Abstract
Growth hormone (GH)-producing adenomas (GHomas) are one of the most frequently-occurring pituitary adenomas. Differentiation of hormone-producing cells in the pituitary gland is regulated by transcription factors and co-factors. The transcription factors include Pit-1, Prop-1, NeuroD1, Tpit, GATA-2, SF-1. Aberrant expression of transcription factors such as Pit-1 results in translineage expression of GH in adrenocorticotropic hormone-producing adenomas (ACTHomas). This situation has been substantiated by GFP-Pit-1 transfection expression in the AtT20 cell line. Experimentally, GHomas have been induced in GH-releasing hormone (GHRH) or Prop-1 transgenic animals. Immunohistochemical detection of somatostatin receptor (SSTR2a) has recently emphasized their role in the response of GHomas to somatostatin analogue therapy. In this review, the advances in technology and their contribution to cell biology and medical practice are discussed.
Keywords: pituitary adenoma, transcription factor, cell lineage, functional differentiation, pituitary hormones
I. Introduction
It is well known that the pituitary glands produce and secrete six different hormones: growth hormone (GH), prolactin (PRL), thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH) and luteinizing hormone (LH). While FSH and LH are produced in the same cells, GH, PRL, TSH and ACTH are generally produced in different cells. In some cell types, the coexpression of GH and PRL in the same cells has been detected by immunohistochemistry. The regulation of the genes encoding these hormones is known to be carried out by transcription factors and co-factors (Fig. 1, Fig. 2, Table 1). Immunohistochemistry has shown that GH-producing cells (GH cells) predominate in the pituitary glands both in humans and rodents. In humans, GH cells frequently also produce the alpha subunit of glycoprotein hormones, TSH, FSH/LH. GHomas are one of the most frequent adenomas treated by transsphenoidal approaches. They are frequently associated with acromegaly and other clinical complications. Interesting pathological characteristics of GHomas include (1) fibrous (keratin) bodies, (2) occasional concomitant production of ACTH, and (3) gangliocytoma (GHRH-GH production).
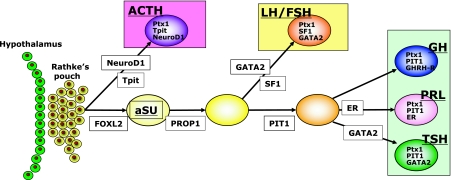
Fig. 1.
Schematic illustration of three cell lineages, GH-PRL-TSH, ACTH and FSH/LH, which are regulated by a combination of transcription factors and co-factors (rodents). GH, growth hormone; PRL, prolactin; FSH, follicle-stimulating hormone; LH, luteinizing hormone; TSH, thyroid-stimulating hormone; ACTH, adrenocorticotropic hormone (modified from Osamura et al.) [22].
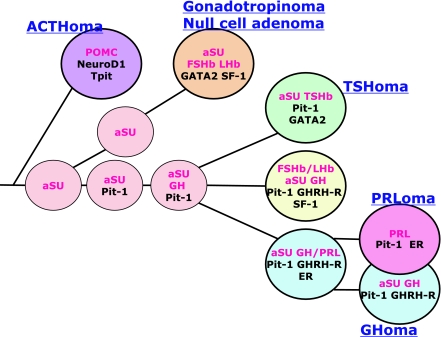
Fig. 2.
Schematic illustration of three cell lineages in human pituitary glands, GH-PRL-TSH, ACTH and FSH/LH, which are similarly defined by a combination of transcription factors and co-factors to those in rodents (Fig. 1). Note that ACTH is the first hormone to appear and that the human pituitary adenomas express the functional differentiation according to the normal process of development. It is also characteristic to see the co-localization of GH and αSU in the pituitary cells and in the adenomas (modified from Osamura et al.) [24].
Table 1.
Pituitary related transcription factors in development
| Transcription Factor | Related Hormone | Predicted Function |
|---|---|---|
| PTX1 | ACTH, LH/FSH/GH, PRL, TSH | Important for early pituitary development and later activation of hormone gene |
| PROP1 | GH, PRL, TSH, LH/FSH | Important for somatotrope, lactotrope, thyrotrope and gonadotrope development |
| FOXL2 | αSU, | Express in early pituitary and related to differentiation of αSU positive cells |
| EGR1 | LH/FSH | Important in gonadotrope and somatotrope development |
| NEUROD1 | ACTH | Important in POMC gene regulation in corticotropes |
| TPIT | ACTH | Important for POMC gene expression in the corticotrope and melanotrope |
| PIT1 | GH, PRL, TSH | Need for somatotrope, lactotrope and thyrotrope development |
| GATA2 | LH/FSH, TSH | Regulation of gonadotrope and thyrotrope cell differentiation |
| SF1 | LH/FSH | Required for gonadotrope development |
| ERα | PRL | Important in PRL and gonadotropin gene expression and lactotrope cell growth |
| GHRH-R | GH | Regulation of somatotrope differentiation |
Modified from Savage et al. 2003 [28].
This review will outline the role of GH cells and GHomas as models for the implementation of recent technical advances in the areas of pituitary pathology and research.
II. Morphology of GH Cells
In rodents and humans, GH cells are distributed in the lateral lobes of the pituitary and possess a characteristic round shape. They contain eosinophilic cytoplasm as demonstrated by H&E staining (Fig. 3A). Immunohistochemistry (IHC) shows that frequently exhibit dense cytoplasmic staining in the cytoplasm (Fig. 3B). Moreover, electron microscopy shows many large round electron dense secretory granules (SG) in these cells [21]. Nakane first showed that GH cells are in close contact with ACTH cells in the rat pituitary glands [17], and we have also observed a similar structural relationship in human pituitary glands (Fig. 3C). Matsuno et al. have reported, by double staining with IHC and non-radioisotopic in situ hybridization (ISH), that GH mRNA is localized in the polyribosomes on the rough endoplasmic reticulum (RER) and GH protein in the predominant SGs in the cytoplasm [13]. In contrast, PRL-expressing cells possess prominent RER and smaller numbers of SGs. It is now generally agreed that GH cells produce smaller amounts of GH and secrete very gradually, whereas PRL-positive cells can produce and secrete PRL very rapidly by turning-over the SG. General questions which arise from these observations include (1) how specific cells produce GH and other hormones and (2) how GH-expressing cells result in one of the most frequently occurring pituitary adenomas in humans. One of the characteristic features of the human pituitary glands is that many adult GH cells also contain α-subunit (SU) in the cytoplasm and in the same SG [19], and occasionally GH cells are also co-localized with FSHβSU. Therefore, it appears that human GH cells may contain factors required for the differentiation toward gonadotropin-producing cells. GH and PRL are also occasionally co-expressed in GH cells as mentioned earlier.
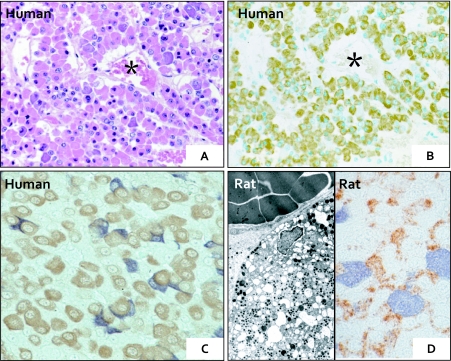
Fig. 3.
Demonstration of hormone-producing cells in the normal pituitary glands, human (A–C) and rat (D). A: H&E stained anterior lobe with many round hormone-producing cells. B: The same section as A. The perivascular eosinophilic cells (in A) are positively stained for GH. C: GH cells (brown) and ACTH cells (blue) are present in close proximity to one another. D: FSH/LH cells (brown) and PRL cells (blue) are present in tight conjunction (left). These hormone-producing cells contain many dense-cored secretory granules (right).
III. Production of GH by Specific Pituitary Cells: Transcriptional Mechanism for GH in Development and Differentiation
The mechanism of this differentiation toward GH was first suggested by the cloning of the pituitary-specific transcription factor-1 (Pit-1) by Adler et al. and Bodner and Karin [1, 2]. Pit-1 was shown to regulate the functional differentiation of GH-, PRL- and TSH-producing cells. This stimulated the cloning of many pituitary-related transcription factors, such as Prop-1, NeuroD1 [32] and Tpit [11]. Known pituitary-related transcription factors are shown in Table 1. These transcription factors mediate the differentiation of pituitary cells into one of three cell lineages: (1) the Pit-1 cell lineage (GH, PRL, TSH), (2) the SF-1 lineage (FSH/LH); and (3) the NeuroD1 lineage (ACTH, proopiomelanocortin (POMC)) [20]. αSU is one of the earliest hormones to play a role in the development of the pituitary (embryonic day 11) [34]. FOXL2 [7], and possibly Lhx3 play a role in regulating the expression of αSU in rodents.
As the expression profile of transcription factors in pituitary adenomas is generally similar to those in the normal anterior pituitary cells, GHomas, as will be described later, express Pit-1.
IV. Pathology of GH-producing Pituitary Adenomas
GH-producing adenomas are one of the most frequent human pituitary adenomas, and are commonly associated with acromegaly. Occasionally, however, they occur without clinical symptoms and are designated as “silent” GH cell adenomas. The 2004 World Health Organization (WHO) classification classifies them into densely granulated or sparsely granulated GHomas. Morphologically, the tumor cells are round and eosinophilic (Fig. 4A), and electron microscopy shows the presence of many electron-dense granules (Fig. 4D).
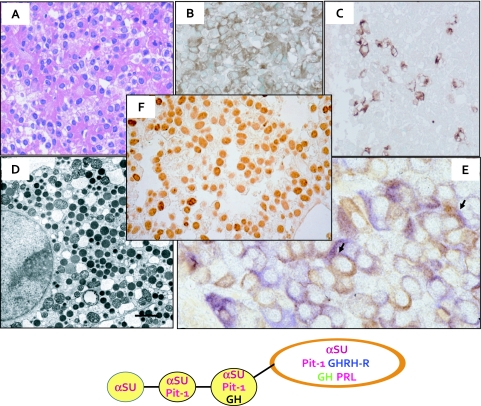
Fig. 4.
Demonstration of hormones and transcription factor Pit-1 in GH-producing pituitary adenoma (GHoma). A: H&E stained GHoma demonstrating many eosinophilic round tumor cells. These tumor cells are positive for GH (B), PRL (C). D: Many round dense secretory granules are observed in the cytoplasm. E: Double immunohistochemical staining indicates the GH+ tumors cells and αSU+ tumor cells. Some tumor cells demonstrate co-localization of GH and αSU (arrows). F: Many tumor cells show intense nuclear staining for Pit-1. The lower scheme indicates the proposed development of GH+αSU+cells.
Immunohistochemistry
Many GHomas exhibit diffuse GH staining in their cytoplasm (Fig. 4B). Approximately 60% of the cases show the concomitant localization of αSU and PRL (Fig. 4C, 4E), as shown by immunohistochemical double staining [21] (Fig. 4E). Many of the cases of sparsely granulated adenomas demonstrate fibrous bodies as demonstrated next.
Fibrous bodies
Immunohistochemistry of sparsely granulated GHomas frequently shows spherical aggregates (inclusions) of intermediate filaments and it has been demonstrated that these fibrous bodies are composed of various types of keratin [27]. Takei et al. reported that GHomas with fibrous bodies are frequently macroadenomas (diameter >1 cm) [29].
Gangliocytomas & metastatic GHRH-producing pancreatic neuroendocrine carcinoma
On rare occasions, microscopy of intrasellar tumors identifies a mixture of pituitary adenoma cells and ganglion cells. Immunohistochemically, these ganglion cells are positive for GHRH and the pituitary adenoma cells are positive for GH [9]. Some of the ganglion cells appear to be also positive for GH.
In some cases, GHRH-producing neuroendocrine tumors occur in the pancreas and bronchus. We have previously reported a case of GHRH-producing metastatic pancreatic neuroendocrine carcinoma (NEC) showing hyperplastic GH cell in an enlarged pituitary gland [26]. While similar cases have been reported in the literature, the high GHRH in these cases was caused by remote tumors in the lung and pancreas [5].
V. Transcription Factors and Functional Differentiation in GHomas
We, as well as other investigators, have reported that human GHomas express a combination of transcription factors similar to those in the non-neoplastic “normal” pituitary glands, which have been reported in rodents and human. It has been elucidated that human GHomas express Pit-1 by immunohistochemistry (IHC) and in situ hybridization (ISH) and other molecular methods such as RT-PCR or real time RT-PCR. GHRH-receptor (GHRH-R) and other co-factors have also been reported in GHomas. Pit-1 has been identified in PRLomas and TSHomas [15], and estrogen receptor (ER) and GATA-2 have been demonstrated in PRLomas and TSHomas, respectively. We also have reported that non-canonical Wnt4 signaling is related to the Pit-1 lineage in human adenomas, GHomas, PRLomas and TSHomas [16]. In the case of GHomas, our questions were as follows: (1) Does Pit-1 expression correlate with GH production? (2) What is the mechanism for the production of PRL in GHomas? (3) How are ACTH and GH expressed aberrantly in human adenomas?
Does the amount of Pit-1 expression correlate with GH production? Use of siRNA technology
IHC and in situ hybridization have been used to demonstrate that, GHomas express Pit-1, which in turn regulates PRL and TSH expression. Using siRNA technology, Miyai et al. showed that the amount of GH protein expression is correlated with the amount of Pit-1 protein in GHomas [14]. The other co-factors for Pit-1 include GHRH-R, the gene for which has a Pit-1 binding site in its promoter region. This co-factor is probably related with the more specific expression of GH. In the case of the TSH gene, the synergistic action between GATA-2 and Pit-1 has been demonstrated [33].
What is the mechanism for the production of PRL in GHomas? Use of Laser capture microdissection (LCM) technology
Laser capture microdissection (LCM) has been used to analyze the expression of ERα and ERβ in GHomas. GHoma cells which exhibited PRL expression by IHC showed ERα mRNA expression by RT-PCR, which may indicate the involvement of ERα in the expression of PRL in GHomas cells. It is also known that one of eight Pit-1 binding sites in the promoter region of PRL gene is in very close vicinity to an ER binding site [31].
How are trans-lineage ACTH and GH expressed in the same human adenomas? Aberrant transcription factors and pit-1 transfection with GFP technology
Aberrant expression transcription factors
Multiple hormone production usually occurs for hormones or hormone subunits in the same lineages: i.e. the Pit-1 lineage (GH, PRL, TSH), the SF-1 lineage (FSH/LH) and the NeuroD1/Tpit lineage (ACTH/POMC). Very rarely, GHomas produce ACTH or ACTHomas produce GH, and this phenomenon has been designated as “aberrant” translineage differentiation of the tumor cells.
GHomas with ACTH production
On rare occasions, patients with acromegaly induced by GHomas also show elevated serum ACTH levels. In this situation, the tumor cells show co-expression of Pit-1 and NeuroD1, which also constitutes “aberrant” expression of transcription factors resulting in the translineage differentiation of the cells, and is probably related to the neoplastic process. On the other hand, ACTHomas in patients with Cushing’s disease occasionally show GH production. This is also induced by the aberrant expression of Pit-1 in the NeuroD1-regulated tumor cells (Fig. 5). The latter condition was reproduced by transfection of Pit1 in the ACTH-producing cell line, AtT20 [10] as described below.
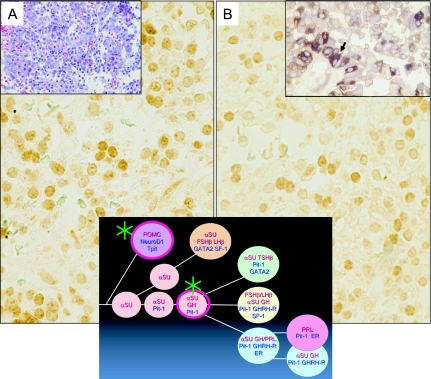
Fig. 5.
A case of pituitary adenoma in Cushing’s disease. Translineage expression of ACTH and GH. By H&E staining, many smaller and compact tumor cells are observed (Inset A). Many tumor cells show immunohistochemical co-localization of ACTH and GH (Inset B arrow). Immunohistochemically, many tumor cells show nuclear staining for NeuroD1 (A- for ACTH differentiation) and Pit-1 (B- for GH differentiation). Schematic drawing indicates the “translineage” differentiation of ACTH and GH in the pituitary adenoma (*).
Induction of GH mRNA or GH protein by transfection of Pit-1: Use of GFP technology in conjunction with laser capture microdissection technology
In order to reproduce the human condition of the aberrant expression of GH in ACTH adenomas, an in vitro experimental model was designed by transfecting a Pit1-GFP construct into the Pit-1-negative/GH negative AtT20 cell line [10]. AtT20 cells with nuclear GFP expression showed Pit-1 mRNA and GH mRNA by RT-PCR indicating that Pit-1 binding to DNA induced GH expression (Fig. 6).
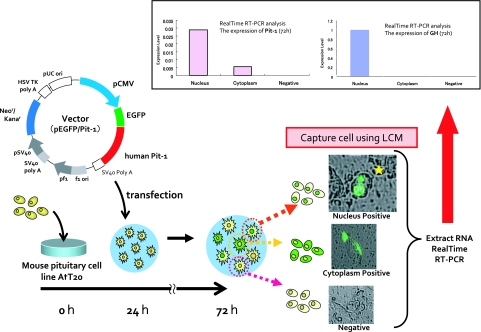
Fig. 6.
Experimental induction of GH by GFP-Pit-1 gene transfection in AtT20 cells. The cells with nuclear fluorescence were collected in “live state” by laser microdissection (PALM Carl Zeiss). Compared with those having cytoplasmic fluorescence, the cells with nuclear fluorescence (yellow star) demonstrated exclusive expression of Pit-1mRNA and GHmRNA by quantitative RT-PCR (upper panel).
By combining GFP and “live cell” laser capture microdissection (LCM) (PALM, Carl Zeiss) technologies, cells exhibiting GFP in the nuclei expressed higher amounts of GH mRNA than those with cytoplasmic GFP, as measured by real time RT-PCR. These experiments indicate that aberrant expression of additional transcription factors could induce translineage expression of hormones in functionally committed tumor cells. The molecular mechanism for the aberrant expression of transcription factors remains to be further investigated.
VI. Experimental GH-producing Adenomas
Experimental models for GHomas have been reported by transgenic animal models, one for GHRH and the other for Prop-1.
GHRH transgenic mice
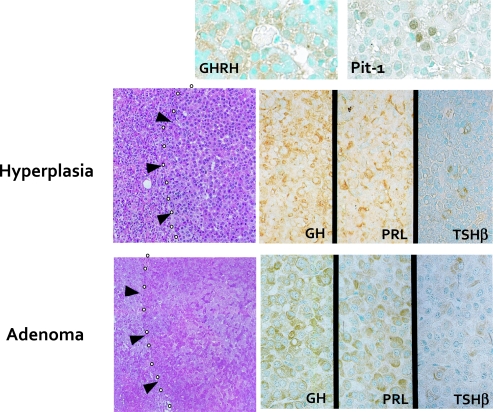
In human GHRH transgenic mice, the pituitaries were enlarged and histologically exhibited GH-producing adenomas and diffusely increased hyperplastic GH cells in the pituitary glands [23]. In particular, the tumors contained nodules which express only GH and PRL and not ACTH or FSH/LH (Fig. 7). Using a sensitive IHC method, Matsuno et al. have reported that 15 of 25 GHomas were positive for GHRH [12]. These findings indicate that at least some proportion of GHomas could overexpress GHRH, resulting in tumor formation.
Fig. 7.
Experimental model for GH-producing pituitary adenoma induced in the hGHRH transgenic mice. The pituitary tumor cells in hGHRH transgenic mice are immunohistochemically positive for GH, PRL and TSH. They are also positive for GHRH and Pit-1 [23].
Prop-1 transgenic mice
Camper et al. have reported the induction of pituitary tumors, TSH producing as well as non-functional adenomas [4]. In collaboration with Dr. Sally Camper (University of Michigan USA), we have shown that Prop-1 transgenic mice develop small adenomas in the pituitary glands, which were positive for GH, PRL or TSHβ [6]. LCM showed that Pit-1 was overexpressed in the adenoma, indicating that it may be related to the functional differentiation of the adenomas. The question as to why some adenomas were GH positive and other tumors were PRL- or TSH-positive still remains to be further investigated. The non-neoplastic pituitary cells were hyperplastic and expressed Prop-1 mRNA, even though the adenoma cells did not express Prop-1. The disappearance of Prop-1 mRNA may be attributable to the loss of αSU in the adenoma cells during the progress of tumorigenesis, since the construct was composed of Prop-1 and αSU.
VII. Medical Treatment of GHomas: Detection of Somatostatin Receptor (SSTRs)
GHomas are treated initially by transsphenoidal surgery. In the cases of macroadenomas and residual tumors, somatostatin analogs (SAs), octreotide or lanreotide, are administered [3]. For this treatment, immunohistochemical detection is typically performed in order to predict the response of treatment. The somatostatin receptor (SSTR) family is composed of five molecules: SSTR1, SSTR2a, SSTR3, SSTR4, and SSTR5. The presence of SSTR2a has been considered to be most important for the success of SA treatment [25]. Immunohistochemically, SSTR2a has been demonstrated on the cell membrane of the tumor cells (Fig. 8A–8C). Moreover, Takei et al. have reported that the association between an improved response to the preoperative SA (octreotide) and enhanced immunohistochemical SSTR2a positivity in patients with GHomas [30]. It has been also shown that SSTR2a mRNA can be detected in formalin fixed paraffin embedded (FFPE) sections [8].
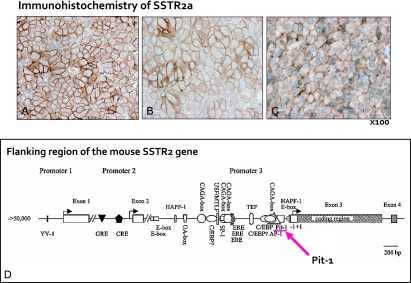
Fig. 8.
Immunohistochemical demonstration of somatostatin receptor2a (SSTR2a) in the GHoma (A), silent GHoma (B) and TSHoma (C). Note the distinct staining of SSTR2a on the cell membrane of many tumor cells. These tumor cells are expected to respond to somatostatin analogue treatment. The bottom scheme (D) demonstrates Pit-1 binding site in the promoter region of the mouse Sstr2 gene (modified from Olias G et al.) [18].
VIII. Conclusions
In this review, the pathology, transcriptional mechanism in the pituitary cells and pathogenesis of GH-producing adenomas (GHomas) were shown and discussed. It has also been emphasized that the recent development of technologies such as GFP-transfection, transgenic animals and laser capture microdissection has greatly contributed to the elucidation of the molecular mechanisms of the expression of particular genes. The treatment of GHomas with somatostatin analogues has been shown to be of benefit in tumors which display immunohistochemical positive staining for SSTR2A on the cell membrane. It is expected that histochemical techniques will contribute further to clarify the molecular biology and pathology of GHomas, as well as to stimulate therapeutic approaches.
IX. Acknowledgments
Portions of this article were presented at the 49th Annual meeting of the Japan Society of Histochemistry and Cytochemistry, in Nagasaki, as the Takamatsu Award Lecture. The authors wish to thank the members of JSHC for giving us this opportunity to publish this review in memory of Dr. Hideo Takamatsu. The authors also wish to thank Dr. Sally Camper, University of Michigan, for scientific collaboration.
Footnotes
This paper was presented as Takamatsu Prize Lecture in 49th Annual Meeting of Japan Society of Histochemistry and Cytochemistry (Nagasaki 2008).
X. References
- 1.Adler S., Waterman M. L., He X., Rosenfeld M. G. Steroid receptor-mediated inhibition of rat prolactin gene expression does not require the receptor DNA-binding domain. Cell. 1988;52:685–695. doi: 10.1016/0092-8674(88)90406-0. [DOI] [PubMed] [Google Scholar]
- 2.Bodner M., Karin M. A pituitary-specific trans-acting factor can stimulate transcription from the growth hormone promoter in extracts of nonexpressing cells. Cell. 1987;50:267–275. doi: 10.1016/0092-8674(87)90222-4. [DOI] [PubMed] [Google Scholar]
- 3.Bronstein M. D. Acromegaly: molecular expression of somatostatin receptor subtypes and treatment outcome. Front. Horm. Res. 2006;35:129–134. doi: 10.1159/000094315. [DOI] [PubMed] [Google Scholar]
- 4.Cushman L. J., Watkins-Chow D. E., Brinkmeier M. L., Raetzman L. T., Radak A. L., Lloyd R. V., Camper S. A. Persistent Prop1 expression delays gonadotrope differentiation and enhances pituitary tumor susceptibility. Hum. Mol. Genet. 2001;10:1141–1153. doi: 10.1093/hmg/10.11.1141. [DOI] [PubMed] [Google Scholar]
- 5.de Jager C. M., de Heide L. J., van den Berg G., Wolthuis A., van Schelven W. D. Acromegaly caused by a growth hormone-releasing hormone secreting carcinoid tumour of the lung: the effect of octreotide treatment. Neth. J. Med. 2007;65:263–266. [PubMed] [Google Scholar]
- 6.Egashira N., Minematsu T., Miyai S., Takekoshi S., Camper S. A., Osamura R. Y. Pituitary changes in Prop1 transgenic mice: hormone producing tumors and signet-ring type gonadotropes. Acta Histochem. Cytochem. 2008;41:47–57. doi: 10.1267/ahc.08007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellsworth B. S., Egashira N., Haller J. L., Butts D. L., Cocquet J., Clay C. M., Osamura R. Y., Camper S. A. FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Mol. Endocrinol. 2006;20:2796–2805. doi: 10.1210/me.2005-0303. [DOI] [PubMed] [Google Scholar]
- 8.Kajiya H., Serizawa A., Itoh H., Minematsu T., Takekoshi S., Osamura R. Y. Morphology-based molecular diagnosis for targeted cancer therapy using formalin-fixed paraffin embedded (FFPE) sections. Mod. Pathol. 2007;20:351A. [Google Scholar]
- 9.Kurosaki M., Saeger W., Ludecke D. K. Intrasellar gangliocytomas associated with acromegaly. Brain Tumor Pathol. 2002;19:63–67. doi: 10.1007/BF02478929. [DOI] [PubMed] [Google Scholar]
- 10.Kurotani R., Yoshimura S., Iwasaki Y., Inoue K., Teramoto A., Osamura R. Y. Exogenous expression of Pit-1 in AtT-20 corticotropic cells induces endogenous growth hormone gene transcription. J. Endocrinol. 2002;172:477–487. doi: 10.1677/joe.0.1720477. [DOI] [PubMed] [Google Scholar]
- 11.Lamolet B., Pulichino A. M., Lamonerie T., Gauthier Y., Brue T., Enjalbert A., Drouin J. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104:849–859. doi: 10.1016/s0092-8674(01)00282-3. [DOI] [PubMed] [Google Scholar]
- 12.Matsuno A., Katakami H., Nagashima T., Teramoto A., Osamura R. Y., Kirino T. Growth hormone-releasing hormone expression in pituitary somatotroph adenomas, studied by immunohistochemistry and in situ hybridization using catalyzed signal amplification system. Hum. Pathol. 2000;31:789–794. doi: 10.1053/hupa.2000.8450. [DOI] [PubMed] [Google Scholar]
- 13.Matsuno A., Mizutani A., Takekoshi S., Itoh J., Okinaga H., Nishina Y., Takano K., Nagashima T., Osamura R. Y., Teramoto A. Analyses of the mechanism of intracellular transport and secretion of pituitary hormone, with an insight of the subcellular localization of pituitary hormone and its mRNA. Brain Tumor Pathol. 2006;23:1–5. doi: 10.1007/s10014-005-0189-y. [DOI] [PubMed] [Google Scholar]
- 14.Miyai S., Itoh J., Kajiya H., Takekoshi S., Osamura R. Y. Pit-1 gene inhibition using small interfering RNAs in rat pituitary GH secreting cell line. Acta Histochem. Cytochem. 2005;38:107–114. [Google Scholar]
- 15.Miyai S., Yoshimura S., Iwasaki Y., Takekoshi S., Lloyd R. V., Osamura R. Y. Induction of GH, PRL, and TSH beta mRNA by transfection of Pit-1 in a human pituitary adenoma-derived cell line. Cell Tissue Res. 2005;322:269–277. doi: 10.1007/s00441-005-0033-z. [DOI] [PubMed] [Google Scholar]
- 16.Miyakoshi T., Takei M., Kajiya H., Egashira N., Takekoshi S., Teramoto A., Osamura R. Y. Expression of Wnt4 in human pituitary adenomas regulates activation of the beta-catenin-independent pathway. Endocr. Pathol. 2008;19:261–273. doi: 10.1007/s12022-008-9048-9. [DOI] [PubMed] [Google Scholar]
- 17.Nakane P. K. Classifications of anterior pituitary cell types with immunoenzyme histochemistry. J. Histochem. Cytochem. 1970;18:9–20. doi: 10.1177/18.1.9. [DOI] [PubMed] [Google Scholar]
- 18.Olias G., Viollet C., Kusserow H., Epelbaum J., Meyerhof W. Regulation and function of somatostatin receptors. J. Neurochem. 2004;89:1057–1091. doi: 10.1111/j.1471-4159.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- 19.Osamura R. Y. Immunoelectron microscopic studies of GH and alpha subunit in GH secreting pituitary adenomas. Pathol. Res. Pract. 1988;183:569–571. doi: 10.1016/s0344-0338(88)80012-8. [DOI] [PubMed] [Google Scholar]
- 20.Osamura R. Y., Egashira N., Miyai S., Yamazaki M., Takekoshi S., Sanno N., Teramoto A. Molecular pathology of the pituitary. Development and functional differentiation of pituitary adenomas. Front. Horm. Res. 2004;32:20–33. doi: 10.1159/000079036. [DOI] [PubMed] [Google Scholar]
- 21.Osamura R. Y., Kajiya H., Takei M., Egashira N., Tobita M., Takekoshi S., Teramoto A. Pathology of the human pituitary adenomas. Histochem. Cell Biol. 2008;130:495–507. doi: 10.1007/s00418-008-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osamura R. Y., Miyai S., Egashira N., Takekoshi S., Yamazaki M., Sanno N., Teramoto A. Application of genetic engineering technologies for the study of pituitary development and neoplasms. Acta Histochem. Cytochem. 2003;36:249–254. [Google Scholar]
- 23.Osamura R. Y., Oda K., Utsunomiya H., Inada K., Umemura S., Shibuya M., Katakami H., Voss J. W., Mayo K. E., Rosenfeld M. G. Immunohistochemical expression of PIT-1 protein in pituitary glands of human GRF transgenic mice: its relationship with hormonal expressions. Endocr. J. 1993;40:133–139. doi: 10.1507/endocrj.40.133. [DOI] [PubMed] [Google Scholar]
- 24.Osamura R. Y., Tahara S., Komatsubara K., Itoh Y., Kajiwara H., Kurotani R., Sanno N., Teramoto A. Pit-1 positive alpha-subunit positive nonfunctioning human pituitary adenomas: a dedifferentiated GH cell lineage? Pituitary. 1999;1:269–271. doi: 10.1023/a:1009954409469. [DOI] [PubMed] [Google Scholar]
- 25.Ren S. G., Taylor J., Dong J., Yu R., Culler M. D., Melmed S. Functional association of somatostatin receptor subtypes 2 and 5 in inhibiting human growth hormone secretion. J. Clin. Endocrinol. Metab. 2003;88:4239–4245. doi: 10.1210/jc.2003-030303. [DOI] [PubMed] [Google Scholar]
- 26.Sanno N., Teramoto A., Osamura R. Y., Genka S., Katakami H., Jin L., Lloyd R. V., Kovacs K. A growth hormone-releasing hormone-producing pancreatic islet cell tumor metastasized to the pituitary is associated with pituitary somatotroph hyperplasia and acromegaly. J. Clin. Endocrinol. Metab. 1997;82:2731–2737. doi: 10.1210/jcem.82.8.4175. [DOI] [PubMed] [Google Scholar]
- 27.Sano T., Yamada S., Hirose T., Hizawa K. Cytokerayin distribution and functional properties of growth hormone-producing pituitary adenomas. Endocr. Pathol. 1994;5:107–113. doi: 10.1007/BF02921378. [DOI] [PubMed] [Google Scholar]
- 28.Savage J. J., Yaden B. C., Kiratipranon P., Rhodes S. J. Transcriptional control during mammalian anterior pituitary development. Gene. 2003;319:1–19. doi: 10.1016/s0378-1119(03)00804-7. [DOI] [PubMed] [Google Scholar]
- 29.Takei M., Kajiya H., Egashira N., Takekoshi S., Tahara S., Teramoto A., Osamura R. Y. Clinical and morphological characteristics of functioning pituitary microadenomas and macroadenomas: analysis of large series. Mod. Pathol. 2007;20:104A. [Google Scholar]
- 30.Takei M., Suzuki M., Kajiya H., Ishii Y., Tahara S., Miyakoshi T., Egashira N., Takekoshi S., Sanno N., Teramoto A., Osamura R. Y. Immunohistochemical detection of somatostatin receptor (SSTR) subtypes 2A and 5 in pituitary adenoma from acromegalic patients: good correlation with preoperative response to octreotide. Endocr. Pathol. 2007;18:208–216. doi: 10.1007/s12022-007-9004-0. [DOI] [PubMed] [Google Scholar]
- 31.Takekoshi S., Yasuda M., Itoh Y., Umemura S., Osamura R. Y. Usefulness of laser capture microdissection for analysis of nucleic acids. Acta Histochem. Cytochem. 2002;35:135. [Google Scholar]
- 32.Therrien M., Drouin J. Cell-specific helix-loop-helix factor required for pituitary expression of the pro-opiomelanocortin gene. Mol. Cell Biol. 1993;13:2342–2353. doi: 10.1128/mcb.13.4.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umeoka K., Sanno N., Osamura R. Y., Teramoto A. Expression of GATA-2 in human pituitary adenomas. Mod. Pathol. 2002;15:11–17. doi: 10.1038/modpathol.3880484. [DOI] [PubMed] [Google Scholar]
- 34.Voss J. W., Rosenfeld M. G. Anterior pituitary development: short tales from dwarf mice. Cell. 1992;70:527–530. doi: 10.1016/0092-8674(92)90422-9. [DOI] [PubMed] [Google Scholar]