Abstract
The effect of new NOP receptor agonists and antagonists in the rat chronic constriction injury model was investigated. Intraperitoneally administered NOP receptor agonist SR14150 and antagonists SR16430 and SR14148, had no effect on mechanical allodynia when given alone. The nonselective NOP/mu-opioid receptor agonist SR16435, however, produced an anti-allodynic response, similar to morphine and reversible by naloxone. Notably, co-administration of the NOP receptor antagonists potentiated the anti-allodynic activity of both morphine and SR16435. Increased levels of the NOP receptor are implicated in the reduced efficacy of morphine in neuropathic pain. Our results suggest the utility of NOP receptor antagonists for potentiating opioid efficacy in chronic pain.
Keywords: N/OFQ, NOP, chronic pain, chronic constriction injury, NOP antagonist, neuropathy
1. Introduction
There are limited therapeutic options for the treatment of chronic pain conditions such as inflammatory, neuropathic, and cancer-related pain. Although opioids have shown efficacy in clinical trials and in animal models of chronic pain, they are used as second-line treatment due to concerns associated with their long-term use, such as adverse systemic effects, dependence, and tolerance-associated hyperalgesia (Ballantyne and Mao, 2003; Dworkin et al., 2007). Despite these concerns, the use of opioids for chronic noncancer pain remains very popular. Therefore, molecular targets and drugs that modulate opioid analgesia and their side effects remain of interest.
Nociceptin/orphanin FQ (N/OFQ), a heptadecapeptide from the opioid family, and its cognate receptor NOP (previously called the opioid receptor-like receptor, ORL1), are present in nociceptive pathways in brain and spinal cord. The role of the NOP-N/OFQ system in pain modulation is quite complex, as suggested by the disparate results obtained in several reported studies (Zeilhofer and Calo, 2003). From the contradictory results, it appears that N/OFQ modulates nociception differentially depending upon site of administration, assay, and dose. N/OFQ was originally thought to be pro-nociceptive, since it produced a decrease in latency in the hot-plate and tail-flick assays when injected intracerebroventricularly (i.c.v) (Meunier et al., 1995; Reinscheid et al., 1995). This effect was subsequently shown to be due to inhibition of stress-induced analgesia mediated by endogenous opioids. This anti-opioid effect of N/OFQ was further confirmed when i.c.v. N/OFQ was found to block morphine analgesia in the tail-flick test (Mogil et al., 1996). However, when administered intrathecally (i.t.), N/OFQ has acute antinociceptive activity in the tail-flick test (Xu et al., 1996) and potentiates morphine analgesia (Tian et al., 1997). Similarly, in models of neuropathic and inflammatory pain, i.t. injections of N/OFQ have also been shown to produce anti-allodynic and anti-hyperalgesic effects and to potentiate morphine anti-hyperalgesia (Courteix et al., 2004; Hao et al., 1998; Yamamoto et al., 1997a; b). These results suggest differential modulation of pain at supraspinal and spinal sites, by the NOP-N/OFQ system.
The effects of synthetic NOP receptor ligands on nociception are also rather confounding and depend on the route of administration and model used. Among peptide-based NOP receptor ligands, we showed that the peptide NOP receptor agonist Syn1020 (Ac-RY(3-Cl)YRWR-NH2) had anti-allodynic effects in the rat chronic constriction injury (CCI) model of neuropathic pain, when given intraperitoneally (i.p.) (Khroyan et al., 2007a). The peptide NOP receptor antagonist [NPhe1]NC(1-13)NH2 was ineffective on its own when administered i.t. in CCI rats and did not modify intrathecal morphine analgesia (Corradini et al., 2001). Among small-molecule NOP receptor ligands, the NOP receptor agonist Ro 64-6198 decreased CCI-induced allodynia when given i.t., but not subcutaneously (s.c.) (Obara et al., 2005). Interestingly, however, unlike peptide NOP receptor antagonists, small-molecule NOP receptor antagonists have been shown to have anti-nociceptive activity in models of neuropathic and inflammatory pain when administered systemically. For example, the highly-selective antagonist SB-612111 attenuates hyperalgesia in the carrageenan model of inflammatory pain (Zaratin et al., 2004), and JTC-801, a less selective NOP receptor antagonist, alleviated hyperalgesia in CCI rats when given intravenously (Suyama et al., 2003). These results further point to differential modulation of pain transmission by the N/OFQ-NOP receptor system at supraspinal and spinal sites, and the effect of the route of administration and type of assay used (Heinricher, 2005).
In the present study, we examined the effects of a series of modestly-selective small-molecule NOP receptor agonists (SR14150) and antagonists (SR14148 and SR16430), and a non-selective NOP/mu-opioid receptor agonist SR16435, on tactile allodynia, in the rat CCI model of neuropathic pain (Bennett and Xie, 1988). Since the upregulation in the NOP receptor and N/OFQ levels have been implicated in the reduced opioid efficacy in chronic states (Briscini et al., 2002; Mika et al., 2004), we also investigated the effect of co-administration of NOP receptor antagonists on the anti-allodynic activity of the mu-opioid receptor agonist morphine and of the NOP/mu-opioid receptor agonist SR16435.
2. Materials and Methods
2.1. Animals
Male Sprague Dawley rats weighing 250-300g were used. Animals were housed three/cage under standard laboratory conditions and were kept on a 12:12 hr day-night cycle (lights on at 07:00). All procedures were approved by the Institutional Animal Care and Use Committee, according to the SRI Animal Welfare guidelines.
2.2. Drugs
NOP receptor ligands SR16430 (1-cyclooctylmethyl-4-hydroxy-4-(3-trifluoromethylphenyl) piperidine), SR14150 (1-(1-cyclooctylpiperidin-4-yl)indolin-2-one), SR14148 (1-(1-cyclooctylmethylpiperidin-4-yl)indolin-2-one), and SR16435, (1-(1-bicyclo[3.3.1]nonan-9-yl) piperidin-4-yl)indolin-2-one), were synthesized in our laboratory (Zaveri et al., 2004). These compounds as well as standards morphine hydrochloride (Eli Lilly & Co.), naloxone (Sigma-Aldrich), and gabapentin were dissolved in 1-2% DMSO and diluted with 0.5% hydroxypropylmethylcellulose, or in water. Drugs were injected in a volume of 1 ml/kg (i.p.). Controls received 1 ml/kg of the appropriate vehicle.
2.3. Surgical induction of CCI in rats
CCI was induced by a ligation of the sciatic nerve using the method developed by Bennett and Xie (Bennett and Xie, 1988). Briefly, an incision was made underneath the right hind limb. The subcutaneous tissue and connective tissue were teased apart to isolate the sciatic nerve and four loose suture ligations were made around the sciatic nerve. The nerve was placed back underneath the connective tissue and the closure was stapled.
2.4. Tactile allodynia testing procedure (Von Frey)
Animals received an i.p. injection of their respective drug and were tested 60-min postinjection for tactile allodynia with von Frey filaments using the modified up-down method (Chaplan et al., 1994). Briefly, a von Frey filament that had a buckling weight of 2.0 g was applied to the right hind paw of the animal with continuous pressure for about 5 seconds. If the animal lifted its paw, the next filament with lower force was then applied. If the animal did not lift its paw, the next filament with higher force was used. Each response was recorded and the experiment ended once the animal had made five responses. One group of CCI-induced animals served as controls and received injections of vehicle, after which they were tested for tactile allodynia in an identical fashion. The average baseline paw withdrawal threshold following CCI surgery prior to testing was 4.3 ± 0.9.
2.5. Experimental design
Animals were tested for tactile allodynia beginning one week following CCI surgery. All drugs were first tested alone. The anti-allodynic effects of morphine and the mixed NOP/muopioid receptor partial agonist SR16435 were also evaluated after co-administration with the NOP receptor antagonists (SR16430 or SR14148) or the opioid antagonist naloxone. Doses of antagonists were chosen from previous experiments in our laboratory (Khroyan et al., 2007b). The 60-min test period was chosen, based on previous studies in our laboratory showing that the maximal effects of gabapentin, morphine, and NOP/mu agonists are still present at 60-min (Khroyan et al., 2007a; Khroyan et al., 2007b). This time point was also suitable since the sedative effects of NOP/mu agonists SR16435 and SR14150 had generally dissipated by 60-min postinjection. When SR16435 was co-administered with SR16430, there was still some decrease in motor activity with 10 mg/kg dose of SR16435 60-min following the injection; however, animals that were sedated and had no muscle tone were not included in the study. There was an N=7-13 animals per group. The control group of animals received injections of vehicle.
2.6. Statistical analyses
The 50% paw withdrawal threshold was calculated (Chaplan et al., 1994) using the formula: 10 (Xf + κδ)/10000 where Xf is the final von Frey filament used (log units), K is a value that analyzes the response pattern (taken from a published table (Chaplan et al., 1994) mean difference between stimuli (log units).
Behavioral results were analyzed by ANOVA using gabapentin, morphine, SR16435, SR14150, SR16430, SR14148, and naloxone as the between group variables followed by Student Newman-Keuls post-hoc tests where appropriate. The level of significance was P<0.05.
3. Results
The in vitro binding affinities and functional activities of the NOP receptor agonists and antagonists used in this study have been characterized previously (Spagnolo et al., 2007; Zaveri et al., 2004) and are shown in Table 1. The NOP receptor agonist SR14150 is 20-fold selective for NOP receptor versus mu-opioid receptors, and has partial agonist activity at both sites. The NOP receptor antagonist SR16430 is 10-fold selective for NOP receptor over the mu-opioid receptor, and has no efficacy at the mu-opioid receptor, whereas antagonist SR14148 about 2-fold selective for the NOP receptor and has very low efficacy at the mu-opioid receptor. The nonselective mixed ligand SR16435 has equal and high affinity at both the NOP receptor and mu-opioid receptors and partial agonist activity at both sites.
Table 1.
Binding affinities and functional activities of SRI compounds at NOP and opioid receptors compared to N/OFQ and morphine.a
| NOP receptor | Mu-opioid receptor | Kappa-opioid receptor | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Receptor Binding |
Functional activity [35S]GTPγS |
Receptor Binding |
Functional activity [35S]GTPγS |
Receptor Binding |
Functional activity [35S]GTPγS |
||||
| Compound | Ki (nM) | EC50 (nM) | % Stim |
Ki (nM) | EC50 (nM) | % Stim |
Ki (nM) | EC50 (nM) | % Stim |
| N/OFQ | 0.2 ± 0.0 | 4.0 ± 0.1 | 100 | 437 ± 12 | >10K | 147 ± 3 | >10K | ||
| Morphineb | >10K | 1.1 ± 0.1 | 15.6 ± 0.5 | 93 | 46.9 ± 14.5 | 484 ± 213 | 62 | ||
| SR16430b | 6.5 ± 1.4 | >10K | 61.0 ± 15.0 | >10K | 219.5 ± 18.0 | >10K | |||
| SR 14148c | 6.0 ± 0.4 | >10K | 14.4 ± 1.1 | 239.0 ± 43.0 | 25.9 | 228.7 ± 33.5 | >10K | ||
| SR 14150c | 1.4 ± 0.4 | 20.8 ± 3.1 | 54.2 | 29.9 ± 2.1 | 98.9 ± 12.5 | 23.4 | 42.7 ± 1.0 | 276 ± 75.8 | 38 |
| SR 16435c | 7.5 ± 0.8 | 28.7 ± 0.6 | 45.0 | 2.70 ± 0.1 | 29.5 ± 10.0 | 30 | 31.7 ± 4.8 | >10K | |
Receptor binding and [35S]GTPγS binding was conducted in CHO cell membranes containing the appropriate human receptor, as described in Dooley et al. (Dooley et al., 1997). Binding constants are shown in Ki ± S.D. for each compound, which are derived from at least two individual experiments conducted in triplicate. An EC50 value of >10,000 for [35S]GTPγS binding indicates no significant stimulation at that concentration. N.D. indicates that it was not determined.
Taken from Khroyan et al. (Khroyan et al., 2007b).
Taken from Zaveri et al. (Zaveri et al., 2004).
3.1. NOP receptor ligands and morphine alone on tactile allodynia
The effects of the NOP receptor ligands on tactile allodynia, when administered alone, are shown in Figure 1. The effect of the positive controls morphine and gabapentin are also shown in Figure 1. As expected, gabapentin (60.0 mg/kg) reversed allodynia 60 min following administration as evidenced by the increase in response threshold relative to control animals [F(1,24)=35.8, P<0.05]. The selective NOP receptor antagonists SR16430 and SR14148, and NOP receptor agonist SR14150 were ineffective in attenuating the allodynia induced by CCI [F(2,23)=2.0, n.s., F(2,24)=2.8, n.s. F(3,54)=1.3, n.s; respectively]. On the other hand, the mixed NOP/mu-opioid receptor partial agonist SR16435 significantly reduced CCI-induced allodynia at the 10.0 mg/kg dose ([F(2,26)=15.1; P<0.05]), whereas morphine produced significant anti-allodynic effects at the 3.0 and 10.0 mg/kg doses ([F(2,42)=18.6, P<0.05]).
Figure 1.
Effects of NOP receptor antagonists SR16430 and SR14148 and agonists SR14150 and SR16435 on mechanical allodynia induced by CCI, compared to vehicle controls, positive control gabapentin, and morphine. All drugs were given i.p. Data are means (± S.E.M.) where * P<0.05 represents a significant difference from vehicle control.
3.2. Co-administration of NOP receptor antagonists or the opioid antagonist naloxone on the anti-allodynic effect of SR16435 and morphine
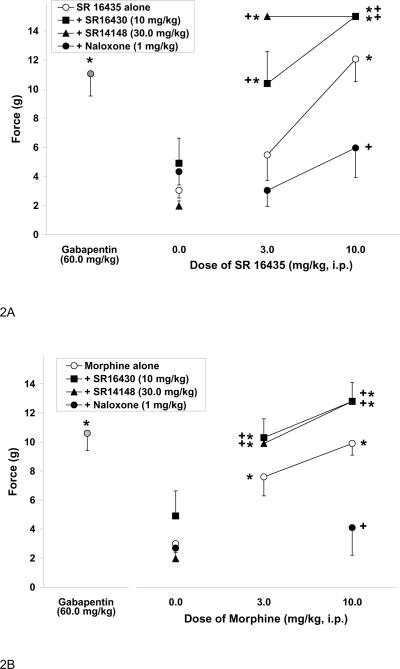
To determine if the anti-allodynic effect of the mixed NOP/mu-opioid receptor ligand SR16435 was through the NOP receptor or mu-opioid receptors, the anti-allodynic activity of SR16435 was determined in the presence of NOP receptor antagonists SR16430 and SR14148, and opioid antagonist naloxone. The effects of SR16430, SR14148 and naloxone on SR16435-induced anti-allodynia are shown in Figure 2A. Unexpectedly, co-administration of the NOP receptor antagonist SR16430 with SR16435 produced a significant increase in response threshold relative to that produced by SR16435 alone [F(1,26)=5.8, P<0.05] resulting in a potentiation of SR16435-induced anti-allodynic activity. The antagonist SR14148 also potentiated the effects of SR16435 [F(1,22)=4.8, P<0.05]. However, co-administration of SR16435 with opioid antagonist naloxone, however, attenuated the anti-allodynic effects of SR16435 [F(1,28)=6.7, P<0.05]. Naloxone alone did not have any anti- or pro-allodynic effects relative to controls. These results suggest that the anti-allodynic activity of SR16435 may be mediated by the mu-opioid agonist activity of the molecule, and not by its NOP receptor agonist activity.
Figure 2.
Effect of SR16435 (A) or morphine (B) administered alone, or co-administered with NOP receptor antagonist SR16430 or SR14148 or opioid antagonist naloxone, on mechanical allodynia induced by CCI. Gabapentin is included as the positive control. Data are means (± S.E.M.) where *P<0.05 represents a significant difference from vehicle control, whereas +P<0.05 represents a significant difference from SR16435 or morphine alone.
Since the NOP receptor antagonists potentiated the anti-allodynic activity of SR16435, we investigated the effect of co-administration of the NOP receptor antagonists on the anti-allodynic activity of the opioid agonist morphine. The effect of NOP receptor antagonists and naloxone on morphine-induced anti-allodynia are shown in Figure 2B. Interestingly, co-administration with NOP receptor antagonist SR16430 [F(1,65)=4.5, P<0.05] or SR14148 [F(1,67)=4.7, P<0.05] also significantly potentiated the anti-allodynic effects of morphine. On the other hand, as expected, the anti-allodynic effect of morphine was attenuated by naloxone [F(1,32) = 9.6, P<0.05]. These results suggest that the NOP receptor antagonists potentiate muopioid receptor-mediated anti-allodynic activity of morphine. These results are also consistent with our observation with SR16435, the anti-allodynic activity of which appears to be mediated by its mu-opioid receptor activity and is reversed by naloxone and potentiated by both the NOP receptor antagonists.
4. Discussion
In the present study, we found that the moderately-selective NOP receptor agonist SR14150 and NOP receptor antagonists SR16430 and SR14148 were ineffective on their own, in reversing tactile allodynia induced by chronic constriction injury of the sciatic nerve in rats. However, our major finding was that the NOP receptor antagonists were effective in potentiating the anti-allodynic activity of morphine and the mixed NOP/mu-opioid receptor agonist SR16435.
SR14150 is a high affinity NOP receptor partial agonist with 20-fold selectivity for NOP receptor versus mu-opioid receptors (see Table 1). The lack of anti-allodynic activity of SR14150, administered s.c., is in agreement with the result obtained with the selective small-molecule NOP receptor agonist Ro 64-6198, which showed no anti-allodynic activity upon s.c. administration (Obara et al., 2005). However, SR14150 does have naloxone-reversible acute antinociceptive activity in the tail-flick assay in mice (Spagnolo et al., 2007). Apparently, the low level of mu-opioid receptor-mediated antinociceptive activity of SR14150 is better manifested in the acute tail-flick thermal assay than in the injury-induced neuropathic pain assay. This is not entirely unexpected since it is well known that, unlike acute pain, neuropathic pain is less sensitive to the mu-opioid receptor-mediated antinociceptive efficacy of morphine (Ossipov et al., 1995; Przewlocki and Przewlocka, 2001). Several adaptive mechanisms have been suggested for the reduced efficacy of morphine in neuropathic pain, including a reduced number of opioid receptors (Ossipov et al., 1995) and increased activity of antiopioidergic systems such as dynorphin (Nichols et al., 1997).
In contrast, NOP/mu-opioid receptor partial agonist SR16435, which is non-selective and has high affinity for both the NOP receptor and mu-opioid receptors, has potent anti-allodynic activity at doses significantly lower than the positive control gabapentin (Figure 1). The anti-allodynic activity of SR16435 is blocked by naloxone (Figure 2A), suggesting that it is mediated through the mu-opioid receptor. Even though SR16435 has equal binding affinity at NOP receptor and mu-opioid receptor, it has lower efficacy at the NOP receptor than the NOP receptor-selective agonist SR14150 (Table 1). Therefore, SR16435 exhibits significant muopioid receptor-mediated anti-allodynic activity that is not greatly affected by its NOP receptor partial agonist activity.
Our main finding is that the systemic administration of NOP receptor antagonists potentiated the anti-allodynic activity of morphine and the NOP/mu-opioid receptor agonist SR16435 (Figure 2A and 2B). The anti-opioidergic activity of the NOP receptor system and an up-regulation of the N/OFQ-NOP receptor system during chronic pain could underlie the ability of NOP receptor antagonists to potentiate mu-opioid receptor-mediated anti-allodynia. An upregulation of NOP receptor and preproN/OFQ mRNA has been reported in the spinal cord and dorsal root ganglia of neuropathic CCI rats (Briscini et al., 2002; Mika et al., 2004). A significant increase in brain N/OFQ immunoreactivity has also been demonstrated in spinal nerve-ligated rats (Sun et al., 2001). Furthermore, increased levels of N/OFQ have also been reported in the cerebrospinal fluid of chronic pain patients (Raffaeli et al., 2006). The upregulation of the NOP-N/OFQ system in chronic pain may also be responsible for the decreased efficacy of mu opioid agonists such as morphine in chronic pain, since other anti-opioidergic systems have been shown to have similar effects on opioid efficacies in chronic pain (Bian et al., 1999; Coudore-Civiale et al., 2000a; Coudore-Civiale et al., 2000b). Together with these studies, our results on the potentiation of morphine anti-allodynic activity by NOP antagonists suggest that NOP receptor antagonists may be useful in combination with morphine in chronic pain.
Our findings are consistent with previous results with the peptide NOP receptor antagonist Phe1ψN/OFQ (1-13)NH2 which, when administered i.t., potentiated the anti-allodynic effects of i.t. administered morphine in CCI rats (Mika et al., 2004). Among small-molecule NOP receptor antagonists, J-113397, administered i.p. potentiated the anti-hyperalgesic activity of i.p. buprenorphine, in the rat formalin model of inflammatory pain (Yamamoto et al., 2006). Even when administered i.c.v,, J-113397 potentiated the anti-hyperalgesic activity of i.p. buprenorphine in the same model. Buprenorphine, a well-recognized opioid analgesic, clinically used in acute and chronic pain treatment (Heit and Gourlay, 2008), has a complex pharmacological profile at the opioid receptors. In vitro characterization shows that buprenorphine is a partial agonist at the mu opioid receptor and an antagonist at the kappa and delta opioid receptors, whereas it has partial agonist activity at the NOP receptor (Bloms-Funke et al., 2000; Huang et al., 2001). Buprenorphine's analgesic activity has been characterized in several acute and chronic pain models and shows an inverted U-shaped dose-response curve (Christoph et al., 2005), that has been postulated to be due to suppression by its NOP partial agonist activity at higher doses, at least in acute pain assays (Lutfy et al., 2003). The results of Yamamoto et al. (2006) in the inflammatory pain model with buprenorphine, and potentiation by the NOP receptor antagonist J-113397, appears to suggest that the analgesic activity of buprenorphine in inflammatory pain might also be compromised by its partial agonist activity at the NOP receptor. However, the effects of NOP receptor antagonists on buprenorphine activity in neuropathic pain models have not yet been shown. Our results are the first to show that in the neuropathic pain state, systemic administration of small-molecule NOP receptor antagonists can potentiate mu-opioid receptor-mediated anti-allodynia. Given our results, a similar study with buprenorphine in a neuropathic pain model seems warranted.
Our results have significant implications for the potential utility of NOP receptor antagonists for the treatment of neuropathic pain, in combination with opioids. Although opioids are widely prescribed for these conditions, clinical studies have shown that neuropathic pain is not very responsive to morphine (Przewlocki and Przewlocka, 2005). Larger doses are generally used, which lead to other undesirable side effects. NOP receptor antagonists may provide a favorable therapeutic combination for neuropathic pain treatment that would allow for a reduction in opioid dosage. In addition, given the modulation of mu-opioid receptor-mediated analgesia by NOP receptors in a chronic pain state, NOP/mu-opioid receptor `dual' ligands with a profile of mu-opioid receptor agonist and NOP receptor antagonist activity may be particularly useful for the treatment of chronic pain.
Acknowledgements
This work was supported by a grant from the National Institute On Drug Abuse (R01DA014026) to N.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N. Engl. J. Med. 2003;349:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bian D, Ossipov MH, Ibrahim M, Raffa RB, Tallarida RJ, Malan TP, Jr., Lai J, Porreca F. Loss of antiallodynic and antinociceptive spinal/supraspinal morphine synergy in nerve-injured rats: restoration by MK-801 or dynorphin antiserum. Brain Res. 1999;831:55–63. doi: 10.1016/s0006-8993(99)01393-1. [DOI] [PubMed] [Google Scholar]
- Bloms-Funke P, Gillen C, Schuettler AJ, Wnendt S. Agonistic effects of the opioid buprenorphine on the nociceptin/OFQ receptor. Peptides. 2000;21:1141–1146. doi: 10.1016/s0196-9781(00)00252-7. [DOI] [PubMed] [Google Scholar]
- Briscini L, Corradini L, Ongini E, Bertorelli R. Up-regulation of ORL-1 receptors in spinal tissue of allodynic rats after sciatic nerve injury. Eur. J. Pharmacol. 2002;447:59–65. doi: 10.1016/s0014-2999(02)01833-2. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Christoph T, Kogel B, Schiene K, Meen M, De Vry J, Friderichs E. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur. J. Pharmacol. 2005;507:87–98. doi: 10.1016/j.ejphar.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Corradini L, Briscini L, Ongini E, Bertorelli R. The putative OP(4) antagonist, [Nphe(1)]nociceptin(1-13)NH(2), prevents the effects of nociceptin in neuropathic rats. Brain Res. 2001;905:127–133. doi: 10.1016/s0006-8993(01)02520-3. [DOI] [PubMed] [Google Scholar]
- Coudore-Civiale MA, Courteix C, Boucher M, Meen M, Fialip J, Eschalier A, Ardid D. Potentiation of morphine and clomipramine analgesia by cholecystokinin -B antagonist CI-988 in diabetic rats. Neurosci. Lett. 2000a;286:37–40. doi: 10.1016/s0304-3940(00)01080-6. [DOI] [PubMed] [Google Scholar]
- Coudore-Civiale MA, Courteix C, Fialip J, Boucher M, Eschalier A. Spinal effect of the cholecystokinin-B receptor antagonist CI-988 on hyperalgesia, allodynia and morphineinduced analgesia in diabetic and mononeuropathic rats. Pain. 2000b;88:15–22. doi: 10.1016/S0304-3959(00)00304-3. [DOI] [PubMed] [Google Scholar]
- Courteix C, Coudore-Civiale MA, Privat AM, Pelissier T, Eschalier A, Fialip J. Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain. Pain. 2004;110:236–245. doi: 10.1016/j.pain.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Dooley CT, Spaeth CG, Berzetei-Gurske IP, Craymer K, Adapa ID, Brandt SR, Houghten RA, Toll L. Binding and in vitro activities of peptides with high affinity for the nociceptin/orphanin FQ receptor, ORL1. J. Pharmacol. Exp. Ther. 1997;283:735–741. [PubMed] [Google Scholar]
- Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice AS, Stacey BR, Treede RD, Turk DC, Wallace MS. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Hao JX, Xu IS, Wiesenfeld-Hallin Z, Xu XJ. Anti-hyperalgesic and anti-allodynic effects of intrathecal nociceptin/orphanin FQ in rats after spinal cord injury, peripheral nerve injury and inflammation. Pain. 1998;76:385–393. doi: 10.1016/S0304-3959(98)00071-2. [DOI] [PubMed] [Google Scholar]
- Heinricher MM. Nociceptin/orphanin FQ: pain, stress and neural circuits. Life Sci. 2005;77:3127–3132. doi: 10.1016/j.lfs.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Heit HA, Gourlay DL. Buprenorphine: new tricks with an old molecule for pain management. Clin. J. Pain. 2008;24:93–97. doi: 10.1097/AJP.0b013e31815ca2b4. [DOI] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J. Pharmacol. Exp. Ther. 2001;297:688–695. [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Orduna J, Zaveri NT, Judd AK, Tuttle DJ, Sanchez A, Toll L. Anti-nociceptive and anti-allodynic effects of a high affinity NOP hexapeptide [Ac-RY(3-Cl)YRWR-NH2] (Syn 1020) in rodents. Eur. J. Pharmacol. 2007a;560:29–35. doi: 10.1016/j.ejphar.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Zaveri NT, Polgar WE, Orduna J, Olsen C, Jiang F, Toll L. SR 16435 [1-(1-(bicyclo[3.3.1]nonan-9-yl)piperidin-4-yl)indolin-2-one], a novel mixed nociceptin/orphanin FQ/mu-opioid receptor partial agonist: analgesic and rewarding properties in mice. J. Pharmacol. Exp. Ther. 2007b;320:934–943. doi: 10.1124/jpet.106.111997. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, Evans CJ. Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J. Neurosci. 2003;23:10331–10337. doi: 10.1523/JNEUROSCI.23-32-10331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mika J, Schafer MK, Obara I, Weihe E, Przewlocka B. Morphine and endomorphin-1 differently influence pronociceptin/orphanin FQ system in neuropathic rats. Pharmacol. Biochem. Behav. 2004;78:171–178. doi: 10.1016/j.pbb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. Orphanin FQ is a functional anti-opioid peptide. Neuroscience. 1996;75:333–337. doi: 10.1016/0306-4522(96)00338-7. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Lopez Y, Ossipov MH, Bian D, Porreca F. Enhancement of the antiallodynic and antinociceptive efficacy of spinal morphine by antisera to dynorphin A (1-13) or MK-801 in a nerve-ligation model of peripheral neuropathy. Pain. 1997;69:317–322. doi: 10.1016/S0304-3959(96)03282-4. [DOI] [PubMed] [Google Scholar]
- Obara I, Przewlocki R, Przewlocka B. Spinal and local peripheral antiallodynic activity of Ro64-6198 in neuropathic pain in the rat. Pain. 2005;116:17–25. doi: 10.1016/j.pain.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lopez Y, Nichols ML, Bian D, Porreca F. Inhibition by spinal morphine of the tail-flick response is attenuated in rats with nerve ligation injury. Neurosci. Lett. 1995;199:83–86. doi: 10.1016/0304-3940(95)12026-z. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Przewlocka B. Opioids in chronic pain. Eur. J. Pharmacol. 2001;429:79–91. doi: 10.1016/s0014-2999(01)01308-5. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Przewlocka B. Opioids in neuropathic pain. Curr. Pharm. Des. 2005;11:3013–3025. doi: 10.2174/1381612054865055. [DOI] [PubMed] [Google Scholar]
- Raffaeli W, Samolsky Dekel BG, Landuzzi D, Caminiti A, Righetti D, Balestri M, Montanari F, Romualdi P, Candeletti S. Nociceptin levels in the cerebrospinal fluid of chronic pain patients with or without intrathecal administration of morphine. J. Pain. Symptom Mgmt. 2006;32:372–377. doi: 10.1016/j.jpainsymman.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr., Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Spagnolo B, Calo G, Polgar WE, Jiang F, Olsen CM, Berzetei-Gurske I, Khroyan TV, Husbands SM, Lewis JW, Toll L, Zaveri NT. Activities of mixed NOP and muopioid receptor ligands. Br. J. Pharmacol. 2007;153:609–619. doi: 10.1038/sj.bjp.0707598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun RQ, Wang Y, Zhao CS, Chang JK, Han JS. Changes in brain content of nociceptin/orphanin FQ and endomorphin 2 in a rat model of neuropathic pain. Neurosci. Lett. 2001;311:13–16. doi: 10.1016/s0304-3940(01)02095-x. [DOI] [PubMed] [Google Scholar]
- Suyama H, Kawamoto M, Gaus S, Yuge O. Effect of JTC-801 (nociceptin antagonist) on neuropathic pain in a rat model. Neurosci Lett. 2003;351:133–136. doi: 10.1016/s0304-3940(03)00502-0. [DOI] [PubMed] [Google Scholar]
- Tian JH, Xu W, Fang Y, Mogil JS, Grisel JE, Grandy DK, Han JS. Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: antagonism in brain and potentiation in spinal cord of the rat. Br. J. Pharmacol. 1997;120:676–680. doi: 10.1038/sj.bjp.0700942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XJ, Hao JX, Wiesenfeld-Hallin Z. Nociceptin or antinociceptin: potent spinal antinociceptive effect of orphanin FQ/nociceptin in the rat. Neuroreport. 1996;7:2092–2094. [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, in the rat formalin test. Neuroscience. 1997a;81:249–254. doi: 10.1016/s0306-4522(97)00166-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. Effects of intrathecally administered nociceptin, an opioid receptor-like1 (ORL1) receptor agonist, on the thermal hyperalgesia induced by unilateral constriction injury to the sciatic nerve in the rat. Neurosci. Lett. 1997b;224:107–110. doi: 10.1016/s0304-3940(97)13475-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shono K, Tanabe S. Buprenorphine activates mu and opioid receptor like-1 receptors simultaneously, but the analgesic effect is mainly mediated by mu receptor activation in the rat formalin test. J. Pharmacol. Exp. Ther. 2006;318:206–213. doi: 10.1124/jpet.105.100859. [DOI] [PubMed] [Google Scholar]
- Zaratin PF, Petrone G, Sbacchi M, Garnier M, Fossati C, Petrillo P, Ronzoni S, Giardina GA, Scheideler MA. Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9- tetrahydro-5H-benzocyclohepten-5-ol (SB-612111) J. Pharmacol. Exp. Ther. 2004;308:454–461. doi: 10.1124/jpet.103.055848. [DOI] [PubMed] [Google Scholar]
- Zaveri NT, Jiang F, Olsen CM, Deschamps JR, Parrish D, Polgar W, Toll L. A novel series of piperidin-4-yl-1,3-dihydroindol-2-ones as agonist and antagonist ligands at the nociceptin receptor. J. Med. Chem. 2004;47:2973–2976. doi: 10.1021/jm034249d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Calo G. Nociceptin/orphanin FQ and its receptor--potential targets for pain therapy? J. Pharmacol. Exp. Ther. 2003;306:423–429. doi: 10.1124/jpet.102.046979. [DOI] [PubMed] [Google Scholar]