Abstract
Microsomal prostaglandin E2 synthase-1 (mPGES-1) is an inducible enzyme that catalyzes the conversion of prostaglandin (PG) H2 to PGE2 in downstream of cyclooxygenase-2 (COX-2). Recent studies have obtained in vitro evidence that PGE2 participates in carcinogenesis, angiogenesis, and induction of matrix metalloproteinase-9 (MMP-9), which plays a crucial role in cancer invasion. However, implications for mPGES-1 in thyroid carcinomas remain to be determined. To address this issue, we performed an immunohistochemical analysis for mPGES-1, COX-2 and MMP-9 in 20 papillary thyroid carcinoma (PTC) patients. mPGES-1 immunoreactivity was localized in the cytoplasm of carcinoma cells in 19 cases, with an intensity that tended to be distinct at the interface between the tumor and the surrounding non-neoplastic tissue. Staining was more intense in regions with papillary arrangement, while it was less intense in regions with trabecular or solid arrangement. In many cases, immunohistochemical localization of COX-2 and MMP-9 resemble that of mPGES-1. Taken together, our results suggest the involvement of mPGES-1 in proliferation and differentiation of PTC as well as local invasion of PTC.
Keywords: cyclooxygenase-2, immunohistochemistry, matrix metalloproteinase, papillary thyroid carcinoma, prostaglandin E synthase
I. Introduction
Recent studies have obtained in vitro evidence that prostaglandin E2 (PGE2) participates in carcinogenesis [1, 14, 17, 20] and angiogenesis [16, 18]. The biosynthesis of PGE2 requires three sequential enzymic reactions: the release of arachidonic acid from membrane glycerophospholipids by phospholipase A2, the conversion of arachidonic acid to the unstable intermediate PGH2 by cyclooxygenase-1 (COX-1) or cyclooxygenase-2 (COX-2), and the isomerization of PGH2 to PGE2 by PGE2 synthase (PGES) [EC 5.3.99.3] (Fig. 1). COX-2 and PGES collaboratively mediate the induction of matrix metalloproteinase-9 (MMP-9) [2], which plays a crucial role in cancer invasion by basement membrane degradation [8].
Fig. 1.
Schematic diagram of the COX-2/PGES-1 catalyzed PGE2 biosynthesis pathway in carcinoma invasion. COX-2, cyclooxygenase-2; MMP-9, matrix metalloproteinase-9; PGE2, prostaglandin E2; PGES, membrane-bound prostaglandin E2 synthase.
It has been shown that COX-2 is involved in the pathomechanisms of thyroid carcinomas and chronic thyroiditis (CT) [4, 11]. However, implications for PGES in thyroid carcinomas remain to be determined. To address this issue, we performed an immunohistochemical analysis for membrane-bound PGES-1 (mPGES-1), a well characterized isoform of PGES, as well as COX-2 and MMP-9, in surgically resected thyroid gland tissues including papillary thyroid carcinoma (PTC).
II. Materials and Methods
Subjects and tissue preparation
This investigation was carried out on archival, formalin (20%)-fixed, paraffin-embedded materials of 20 sporadic PTC patients who underwent thyroidectomy, at Tokyo Women’s Medical University Hospital. This study was performed after obtaining written informed consent from the patients examined.
Primary antibodies
The primary antibodies employed in immunohistochemistry were rabbit polyclonal IgG against mPGES-1 (Cayman Chemical, Ann Arbor, MI, USA; diluted 1:300), rabbit polyclonal IgG against COX-2 (Cayman Chemical; diluted 1:300), and mouse monoclonal IgG against MMP-9 (Daiichi Fine Chemical, Toyama, Japan; diluted 1:500).
Immunohistochemical analysis
Multiple 3-µm-thick sections of each material were used for hematoxylin-eosin staining and immunohistochemical staining. For the latter staining, sections were deparaffinized, rehydrated, quenched for 5 min at room temperature with 3% H2O2, rinsed in phosphate-buffered saline (PBS), pH 7.6, processed with microwaving (95°C, 400 W, 20 min) in 10 mM citrate buffer, pH 6.0 for mPGES-1 and MMP-9 staining and 1 mM ethylenediamine N,N,N’,N’-tetraacetic acid, pH 8.0 for COX-2, pretreated for 30 min at room temperature with 3% nonimmune animal serum in PBS, and then incubated overnight at 4°C with the primary antibodies. Antibody binding was visualized by the avidin-biotin-immunoperoxidase complex method using the appropriate Vectastain ABC kits (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions, with 3,3'-diaminobenzidine tetrahydrochloride and hematoxylin as the chromogen and the counterstain, respectively. Sections from which the primary antibodies were omitted or sections which were incubated with nonimmune serum derived from the same animal species as those producing the antibodies served as negative reaction controls.
III. Results
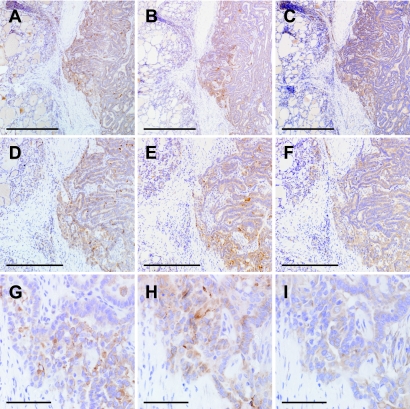
Of the 20 PTC cases, 19 showed focal strong mPGES-1 immunoreactivity, while the other one showed diffuse weak immunoreactivity. The immunoreactivity was localized in the cytoplasm of carcinoma cells in all of the PTC cases, and was prominent at the interface between the tumor and the surrounding non-neoplastic parenchyma (Fig. 2B, E, H). Staining was more intense in regions displaying stromal invasion with papillary arrangement (Fig. 3A), and by contrast it was less intense in regions displaying trabecular arrangement (Fig. 3B) and solid nest formation (Fig. 3C). In many cases, immunohistochemical localization of COX-2 (Fig. 2A, D, G) and MMP-9 (Fig. 2C, F, I) resembled that of mPGES-1. However, immunoreactivities for COX-2 and MMP-9 were diffuse and uniform in PTC cells of 13 cases and four cases, respectively; of the latter four cases, the mPGES-1 immunoreactivity was weak in one case and strong in three cases. Of the 20 PTC cases, seven were with CT, and the rest were not associated with any other thyroid disease. In the seven CT cases, non-neoplastic thyroid parenchyma demonstrated scattered formation of lymph follicles and swelling of follicular epithelial cells that often had eosinophilic cytoplasm and showed papillary arrangement. Immunoreactivities for mPGES-1 and MMP-9 were detected in germinal center lymphocytes and papillary-arranged follicular epithelial cells in five cases (Fig. 4A–C), but were undetectable in intact follicular epithelial cells in all of the CT cases. Immunohistochemical localization of COX-2 was similar to that of mPGES-1 and MMP-9 in three CT cases (Fig. 4A–C). Normal thyroid parenchyma did not show any marked staining for mPGES-1, COX-2 or MMP-9 (Fig. 4A–C).
Fig. 2.
Photomicrographs of PTC tissue sections immunostained for COX-2 (A, D, G), mPGES-1 (B, E, H) and MMP-9 (C, F, I). Series of panels (A–C), (D–F) and (G–I) indicate the same regions including the interface between PTC tissue (each right half) and non-neoplastic thyroid parenchyma (each left half) on consecutive sections, respectively. Bars=1 mm (A–C), 500 µm (D–F), 100 µm (G–I). ABC method using DAB. ABC, avidin-biotin-immunoperoxidase complex; DAB, 3,3'-diaminobenzidine tetrahydrochloride; PTC, papillary thyroid carcinoma.
Fig. 3.
Photomicrographs of PTC lesions immunostained for PGES-1. Panels (A), (B) and (C) indicate foci of papillary arrangement, trabecular arrangement, and solid nest formation of PTC, respectively. Panel (A) is from the interface of stromal invasion, and panels (B) and (C) are from the central core of a tumor. Bars=100 µm. ABC method using DAB. ABC, avidin-biotin-immunoperoxidase complex; DAB, 3,3'-diaminobenzidine tetrahydrochloride; PTC, papillary thyroid carcinoma.
Fig. 4.
Photomicrographs of CT lesions immunostained for COX-2 (A), mPGES-1 (B) and MMP-9 (C). Immunoreactivities for COX-2 (A), mPGES-1 (B) and MMP-9 (C) were detected in germinal center lymphocytes (asterisks) and papillary-arranged follicular epithelial cells (arrows) but undetectable in intact follicular epithelial cells (each left upper side). Bars=200 µm. ABC method using DAB. ABC, avidin-biotin-immunoperoxidase complex; CT, chronic thyroiditis; DAB, 3,3'-diaminobenzidine tetrahydrochloride; PTC, papillary thyroid carcinoma.
IV. Discussion
To date, three isoforms of PGES have been identified in mammalians: mPGES-1, mPGES-2 and cytosolic PGES (cPGES) [7, 9]. mPGES-1 is localized in the microsomal membrane, induced by proinflammatory stimuli, and acts in concert with COX-2 to maintain and amplify inflammatory activity. mPGES-2 is localized in the Golgi apparatus membrane, constitutively expressed, and functionally coupled with COX-1 and COX-2. cPGES is localized in the cytosol, constitutively expressed, and functionally linked to COX-1. Thus, as our results indicated, it is conceivable that the inducible isoform mPGES-1 immunoreactivity was focally prominent in PTC lesions, suggesting that the levels of mPGES-1 induction stimuli vary in the sites of the lesions. Previous studies have indicated the overexpression of mPGES-1 in cancers of the lung [21], colon [22], stomach [19], head and neck [3], endometrium [6], and penis [5]. However, there is no precedent showing immunohistochemical detection of PGES in thyroid tissues, and this is the first report showing mPGES-1 expression in PTC. Several studies have documented the pathological actions of PGE2 as a PGES product. PGE2 activates epidermal growth factor receptor [14], peroxisome proliferator-activated receptor δ [20], and Wnt signaling [1] in carcinogenesis. PGE2 also induces expression of vascular endothelial growth factor, basic fibroblast growth factor, and MMP-9 in carcinoma cells and stromal cells through PGE receptor EP2 that promotes cancer angiogensis [16, 18]. Thus, the COX-2/mPGES-1-catalyzed PGE2 production cascade has been of great interest to oncologists as a possible therapeutic target.
It is known that CT increases risk of the pathogenesis of PTC in a proportion varying from 10 to 40% [12, 13]. A recent study demonstrated the upregulation of COX-2 in both PTC and CT lesions, suggesting the involvement of inflammatory processes in carcinogenesis [4, 11]. In relation to this, it is noteworthy that our study showed that both COX-2 and mPGES-1 were restricted to these lesions but undetectable in morphologically intact thyroid parenchyma. Our finding of the colocalization of mPGES-1 with MMP-9 at the interface between the tumor and the surrounding non-neoplastic tissue suggests a close link between these two enzymes. This supports in vitro evidence that the mPGES-1 product PGE2 induces MMP-9 [2], which contributes to carcinoma invasion [8].
Another intriguing finding that mPGES-1 expression levels were greater in PTC cells displaying papillary arrangement compared to those showing trabecular or solid arrangement points to the possibility that mPGES-1 upregulation may be involved in the differentiation of PTC; papillary arrangement may indicate the state of well differentiation of carcinoma, while trabecular or solid arrangement may indicate the state of poor differentiation. It has been shown that RET/PTC rearrangement is detectable in approximately 30% of the adult sporadic PTC cases [10]. Intracellular tyrosine kinase domain of RET is coupled with an N-terminal fragment of various unrelated genes, and RET/PTC rearrangement occurs in the early step of carcinogenesis of PTC. The two most common rearrangement types, RET/PTC1 and RET/PTC3, upregulate COX-2 and mPGES-1 expression levels in cultured thyroid cells [15]. These observations suggest that mPGES-1 expression triggers PTC carcinogenesis. Finally, it remains to be determined whether mPGES-1 expression levels may have relevance to the prognosis of PTC via direct invasion or hematogenous or lymphogenous metastasis. The answers to this and other questions concerning the establishment of therapeutic strategies will require further investigations.
V. Acknowledgments
We wish to thank N. Sakayori and H. Takeiri for technical assistance.
VI. References
- 1.Castellone M. D., Teramoto H., Williams B. O., Druey K. M., Gutkind J. S. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 2.Cipollone F., Prontera C., Pini B., Marini M., Fazia M., De Cesare D., Iezzi A., Ucchino S., Boccoli G., Saba V., Chiarelli F., Cuccurullo F., Mezzetti A. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E2-dependent plaque instability. Circulation. 2001;104:921–927. doi: 10.1161/hc3401.093152. [DOI] [PubMed] [Google Scholar]
- 3.Cohen E. G., Almahmeed T., Du B., Golijanin D., Boyle J. O., Soslow R. A., Subbaramaiah K., Dannenberg A. J. Microsomal prostaglandin E synthase-1 is overexpressed in head and neck squamous cell carcinoma. Clin. Cancer Res. 2003;9:3425–3430. [PubMed] [Google Scholar]
- 4.Cornetta A. J., Russell J. P., Cunnane M., Keane W. M., Rothstein J. L. Cyclooxygenase-2 expression in human thyroid carcinoma and Hashimoto’s thyroiditis. Laryngoscope. 2002;112:238–242. doi: 10.1097/00005537-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Golijanin D., Tan J. Y., Kazior A., Cohen E. G., Russo P., Dalbagni G., Auborn K. J., Subbaramaiah K., Dannenberg A. J. Cyclooxygenase-2 and microsomal prostaglandin E synthase-1 are overexpressed in squamous cell carcinoma of the penis. Clin. Cancer Res. 2004;10:1024–1031. doi: 10.1158/1078-0432.ccr-1032-3. [DOI] [PubMed] [Google Scholar]
- 6.Jabbour H. N., Milne S. A., Williams A. R., Anderson R. A., Boddy S. C. Expression of COX-2 and PGE synthase and synthesis of PGE2 in endometrial adenocarcinoma: a possible autocrine/paracrine regulation of neoplastic cell function via EP2/EP4 receptors. Br. J. Cancer. 2001;85:1023–1031. doi: 10.1054/bjoc.2001.2033. [DOI] [PubMed] [Google Scholar]
- 7.Kudo I., Murakami M. Prostaglandin E synthase, a terminal enzyme for prostaglandin E2 biosynthesis. J. Biochem. Mol. Biol. 2005;38:633–638. doi: 10.5483/bmbrep.2005.38.6.633. [DOI] [PubMed] [Google Scholar]
- 8.Maeta H., Ohgi S., Terada T. Protein expression of matrix metalloproteinase 2 and 9 and tissue inhibitors of metalloproteinase 1 and 2 in papillary thyroid carcinomas. Virchows Arch. 2001;438:121–128. doi: 10.1007/s004280000286. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M., Kudo I. Prostaglandin E2 synthase: a novel drug target for inflammation and cancer. Curr. Pharm. Des. 2006;12:943–954. doi: 10.2174/138161206776055912. [DOI] [PubMed] [Google Scholar]
- 10.Nikiforov Y. E. RET/PTC rearrangement in thyroid tumors. Endocr. Pathol. 2002;13:3–16. doi: 10.1385/ep:13:1:03. [DOI] [PubMed] [Google Scholar]
- 11.Nose F., Ichikawa T., Fujisawa M., Okayasu I. Up-regulation of cyclooxygenase-2 expression in lymphocytic thyroiditis and thyroid tumors. Am. J. Clin. Pathol. 2002;117:546–551. doi: 10.1309/9CCJ-XQ8P-PMFM-M65K. [DOI] [PubMed] [Google Scholar]
- 12.Okayasu I., Fujiwara M., Hara Y., Tanaka Y., Rose N. R. Association of chronic lymphocytic thyroiditis and thyroid papillary carcinoma. A study of surgical cases among Japanese, and white and African Americans. Cancer. 1995;76:2312–2318. doi: 10.1002/1097-0142(19951201)76:11<2312::aid-cncr2820761120>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Ott R. A., McCall A. R., McHenry C., Jarosz H., Armin A., Lawrence A. M., Paloyan E. The incidence of thyroid carcinoma in Hashimoto’s thyroiditis. Am. Surg. 1987;53:442–445. [PubMed] [Google Scholar]
- 14.Pai R., Soreghan B., Szabo I. L., Pavelka M., Baatar D., Tarnawski A. S. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat. Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 15.Puxeddu E., Mitsutake N., Knauf J. A., Moretti S., Kim H. W., Seta K. A., Brockman D., Myatt L., Millhorn D. E., Fagin J. A. Microsomal prostaglandin E2 Synthase-1 is induced by conditional expression of RET/PTC in thyroid PCCL3 cells through the activation of the MEK-ERK pathway. J. Biol. Chem. 2003;278:52131–52138. doi: 10.1074/jbc.M306003200. [DOI] [PubMed] [Google Scholar]
- 16.Seno H., Oshima M., Ishikawa T., Oshima H., Takaku K., Chiba T., Narumiya S., Taketo M. M. Cyclooxygenase 2- and prostaglandin E(2) receptor EP(2)-dependent angiogenesis in Apc(Delta716) mouse intestinal polyps. Cancer Res. 2002;62:506–511. [PubMed] [Google Scholar]
- 17.Sonoshita M., Takaku K., Sasaki N., Sugimoto Y., Ushikubi F., Narumiya S., Oshima M., Taketo M. M. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat. Med. 2001;7:1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- 18.Tsujii M., Kawano S., Tsuji S., Sawaoka H., Hori M., Dubois R. N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 19.van Rees B. P., Sivula A., Thorén S., Yokozaki H., Jakobsson P. J., Offerhaus G. J., Ristimäki A. Expression of microsomal prostaglandin E syntahse-1 is in intestinal type gastric adenocarcinoma and in gastric cancer cell lines. Int. J. Cancer. 2003;107:551–556. doi: 10.1002/ijc.11422. [DOI] [PubMed] [Google Scholar]
- 20.Wang D., Wang H., Shi Q., Katkuri S., Walhi W., Desvergne B., Das S. K., Dey S. K., Dubois R. N. Prostaglandin E (2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004;6:285–281. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Wang H. W., Hsueh C. T., Lin C. F., Chou T. Y., Hsu W. H., Wang L. S., Wu Y. C. Clinical implications of microsomal prostaglandin E synthase-1 overexpression in human non-small-cell lung cancer. Ann. Surg. Oncol. 2006;13:1224–1234. doi: 10.1245/s10434-006-9001-4. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimatsu K., Golijanin D., Paty P. B., Soslow R. A., Jakobsson P. J., DeLellis R. A., Subbaramaiah K., Dannenberg A. J. Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenomas and cancer. Clin. Cancer Res. 2001;7:3971–3976. [PubMed] [Google Scholar]