects (1M) had past dysthymia, one female subject had past GAD and PD, and one female subject had past dysthymia and PD. All these diagnoses were not current. The MDD subjects were compared to fifteen medically healthy subjects (3M, mean age 23.9±5.2) who also completed SCID-P (34) and showed no history of MDD or other Axis I psychiatric disorder. The non-MDD subjects were matched to the MDD subjects for gender, age and level of education (Table 2). Subjects were excluded from the study if they: 1) met DSM-IV criteria for lifetime alcohol or substance dependence; 2) met DSM-IV criteria for alcohol or substance abuse within the past 30 days; 3) were experiencing active suicidal ideation; 4) had a history of bipolar or psychotic disorder; 5) had an active medical problem; 6) had a history of chronic pain. In addition to the BDI-II, all subjects completed: 1) the Multidimensional Fatigue Symptom Inventory – Short Form (MFSI-SF) (i.e., a self-report 30-item questionnaire which assesses general, physical, emotional, and mental manifestations of fatigue as well as vigor- an estimate of energy level) (36); 2) the neuroticism module of the NEO-FFI (i.e., a self-report 60-item version of NEO personality inventory that assesses five domains of adult personality, including neuroticism, extraversion, openness to experience, agreeableness, and conscientiousness) (37).
Table 1.
Patients Characteristics
| ID | Sex | Age | MDD Onset | # Lifetime MDD Episodes | BDI-II | Diagnosis (DSM-IV) | Lifetime Co-morbidities |
|---|---|---|---|---|---|---|---|
| 1 | F | 20 | 18 | 3 | 25 | MDD | None |
| 2 | F | 19 | 17 | 2 | 15 | MDD | None |
| 3 | F | 19 | 16 | 3 | 22 | MDD | PTSD$ |
| 4 | F | 18 | 17 | 3 | 33 | MDD | PTSD$ |
| 5 | F | 19 | 16 | 3 | 18 | MDD | None |
| 6 | M | 29 | 13 | 3 | 43 | MDD | Dysthymia$ |
| 7 | F | 21 | 13 | 5 | 23 | MDD | None |
| 8 | F | 25 | 15 | 8 | 32 | MDD | GAD$, PD$ |
| 9 | F | 24 | 12 | 9 | 26 | MDD | Dysthymia$, PD$ |
| 10 | F | 28 | 25 | 2 | 24 | MDD | None |
| 11 | M | 34 | 28 | 1 | 34 | MDD | None |
| 12 | F | 35 | 31 | 12 | 26 | MDD | Dysthymia$ |
| 13 | M | 20 | 18 | 6 | 30 | MDD | None |
| 14 | F | 28 | 11 | 4 | 26 | MDD | Dysthymia$ |
| 15 | F | 22 | 14 | 3 | 40 | MDD | None |
MDD = major depressive disorder; PTSD = post-traumatic stress disorder; GAD = general anxiety disorder; PD = panic disorder; BDI-II = Beck Depression Inventory II;
= lifetime, not current diagnosis
Table 2.
Demographics & Behavioral Measures
| MDD | Comparison Group | ||||
|---|---|---|---|---|---|
| Gender | 3M, 12F | 3M, 12F | |||
| Mean | SD | Mean | SD | T-value | |
| Age | 24.1 | 5.6 | 23.9 | 5.2 | 0.1n.s. |
| Education | 15.7 | 1.7 | 15.4 | 2.0 | 0.4n.s. |
| BDI-II | 27.8 | 7.6 | 1.3 | 1.7 | 13.1** |
| MFSI-SF | 43.5 | 15.1 | −8.1 | 8.6 | 11.3** |
| Neuroticism | 70.5 | 8.3 | 41.4 | 9.8 | 8.4** |
| Heat Pain Threshold | 41.7 | 3.3 | 43.9 | 1.2 | 2.4* |
p < 0.01,
p < 0.05,
n.s. – non significant; BDI-II – Beck Depression Inventory; MFSI-SF – short form of the multidimensional fatigue symptoms inventory
Temperature Sensitivity
The method of constant stimuli was used to measure subjects’ sensitivity to heat. Heat stimulation started from a baseline of 32°C and rose linearly at a rate of 1.5°/C to one of five predetermined temperatures (40, 42, 44, 46, 48°C). The duration of each stimulus was 5 sec, excluding the rise/fall time. Each temperature was presented twice, and the order of presentation was randomized and unknown to the subjects and the experimenter. A 9cm2 thermode (Medoc TSA-II, Ramat-Yishai, Israel) was applied to each subjects’ volar forearm, and the site of stimulation on the forearm skin was varied slightly to avoid sensitization. The skin under the thermode was adapted to the baseline thermode temperature before the start of stimulation. The interval between successive stimuli was at least 30 sec, and the minimum interval between stimulation of the same skin site was at least 1 min. Subjects were asked to rate the intensity and unpleasantness of each stimulus using three validated visual scales (6). After each temperature stimulus, subjects were asked to rate the maximum sensation of pain using a scale that ranged from 0 (“no pain sensation”) to 10 (“extremely intense pain sensation”). If the stimulus produced no painful sensation, subjects were asked to rate how far from painful the sensation was using a scale that ranged from 0 (“extremely warm” – the pain threshold) to −10 (“no sensation”). Furthermore, subjects rated the maximum unpleasantness evoked by each temperature stimulus, even if it was perceived as non-painful, using a scale that ranged from 0 (“not at all unpleasant”) to 10 (“extremely unpleasant”). Each subject’s pain threshold was estimated by fitting linear functions to the non-painful/painful intensity response curves (7, 38). In order to specifically evaluate affective bias, all unpleasantness ratings were weighted by the heat intensity ratings at that temperature (affective bias = unpleasantness rating/intensity rating). This transformation was used to control for possible group differences in subjects’ response bias (39). Intensity ratings were rescaled from −10 to 10 to 0 to 10 before affective bias was calculated.
Statistical Analyses
We used Pearson correlations to explore how the affective bias measure related to other common psychophysiological variables. Specifically, we tested the relationship between affective bias (averaged across all stimulus temperatures), BDI, MFSI-SF and the neuroticism score of the NEO-FFI (significance level was adjusted based on Bonferroni correction for multiple comparison). These measures were selected since MDD has been associated with high neuroticism and fatigue (40, 41). In order to specifically test the hypothesis that MDD relative to non-MDD subjects show bias towards the affective component of brief heat stimuli we ran repeated measures ANOVA on the affective bias ratings (group as between-subject factor; temperature as within-subject factor). To determine the individual contrasts that powered the group by temperature interaction effects we did exploratory post-hoc t-tests at each temperature (40°, 42°, 44°, 46°, and 48°). The slopes and intercepts of the individual affective bias curves were determined by linear regression and the coefficients were compared between groups after correcting for multiple comparisons. Differences in slopes reflected between group differences in discrimination, whereas differences in the intercept of the affective bias curve reflected between group differences in the initial reactivity to temperature stimuli. The relationship between affective bias (averaged across all stimulus temperatures) and sensory pain thresholds was tested using Pearson correlations. The between group difference in the strength of these correlations was tested by contrasting the Fisher Z-transform of the correlation values in each group. In addition, we ran two exploratory follow-up repeated measures ANOVAs for the intensity and unpleasantness ratings separately to provide further details about how depression affects these variables. Finally, to explore which behavioral measure, i.e., intensity, unpleasantness, or affective bias, explained the greatest degree of variance between the groups at each temperature level (40°, 42°, 44°, 46°, and 48°) we performed five exploratory step-wise linear regressions where all three measures were entered simultaneously into the model. All statistical analyses were done with SPSS 12.0 (SPSS. Inc, Chicago, IL).
Results
Demographics and Psychophysiological Variables
As expected, MDD subjects exhibited significantly higher levels of: 1) depression, as measured by the BDI-II, 2) fatigue, as measured by the MFSI-SF, and 3) neuroticism, as measured by the NEO-FFI (Table 2). In addition, sensory pain thresholds were significantly lower in the MDD relative to the non-MDD subjects (t = 2.4, p < 0.05, Table 2).
Best Measure to Differentiate the Groups
The results of exploratory step-wise linear regression analysis with group as a dependent variable and three predicting factors (subjective stimulus intensity, unpleasantness, and affective bias ratings, i.e., unpleasantness rating/intensity rating) are shown in Table 3 for each stimulus temperature. Group membership was significantly predicted by the affective bias measure at all temperatures tested, except for 48°C. In other words, at all temperature stimuli, except for 48°C, the affective bias measure explained most of the between-group variance; adding unpleasantness and intensity ratings did not improve the model. It is important to note that although intensity and unpleasantness ratings were used to calculate the affective bias ratio, it was the ratio that best explained the variance between MDD and non-MDD patients whereas the linear combination of the intensity and unpleasantness ratings did not. At 48°C all factors were needed to predict the group differences.
Table 3.
Linear Regression Analysis (n=30)
| Temp | AB1 | AB & Unpl | AB & Unpl & Int |
|---|---|---|---|
| 40 | .431** | .452 | .535 |
| 42 | .536** | .568 | .609 |
| 44 | .643** | .644 | .645 |
| 46 | .621** | .643 | .667 |
| 48 | .296 | .383 | .522* |
: R-coefficients are shown;
: p < 0.05;
: p < 0.01; stepwise linear regression model with group as dependent variable and three predicting factors: affective bias (AB), unpleasantness (Unpl), and intensity (Int);
Relationship between Affective Bias and Psychophysiological Variables
The results of Pearson correlations between average affective bias and behavioral measures are shown in Table 4. Following Bonferroni correction for multiple comparisons significant positive relationships were observed between all variables tested, including BDI-II, MFSI-SF and neuroticism scores in the combined group (r’s > 0.5, p’s < 0.05, Table 4). These positive relationships between the BDI-II, MFSI-SF, neuroticism and the affective bias remained significant after controlling for sensory pain thresholds in the combined group (r’s > 0.4, p’s < 0.05, Table 4).
Table 4.
Pearson Correlations
| AB | AB co-v. Th1 | |
|---|---|---|
| Measure | r-coeff | r-coeff |
| BDI-II | .575** | .458* |
| MFSI-SF3 | .615** | .473* |
| Neuroticism2,3 | .555** | .457* |
p<0.01;
p<0.05 (Bonferroni correction for multiple comparisons); affective bias (AB),
- correlation coefficients after co-varying for sensory pain thresholds; BDI-II – Beck Depression Inventory, MFSI-SF – short form of the multidimensional fatigue symptom inventory;
– from NEO-FFI five-factor personality inventory;
– missing data on 2/30 subjects
Increased Pain Affect in MDD
Figure 1A shows ratings of the perceived intensity and unpleasantness to brief heat stimuli in MDD and non-MDD subjects. Repeated measures ANOVAs revealed significant main effects of both group (p < 0.01) and temperature (p < 0.01) on the perceived intensity (p < 0.01) and unpleasantness (p < 0.01) rating to brief temperature stimuli. Additionally, a significant group by temperature interaction on the perceived unpleasantness rating was observed (p < 0.01). Post-hoc analyses showed that MDD subjects rated the heat stimuli as more intense at 42°C (p < 0.05), 44°C (p<0.01) and 46°C (p<0.01), but not at 40°C (p=0.06) or 48°C (p=0.2) (Figure 1A, left). Similarly, MDD subjects rated the stimuli as more unpleasant at 40°C (p<0.05), 42°C (p < 0.01), 44°C (p<0.01) and 46°C (p<0.01), but not at 48°C (p=0.07) (Figure 1A, right). Figure 1B shows ratings of the affective bias to brief heat stimuli. Repeated measures ANOVAs showed significant main effects of group (p < 0.01) and of temperature (p<0.01) and a significant group × temperature interaction (p<0.01) on the affective bias rating. For both groups, as intensity ratings increased, unpleasantness ratings also increased. Post-hoc analyses showed a greater affective bias rating in the MDD relative to the non-MDD group at 40°C (p<0.05), 42°C (p<0.01), 44°C (p<0.01), and 46 °C (p<0.01), but not at 48 °C (p=0.1) - the temperature at which the affective bias ratings did not differ between the groups (see Figure 1B). There were no significant differences in the slopes of the stimulus-response functions related to the different temperature stimuli (t= −1.0, p = 0.3), suggesting that there were no differences in scaling of heat stimuli. However, a significant difference between the intercepts of the stimulus-response functions was observed between the groups (MDD: 36.9°C ± 1.6, non-MDD: 40.9°C ± 0.5, t= 2.5, p <0.05), suggesting that there were differences in the initial reactivity to the temperature stimuli. Interestingly, in the MDD group, the affective bias ratings at 40°C were as high as the ratings at 46°C in the non-MDD group (t = −0.26, p = 0.79).
Figure 1. Psychometric Functions. A. Intensity (left) and unpleasantness (right) ratings to temperature stimuli in MDD (closed shapes) and Comparison (CON, open shapes) Subjects.
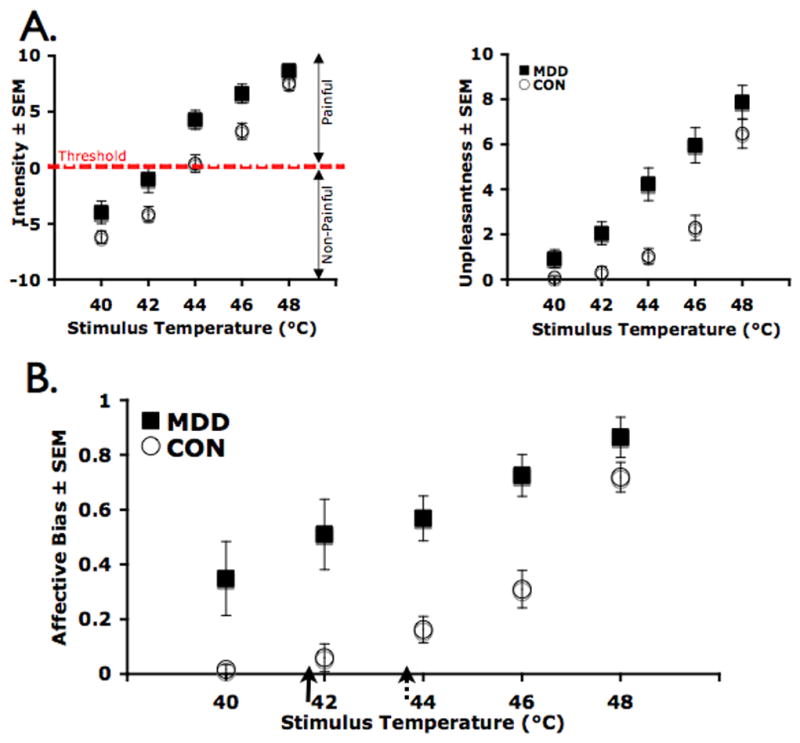
Repeated measures ANOVA showed significant effect of group and temperature for both measures, and significant group × temperature interaction for the unpleasantness measure (p’s < 0.01). B. Affective Bias ratings during temperature stimuli in MDD (closed shapes) and Comparison (CON, open shapes) Subjects. Affective bias to heat stimulation was calculated by dividing the individual subject’ unpleasantness scores by the corresponding intensity scores (See Methods). Repeated measures ANOVA showed significant main effect of group, temperature and significant group × temperature interaction (p’s<0.05). Arrows indicate heat pain intensity thresholds for MDD (solid) and CON (broken) group (MDD: 41.7±3.3; CON: 43.9±1.1, p<0.05, t(28)=2.4).
Divergence of subjective pain experiences in MDD
Heat pain thresholds were slightly lower in the MDD compared to the non-MDD subjects (t=2.4, p < 0.05, Table 2). We investigated whether increased affective bias in MDD was predicted by the decreased sensory pain by examining the correlation between the two measures. In healthy comparison subjects, the average affective bias showed an almost perfect negative correlation with sensory pain thresholds (r=−0.9, p < 0.01, t=−6.5), i.e., subjects with higher affective bias scores experienced pain at lower stimulus temperatures. In comparison, there was no significant correlation between the mean affective bias and the sensory pain thresholds in the MDD group (r=−0.3, p=0.2, t=−1.2). Furthermore, there was a significant difference between the groups in the strengths of correlation coefficients (p < 0.01, Fisher Z-transform). In summary, whereas pain thresholds were related to the degree of affective bias in healthy comparison subjects, no such relationship was observed in individuals with MDD. This supports the notion that physiological thresholds are at least partially disconnected from the psychological assessment in these subjects.
Discussion
This investigation tested the hypothesis that young adults with MDD (without co-morbid pain conditions) have altered affective processing that can be revealed by their responses to experimental non-noxious and noxious heat stimuli. Our results indicate that MDD individuals, when compared to age- and gender-matched healthy comparison subjects, perceive heat stimuli as both more intense and more unpleasant, suggesting that MDD is associated with hypersensitivity in both the sensory and the affective dimensions of heat pain. Interestingly, after controlling for the sensory dimension, MDD individuals still displayed an increased bias in the affective dimension of heat stimuli that was particularly apparent in the non-noxious heat range. Furthermore, in our data the decreased sensory thresholds to pain did not account for the increased affective bias in these patients. These results are in line with previous reports that associated depressive symptoms with alterations in the affective aspect of self-reported pain experience (14, 16, 25, 42), and they suggest independent neural pain-processing networks of the affective and sensory pain dimensions in MDD (15).
Our results indicate that the increased affective bias revealed by heat stimuli (i.e., the affective-motivational divided by the sensory processing of pain) in MDD is not due to between group differences in the ability to distinguish heat stimuli, because there were no differences between the slopes of the stimulus-response functions. Instead, our results suggest that this increased affective bias in MDD is due to a leftward shift of this function, which suggests that MDD is associated with earlier engagement of the affective-motivational processing system. In fact, the affective bias for the MDD subjects at 40°C (a heat stimulus that is normally well below pain threshold) is as large as that at 46°C (a stimulus that is clearly supra-threshold for pain). Both groups showed similar affective biases at the highest temperature. The latter observation suggests that both MDD and non-MDD subjects showed a strong affective bias when there is a real notion that potential damage to the body is taking place (43). However, in MDD individuals, this motivational component seems to be already active at stimulation intensities well below normal pain threshold, and strikingly, even below the heat intensity that they report as distinctly painful.
The fact that MDD subjects showed increased affective bias in the range of non-painful stimuli is reminiscent of the concept of sensory allodynia, which refers to the condition in which ordinarily non-painful stimuli evoke the sensation of pain. Allodynia in pain sensation is commonly observed following acute (e.g., sunburn) or chronic (e.g., fibromyalgia) tissue injury. People with such injuries are hypersensitive to light touch, warmth, cold, or light pressure stimuli (44). Because the difference in afective bias that we observed between MDD and comparison subjects is strongest in the non-noxious range, we would like to suggest that the concept of allodynia can be extended to the affective realm; thus, “emotional allodynia” is the abnormal elicitation by sub-threshold stimuli of the affective-motivational component associated with the perception of pain. Emotional allodynia in depressed individuals appears to be due to increased baseline negative affect (45, 46) or to higher reactivity to potentially aversive stimuli (47, 48). Like sensory allodynia, emotional allodynia may be a consequence of psychological injury or the presence of a psychiatric condition. This notion is useful because it suggests particular processing abnormalities and specific differences in neural activation patterns that one may expect to find in individuals with MDD. Moreover, it provides a conceptual bridge between disorders that have been described within the pain field and those within psychiatry.
Prior studies that examined experimental pain perception in MDD reported conflicting results (19–28, 30–33). For example, based on findings of higher heat pain and electrical pain thresholds, it was suggested that depressed individuals do not feel pain, and that they experienced what was described as “perceptual unresponsiveness” and “affective indifference” (reviewed in (23)). Others, however, found no differences in electrical pain tolerance or found decreased cold-pressor tolerance in depression (26, 27, 33). Recent studies suggest that depressed individuals might have different reactions to “superficial” (i.e., cutaneous) versus “deep” (i.e., muscle, joint, viscera) pain (28, 29, 49), with decreased sensitivity to the former and increased sensitivity to the latter. That finding is particularly interesting, because one of the main psychophysical differences between “deep” and “superficial” pain is the greater unpleasantness of the former (6, 7).
Neurophysiologically, it is the different amounts of signaling via unmyelinated small diameter fibers (C-fibers) that may distinguish different sensations arising from “deep” or “superficial” tissues. Both visceral afferents (e.g., stomach ache) and muscle afferents (e.g., ischemic pain) have a high proportion of C-fibers (50, 51), which convey a strong affective component of pain in these areas. Consistent with this idea, investigators concluded from a recent study of ischemic pain that sensitivity to the afferent activation of C-fibers is especially enhanced during depression (49). The small-diameter sensory C-fibers can be viewed as homeostatic, based on the idea that these afferents constantly relay information about the tissue status (reviewed in (52)). Thus, one might speculatively associate depression with a homeostatic dysfunction (53) characterized by abnormal cortical interpretation of the homeostatic afferent C-fiber activity, resulting in a heightened baseline affective bias, or emotional allodynia. In fact, deficits in nocturnal thermoregulation have been observed in MDD, which may underlie abnormal sleep patterns in this disorder (54). Furthermore, C-fibers play a critical role in central sensitization, which is one of the mechanisms responsible for sensory allodynia (55).
Our results showing increased affective bias to experimental heat stimuli in young adults with current MDD and no co-morbid chronic pain conditions have direct clinical relevance. As mentioned in the introduction, individuals with chronic pain are more likely to emotionally bias their daily self-reports of pain if they have a history of clinical depression (16–18). Even though our findings cannot determine whether affective biasing is a predisposition to develop chronic pain, longitudinal studies on depressed patients like the ones examined in this study will be able answer this question. Likewise, measuring affective bias to experimental pain in healthy controls may decipher the relationship between affective biasing and predisposition to depression.
We would like to acknowledge that our findings are based on a relatively small sample size. Even though we observed large statistical differences between MDD and non-MDD groups further studies conforming our results would aid in generalizing present findings. Another possible limitation of our study is the relatively young age of our participants, which might question the applicability of our findings to older patients with MDD. Because chronic pain conditions are common in older patients with depressive disorders (1, 56) identifying older MDD patients without chronic pain co-morbidities is more challenging. However, it is imperative to select individuals who are not currently co-morbid with chronic pain to examine alterations of pain processing in this population that are specifically related to MDD. Future studies examining responses to brief heat stimuli in older MDD patients without chronic pain as well as in patients with co-morbid chronic pain and MDD conditions would aid in clarifying the relationship between pain and depression.
One interesting observation in our study that merits further investigation is the role of personality traits, and neuroticism in particular, in affective pain processing. It is widely accepted that people with high levels of neuroticism have a general tendency to experience negative affect and to report more somatic complaints (57, 58), as well as showing a heightened suffering component of chronic pain (59). Future studies should examine whether, in a non-clinical population, neuroticism affectively biases responses to experimental pain or leads to the expression of emotional allodynia.
To summarize, unmedicated young MDD adults showed increased affective bias towards brief heat stimuli, which was independent of sensory pain thresholds in these patients. These results extend previous findings of increased sensitivity to deep pain in depressed patients, and suggest that increased pain sensitivity in MDD may be influenced by increased affective bias (or emotional allodynia). The increased affective bias is particularly striking during cutaneous heat stimuli at temperatures that are below pain threshold, which MDD patients abnormally report as unpleasant. To our knowledge, this is the first investigation to link abnormalities in experimental pain processing with abnormalities of affect processing in young adults with clinical depression without comorbid chronic pain condition. Future investigations may provide new insights that could improve treatments for both chronic pain and depression.
Acknowledgments
This study was supported by Barrow Neurological Foundation (IAS and ADC), by the National Institute of Mental Health (1K99MH080003; IAS), by the National Association for Research in Schizophrenia and Depression (NARSAD) (AIS, ANS and SCM), and by the UCSD Center of Excellence for Stress and Mental Health (MPP and ANS). Authors would like to thank Prof. Alex J. Zautra for his invaluable comments on the final version of the manuscript and Ms. Sarah Jacobson for assistance in data collection.
List of Abbreviations
- MDD
major depressive disorder
References
- 1.Lepine JP, Briley M. The epidemiology of pain in depression. HumPsychopharmacol. 2004;19 (Suppl 1):S3–S7. doi: 10.1002/hup.618. [DOI] [PubMed] [Google Scholar]
- 2.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. ArchInternMed. 2003;163:2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 3.Gameroff MJ, Olfson M. Major depressive disorder, somatic pain, and health care costs in an urban primary care practice. The Journal of clinical psychiatry. 2006;67:1232–9. doi: 10.4088/jcp.v67n0809. [DOI] [PubMed] [Google Scholar]
- 4.Melzack R, Casey KL, Kenshalo DR. The Skin Senses. Vol. 1. Springfield, Ill: Thomas; 1968. Sensory, motivational and central control determinants of pain: A new conceptual model; pp. 423–43. [Google Scholar]
- 5.Merskey H, Bogduk N. IASP Task Force on Taxonomy: Classification of Chronic Pain: Description of Chronic Pain Syndromes and Definition of Pain Terms. Seattle: IASP Press; 1994. [Google Scholar]
- 6.Strigo IA, Bushnell MC, Boivin M, Duncan GH. Psychophysical analysis of visceral and cutaneous pain in human subjects. Pain. 2002;97:235–46. doi: 10.1016/S0304-3959(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 7.Rainville P, Feine JS, Bushnell MC, Duncan GH. A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. SomatosensMot Res. 1992;9:265–77. doi: 10.3109/08990229209144776. [DOI] [PubMed] [Google Scholar]
- 8.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–72. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 9.Fields HL. Pain modulation: expectation, opioid analgesia and virtual pain. ProgBrain Res. 2000;122:245–53. doi: 10.1016/s0079-6123(08)62143-3. [DOI] [PubMed] [Google Scholar]
- 10.Villemure C, Slotnick BM, Bushnell MC. Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain. 2003;106:101–8. doi: 10.1016/s0304-3959(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 11.Strigo IA, Carli F, Bushnell MC. Effect of ambient temperature on human pain and temperature perception. Anesthesiology. 2000;92:699–707. doi: 10.1097/00000542-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Erickson K, Drevets WC, Clark L, Cannon DM, Bain EE, Zarate CA, Jr, Charney DS, Sahakian BJ. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. AmJPsychiatry. 2005;162:2171–3. doi: 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- 13.Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195–9. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 14.Zelman DC, Howland EW, Nichols SN, Cleeland CS. The effects of induced mood on laboratory pain. Pain. 1991;46:105–11. doi: 10.1016/0304-3959(91)90040-5. [DOI] [PubMed] [Google Scholar]
- 15.Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–84. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- 16.Conner TS, Tennen H, Zautra AJ, Affleck G, Armeli S, Fifield J. Coping with rheumatoid arthritis pain in daily life: within-person analyses reveal hidden vulnerability for the formerly depressed. Pain. 2006;126:198–209. doi: 10.1016/j.pain.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Tennen H, Affleck G, Zautra A. Depression history and coping with chronic pain: a daily process analysis. Health Psychol. 2006;25:370–9. doi: 10.1037/0278-6133.25.3.370. [DOI] [PubMed] [Google Scholar]
- 18.Zautra AJ, Parrish BP, Van Puymbroeck CM, Tennen H, Davis MC, Reich JW, Irwin M. Depression history, stress, and pain in rheumatoid arthritis patients. Journal of behavioral medicine. 2007;30:187–97. doi: 10.1007/s10865-007-9097-4. [DOI] [PubMed] [Google Scholar]
- 19.Adler G, Gattaz WF. Pain perception threshold in major depression. BiolPsychiatry. 1993;34:687–9. doi: 10.1016/0006-3223(93)90041-b. [DOI] [PubMed] [Google Scholar]
- 20.Bar KJ, Greiner W, Letsch A, Kobele R, Sauer H. Influence of gender and hemispheric lateralization on heat pain perception in major depression. JPsychiatrRes. 2003;37:345–53. doi: 10.1016/s0022-3956(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 21.Davis GC, Buchsbaum MS, Bunney WE., Jr Analgesia to painful stimuli in affective illness. AmJPsychiatry. 1979;136:1148–51. doi: 10.1176/ajp.136.9.1148. [DOI] [PubMed] [Google Scholar]
- 22.Dickens C, McGowan L, Dale S. Impact of depression on experimental pain perception: a systematic review of the literature with meta-analysis. PsychosomMed. 2003;65:369–75. doi: 10.1097/01.psy.0000041622.69462.06. [DOI] [PubMed] [Google Scholar]
- 23.Lautenbacher S, Krieg JC. Pain perception in psychiatric disorders: a review of the literature. JPsychiatrRes. 1994;28:109–22. doi: 10.1016/0022-3956(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 24.Lautenbacher S, Spernal J, Schreiber W, Krieg JC. Relationship between clinical pain complaints and pain sensitivity in patients with depression and panic disorder. PsychosomMed. 1999;61:822–7. doi: 10.1097/00006842-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Walsh T. Pain Correlates of Depressed Mood in Young Adalts. Pain research & management. 1998;3:135–44. [Google Scholar]
- 26.Merskey H. The effect of chronic pain upon the response to noxious stimuli by psychiatric patients. JPsychosomRes. 1965;148:405–19. doi: 10.1016/0022-3999(65)90083-8. [DOI] [PubMed] [Google Scholar]
- 27.Gormsen L, Ribe AR, Raun P, Rosenberg R, Videbech P, Vestergaard P, Bach FW, Jensen TS. Pain thresholds during and after treatment of severe depression with electroconvulsive therapy. EurJPain. 2004;8:487–93. doi: 10.1016/j.ejpain.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Bar KJ, Brehm S, Boettger MK, Boettger S, Wagner G, Sauer H. Pain perception in major depression depends on pain modality. Pain. 2005;117:97–103. doi: 10.1016/j.pain.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Pinerua-Shuhaibar L, Prieto-Rincon D, Ferrer A, Bonilla E, Maixner W, Suarez-Roca H. Reduced tolerance and cardiovascular response to ischemic pain in minor depression. JAffectDisord. 1999;56:119–26. doi: 10.1016/s0165-0327(99)00051-8. [DOI] [PubMed] [Google Scholar]
- 30.Otto MW, Dougher MJ, Yeo RA. Depression, pain, and hemispheric activation. JNervMentDis. 1989;177:210–8. doi: 10.1097/00005053-198904000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Carter GT, Sullivan MD. Antidepressants in pain management. CurrOpinInvestigDrugs. 2002;3:454–8. [PubMed] [Google Scholar]
- 32.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105:403–13. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 33.von Knorring L. An experimental study of visual averaged evoked responses (V. AER) and pain measures (PM) in patients with depressive disorders. BiolPsychol. 1978;6:27–38. doi: 10.1016/0301-0511(78)90004-2. [DOI] [PubMed] [Google Scholar]
- 34.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. ArchGenPsychiatry. 1992;49:624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 35.Beck AT, Rial WY, Rickels K. Short form of depression inventory: cross-validation. PsycholRep. 1974;34:1184–6. [PubMed] [Google Scholar]
- 36.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. JPain SymptomManage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa; FL: 1992. [Google Scholar]
- 38.Stevens SS. On the psychophysical low. Psychological Reviews. 1957;64:153–81. doi: 10.1037/h0046162. [DOI] [PubMed] [Google Scholar]
- 39.Clark WC. Pain sensitivity and the report of pain: an introduction to sensory decision theory. Anesthesiology. 1974;40:272–87. doi: 10.1097/00000542-197403000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Bienvenu OJ, Samuels JF, Costa PT, Reti IM, Eaton WW, Nestadt G. Anxiety and depressive disorders and the five-factor model of personality: a higher- and lower-order personality trait investigation in a community sample. Depression and anxiety. 2004;20:92–7. doi: 10.1002/da.20026. [DOI] [PubMed] [Google Scholar]
- 41.Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2005;8:93–105. doi: 10.1017/S1461145704004729. [DOI] [PubMed] [Google Scholar]
- 42.Ackerman MD, Stevens MJ. Acute and chronic pain: pain dimensions and psychological status. JClinPsychol. 1989;45:223–8. doi: 10.1002/1097-4679(198903)45:2<223::aid-jclp2270450208>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 43.Price DD. Central neural mechanisms that interrelate sensory and affective dimensions of pain. MolInterv. 2002;2:392–403. 339. doi: 10.1124/mi.2.6.392. [DOI] [PubMed] [Google Scholar]
- 44.Price DD, Staud R. Neurobiology of fibromyalgia syndrome. JRheumatolSuppl. 2005;75:22–8. [PubMed] [Google Scholar]
- 45.Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. JAbnormPsychol. 1988;97:346–53. doi: 10.1037//0021-843x.97.3.346. [DOI] [PubMed] [Google Scholar]
- 46.Snaith P. Anhedonia: a neglected symptom of psychopathology. Psychol Med. 1993;23:957–66. doi: 10.1017/s0033291700026428. [DOI] [PubMed] [Google Scholar]
- 47.Gatchel RJ, McKinney ME, Koebernick LF. Learned helplessness, depression, and physiological responding. Psychophysiology. 1977;14:25–31. doi: 10.1111/j.1469-8986.1977.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 48.Lewinsohn PM, Teri L. Selection of depressed and nondepressed subjects on the basis of self-report data. JConsult ClinPsychol. 1982;50:590–1. doi: 10.1037//0022-006x.50.4.590. [DOI] [PubMed] [Google Scholar]
- 49.Suarez-Roca H, Pinerua-Shuhaibar L, Morales ME, Maixner W. Increased perception of post-ischemic paresthesias in depressed subjects. JPsychosomRes. 2003;55:253–7. doi: 10.1016/s0022-3999(02)00498-1. [DOI] [PubMed] [Google Scholar]
- 50.Cervero F, Laird JM. Visceral pain. Lancet. 1999;353:2145–8. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- 51.Mense S, Schmidt RF. Activation of group IV afferent units from muscle by algesic agents. Brain Res. 1974;72:305–10. doi: 10.1016/0006-8993(74)90870-1. [DOI] [PubMed] [Google Scholar]
- 52.Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. AnnuRevNeurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 53.Naisberg Y. Homeostatic disruption and depression. MedHypotheses. 1996;47:415–22. doi: 10.1016/s0306-9877(96)90152-8. [DOI] [PubMed] [Google Scholar]
- 54.Avery DH, Shah SH, Eder DN, Wildschiodtz G. Nocturnal sweating and temperature in depression. Acta PsychiatrScand. 1999;100:295–301. doi: 10.1111/j.1600-0447.1999.tb10864.x. [DOI] [PubMed] [Google Scholar]
- 55.Torebjork E, Devor M, Rowbotham MC, Wiesenfeld-Hallin Z. Subpopulation of human C-nociceptors and their sensory correlates; Proceedings of the 9th Congress on Pain; Seattle: IASP Press; 2000. pp. 199–206. [Google Scholar]
- 56.Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, Kraemer HC, Dea R, Robinson R, Hayward C. Comorbid depression, chronic pain, and disability in primary care. Psychosomatic medicine. 2006;68:262–8. doi: 10.1097/01.psy.0000204851.15499.fc. [DOI] [PubMed] [Google Scholar]
- 57.Watson D, Pennebaker JW. Health complaints, stress, and distress: exploring the central role of negative affectivity. Psychological review. 1989;96:234–54. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- 58.Costa PT, Jr, McCrae RR. Neuroticism, somatic complaints, and disease: is the bark worse than the bite? Journal of personality. 1987;55:299–316. doi: 10.1111/j.1467-6494.1987.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 59.Wade JB, Dougherty LM, Hart RP, Rafii A, Price DD. A canonical correlation analysis of the influence of neuroticism and extraversion on chronic pain, suffering, and pain behavior. Pain. 1992;51:67–73. doi: 10.1016/0304-3959(92)90010-9. [DOI] [PubMed] [Google Scholar]


