Abstract
Surface modification enables the creation of bioactive implants using traditional material substrates without altering the mechanical properties of the bulk material. For applications such as bone plates and stents, it is desirable to modify the surface of metal alloy substrates to facilitate cellular attachment, proliferation, and possibly differentiation. In this work we present a general strategy for altering the surface chemistry of nickel-titanium shape memory alloy (NiTi) in order to covalently attach self-assembled peptide amphiphile (PA) nanofibers with bioactive functions. Bioactivity in the systems studied here includes biological adhesion and proliferation of osteoblast and endothelial cell types. The optimized surface treatment creates a uniform TiO2 layer with low levels of Ni on the NiTi surface, which is subsequently covered with an aminopropylsilane coating using a novel, lower temperature vapor deposition method. This method produces an aminated surface suitable for covalent attachment of PA molecules containing terminal carboxylic acid groups. The functionalized NiTi surfaces have been characterized by X-ray photoelectron spectroscopy (XPS), time-of-flight secondary ion mass spectroscopy (ToF-SIMS), and atomic force microscopy (AFM). These techniques offer evidence that the treated metal surfaces consist primarily of TiO2 with very little Ni, and also confirm the presence of the aminopropylsilane overlayer. Self-assembled PA nanofibers presenting the biological peptide adhesion sequence Arg-Gly-Asp-Ser are capable of covalently anchoring to the treated substrate, as demonstrated by spectrofluorimetry and AFM. Cell culture and scanning electron microscopy (SEM) demonstrate cellular adhesion, spreading, and proliferation on these functionalized metal surfaces. Furthermore, these experiments demonstrate that covalent attachment is crucial for creating robust PA nanofiber coatings, leading to confluent cell monolayers.
Keywords: Peptide amphiphile nanofibers, self-assembly, titanium, nickel-titanium APTES, biofunctionalization, MC3T3-E1, CPAE, covalent attachment
Introduction
By incorporating recent advances in cellular biology into implant design, materials surfaces that are bioactive have been created to promote specific biological responses from host tissues [1–3]. Some important examples of surface biofunctionalization include apatite coatings for osteogenic applications [4, 5], immobilization of proteins and peptides for directed cellular response [6, 7], immobilization of gene vectors [8], and immobilization of antibodies for cellular adhesion [9].
Among the different functionalization techniques, covalent attachment of molecules on the surface of a substrate provides several distinct advantages. They include control of molecular orientation, minimization of non-specific interactions, and greater stability of the functional surface by preventing dissolution, desorption, and degradation of molecules [10, 11]. In order to covalently immobilize molecules on substrates, aminosilanes have been used commonly because of their ability to bind to hydroxyl groups on oxide surfaces [7, 12, 13], and the versatility of primary amine groups for subsequent coupling reactions [12, 14]. The aminosilanes have been typically deposited onto substrates using liquid [12, 14] and vapor [15, 16] deposition techniques. Vapor deposition offers several benefits over liquid deposition, including improved coating uniformity and thickness [17], improved ordering of overlayers [17], and typically simpler experimental setups [18].
The metals used currently as implants include stainless steels, cobalt-chrome (CoCr) alloys, titanium alloys, and nickel-titanium (NiTi) alloys, among others. Nearly equimolar NiTi alloys are of particular interest for certain biomedical applications such as vascular stents, bone plates, and artificial joints, because of their inherent shape memory effect (SME) and resulting superelasticity [19–21]. For example, shape memory may facilitate improved fitting of bone plates and joint replacements, while the superelastic effect enables superior stent expansion at lower stresses, resulting in less damage to the arterial wall [22]. Due to an inert protective TiO2 oxide layer on the surface, NiTi has demonstrated biocompatibility that is as good or better than stainless steel, and comparable to titanium implants [23–27]. Furthermore, NiTi has a fairly low elastic modulus, which can limit stress shielding in bone applications, as well as a high damping coefficient and good fatigue resistance [21, 22]. As NiTi offers several structural advantages over other metallic implants, it would be appealing to develop a versatile method to modulate its bioactivity through covalent attachment of biomolecules on its surface.
In this work we have studied the covalent attachment of self-assembled peptide amphiphile (PA) nanofibers to NiTi substrates. This highly versatile class of self-assembling bioactive nanofibers has been previously used to template the mineralization of hydroxyapatite [28], promote rapid and selective neural progenitor cell differentiation [29], present bioactive epitopes for integrin-mediated cellular interactions [30, 31], and induce rapid blood vessel growth [32]. Bioactive PA nanofibers are therefore interesting motifs for surface functionalization of implant materials. Here, we have used a model system of PA nanofibers containing the RGDS cellular adhesion sequence [33] to functionalize NiTi substrates via an aminosilane linker. We have characterized the functionalized substrates using XPS, SIMS, AFM, fluorimetry, and SEM techniques. Furthermore, we have performed microscopy and a cell quantification assay with pre-osteoblastic cells and pulmonary artery endothelial cells in order to biologically probe the modified substrates.
Materials and Methods
All chemical reagents, unless otherwise noted, were purchased from Sigma-Aldrich (St. Louis, MO). Solvents were purchased from Fisher Scientific (Hanover Park, IL). Aminopropyltriethoxy silane (APTES) was purchased from Gelest Inc. (Morrisville, PA), and amino acids from EMD Biosciences (San Diego, CA). Nickel-titanium alloy (NiTi) strip was generously donated by Nitinol Devices & Components, Inc. (Fremont, CA). CyQuant Cell Proliferation Assay Kit was purchased from Molecular Probes (Carlsbad, CA) and other cell culture supplies from VWR (West Chester, PA). 8-well chamber slides were purchased from Nunc Lab-Tek II (Rochester, NY).
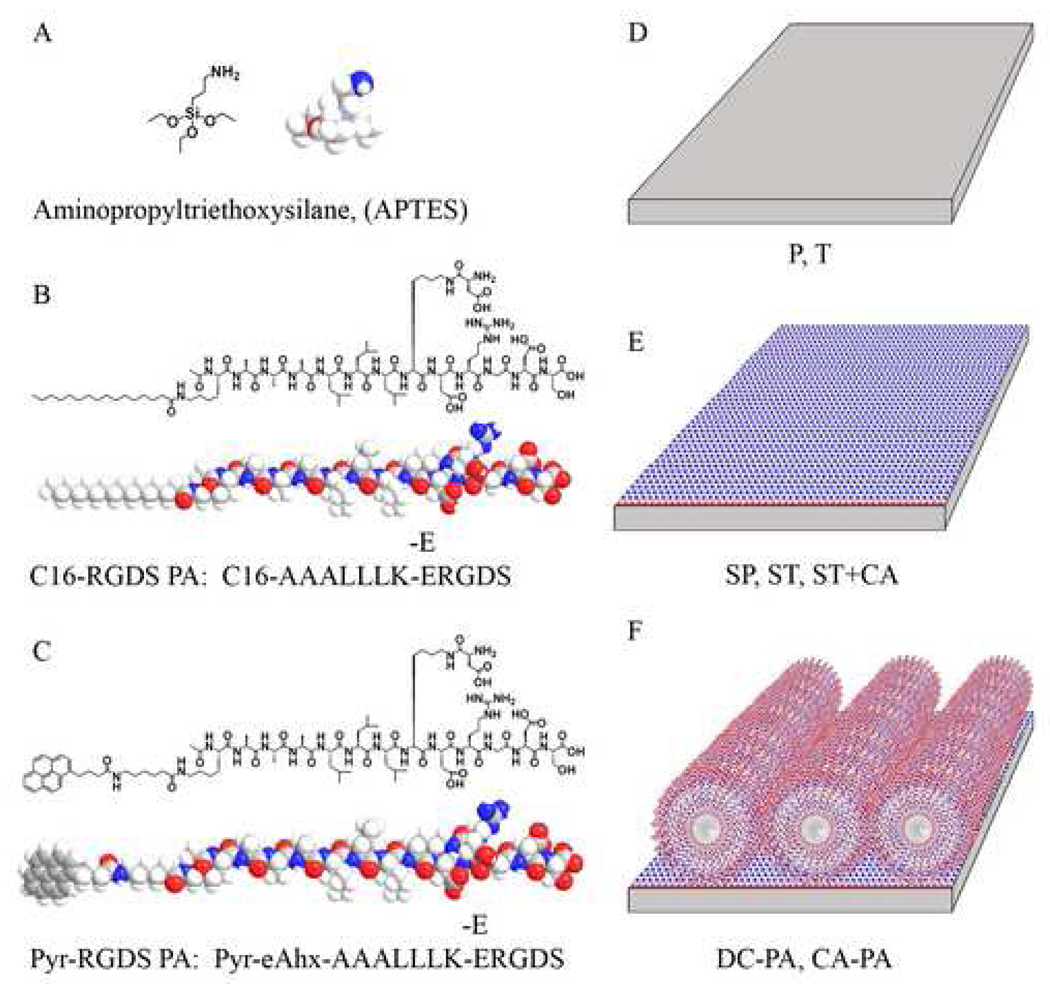
NiTi substrates were treated under different conditions to modify their physical and chemical surface properties. Table 1 lists the nomenclature used throughout the text for each sample type. Figure 1 shows the chemical structures of the silane and PAs used, as well as schematics of the substrates used. The subsequent sections describe the preparation of each substrate type.
Table 1.
Nomenclature for modified NiTi surfaces.
| Nomenclature | Sample Description |
|---|---|
| P | Polished NiTi |
| T | P treated with HF, HNO3 and boiling water |
| SP | P followed by silane vapor deposition |
| ST | T followed by silane vapor deposition |
| ST+CA | ST subjected to covalent attachment procedure w/out PA |
| T+PA | Drop-cast PA nanofiber layer on T samples |
| DC-PA | Drop-cast PA nanofiber layer on ST samples |
| CA-PA | Covalently attached PA nanofiber layer on ST samples |
Figure 1.
Chemical structures of APTES (A), the peptide amphiphile (PA) used for AFM, SEM, and biological assays (B), the peptide amphiphile (pyr-PA) used for the fluorimetry assay and AFM (C), and schematics for the various NiTi surfaces obtained in the process to create covalently bound PA nanofibers substrates (D–F).
Preparation of NiTi Substrates
Polished NiTi (P): NiTi with a composition of 55.8 wt % Ni, <0.05 wt % O, <0.02 wt % C, and balance Ti was used for this study (information obtained from the supplier). The cold-rolled NiTi had an austenitic transformation temperature of 5–10 °C, but was not heat treated to give superelasticity. Samples for spectroscopy were cut to dimensions of 8.50 mm × 8.50 mm × 1.60 mm, while samples for biological assay and fluorimetry measurements were cut to 8.70 mm × 10.65 mm × 1.60 mm. All NiTi samples were mechanically polished with silicon carbide coated papers up to 320 grit, followed by water-based diamond paste polishing up to 1 µm to produce a mirror-like finish. The samples were cleaned ultrasonically using dichloromethane, acetone, and deionized (DI) water for 3 × 5 minutes each, and dried under vacuum.
Acid and Boiling Water Treated NiTi (T): The above polished samples were subjected to a 10 second treatment with a solution containing 1 vol % HF and 4 vol % HNO3, followed by a 30 minute treatment in boiling water, and dried under vacuum.
Vapor Deposition of Aminopropyltriethoxy Silane (APTES) on NiTi Substrates
A custom-designed Teflon® sample holder with a matching airtight Teflon® vessel was used to deposit APTES on NiTi substrates. 2 mL of neat APTES precursor was placed at the bottom of the vessel, following which, NiTi samples were exposed to APTES vapors for a period of 24 hours at 50 °C. The samples were then removed from the vessel and heat treated at 50 °C for 12 hours and then at 75 °C for an additional 12 hours. Subsequently, the silanized samples were cleaned ultrasonically with reagent grade acetone for 10 minutes and dried under nitrogen gas. This process was repeated once and the samples were then stored under vacuum (ca. 25 in. Hg) until further use. This process was used to prepare both SP and ST sample types.
Synthesis of Peptide Amphiphiles
Peptide amphiphiles (PAs) were synthesized using solid phase peptide synthesis (SPPS) on a Rink amide MBHA resin, using standard 9-Fluorenylmethoxycarbonyl (Fmoc) protected amino acids in N,N-dimethylformamide (DMF) with diisopropylethylamine (DIEA) and 2-(1H-Benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU). To create the branched PA architecture, palmitic acid was first coupled to the ɛ-amine on a lysine to create a hydrophobic tail group. The peptide group was then synthesized using orthogonal protecting group chemistry. Selective deprotection of Fmoc, Boc, and 4-methyl trityl (Mtt) protecting groups allowed for control over the design of the PA. After its synthesis, the PA was cleaved from the resin using TFA, and purified by prep-scale high performance liquid chromatography (HPLC) using a Phenomenex column (purchased from Torrance, CA). The product was then analyzed by electrospray ionization mass spectrometry (ESI-MS) and analytical HPLC for product confirmation.
Covalent Attachment of Self-Assembled Peptide Amphiphile Nanofibers on Silanized NiTi Substrates
For all experiments, a 0.05 % solution by weight of the appropriate peptide amphiphile was prepared in distilled water and the pH adjusted to 7.4 with ammonium hydroxide. To create PA coatings, this solution was drop-cast onto NiTi before or after silanization (T or ST) and allowed to dry overnight to produce T+PA and DC-PA samples. Separately, silanized NiTi with or without PA (DC-PA and ST) were then subjected to the covalent attachment procedure to create CA-PA and ST+CA samples, respectively. This was achieved by immersing the samples in a solution of 0.1 M N-(3-Dimethylaminopropyl)-N '-ethylcarbodiimide (EDC) and 5 mM N-hydroxysulfosuccinimide sodium salt (sulfo-NHS) in distilled water for 2 hours. Samples were then rinsed with fresh distilled water, and dried in a desiccator. Throughout the procedure, pyr-RGDS PA (shown in Figure 1C) was protected from light to prevent loss of fluorescence from pyrene.
X-ray photoelectron spectroscopy (XPS)
XPS analysis was performed on NiTi before and after chemical treatment or silanization (P, T, SP, and ST samples) using an Omicron ESCA Probe equipped with an EA125 energy analyzer. Spectra were obtained using a monochromatized Al Kα1 radiation (1486.6 eV) at 10 kV with a current of 7.74 mA under UHV. EIS software (Omicron, Taunusstein, Germany) was used to collect and evaluate survey and high resolution scans with pass energies of 50 and 25 eV, respectively. The system was calibrated using the C1s peak at 284.8 eV. The data was quantified using the following formula:
| (1) |
The intensity, Ii, for each element was obtained from the raw data using EIS software, and the sensitivity factor, Si, (specific to the instrument) was used to calculate the concentrations of each element detected. Gaussian peak fitting was used to determine the peak locations for each element with Microcal Origin 6.0 software.
Time-of-Flight Secondary Ion Mass Spectroscopy (ToF-SIMS)
SIMS analysis was performed on NiTi before and after chemical treatment or silanization (P, T, SP, and ST samples) using a PHI TRIFT III mass spectrometer (Physical Electronics, Chanhassen, MN) in pulse mode at 15 kV using a Ga source at a pressure of 10−9 mbar. Samples were analyzed three times each in random locations to ensure homogeneous readings. WinCadence software was used to analyze the data and evaluate the ions detected. The system was calibrated in positive mode using CH3+, C2H5+, and C3H7+ hydrocarbons.
Atomic Force Microscopy (AFM)
AFM was performed on all NiTi surfaces (P, T, SP, ST, DC-PA, CA-PA, and ST+CA) using a Digital Instruments Multimode Scanning Probe Microscope in tapping mode with a Nanoprobe SPM tip. A scan size of 5 mm was used with a scan rate of 2 Hz. Images were collected with respect to height, amplitude, and phase for each sample. Line scans were performed to illustrate surface roughness, and 3D reconstructions were made using both height and amplitude information. Both the pyrene-PA (pyr-RGDS PA) and non-pyrene-PA (C16-RGDS PA) were used to create nanofiber coatings for AFM.
Fluorimetric Analysis of PA Nanofibers Attached on Silanized NiTi
NiTi samples with PA coatings before and after covalent attachment (DC-PA and CA-PA) made with pyr-RGDS PA were subjected to controlled rinsing to evaluate the robustness of the PA nanofibers attached to the substrates. The samples were ultrasonically washed in 1X Hanks’ balanced salt solution (HBSS) containing 1.26 mM CaCl2 and 0.493 mM MgCl2 for 20 minutes, followed by a quick rinse in ultrapure H2O. Fluorescence measurements were made on the samples before PA coating, and before and after ultrasonic rinsing. While performing this work, the samples were protected from light to prevent degradation of pyrene fluorescence.
Photoluminescence spectra were measured using a Horiba Jobin-Yvon (Edison, NJ) Nanolog-3 spectrofluorimeter with a double excitation-side and a single emission-side monochromator, both set to band pass slit widths of 2 nm. Photoluminescence was monitored using a room-temperature PMT detector. Xenon lamp excitation was incident normal to the sample surface on a spot size of ca. 0.5 cm × 0.2 cm, and photoluminescence was detected in front-face (reflectance) mode, at 22.5 deg. off the angle of incidence. Single-point excitation/emission measurements were collected until variation was less than 1%, up to 10 seconds. Full spectra for the same surface region of each sample were obtained with excitation at 346 nm and emission collection between 355 and 600 nm. A step size of 1 nm and less than 10 sec. integration time was used with the sample oriented at 0 and 180 degrees. Measured intensities were dark-count subtracted and normalized using a reference detector to account for lamp fluctuations.
Cell Quantification Assays on Modified NiTi substrates
To evaluate cell proliferation of two cell types on substrates, primary bovine pulmonary artery endothelial cells (CPAE) and a mouse calvarial pre-osteoblastic cell line (MC3T3-E1) were measured on all silane and PA variations of chemically treated NiTi (T, ST, ST+CA, DC-PA, CA-PA, and T+PA) after 7 days in culture. The PA used for this experiment was the C16-RGDS PA (Figure 1B). The proliferation assay was performed using a CyQuant Cell Proliferation Assay Kit as per protocol. Briefly, cells were cultured on sample substrates cut to fit an 8-well chamber slide. CPAE cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 20% Fetal Bovine Serum (FBS), 1% Penicillin/Streptomycin (P/S), 1 mM sodium pyruvate, 3.6 mM L-glutamine, and 0.02 mM non-essential amino acids. MC3T3-E1 cells were cultured in Minimum Essential Medium α (MEMα) supplemented with 10% FBS, 1% P/S, 10 mM β-glycerophosphate, and 10 µg/ml L-ascorbic acid. Half the media was replaced every 2–3 days during culture. After 7 days of culture the media was removed from the wells and the entire substrate was frozen to −80 °C. Samples were then thawed, subjected to CyQuant reagent for 5 minutes and transferred to a 96-well plate. The fluorescence was then measured using a multi-well plate reader. To quantify results, a standard curve was prepared using a known quantity of cells as per protocol. In a second experiment, the number of cells detaching during media exchange was evaluated for bare NiTi (T), and PA coated NiTi before or after covalent attachment (DC-PA or CA-PA). In order to do this, the same experiment mentioned above was performed, with the exception that media was fully exchanged at days 1, 3, and 5. The collected media was then transferred to a standard tissue culture plate and incubated for 5 hours to allow any viable cells to adhere. This media was then removed and plates were subjected to the CyQuant assay as described above.
Cell Morphology Assessment by Scanning Electron Microscopy (SEM)
SEM was used to evaluate the cell morphology on each sample type used in the proliferation assay at day 1, as well as T, CA-PA, and DC-PA samples at days 3 and 7. For SEM preparation, samples were removed from culture and rinsed with PBS, fixed with 10% formalin for 1 hour, dehydrated with graded ethanol, critical point dried, and coated with 3 nm Au-Pd layer using a spin coater. SEM was performed using a Hitachi S-4500 with a cold field emission electron gun at 3 kV with a current of 20 mA. A secondary electron detector was used for high-resolution imaging.
Statistical Analysis
Statistics for XPS and CyQuant Assay are shown using 95% confidence error bars. T-test with unknown variance was used to determine two-tailed P-values.
Results and Discussion
Silanization of NiTi
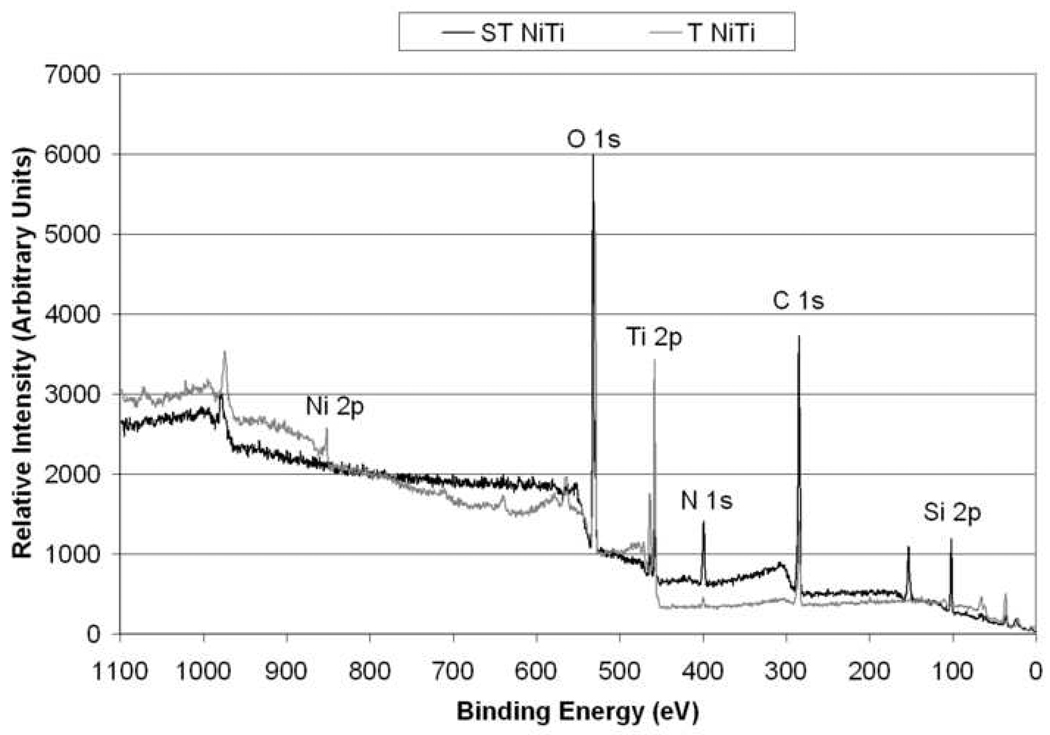
The chemical and physical differences in the surface of NiTi samples before and after silanization procedure were evaluated with XPS, SIMS and AFM characterization techniques. XPS data was obtained from two indiscriminate areas of each sample type (n = 4) in order to obtain representative results. The spectral curves for NiTi before and after chemical treatment (P and T samples) were similar and exhibited low standard deviation values. A similar trend was observed for the two silanized samples (SP and ST). Typical spectra for T and ST are shown in Figure 2. The quantitative compositional results for all sample types are shown in Table 2. Spectra for the bare NiTi samples (P and T) samples showed peaks for C, N, O, Ti, and Ni, while silanized samples (SP and ST) showed peaks for Si in addition to those observed for P and T samples. A Ti:Ni ratio of approximately 3:1 is observed for the P sample, while a ratio of 11:1 is observed for T samples, indicating a decrease in Ni content due to the chemical treatment procedure employed for the NiTi samples. The levels of C and O are comparable for both of these sample types. Polished NiTi (P) samples showed greater traces of N than chemically treated NiTi (T) and neither sample showed the presence of Si as expected. For both P and T samples, all elements except for O were statistically different (P<0.01). Comparing the silanized samples SP and ST, only very low levels of Ti were observed, and Ni was undetectable. In addition to the Si peaks, C levels were found to increase and O levels were found to decrease as compared to bare NiTi samples, indicating the deposition of APTES layer on the NiTi surface. No significant difference was observed for any element between the two silanized sample types (P<0.05).
Figure 2.
Typical XPS survey spectra of T and ST NiTi surface showing observed elements.
Table 2.
XPS characterizations of P and T NiTi, both before and after silanization (SP and ST). Numbers in brackets indicate 95% confidence interval.
| Sample | C | N | Ni | O | Si | Ti |
|---|---|---|---|---|---|---|
| P NiTi | 34.1 (2.6) | 3.6 (0.4) | 3.8 (0.7) | 43.3 (2.2) | 0.0 (0.0) | 15.2 (1.0) |
| T NiTi | 35.9 (0.9) | 1.1 (0.3) | 1.4 (0.3) | 43.2 (0.8) | 0.0 (0.0) | 18.4 (0.4) |
| SP NiTi | 52.6 (2.4) | 7.9 (1.1) | 0.0 (0.0) | 24.6 (1.4) | 13.7 (1.7) | 1.3 (0.6) |
| ST NiTi | 51.7 (1.2) | 7.7 (0.9) | 0.0 (0.0) | 26.2 (1.3) | 12.8 (1.1) | 1.5 (0.5) |
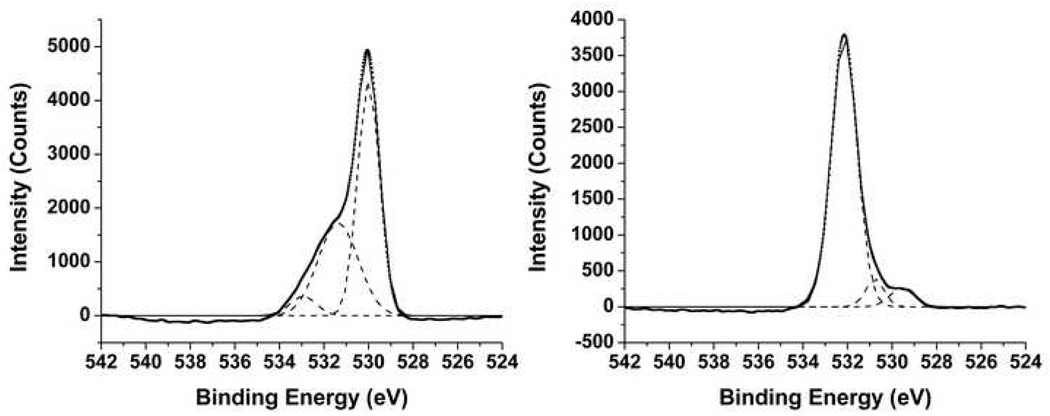
As shown in Table 3, XPS was also used to determine the oxidative state of each element. The single Ti 2p3/2 peak corresponding to TiO2 was determined to be 458.8 eV for bare NiTi samples (P and T) and 458.2 for silanized samples (SP and ST) [7, 34, 35]. Multiple Ni 2p3/2 peaks were determined for P and T samples measured at 852.2 eV, 852.9 eV, and 855.8 eV. P had an additional peak at 857 eV. These peaks correspond to Ni0 and Ni2+, as well as their corresponding shake up satellite peaks [36, 37]. Ni peaks were not observed for both silanized samples, and three O 1s peaks were present for P, T, SP, and ST samples. For bare NiTi samples, two large peaks were located at 529.9 eV and 531.4 eV, with a smaller peak at 532.9 eV. These peaks are assigned to TiO2, TiOH, and hydrated Ti or basic OH, respectively [7, 35, 38]. Silanized samples had one dominant peak at 532.2 eV, with two smaller peaks located at 529.6 eV and 530.8 eV. These peaks are assigned to Ti-O-Si, TiO2, and TiOH, respectively [7, 35, 38]. Typical high resolution O 1s spectra for T and ST with deconvoluted peaks are shown in Figure 3. The average peak locations, their assignments, and the corresponding percentages for these three elements are shown in Table 3.
Table 3.
Characteristic XPS binding energies of Ni, O, and Ti for P, T, SP, and ST NiTi surfaces, and the proposed assignments to surface functionalities. Numbers in brackets indicate standard deviation.
| Sample | Ni 1s3/2 region BE(eV), %, assignments |
Ti 2p3/2 region BE(eV), %, assignments |
O 1s region BE(eV), %, assignments |
|---|---|---|---|
| P | 852.3, (49), Ni0, 855.5, (51), Ni2+ |
458.5, (100), TiO2 | 529.9, (44), TiO2 531.3, (49), TiOH 533.0, (7), hydrated Ti, basic OH |
| T | 852.3, (100), Ni0 | 458.6, (100), TiO2 | 530.0, (55), TiO2 531.4, (39), TiOH 532.9, (5), hydrated Ti, basic OH |
| SP | ---* | 458.2,(100), TiO2 | 529.6, (8), TiO2 530.7, (4), TiOH 532.2, (88), Ti-O-Si |
| ST | ---* | 458.3, (100), TiO2 | 529.6, (10), TiO2 530.9, (7), TiOH 532.2, (83), Ti-O-Si |
Indicates peak was undetectable.
Figure 3.
High resolution XPS spectra for Oxygen 1s for T NiTi surface (left) and ST NiTi surface (right). Dotted lines indicate smoothed spectra, dashed lines indicate deconvoluted peaks, and solid lines indicate sum of deconvoluted peaks.
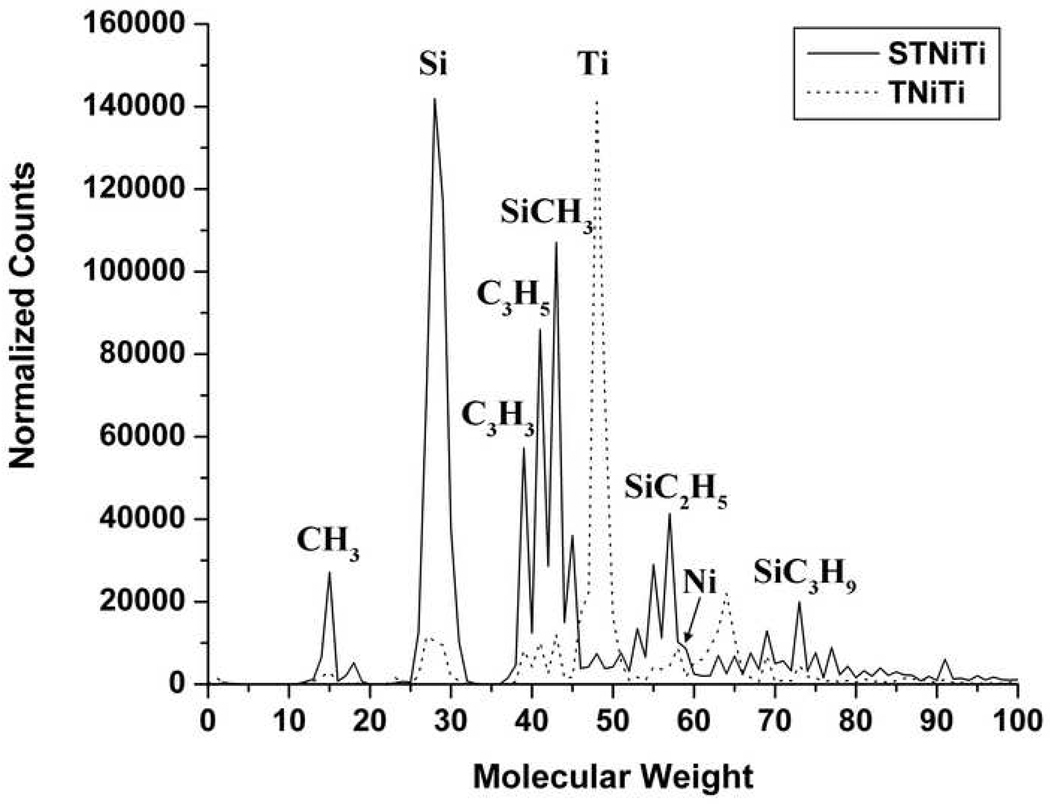
SIMS was performed in conjunction with XPS to confirm the nature of the elements present. SIMS spectra obtained in positive mode for bare NiTi samples (P and T) showed peaks for Ti, Ni, TiO, and low levels of hydrocarbons. Peaks indicative of the presence of silane molecules were not observed. Spectra for silanized samples (SP and ST) showed peaks for the typical fragmented species for APTES, including Si, SiCH3, and SiC3H9. Very small intensity peaks for Ti were present, and Ni was not detected at all. Typical spectra for chemically treated NiTi surfaces before and after silanization (T and ST) are shown in Figure 4.
Figure 4.
SIMS spectra of T NiTi surface (dashed) and ST NiTi surface (solid) normalized so maximum counts are equal for comparison.
The mechanical polishing and cleaning technique used herein (sample P) reduced the nickel content to low levels (<4%) and produced an oxide layer with a Ti to Ni ratio of 3:1. Acid-treatment of this surface with a mixture of HF and HNO3, followed by exposure of the sample to boiling water, further reduced the nickel level to create a surface oxide that is primarily titanium oxide. These results correlate well to Shabalovskaya [21] who attributes the observed reduction in Ni content to Ni diffusion into water. For P samples, polishing was performed with water-based solutions. As the surface gets removed, the newly exposed Ni in metallic form is more susceptible to diffusion. The chemically treated samples (T) were obtained by treating P samples with acid followed by boiling water. The HF and HNO3 mixture removes the existing oxide and preferentially leaches out Ni to help create a new Ti rich oxide grown during the boiling water treatment. The metallic Ni near the surface is susceptible to diffusion in the boiling water, leaving only a very small amount of Ni in the oxide layer [21]. In P samples, Ni is observed to be present in almost equal percentages in metallic and ionic forms, while for T samples, Ni2+ is observed to be present in a greater quantity compared to Ni0. This indicates oxidation of Ni0 to Ni2+, and an increased extent of diffusion of Ni0 compared to Ni2+. The excess Ni2+ has a lower diffusion rate and therefore might improve biocompatibility of T samples [39, 40]. The single peak at 485.5 eV for Ti indicates the presence of TiO2, which is ideal for silane deposition. The TiO2 layer is known to provide hydroxyl groups on the surface for the ethoxy groups of APTES to form a covalent bond as shown in Scheme 1. In addition, our sample surfaces contain no other trace elements than C and N (attributed to hydrocarbon contamination in XPS), and in fact, seem comparable to those of Nanci et al. [41], who reported preparation of Ti substrates for silane deposition.
To deposit the silane layer on NiTi, we have used a moderately low temperature condition and neat aminosilane precursor without any solvent as the starting solution.Previous work by Ek et al. have shown the importance of vapor deposition below 150 °C [18] and the improvement in silane coating density with repeated depositions [42]. Our method uses a 50 °C deposition temperature, followed by 50 °C heat treatment to allow for molecular orientation, and then a 75 °C curing temperature to crosslink the deposited silane molecules. The advantages of using neat precursor without any solvent are an increased density of aminosilane molecules on the substrate due to absence of solvent molecules, and also a shorter time to achieve desired coating thickness.
Characterization of aminosilane deposited NiTi samples (SP and ST) with XPS and SIMS confirmed the presence of a covalently attached silane layer on NiTi substrates. XPS showed increased levels of Si, N, and C, lower levels of Ti and O, and undetected Ni. The observed increase in Si, N, and C signals are expected, as they are components of the APTES molecule. We also expected to see a decrease in Ni and Ti as the NiTi surface would be covered with an aminosilane layer. The decrease in O can be explained by considering that the surface is changing from primarily TiO2 (where O is theoretically 66% of the surface components) to a silane coating, where O is now a much smaller component (theoretically ~30% not including the TiO2 substrate). This is more easily viewed in Figure 3, where we see that the deconvoluted peaks corresponding to TiO2 and TiOH are by far the largest in the O1s spectra for the chemically treated samples (T), whereas for the silanized samples (ST), the peak corresponding to Ti-O-Si is the major feature in the spectrum. Furthermore, this peak shows that the silane layer is covalently attached to the NiTi surface, and not just physically adsorbed. SIMS data confirms the coverage of NiTi by APTES after vapor deposition, as shown in Figure 4. Chemically treated NiTi (T) shows peaks for Ti oxide, Ni oxide, Ti, Ni, O, and a small amount of hydrocarbons that were used to calibrate the system. After vapor deposition, we see these peaks greatly reduced, with larger peaks corresponding to fragments of APTES, including Si, SiCH3, and SiC3H9.
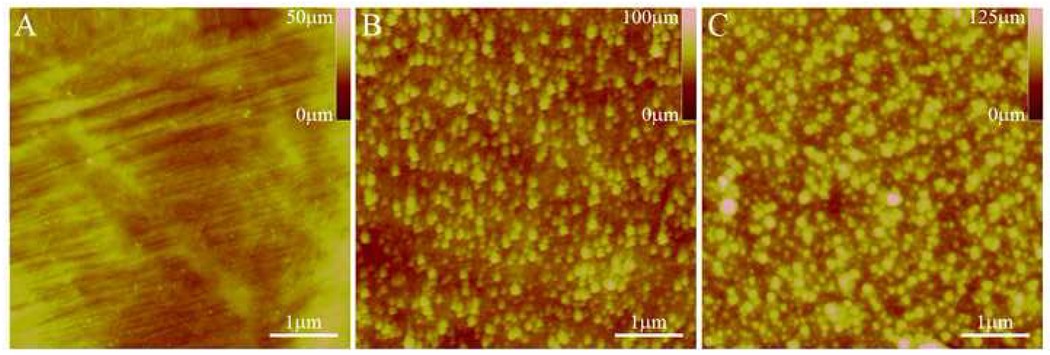
AFM was used to determine the surface topography of bare (P and T) and silanized (ST) samples. Representative images are shown in Figure 5. A noticeable difference in surface topography between the P and T samples is observed, with the chemically treated sample (T) having an increased level of surface roughness. After APTES deposition (ST), the surface roughness is similar in topography to that of chemically treated NiTi (T), with slightly increased roughness. While this is simply an artifact of the procedure used to create the bioactive surface, texturing has been shown to influence cellular behavior in different ways. For example, several studies have shown increased endothelial cell (EC) proliferation, adhesion, and spreading on nano-textured substrates with identical surface chemistries [43–45]. However, Webster [46] has seen minimal effect of nanoscale texture on EC adhesion, while Xu [47] has found that EC proliferation decreased on surfaces with micron-scale roughness (Rms = 1557 nm) compared to “smooth” surfaces (Rms = 135 nm). A similar trend has been observed by studies on the effect of surfaces with different roughness and topography on osteoblast cell (OC) behavior. Surfaces prepared with nanoscale roughness have been shown to correlate well with increased osteoblast adhesion, proliferation, and function [46, 48, 49], whereas studies with larger-scale roughness and texture have shown mixed results [50–53]. Since cellular adhesion occurs via serum proteins absorbed onto a synthetic substrate [43], it appears that the scale of surface roughness plays a significant role in determination of the type and conformation of absorbed proteins, and hence the cellular response [46, 49]. Therefore, it would be expected that the acid treatment would be beneficial for tissue applications due to the resulting chemical composition and topographical features.
Figure 5.
AFM images of P NiTi surface (A), T NiTi (B), and ST NiTi surface (C) showing topography changes after acid treatment and then APTES vapor deposition.
Covalent Attachment of PA Nanofibers
The purpose of silanizing NiTi was to covalently attach biologically relevant moieties on the surface to facilitate adhesion and proliferation of cells and promote tissue integration of implants. Our interest in attaching PA nanofibers to such NiTi surfaces stems from the fact that PA nanofibers have demonstrated their versatility in eliciting cellular response [29, 30] and templating biomineralization [28]. By incorporating these PA nanofibers on a metallic surface, we hope to improve tissue integration and interfacial biocompatibility. In this case, we are using a model RGDS containing PA to evaluate a general strategy to create covalently bound nanofiber coatings on NiTi. Fluorimetry and AFM techniques have been used to characterize these PA nanofiber coatings and demonstrate their covalent attachment to NiTi substrates.
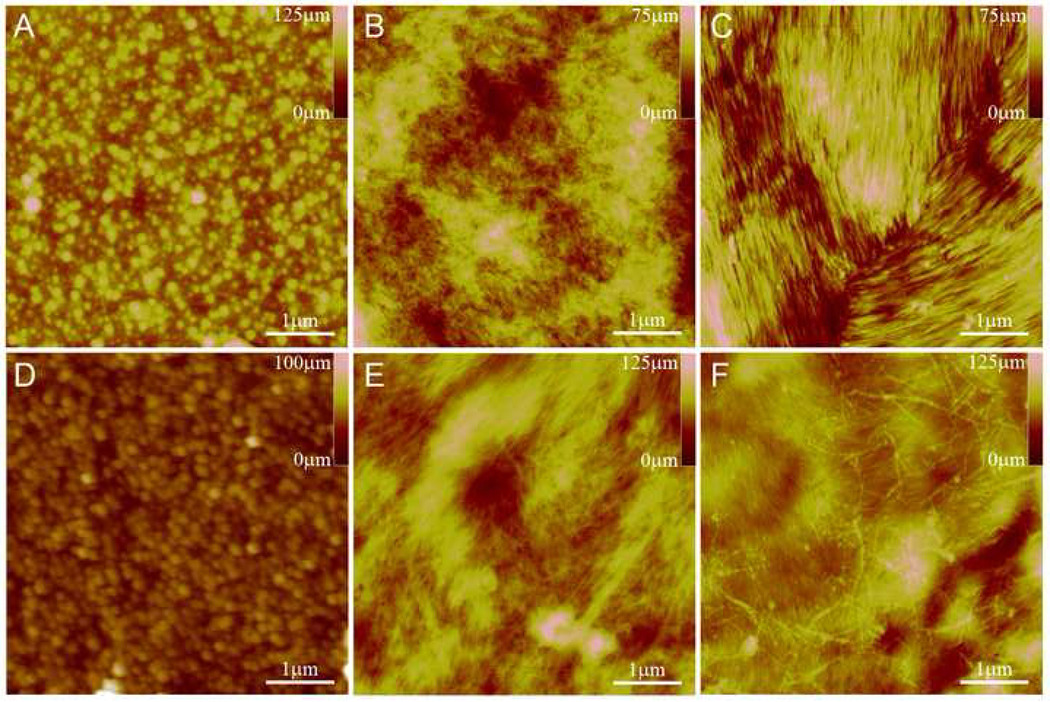
AFM images were taken of PA coated NiTi before and after covalent attachment (DC-PA, and CA-PA), as well as the covalent attachment control (ST+CA) using both the C16-RGDS PA and the pyr-RGDS PA, as shown in Figure 6. There is a marked difference between the silanized surface and the PA nanofiber coated surface, where bundles of fibers can be readily distinguished. After covalent attachment both coatings of PA remain assembled into nanofibers as shown in Figure 6 (B-C and E-F). When the covalent attachment process is performed on silanized NiTi without PA (ST+CA), the surface retains its topography and nanofibers are not observed (Figure 6D), indicating the reaction reagents are not assembling or remaining on the surface after rinsing.
Figure 6.
AFM images of ST (A), C16-RGDS PA as DC-PA (B) and CA-PA (C), ST+CA (D), and pyr-RGDS PA as DC-PA (E) and CA-PA (F). B & C and E & F show that the PA nanofiber structure is maintained after covalent attachment. D is a control to show no change in topography due the covalent attachment process.
Of the several methods investigated, drop-casting PA solution to form nanofibers on the surface followed by their covalent attachment to the substrate resulted in the most reproducible, thin, homogeneous coatings of PA nanofibers on metallic surfaces. Other methods, such as covalently attaching pre-formed nanofibers in solution often result in vary sparse coatings, with individual fibers spaced far apart from one-another. Conversely, by using an appropriate concentration of PA molecules in solution, we were able to create a coating of nanofibers that covered the entire surface. This is clearly seen in AFM images shown in Figure 6, where similar structures are observed for both PAs. Here we see that the nanofibers, often aggregated in patches of aligned areas, cover the surface in a thin layer that masks the topography observed for a silanized NiTi (ST) surface. Furthermore, the self-assembled nanofibers, formed after drop-casting, retain their structural integrity after the covalent attachment procedure.
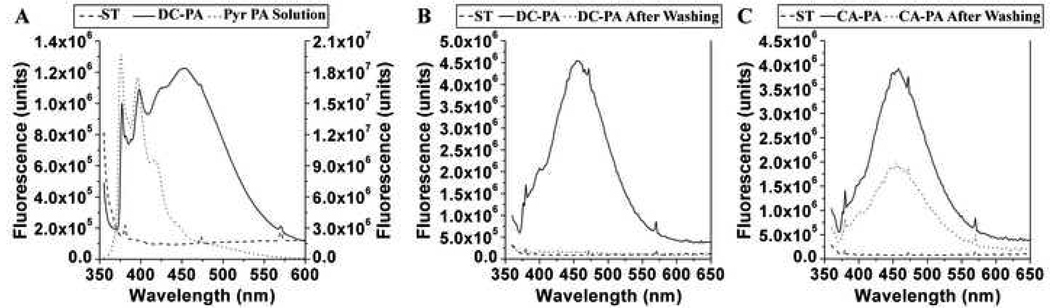
The use of an aminosilane linker to covalently attach PA nanofibers does not allow us to confirm the formation of a peptide bond between the PA and aminosilane molecules. Due of the large number of peptide bonds present in the PA nanofiber, the signal from this additional linkage is undetectable by Fourier Transform infrared spectroscopy (FT-IR). Consequently, we designed a fluorescent PA molecule that enabled us to measure the fluorescence associated with the PA nanofiber coating. We then assessed the robustness of covalent attachment of PA nanofibers to NiTi substrates by measuring the fluorescence before and after rigorous washing. To be consistent with the rest of the work presented, a pyrene containing PA (Figure 1C) that is analogous to the C16-RGDS PA (Figure 1B) was used to create these PA coatings (DC-PA and CA-PA samples). Fluorimetry spectra were obtained on silanized samples before PA drop-casting (ST), after drop-casting or covalent attachment, i.e. DC-PA and CA-PA samples respectively, and then after rigorous washing. A spectrum of 0.05 wt% pyr-RGDS PA solution was also measured to compare against the spectra obtained from the NiTi samples. Spectra of the pyrene solution and silanized NiTi before and after drop-cast PA (ST and DC-PA) are shown in Figure 7A, demonstrating the monomeric signal of the solution, the addition of the excimeric signal in the PA coating, and the lack of signal contributed by the silanized NiTi. A spectrum corresponding to the monomeric species has been shown previously with a similar PA design [54], when the PA self-assembly is not triggered in a solution state. When a PA solution is dried on a substrate and the self-assembly has been triggered by concentration effects, we also see the onset of excimeric emission. Comparing these spectra for PA coated NiTi before and after covalent attachment (DC-PA and CA-PA) before and after washing with HBSS (Figures 7B and C), we see that the signal has almost completely disappeared when the PA is not covalently bound, while the covalently bound sample retains a slightly diminished signal for the excimer as compared to the sample before washing. This clearly demonstrates that PA is retained on the covalently bound PA samples and not on the drop-cast PA samples. Furthermore, the PA molecules are still observed to form excimers, indicating very close proximity of the pyrene moieties. While the PA nanofibers may not remain completely intact under such extreme and harsh conditions, the PA nanofiber coatings would be robust enough for most implant applications. For applications that would require a higher level of robustness, such as stent coatings, PA molecules that can be crosslinked within the nanofibers could be used to ensure the integrity of the PA nanofiber coating [28]. Usually, washing procedures with HBSS result in masking of the electrostatic interactions between the PA molecules and the substrate due to the presence of salts, and the weak physisorption is insufficient to retain any PA coating. This is an important point, and highlights the necessity for covalent attachment to create robust PA coatings for biological applications.
Figure 7.
Fluorimetry spectra of pyr-PA coatings on NiTi. Graph (A) shows the spectra for ST NiTi and a typical DC-PA NiTi sample (left scale bar), and the pyr-PA in solution (right scale bar). Upon self-assembly of the PA molecules when dried on the NiTi, an excimer signal is observed. Graph (B) shows the elimination of the characteristic pyr-PA signal after washing DC-PA, while graph (C) shows conservation of the pyr-PA excimer signal after washing CA-PA, indicating the covalent coupling reaction enabled the retention of assembled pyr-PA on the NiTi.
Cellular Response to PA Coated NiTi Samples
When an implant with a covalently bound coating is placed in situ, the surface of the substrate undoubtedly changes over time. In the present case, the adjacent tissue will initially come in contact with the PA nanofibers, which are designed to elicit an initial response, such as cellular adhesion. As the cells respond to this surface, they will attach to the surface and begin to secrete a host of proteins that will eventually become ECM. The cells will also start to resorb the biodegradable PA nanofibers, and eventually come into contact with the underlying silane layer and NiTi substrate. Consequently, it is of great interest to assess the biological response of relevant cells to these materials in vitro. To evaluate these factors, we used a 7 day cell quantification assay (CyQuant) and SEM imaging to assess any potential negative biological response to the model RGDS containing PA (C16-RGDS PA) and other materials used to create covalently bound PA coatings on silanized NiTi.
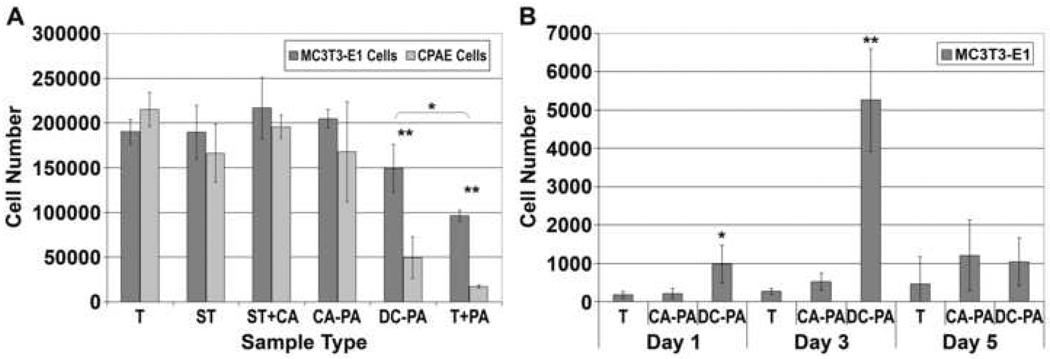
For cell quantification, the samples assayed included the various stages between chemically treated NiTi and NiTi with covalently bound PA (T, ST, ST+CA, CA-PA, DC-PA, and T+PA). The results of the proliferation assay are shown in Figure 8A. All samples demonstrated cell proliferation from the initial seeding density, indicated by the dashed horizontal line. Chemically treated NiTi before and after silanization (T, ST), with covalently bound PA (CA-PA), and the covalent attachment control (ST+CA) showed no statistical difference in cell number at day 7. However, samples with drop-cast PA on bare or silanized NiTi (T+PA and DC-PA) had significantly (P<0.01) lower cell numbers than the other sample types, and significantly different (P<0.05) from one another. To understand the cause of diminished cell proliferation on the non-covalently bound PA samples, the number of viable cells removed during media exchanges was evaluated for chemically treated NiTi (T), and PA coated NiTi before and after covalent attachment (DC-PA and CA-PA), as shown in Figure 8B. There is a statistically significant increase in the number of viable cells removed from the non-covalently bound PA samples (DC-PA) compared to bare NiTi or covalently bound PA samples (T and CA-PA) at 1 day (P<0.05) and 3 days (P<0.01). There was no significant difference at 5 days between the sample types because most of the cells on the DC-PA samples were already removed.
Figure 8.
7-day CyQuant® proliferation assay results for MC3T3-E1 and CPAE cells on various modified NiTi substrates (A) and quantification by CyQuant® assay for the number of cells detached during medium changing (B). In (A), DC-PA and T+PA are both significantly different from T, ST, ST+CA, and CA-PA (P<0.01). T+PA is also significantly different from DC-PA (P<0.05). In (B), DC-PA is significantly different from T and CA-PA at day 1 (P<0.05) and day 3 (P<0.01).
SEM images of MC3T3-E1 and CPAE cells cultured for 1 day on the same samples used for cell quantification (T, ST, DC-PA, CA-PA, and ST+CA), except for PA coated NiTi without silane (T+PA), are shown in Figure 9. Both cells types adhere to and spread on each sample type. The cell morphology indicates normal cellular behavior; rounded cells that would indicate necrosis were not observed. Images were also taken for both cell types on bare NiTi (T) and PA coated NiTi before and after covalent attachment (CA-PA and DC-PA) at days 3 and 7, as shown in Figure 10. At day 3, both cell types have proliferated significantly and cover a majority of bare NiTi and covalently bound PA NiTi samples, while only a few small colonies of cells were observed on the drop-cast PA NiTi samples. By day 7, both cell types were observed to have grown to confluency on bare NiTi (not shown) and covalently bound PA samples, while the drop-cast PA samples (not shown) still resembled the day 3 samples.
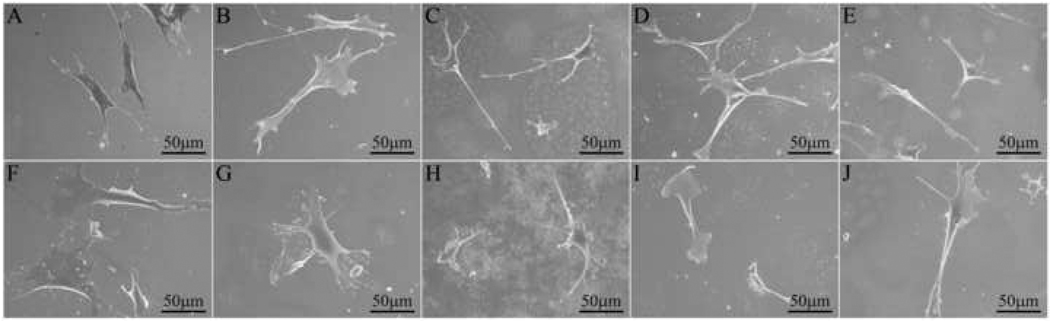
Figure 9.
SEM images of MC3T3-E1 cells (top row) and CPAE cells (bottom row) on NiTi: (A, F) T; (B, G) ST; (C, H) DC-PA; (D, I) CA-PA, and (E, J) ST+CA, all showing initial attachment and spreading after cell seeding.
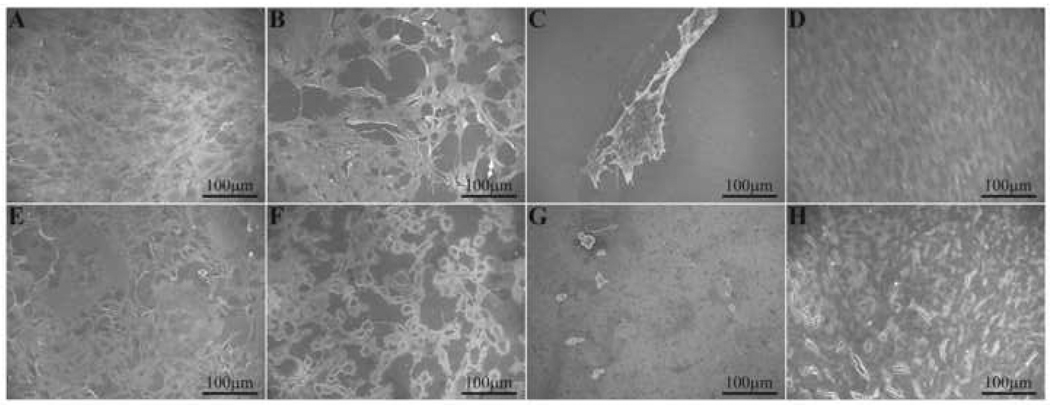
Figure 10.
SEM images of MC3T3-E1 (top row) and CPAE (bottom row) cells on NiTi: (A, E) T, Day 3; (B, F) CA-PA, Day 3; (C, G) DC-PA, Day 3; (D, H) CA-PA, Day 7. There is a drastic reduction in cell attachment when the PA nanofibers are not covalently bound to the silanized NiTi (DC-PA), whereas cells attach and grow to confluency on the CA-PA surfaces.
Several interesting findings can be concluded from these experiments. First, the cell quantification at day 7 was statistically the same for each chemically treated NiTi before and after silanization (T and ST), with covalently bound PA (CA-PA), and for the covalent attachment control (ST+CA). At first, this appears to imply no preference between any of these surfaces; however, upon viewing the day 7 SEM images it is clear that the cells have reached confluency on each of these surfaces. Therefore, the lack of statistical difference is simply due to the fact that both cell types have proliferated to confluency on each of these surfaces, and hence no negative effect on cell viability can be attributed to the NiTi, silane, or the PA molecules. This is also confirmed by the SEM images in Figure 10, which shows the cells to be adhered and spreading to all surfaces, as is typical of healthy cells. In fact, there is no obvious difference of cell morphology among the different substrates. Secondly, we see that there was a decreased number of cells on silanized NiTi with drop-cast PA, and even less on bare NiTi with drop-cast PA.
This effect cannot be attributed to the PA itself because the surface exposed to the cells is effectively identical to the covalently bound PA samples. However, there is a difference in the interaction between the PA nanofibers and the underlying substrate. For the covalently bound PA samples, the PA is bound very strongly to the silane layer; for silanized NiTi with drop-cast PA, there is electrostatic interaction between the negatively charged PA molecule and the positively charged free amines of the silane layer; and for the bare NiTi with drop-cast PA, there are Van der Waals attractive forces but repulsive electrostatic forces between the negatively charged PA and oxide layer. Consequently, it is quite possible that the cells do in fact like the PA, so much so that they might be binding the RGDS epitopes via integrin receptors [55] and pulling the nanofibers from the surface, thereby inhibiting cell spreading and motility, and even possibly detaching from the substrate altogether. Given the complete coverage of nanofibers on the surface, there is sufficient PA for this to occur. To test this hypothesis, chemically treated NiTi (T) and PA coated NiTi before and after covalent attachment (DC-PA and CA-PA) were cultured and the exchanged media was collected and incubated to allow for viable cells to re-plate in a culture dish. The quantitative results shown in Figure 8B confirm this hypothesis. There is a significant increase in the number of cells detaching from the drop-cast PA samples compared to the covalently bound PA samples and bare NiTi samples, while the latter two are not statistically different. This indicates that viable cells are detaching from the substrate when the PA is not strongly bound to the NiTi. This is confirmed by the SEM images in Figure 10, showing good cell proliferation and spreading at day 3 in both the bare NiTi and covalently bound PA samples, with very few cells surviving the media exchange and SEM sample preparation on the drop-cast PA samples. Therefore we can conclude that the cells prefer the PA over the silane or NiTi, as evident by their propensity to bind the PA and detach rather than adhere to the underlying silane or NiTi. Furthermore, it is interesting that the detached cells do not undergo apoptosis after detaching. This may indicate that the detachment is occurring during or media exchange, or possibly that the interaction of the cells with the PA is leading to the release of soluble mediators that cause the inhibition of apoptosis [56]. Third, the results confirm that there is no cytotoxic effect from any residual reagents used for covalent attachment. When the silanized samples underwent the covalent attachment reaction in the absence of PA nanofibers (ST+CA), there was no effect on cell viability as compared to the silanized samples from any residual chemical reagents.
Conclusions
We have developed a method to covalently attach self-assembled PA nanofibers on pre-treated NiTi substrates using an intermediary aminosilane layer. This novel approach uses processing conditions such that the NiTi surfaces have very low Ni and significantly higher TiO2 levels, which favor a homogenous deposition of aminosilane molecules on NiTi substrates. Covalent attachment of PA nanofibers to the intermediary silane layer does not cause disassembly of the nanofiber architecture and leads to retention of the PA nanofibers on the surface under harsh washing conditions at physiological salt concentrations. Biological assay results showed no toxic effect on endothelial and pre-osteoblast cells by any of the materials or reagents used. Furthermore, we have demonstrated the importance of covalently binding the PA nanofibers to the substrate to create robust coatings, leading to a confluent cell monolayer within 7 days. We have developed a general strategy to create modified NiTi surfaces that can be used as biomedical implants with enhanced capabilities to facilitate cell adhesion, proliferation, and potentially a variety of implant-specific cellular responses.
Acknowledgements
The authors gratefully acknowledge funding support from the National Science Foundation through grant DMR-0505772 and the National Institutes of Health through grants 1 R01 DE015920-01A1, 5 R01 DE015920, and 5 R01 EB003806. X-ray Photoelectron Spectroscopy and time-of-flight Secondary Ion Mass Spectroscopy were performed in the Keck Interdisciplinary Surface Science (Keck II) Center at Northwestern University. Atomic force microscopy was performed in the Nanoscale Integrated Fabrication, Testing and Instrumentation (NIFTI) Center at Northwestern University. Electron microscopy was performed in the Electron Probe Instrumentation Center (EPIC) at Northwestern University. Peptide synthesis and purification, fluorimetry, and cell work were performed in the Institute for BioNanotechnology in Medicine (IBNAM) at Northwestern University. We thank Dr. James Hulvat for his technical help with experiments at IBNAM, Dr. Nick Wu for his technical help with experiments at Keck II, Dr. Gajendra Shekhawat for his technical help with experiments at NIFTI, and Mr. Ben Myers for his technical help with experiments at EPIC.
This work was funded by the National Science Foundation, under award no. DMR-0505772 and the National Institutes of Health, under award no.’s 1 R01 DE015920-01A1, 5 R01 DE015920, and 5 R01 EB003806. We thank also Nitinol Devices & Components, Inc. (Fremont, CA) for donation of the NiTi strips used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Timothy D. Sargeant, Department of Materials Science and Engineering, Northwestern University, Evanston, IL 60208-3108 (USA)
Dr. Mukti S. Rao, Department of Materials Science and Engineering, Northwestern University, Evanston, IL 60208-3108 (USA)
Chung-Yan Koh, Department of Chemistry, Northwestern University, Evanston, IL 60208-3108 (USA).
Samuel I. Stupp, Email: s-stupp@northwestern.edu, Departments of Materials Science and Engineering, Chemistry, and Medicine, Northwestern University, Evanston, IL 60208-3108 (USA), Fax: (+1) 847-491-3010.
References
- 1.Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. European Spine Journal. 2001;10:S96–S101. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langer R, Vacanti JP. Tissue Engineering. Science. 1993 May;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Vasita R, Katti DS. Growth factor-delivery systems for tissue engineering: a materials perspective. Expert Review of Medical Devices. 2006;3(1):29–47. doi: 10.1586/17434440.3.1.29. [DOI] [PubMed] [Google Scholar]
- 4.Stupp SI, Ciegler GW. Organoapatites - Materials for Artificial Bone .1. Synthesis and Microstructure. Journal of Biomedical Materials Research. 1992;26(2):169–183. doi: 10.1002/jbm.820260204. [DOI] [PubMed] [Google Scholar]
- 5.Chou YF, Huang WB, Dunn JCY, Miller TA, Wu BM. The effect of biomimetic apatite structure on osteoblast viability, proliferation, and gene expression. Biomaterials. 2005;26(3):285–295. doi: 10.1016/j.biomaterials.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 6.El-Ghannam AR, Ducheyne P, Risbud M, Adams CS, Shapiro IM, Castner D, et al. Model surfaces engineered with nanoscale roughness and RGD tripeptides promote osteoblast activity. Journal of Biomedical Materials Research Part A. 2004;68A(4):615–627. doi: 10.1002/jbm.a.20051. [DOI] [PubMed] [Google Scholar]
- 7.Xiao SJ, Textor M, Spencer ND, Sigrist H. Covalent attachment of cell-adhesive, (Arg-Gly-Asp)-containing peptides to titanium surfaces. Langmuir. 1998;14(19):5507–5516. [Google Scholar]
- 8.Fishbein I, Alferiev IS, Nyanguile O, Gaster R, Vohs JM, Wong GS, et al. Bisphosphonate-mediated gene vector delivery from the metal surfaces ot stents. P Natl Acad Sci USA. 2006;103(1):159–164. doi: 10.1073/pnas.0502945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki J, Serruys PW, van Beusekom H, Ong ATL, McFadden EP, Sianos G, et al. Endothelial progenitor cell capture by stents coated with antibody against CD34 - The HEALING-FIM (healthy endothelial accelerated lining inhibits neointimal growth-first in man) registry. Journal of the American College of Cardiology. 2005;45(10):1574–1579. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 10.Heise C, Bier FF. Immobilization of DNA on microarrays. Immobilisation of DNA on Chips Ii. 2005;261:1–25. [Google Scholar]
- 11.Thakkar H, Davey CL, Medcalf EA, Skingle L, Craig AR, Newman DJ, et al. Stabilization of Turbidimetric Immunoassay by Covalent Coupling of Antibody to Latex-Particles. Clinical Chemistry. 1991;37(7):1248–1251. [PubMed] [Google Scholar]
- 12.Zhang FX, Srinivasan MP. Self-assembled molecular films of aminosilanes and their immobilization capacities. Langmuir. 2004;20(6):2309–2314. doi: 10.1021/la0354638. [DOI] [PubMed] [Google Scholar]
- 13.Puleo DA. Activity of Enzyme Immobilized on Silanized Co-Cr-Mo. Journal of Biomedical Materials Research. 1995;29(8):951–957. doi: 10.1002/jbm.820290806. [DOI] [PubMed] [Google Scholar]
- 14.Moon JH, Shin JW, Kim SY, Park JW. Formation of uniform aminosilane thin layers: An imine formation to measure relative surface density of the amine group. Langmuir. 1996;12(20):4621–4624. [Google Scholar]
- 15.White LD, Tripp CP. Reaction of (3-aminopropyl)dimethylethoxysilane with amine catalysts on silica surfaces. J Colloid Interf Sci. 2000;232(2):400–407. doi: 10.1006/jcis.2000.7224. [DOI] [PubMed] [Google Scholar]
- 16.Petri DFS, Wenz G, Schunk P, Schimmel T. An improved method for the assembly of amino-terminated monolayers on SiO2 and the vapor deposition of gold layers. Langmuir. 1999;15(13):4520–4523. [Google Scholar]
- 17.Ek S, Iiskola EI, Niinisto L. Gas-phase deposition of aminopropylalkoxysilanes on porous silica. Langmuir. 2003;19(8):3461–3471. [Google Scholar]
- 18.Ek S, Iiskola EI, Niinisto L, Pakkanen TT, Root A. New bonding modes of gas-phase deposited gamma-aminopropyltriethoxysilane on silica studied by Si-29 CP/MAS NMR. Chem Commun. 2003;(16):2032–2033. doi: 10.1039/b305534e. [DOI] [PubMed] [Google Scholar]
- 19.Trepanier C, Leung TK, Tabrizian M, Yahia L, Bienvenu JG, Tanguay JF, et al. Preliminary investigation of the effects of surface treatments on biological response to shape memory NiTi stents. Journal of Biomedical Materials Research. 1999;48(2):165–171. doi: 10.1002/(sici)1097-4636(1999)48:2<165::aid-jbm11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Trepanier C, Tabrizian M, Yahia L, Bilodeau L, Piron DL. Effect of modification of oxide layer on NiTi stent corrosion resistance. Journal of Biomedical Materials Research. 1998;43(4):433–440. doi: 10.1002/(sici)1097-4636(199824)43:4<433::aid-jbm11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Shabalovskaya SA. Surface, corrosion and biocompatibility aspects of Nitinol as an implant material. Bio-Med Mater Eng. 2002;12(1):69–109. [PubMed] [Google Scholar]
- 22.Shabalovskaya SA. On the nature of the biocompatibility and on medical applications of NiTi shape memory and superelastic alloys. Bio-Med Mater Eng. 1996;6(4):267–289. [PubMed] [Google Scholar]
- 23.Ryhanen J, Kallioinen M, Tuukkanen J, Junila J, Niemela E, Sandvik P, et al. In vivo biocompatibility evaluation of nickel-titanium shape memory metal alloy: Muscle and perineural tissue responses and encapsule membrane thickness. Journal of Biomedical Materials Research. 1998;41(3):481–488. doi: 10.1002/(sici)1097-4636(19980905)41:3<481::aid-jbm19>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Thierry B, Merhi Y, Bilodeau L, Trepanier C, Tabrizian M. Nitinol versus stainless steel stents: acute thrombogenicity study in an ex vivo porcine model. Biomaterials. 2002;23(14):2997–3005. doi: 10.1016/s0142-9612(02)00030-3. [DOI] [PubMed] [Google Scholar]
- 25.Rocher P, El Medawar L, Hornez JC, Traisnel M, Breme J, Hildebrand HF. Biocorrosion and cytocompatibility assessment of NiTi shape memory alloys. Scripta Mater. 2004;50(2):255–260. doi: 10.1016/s1389-0344(02)00041-2. [DOI] [PubMed] [Google Scholar]
- 26.Rhalmi S, Charette S, Assad M, Coillard C, Rivard CH. The spinal cord dura mater reaction to nitinol and titanium alloy particles: a 1-year study in rabbits. European Spine Journal. 2007;16(7):1063–1072. doi: 10.1007/s00586-007-0329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assad M, Jarzem P, Leroux MA, Coillard C, Chernyshov AV, Charette S, et al. Porous titanium-nickel for intervertebral fusion in a sheep model: Part 1. Histomorphometric and radiological analysis. J Biomed Mater Res B. 2003;64B(2):107–120. doi: 10.1002/jbm.b.10530. [DOI] [PubMed] [Google Scholar]
- 28.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 29.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 30.Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci U S A. 2002;99(8):5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niece KL, Hartgerink JD, Donners JJJM, Stupp SI. Self-assembly combining two bioactive peptide-amphiphile molecules into nanofibers by electrostatic attraction. Journal of the American Chemical Society. 2003;125(24):7146–7147. doi: 10.1021/ja028215r. [DOI] [PubMed] [Google Scholar]
- 32.Rajangam K, Behanna HA, Hui MJ, Han XQ, Hulvat JF, Lomasney JW, et al. Heparin binding nanostructures to promote growth of blood vessels. Nano Letters. 2006;6(9):2086–2090. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 33.Ruoslahti E, Pierschbacher MD. New Perspectives in Cell-Adhesion - Rgd and Integrins. Science. 1987;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 34.Carley AF, Roberts JC, Roberts MW. Dissociative Chemisorption and Localized Oxidation-States at Titanium Surfaces. Surface Science. 1990;225(3):L39–L41. [Google Scholar]
- 35.Viornery C, Chevolot Y, Leonard D, Aronsson BO, Pechy P, Mathieu HJ, et al. Surface modification of titanium with phosphonic acid to improve bone bonding: Characterization by XPS and ToF-SIMS. Langmuir. 2002;18(7):2582–2589. [Google Scholar]
- 36.Endo K. Chemical modification of metallic implant surfaces with biofunctional proteins (Part 1). Molecular structure and biological activity of a modified NiTi alloy surface. Dent Mater J. 1995;14(2):185–198. doi: 10.4012/dmj.14.185. [DOI] [PubMed] [Google Scholar]
- 37.Gu YW, Tay BY, Lim CS, Yong MS. Characterization of bioactive surface oxidation layer on NiTi alloy. Applied Surface Science. 2005;252(5):2038–2049. [Google Scholar]
- 38.Smith NA, Antoun GG, Ellis AB, Crone WC. Improved adhesion between nickel-titanium shape memory alloy and a polymer matrix via silane coupling agents. Composites Part a-Applied Science and Manufacturing. 2004;35(11):1307–1312. [Google Scholar]
- 39.Armitage DA, Parker TL, Grant DM. Biocompatibility and hemocompatibility of surface-modified NiTi alloys. Journal of Biomedical Materials Research Part A. 2003;66A(1):129–137. doi: 10.1002/jbm.a.10549. [DOI] [PubMed] [Google Scholar]
- 40.Shabalovskaya SA. Biological aspects of TiNi alloy surfaces. Journal De Physique Iv. 1995;5(C8):1199–1204. [Google Scholar]
- 41.Nanci A, Wuest JD, Peru L, Brunet P, Sharma V, Zalzal S, et al. Chemical modification of titanium surfaces for covalent attachment of biological molecules. Journal of Biomedical Materials Research. 1998;40(2):324–335. doi: 10.1002/(sici)1097-4636(199805)40:2<324::aid-jbm18>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 42.Ek S, Iiskola EI, Niinisto L, Vaittinen J, Pakkanen TT, Keranen J, et al. Atomic layer deposition of a high-density aminopropylsiloxane network on silica through sequential reactions of gamma-aminopropyltrialkoxysilanes and water. Langmuir. 2003;19(25):10601–10609. [Google Scholar]
- 43.Miller DC, Thapa A, Haberstroh KM, Webster TJ. Endothelial and vascular smooth muscle cell function on poly(lactic-co-glycolic acid) with nano-structured surface features. Biomaterials. 2004;25(1):53–61. doi: 10.1016/s0142-9612(03)00471-x. [DOI] [PubMed] [Google Scholar]
- 44.Chung TW, Liu DZ, Wang SY, Wang SS. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials. 2003;24(25):4655–4661. doi: 10.1016/s0142-9612(03)00361-2. [DOI] [PubMed] [Google Scholar]
- 45.Dalby MJ, Riehle MO, Johnstone H, Affrossman S, Curtis ASG. In vitro reaction of endothelial cells to polymer demixed nanotopography. Biomaterials. 2002;23(14):2945–2954. doi: 10.1016/s0142-9612(01)00424-0. [DOI] [PubMed] [Google Scholar]
- 46.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. Journal of Biomedical Materials Research. 2000;51(3):475–483. doi: 10.1002/1097-4636(20000905)51:3<475::aid-jbm23>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 47.Xu CY, Yang F, Wang S, Ramakrishna S. In vitro study of human vascular endothelial cell function on materials with various surface roughness. Journal of Biomedical Materials Research Part A. 2004;71A(1):154–161. doi: 10.1002/jbm.a.30143. [DOI] [PubMed] [Google Scholar]
- 48.Balasundaram G, Sato M, Webster TJ. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials. 2006;27(14):2798–2805. doi: 10.1016/j.biomaterials.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803–1810. doi: 10.1016/s0142-9612(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 50.Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17(2):137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz Z, Lohmann CH, Vocke AK, Sylvia VL, Cochran DL, Dean DD, et al. Osteoblast response to titanium surface roughness and 1 alpha,25-(OH)(2)D-3 is mediated through the mitogen-activated protein kinase (MAPK) pathway. Journal of Biomedical Materials Research. 2001;56(3):417–426. doi: 10.1002/1097-4636(20010905)56:3<417::aid-jbm1111>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 52.Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials. 2001;22(1):87–96. doi: 10.1016/s0142-9612(00)00174-5. [DOI] [PubMed] [Google Scholar]
- 53.Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J, et al. Effect of Titanium Surface-Roughness on Proliferation, Differentiation, and Protein-Synthesis of Human Osteoblast-Like Cells (Mg63) Journal of Biomedical Materials Research. 1995;29(3):389–401. doi: 10.1002/jbm.820290314. [DOI] [PubMed] [Google Scholar]
- 54.Harrington DA, Cheng EY, Guler MO, Lee LK, Donovan JL, Claussen RC, et al. Branched peptide-amphiphiles as self-assembling coatings for tissue engineering scaffolds. Journal of Biomedical Materials Research Part A. 2006;78A(1):157–167. [Google Scholar]
- 55.Storrie H, Guler MO, Abu-Amara SN, Volberg T, Rao M, Geiger B, et al. Supramolecular crafting of cell adhesion. Biomaterials. 2007;28(31):4608–4618. doi: 10.1016/j.biomaterials.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 56.Bogdanski D, Esenwein SA, Prymak O, Epple M, Muhr G, Koller M. Inhibition of PMN apoptosis after adherence to dip-coated calcium phosphate surfaces on a NiTi shape memory alloy. Biomaterials. 2004;25(19):4627–4632. doi: 10.1016/j.biomaterials.2003.12.001. [DOI] [PubMed] [Google Scholar]