Abstract
Rationale: Debate exists about the immunogenicity and protective efficacy of antibodies produced by the 23-valent pneumococcal polysaccharide vaccine (PPSV23) in chronic obstructive pulmonary disease (COPD). The 7-valent diphtheria-conjugated pneumococcal polysaccharide vaccine (PCV7) induces a more robust immune response than PPSV23 in healthy elderly adults.
Objectives: We hypothesized that serotype-specific IgG antibody concentration and functional antibody activity would be superior after PCV7 vaccination compared with PPSV23 in moderate to severe COPD. We also posited that older age and prior PPSV23 vaccination would be associated with reduced vaccine responsiveness.
Methods: One hundred twenty patients with COPD were randomized to PPSV23 (63 subjects) or PCV7 (57 subjects). IgG concentrations were determined by ELISA; functional antibody activity was assayed with a standardized opsonophagocytosis assay and reported as an opsonization killing index (OPK). Increases in serotype-specific IgG and OPK at 1 month post vaccination were compared within and between vaccine groups.
Measurements and Main Results: Both vaccines were well tolerated. Within each study group, postvaccination IgG and OPK were higher than baseline (P < 0.01) for all serotypes. Adjusted for baseline levels, postvaccination IgG was higher in the PCV7 group than the PPSV23 group for all seven serotypes, reaching statistical significance for five (P < 0.05). PCV7 resulted in a higher OPK for six of seven serotypes (statistically greater for four) compared with PPSV23. In multivariate analyses, younger age, vaccine naivety, and receipt of PCV7 were associated with increased OPK responses.
Conclusions: PCV7 induces a superior immune response at 1 month post vaccination compared with PPSV23 in COPD. Older age and prior PPSV23 reduce vaccine responsiveness.
Clinical trial registered with www.clinicaltrials.gov (NCT00457977).
Keywords: pneumococcal vaccines; vaccination, COPD; immune responses; immunization
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Debate remains about the protective efficacy of antibodies produced in response to the 23-valent free pneumococcal polysaccharide vaccine (PPSV23) in chronic obstructive pulmonary disease (COPD) and no study has accurately examined its immunogenicity in this population. Preliminary data suggest that a protein-conjugate pneumococcal vaccine (PCV7) may elicit superior immune responses to PPSV23 in healthy elderly patients.
What This Study Adds to the Field
PCV7 induces a superior immune response to PPSV23 in COPD at 1 month post vaccination. Both vaccines elicit responses comparable to those previously observed in healthy elderly patients.
Streptococcus pneumoniae is a major cause of morbidity and mortality among older adults and disproportionately affects patients with comorbid illnesses, including chronic obstructive pulmonary disease (COPD) (1). It is estimated to cause 500,000 cases of pneumonia and 40,000 deaths each year and frequently causes COPD exacerbations (1, 2). Consequently, the Centers for Disease Control and Prevention recommend that the 23-valent pneumococcal polysaccharide vaccine (PPSV23) be administered to all patients with COPD (1). The Centers for Disease Control and Prevention Advisory Committee on Immunization Practices has also recently recommended that PPSV23 vaccination be extended to all adult smokers (3). PPSV23 contains the capsular polysaccharide antigens from the 23 most pathogenic pneumococcal serotypes that are responsible for 90% of all invasive infections in adults (4). Reasonable effectiveness for this vaccine has been demonstrated in cohort studies in adults with lung disease (5, 6). Despite evidence that antibodies produced in response to PPSV23 can protect against invasive disease in healthy adults, debate remains about its immunogenicity and effectiveness in COPD.
Four randomized, placebo-controlled trials of pneumococcal polysaccharide vaccination (PPSV) in COPD have failed to show a significant reduction in mortality, hospitalization, or pneumonia in the intention-to-treat population, although these trials were likely underpowered to detect a vaccine effect (7–10). Although prior studies have suggested that patients with COPD can mount an immune response when challenged with PPSV, interpretation of these results is limited as antibody levels were measured with a nonspecific ELISA (11). These early-generation ELISAs routinely measured nonspecific, nonfunctional antibodies to the pneumococcal capsule and cell wall polysaccharide, which overestimated both baseline and postvaccination levels. The assay has since been modified and standardized but requires that the samples be preabsorbed with both cell wall polysaccharide and a pneumococcal capsule other than the one being tested to remove these nonspecific antibodies. Prior studies were also limited by the failure to determine functional antibody activity. Although antibody levels are believed to correlate reasonably well with protective efficacy, data from both animal and human studies suggest that measures of antibody function are better surrogate markers of immunity (11). The primary method by which S. pneumoniae is killed in vivo is by antibody coating, activation of complement, phagocytosis, and cell lysis. This opsonophagocytosis activity can now be assayed and is the method of choice for measuring vaccine immunogenicity.
There has been increasing interest in the use of protein-conjugate vaccines to augment the immunogenicity of polysaccharide antigens (12). Conjugated vaccines were originally intended for young children who respond poorly to polysaccharide antigens. The 7-valent diphtheria-conjugated pneumococcal polysaccharide vaccine (PCV7) (Prevnar; Wyeth, Pearl River, NY) induces a potent immune response in children and reduces the nasopharyngeal carriage of vaccine serotypes, episodes of otitis, and the frequency of invasive disease (13). Although this vaccine is not currently licensed for use in adults, preliminary studies in healthy patients older than age 70 years have shown that PCV7 induces greater functional antibody activity at 1 month post vaccination than does PPSV23, although this response is reduced in those who have been previously vaccinated (14, 15). Jackson and colleagues demonstrated that in these healthy patients the 1.0-ml dose of PCV7 induced a greater immune response than the pediatric 0.5-ml dose. No additional benefit was observed with a 2.0-ml dose (15).
This study was conducted with two hypotheses: (1) the immunogenicity of PCV7 (1.0 ml) vaccination would be superior to that of PPSV23 in patients with moderate to severe COPD, and (2) prior PPSV23 vaccination and older age would reduce vaccine responsiveness.
METHODS
Study Design
This study was a randomized, open-label trial that compared the safety and immunogenicity of PCV7 (1.0 ml) to PPSV23 vaccination in 120 subjects with moderate to severe COPD. The study was conducted by the 10 centers participating in the National Heart, Lung and Blood Institute's COPD Clinical Research Network (CCRN). Randomization was performed after linking to the CCRN coordinating center website and stratified by study center. The study was approved by the CCRN Protocol Review Committee, each of the participating center's Institutional Review Boards, and by the Food and Drug Administration under an Investigational New Drug approval. The study was registered on line as a clinical trial. For full methods, including expanded exclusion criteria, vaccine administration, and safety monitoring, please see the online supplement.
Study Population
Subjects were men and women older than 40 years of age with a 10 or more pack-year cigarette smoking history with a clinical diagnosis of moderate to very severe COPD (as defined by postbronchodilator FEV1/FVC <70% and FEV1 <70% predicted). Subjects were eligible if they had never received PPSV23 or if it was administered more than 5 years before randomization. Exclusion criteria included a diagnosis of asthma, sensitivity to pneumococcal vaccination, bleeding disorder, chronic anticoagulation, or the presence of conditions known to impair pneumococcal vaccine response. Subjects taking oral corticosteroids were not excluded from the trial. Those suffering an acute illness requiring antibiotics or steroids within the past month or not expected to survive 12 months were also excluded.
Serologic Testing
Blood specimens were obtained immediately before and 1 month after vaccination. The capacity of each serum to opsonize S. pneumoniae for ingestion and killing by phagocytes was determined by incubating bacteria in serum and then exposing them in vitro to HL-60 cells (16). Results are reported as an opsonophagocytosis killing index (OPK), which represents the reciprocal of the serum dilution that led to 50% uptake and killing of pneumococci during incubation at 37°C for 1 hour. Total IgG antibody concentrations to the seven PCV7 serotypes were also measured using a WHO-recommended ELISA protocol (www.vaccine.uab.edu).
Statistics
Antibody levels (IgG) and OPK were transformed using natural logarithms for statistical analysis to account for their strongly skewed distributions and are reported as geometric means. A paired t test was used to assess the increase in serotype-specific IgG and OPK from pre- to postvaccination within study groups. An unpaired t test was used for between-group comparisons of postvaccination IgG and OPK. To correct for differences in prevaccination IgG and OPK, we also compared the ratios of 1 month to baseline IgG and OPK between vaccine groups. We performed univariate and multivariate linear regression to determine the relationship between age, sex, vaccine assignment, lung function impairment (FEV1 % predicted), and prior vaccination status with vaccine responsiveness as measured by the number of serotypes to which a subject exhibited a 10-fold increase in OPK or a twofold increase in IgG (17). The proportion of subjects reporting systemic or local adverse reactions during the 7-day diary were compared using Fisher exact test. P values less than 0.05 were considered significant. No adjustments were made for multiple comparisons.
RESULTS
Table 1 shows the demographic characteristics, comorbid illnesses, and lung function of the 120 subjects randomized between April 9, 2007 and May 5, 2008. Forty-four additional subjects signed informed consent and were screened for the study but were excluded primarily because they did not meet spirometric inclusion criteria. Subjects had significant airflow obstruction and were predominantly male and white, although a significant proportion of women and minorities were enrolled compared with prior COPD vaccination studies (7–9). Almost half the subjects had prior episodes of pneumonia and a similar proportion had never received the pneumococcal vaccine. Approximately one-quarter of the subjects had coronary artery disease, but the burden of other comorbid illness was relatively low in both groups. Subjects who had not been previously vaccinated with PPSV23 were younger than those who had received the vaccine (60 vs. 67 yr, P < 0.001).
TABLE 1.
PATIENT CHARACTERISTICS
| PCV7 (n = 57) | PPSV23 (n = 63) | P Value | ||||
|---|---|---|---|---|---|---|
| Age, years | 63 ± 10 | 63 ± 10 | 0.95 | |||
| Male, % | 38 (67) | 37 (59) | 0.45 | |||
| White, % | 43 (75) | 48 (76) | 1.0 | |||
| FEV1, L | 1.40 ± 0.58 | 1.23 ± 0.54 | 0.12 | |||
| FEV1, % predicted | 45 ± 16 | 43 ± 15 | 0.56 | |||
| FEV1/FVC | 0.47± 0.13 | 0.44 ± 0.13 | 0.16 | |||
| Oxygen use, % | 12 (21) | 21 (33) | 0.15 | |||
| Inhaled corticosteroid use, % | 36 (63) | 43 (68) | 0.57 | |||
| Pack-years smoking | 54 ± 32 | 55 ± 34 | 0.82 | |||
| Comorbid illness, % | ||||||
| Coronary artery disease | 16 (28) | 15 (24) | 0.68 | |||
| Congestive heart failure | 3 (5) | 2 (3) | 0.67 | |||
| Stroke | 2 (4) | 6 (9) | 0.28 | |||
| Diabetes mellitus | 5 (9) | 3 (5) | 0.48 | |||
| Prior malignancy | 7 (12) | 8 (13) | 1.00 | |||
| Anemia | 7 (12) | 4 (6) | 0.35 | |||
| Previous pneumonia, % | 26 (46) | 30 (48) | 0.86 | |||
| Exacerbation history (yr before enrollment) | ||||||
| Hospitalized or unscheduled emergency visit, % | 10 (18) | 9 (14) | 0.80 | |||
| Received systemic steroids and/or antibiotics, % | 26 (46) | 26 (41) | 0.71 | |||
| Vaccine naive, % | 30 (53) | 33 (52) | 1.00 | |||
| Years since last vaccination | 7.9 | 8.4 | 0.56 | |||
Definition of abbreviations: PCV7 = 7-valent diphtheria-conjugated pneumococcal polysaccharide vaccine; PPSV23 = 23-valent pneumococcal polysaccharide vaccine.
Both vaccines were well tolerated with only three episodes of grade III toxicity (Table 2). Local and systemic side effects were more common in the PPSV23 group. There was no increased rate of adverse events in subjects who had been previously vaccinated with PPSV23 compared with those who were vaccine naive (data not shown). No subjects required unscheduled visits within the first 7 days after vaccination and only one patient had a persistent complaint at 1 month (muscle soreness). One patient died within 30 days of vaccination due to cardiovascular disease but had not reported any adverse events in their diary or during the Day 7 phone call.
TABLE 2.
SOLICITED ADVERSE EVENTS FROM 7-DAY DIARY
| PCV7 (n = 57)(%) | PPSV23 (n = 63)(%) | P Value | |
|---|---|---|---|
| Fatigue | 0.18 | ||
| Grade I | 22 (39) | 33 (52) | |
| Grade II | 1 (2) | 6 (10) | |
| Muscle aches | 0.74 | ||
| Grade I | 29 (51) | 37 (59) | |
| Grade II | 4 (7) | 5 (8) | |
| Headache | 0.53 | ||
| Grade I | 8 (14) | 12 (19) | |
| Grade II | 0 (0) | 1 (2) | |
| Itching of vaccinated arm | 0.09 | ||
| Grade I | 5 (9) | 13 (21) | |
| Grade II | 0 (0) | 0 (0) | |
| Pain of vaccinated arm | 0.74 | ||
| Grade I | 29 (51) | 35 (56) | |
| Grade II | 2 (4) | 4 (6) | |
| Fever | 0.54 | ||
| Grade I | 3 (5) | 3 (5) | |
| Grade II | 1 (2) | 0 (0) | |
| Limitation of arm movement | 0.35 | ||
| Grade I | 16 (28) | 23 (36) | |
| Grade II | 2 (4) | 6 (10) | |
| Redness or discoloration | 0.06 | ||
| Grade I (≤8 cm) | 9 (16) | 19 (30) | |
| Grade II (>8 cm and ≤15 cm) | 1 (2) | 8 (13) | |
| Grade III (>15 cm) | 0 (0) | 2 (3) | |
| Localized swelling | 0.59 | ||
| Grade I (≤8 cm) | 15 (26) | 18 (29) | |
| Grade II (>8 cm and ≤5 cm) | 0 (0) | 5 (8) | |
| Increase in arm circumference from baseline | 0.74 | ||
| Grade I (<2 cm) | 11 (19) | 14 (22) | |
| Grade II (≥2 cm and <3 cm) | 3 (5) | 3 (5) | |
| Grade III (≥3 cm) | 1 (2) | 0 (0) |
Table 3 shows baseline and 1 month postvaccination IgG antibody levels. Five samples could not be analyzed due to the following: patient death (1), loss to follow-up (1), inability to draw blood (2), and quality control issues for one of the two blood samples (1). Within each study group, postvaccination IgG levels were higher than baseline (P < 0.01) for all serotypes. Absolute IgG levels were higher for those receiving PCV7 compared with PPSV23 for all seven serotypes, reaching statistical significance for four (P < 0.05, shown in bold in Table 3). One month to baseline antibody concentration ratios were also higher in the PCV7 treatment arm for all seven serotypes tested and statistically significant for all serotypes except 14 and 19F (P < 0.05, shown underlined in Table 3). The fraction of subjects exhibiting a twofold increase in serotype-specific IgG antibody was also higher in the PCV7 group for five of the seven serotypes tested: 4 (85 vs. 39%, P < 0.001), 6B (57 vs. 36%, P = 0.03), 9V (74 vs. 55%, P = 0.05), 18C (72 vs. 55%, P = 0.08), 23F (81 vs. 52%, P = 0.001). There was no difference in the fraction of subjects achieving this twofold increase for serotypes 14 (59 vs. 65%, P = 0.57) and 19F (38 vs. 39%, P = 1.0).
TABLE 3.
BASELINE AND 1-MONTH SEROTYPE-SPECIFIC GEOMETRIC MEAN IgG ANTIBODY LEVELS
| Baseline IgG μg/ml (95% CI)
|
1-Month IgG μg/ml (95% CI)
|
|||
|---|---|---|---|---|
| Serotype | PCV7 | PPSV23 | PCV7 | PPSV23 |
| 4 | 0.26 (0.17–0.38) | 0.32 (0.23–0.44) | 2.03 (1.24–3.32) | 0.70 (0.47–1.03) |
| 6B | 0.90 (0.65–1.25) | 0.85 (0.67–1.08) | 2.96 (1.96–4.48) | 1.76 (1.23–2.52) |
| 9V | 0.86 (0.61–1.20) | 0.76 (0.55–1.06) | 4.69 (3.27–6.74) | 2.09 (1.47–2.97) |
| 14 | 2.88 (1.86–4.46) | 2.90 (1.96–4.27) | 17.2 (11.7–25.3) | 11.22 (7.42–16.96) |
| 18C | 1.26 (0.84–1.89) | 1.17 (0.83–1.64) | 9.26 (6.29–13.6) | 3.70 (2.58–5.29) |
| 19F | 3.20 (2.43–4.21) | 3.16 (2.55–3.93) | 7.33 (5.16–10.4) | 6.20 (4.75–8.09) |
| 23F | 0.75 (0.52–1.07) | 0.55 (0.40– 0.76) | 7.31 (4.56–11.7) | 1.52 (0.99–2.32) |
Definition of abbreviations: OPK = opsonophagocytosis killing index; PCV7 = 7-valent diphtheria-conjugated pneumococcal polysaccharide vaccine; PPSV23 = 23-valent pneumococcal polysaccharide vaccine.
Within each study group, postvaccination antibody levels and OPK were higher than baseline (P < 0.01) for all serotypes. Bolded items represent a significant difference in absolute postvaccination IgG levels between vaccine groups and underlined items represent a significant difference in 1 month to baseline IgG ratio.
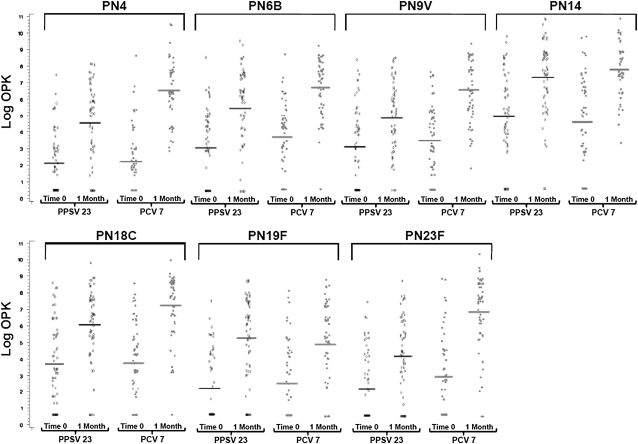
Figure 1 shows the baseline and 1 month post vaccination OPK. Similar to the ELISA results, within both study groups postvaccination OPKs were higher than baseline for all serotypes (P < 0.001). Absolute OPKs were higher in the PCV7 group for six of seven serotypes, reaching statistical significance in five. OPK ratios from 1 month to baseline were also greater in the PCV7 group than in the PPSV23 group for six of the seven serotypes tested, reaching statistical significance for serotypes 4, 9V, 18C, and 23F.
Figure 1.
The serotype-specific baseline and 1-month opsonophagocytosis killing index (OPK) are shown for each patient. The 7-valent diphtheria-conjugated pneumococcal polysaccharide vaccine (PCV7) resulted in statistically significantly higher 1 month to baseline OPK ratios for serotypes 4 (75.5 [95% confidence interval, 43.9–130] vs. 10.3 [6.1–17.6]; P < 0.001), 9V (18.7 [10.3–34.0] vs. 5.7 [3.6–9.2]; P = 0.003), 18C (31.3 [16.2–60.4] vs. 9.5 [5.7–16.1]; P = 0.006), 23F (52.4 [26.2–104] vs. 7.2 [4.1–12.7]; P < 0.001). PCV7 vaccination elicited a nonstatistically significant but superior response for serotypes 6B (20.8 [11.3–38.1] vs. 11.2 [6.3–19.88]; P = 0.09) and 14 (22.8 [9.9–52.2] vs. 10.7 [5.8–19.8]; P = 0.24), whereas 23-valent pneumococcal polysaccharide vaccine (PPSV23) elicited a similar improved response for serotype 19F (20.65 [11.05–38.61] vs. 10.6 [5.5–20.4]; P = 0.20). Horizontal bars indicate geometric mean OPK.
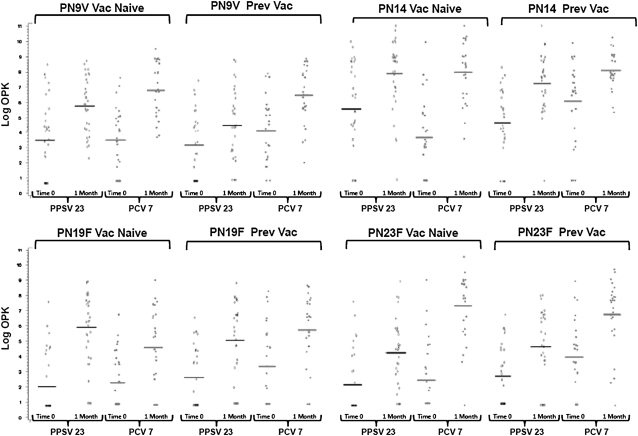
We also separately compared vaccine responses in subjects who were vaccine naive and in those who had been previously vaccinated. Among vaccine-naive subjects, PCV7 elicited a greater 1 month to baseline OPK ratio than PPSV23 for all serotypes (reaching significance in five) except for serotype 19F, for which PPSV23 generated a statistically superior response. Among those previously vaccinated, PCV7 again elicited a superior OPK ratio for all serotypes (reaching significance in four) except for serotype 14, for which PPSV23 elicited a nonsignificantly higher response. Figure 2 highlights the immune responses based on prior vaccination status and vaccine assignment for the four most frequently isolated pneumococcal serotypes in COPD that are also components of PCV7.
Figure 2.
Shown are the serotype-specific baseline and 1-month opsonophagocytosis killing index (OPK) for the four most frequently recovered pneumococcal serotypes in patients with chronic obstructive pulmonary disease (27) divided by prior vaccination status and vaccine assignment. The 7-valent diphtheria-conjugated pneumococcal polysaccharide vaccine (PCV7) elicited statistically superior 1 month to baseline OPK ratios compared with PPSV23 in vaccine-naive subjects for serotypes 9V (P = 0.04), 14 (P = 0.006), and 23F (P < 0.001). The 23-valent pneumococcal polysaccharide vaccine (PPSV23) elicited a superior response for serotype 19F (P = 0.05). Among previously vaccinated subjects, PCV7 elicited superior OPK ratios for serotypes 9V (P = 0.01) and 23F (P = 0.05). There was no statistical difference in OPK responses for serotype 14 (P = 0.22) and 19F (P = 0.94). Horizontal bars indicate geometric mean OPK.
The associations between age, sex, FEV1 % predicted, prior vaccination status, vaccine assignment, and OPK response are shown in Table 4. In total, 42% of subjects exhibited a 10-fold increase in OPK to five or more serotypes. In both univariate and multivariate models younger age, vaccine naivety, and receipt of PCV7 were associated with more frequent 10-fold increases in serotype-specific OPK. There was no significant interaction between age and vaccine assignment (P = 0.64) or between age and prior vaccination status (P = 0.36). In a similar analysis of IgG response, younger age and PCV7 vaccination were associated with twofold increases in antibody levels (model not shown). In univariate analyses, we found no association between OPK responses and patient-reported prior pneumonia (lifetime) (P = 0.77), hospitalization or emergency visit for COPD (P = 0.37), or courses of antibiotics or steroids for COPD exacerbations in the prior 12 months (P = 0.20).
TABLE 4.
UNIVARIATE AND MULTIVARIATE ASSOCIATIONS BETWEEN AGE, SEX, FEV1 % PREDICTED, PRIOR VACCINATION STATUS, AND VACCINE ASSIGNMENT AND IMMUNE RESPONSE
| Univariate
|
Multivariate
|
|||
|---|---|---|---|---|
| Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | |
| PCV7 | 1.25 (0.44, 2.06) | 0.003 | 1.31 (0.58, 2.05) | 0.001 |
| Age (per 10 yr) | −0.84 (−1.23, −0.44) | <0.001 | −0.66 (−1.06, −0.27) | 0.001 |
| Previously vaccinated | −1.37 (−2.17, −0.57) | 0.001 | −1.07 (−1.87, −0.28) | 0.009 |
| FEV1, % predicted | −0.01 (−0.04, 0.01) | 0.36 | −0.02 (−0.04, 0.01) | 0.13 |
| Female sex | −0.38 (−1.24, 0.48) | 0.38 | −0.04 (−0.80, 0.72) | 0.91 |
Definition of abbreviations: CI = confidence interval; OPK = opsonophagocytosis killing index; PCV7 = 7-valent diphtheria-conjugated pneumococcal polysaccharide vaccine.
Immune response was assessed as the number of serotypes to which each subject exhibited a 10-fold increase in OPK.
DISCUSSION
This article is the first to report pneumococcal vaccine responses in people with COPD using measures of functional antibody activity and the latest generation ELISA. We have demonstrated that patients with COPD are able to mount immune responses comparable to those previously reported in healthy elderly patients (14, 15). We showed that PCV7, when given at twice the dose recommended for children, induces a superior immune response to PPSV23 in COPD. We also showed that both older age and prior PPSV23 vaccination impair PCV7 responsiveness.
Our ELISA results demonstrate that PCV7 (1.0 ml) induces a superior response to PPSV23 in patients with COPD. Although both the PCV7 and PPSV23 groups showed statistically significant increases in postimmunization antibody concentrations, PCV7 vaccination resulted in superior responses for all seven of the tested serotypes (statistically superior for all but serotype 14 and 19F) and more frequently resulted in a twofold increase in antibody titer, a traditional marker of adequate vaccine response (17). Similar results were reported by Jackson and colleagues who found higher postvaccination antibody levels in previously vaccinated healthy patients older than 70 years of age using the 1.0-ml PCV7 dose and by de Roux and coworkers who demonstrated the same results using the traditional pediatric dose (0.5 ml) in a vaccine-naive elderly population (14, 15). Taken together, these studies suggest that although protein-conjugate vaccines may induce superior immune responses to the free polysaccharide vaccine, the optimal dose is uncertain and higher doses may be needed in those previously vaccinated with PPSV23.
In our study, both PPSV23 and PCV7 elicited increases in OPK, although this was superior after PCV7 for all serotypes except 19F and reached statistical significance for serotype 4, 9V, 18C, and 23F. These results are comparable to those observed by Jackson and colleagues in healthy elderly patients (15). Only one prior study has examined OPK in people with COPD after PPSV23 with only a single serotype tested and demonstrating a nonsignificant increase from baseline (18). Our OPK results suggest that patients with COPD can mount functional antibody responses to both PPSV23 and PCV7, although the conjugate vaccine is likely to offer greater protection against pneumococcal disease.
We also examined the impact of older age and prior vaccination with PPSV23 on the response to vaccination and found that both were associated with reduced OPK responses in multivariate modeling. It has been repeatedly shown that elderly adults develop antibodies with reduced function and our data suggest that this is not eliminated by the administration of a protein-conjugate vaccine (11, 19). Immune hyporesponsiveness after polysaccharide vaccination has been observed with various vaccines, including meningococcal serogroup C vaccine (20) and in recent comparative trials of sequential vaccination with PPSV23 and PCV7 (14, 21). In one of these studies, subjects who received PPSV23 followed by PCV7 1 year later had threefold lower ELISA and OPK than those who received PCV7/PCV7 or PCV7/PPSV23 vaccination regimens (14). In our study, although all subjects who had been previously vaccinated had received PPSV more than 5 years before enrollment, immunologic hyporesponsiveness was still apparent. Taken together these data suggest that PPSV23 vaccination is associated with a reduction in the availability of memory B cells to respond to subsequent vaccinations, whereas PCV7 may prime the immune system for such reimmunization. Results of the multivariate analysis for IgG response were similar to that for OPK in that PCV7 and younger age were also associated with an increased frequency of twofold increases in serotype-specific IgG. Vaccine naivety was not a significant predictor of response in this model suggesting that prior PPSV23 may reduce postvaccination antibody activity to a greater extent than antibody concentration. Importantly, we also demonstrated that PCV7 was comparably or more immunogenic than PPSV23 regardless of prior vaccination status.
Both PCV7 and PPSV23 were well tolerated and associated with few local or systemic side effects. Although increased reactogenicity has been associated with repeated dosing of polysaccharide vaccines (22), we observed no increase in adverse events in those who had been previously vaccinated compared with those who had not. This may be due to the requirement that prior vaccinations were received more than 5 years before enrollment.
We believe there are several strengths to this study. Prior studies have examined the immunogenicity of PPSV23 in patients with COPD by ELISA, but older methodology did not detect specific immunological responses and measured nonspecific, nonfunctional antibodies to the pneumococcal capsule and cell wall polysaccharide, which overestimated antibody levels (11). Prior studies of PPSV immunogenicity in COPD are also limited by the use of ELISA antibody levels as the only surrogate for immunity. The standard ELISA cannot differentiate between functional and nonfunctional antibodies, and measures of antibody-mediated killing, such as opsonophagocytosis, are superior surrogates of protection against pneumonia and bacteremia (19, 23, 24).
It should be noted that there are limitations to the PCV7 vaccine. First, although the greater immunogenicity of PCV7 may provide improved protection against invasive pneumococcal disease and pneumonia, it is not clear that the vaccine will prevent acute exacerbations of COPD. Prior studies have shown that patients with COPD are frequently colonized with S pneumoniae and that this colonization is a predictor of subsequent exacerbation, but higher levels of anti-pneumococcal IgG and OPK have not been associated with bacterial clearance (25). It is possible that protein-conjugate vaccines may elicit greater IgA antibody responses as compared with polysaccharide vaccines and this may augment the mucosal immune response and better protect against both colonization and exacerbation (26). Second, the serotypes contained within PCV7 account for only about one-third of pneumococcal isolates recovered from patients with COPD suffering an acute exacerbation (27). If the vaccine were to offer protection against cross-reactive serotypes (such as 23A/B and 9L/N) then this could be extended to about two-thirds of exacerbation-associated isolates but this would still be inferior to PPSV23, which theoretically protects against 90% of serotypes. In addition, we did not demonstrate a clearly superior immune response after PCV7 vaccination for serotype 14 and in fact, as in most studies, we demonstrated an inferior response to serotype 19F after PCV7 vaccination as compared with PPSV23 (14, 15). This is of particular concern as these antibodies do not appear to cross-protect against serotype 19A, which is frequently antibiotic resistant and has become a more common cause of invasive disease since the introduction of routine PCV7 vaccination among children (28). A 13-valent pneumococcal conjugate vaccine (PCV13) is in development that will extend the coverage against COPD-associated pneumococcal serotypes to more than 80% and also contains serotype 19A but the phenomenon of replacement disease may ultimately limit the protection offered by any serotype-specific vaccine (29).
There are also limitations to our study. Although the use of a twofold increase in IgG antibody titer has been used previously as a measure of vaccine response (17), it is not clear how this threshold or the 10-fold increase in OPK, which was selected on the basis of the distribution of the data, correlate with clinically relevant outcomes. It should also be noted that in many cases PPSV23 does elicit immune responses that meet these thresholds and yet appears to have limited efficacy against pneumococcal pneumonia and acute exacerbations in patients with COPD. As a result, it cannot be inferred that the more frequent achievement of serologic responses after PCV7 would definitively translate into improved clinical outcomes. In addition, we excluded subjects with comorbid illnesses known to impair vaccine responses, such as diabetes and alcoholic cirrhosis. As these conditions are very common, it is likely that the immune responses we observed would not be replicated fully in clinical practice. Our study was designed to examine the independent effect of the presence of COPD on pneumococcal vaccine responses as this has not been adequately examined previously.
In conclusion, we show that PCV7 induces a superior immune response to PPSV23 in COPD at 1 month post vaccination, and that both vaccines elicit responses comparable to those previously observed in healthy elderly patients. Despite controversy about the clinical efficacy of PPSV23 in preventing pneumonia (11), these findings suggest that patients with COPD respond adequately to the vaccine and support its use to prevent episodes of invasive disease in this population. We have also confirmed that prior PPSV23 vaccination and older age are associated with relative immune hyporesponsiveness. Additional studies are needed to confirm the superior immunogenicity of PCV13 in COPD, to determine the relative duration of the immune response after PPSV23 and conjugate vaccination, and to identify the immunologic correlates of protection against both invasive and noninvasive pneumococcal disease. In addition, we believe our data provide the rationale for further study of the clinical efficacy of protein-conjugate pneumococcal vaccines in the high-risk COPD population.
Supplementary Material
Supported by the National Heart, Lung and Blood Institute of the National Institutes of Health (U10 HL074441, U10 HL074418, U10 HL074428, U10 HL074409, U10 HL074407, U10 HL074422, U10 HL074416, U10 HL074408, U10 HL074439, U10 HL074431, U10 HL074424).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200903-0488OC on June 25, 2009
Conflict of Interest Statement: M.T.D. served on the Advisory Board for GlaxoSmithKline ($1,001–$5,000) and AstraZeneca ($1,001–$5,000). He also received Industry-Sponsored Grants for Contracted COPD Research from Aeris Therapeutics ($10,001–$50,000), Boehringer Ingelheim ($100,000 or more), Novartis ($50,001–$100,000), and GlaxoSmithKline for Contracted Asthma Research ($100,001 or more). M.H.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.K.H. has received a consultancy with Novartis (<$1,000) and served on an advisory board for CSL Behring ($1,001–$5,000). S.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.J.C. has served on an advisory board for Study Design at Philips-respironics ($1,001–$5,000) and for Dey ($1,001–$5,000). F.J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.D.S. has been an investigator for Phase II, III, and IV clinical trials sponsored by Boehringer Ingelheim, Dey LP Pharmaceutical, GlaxoSmithkline, Novartis AG, and Pfizer Inc. He has served as an ad hoc consultant for Inflazyne Pharmaceuticals. P.G.W. has received a research grant from Genentech ($100,001 or more) and Boehringer ($100,001 or more). G.R.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.E.C. has served as an expert witness for Carlson, Caspers, Vandenbergh & Lindquist, receiving $1,001–5,000; he has received a grant from Covidien Surgical Staples worth over $100,000. N.R.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.C.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Advisory Committee on Immunization Practices. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997;46:1–24. [PubMed] [Google Scholar]
- 2.Eller J, Ede A, Schaberg T, Niederman MS, Mauch H, Lode H. Infective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung function. Chest 1998;113:1542–1548. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2008. Meeting of the Advisory Committee on Immunization Practices (ACIP). November 24, 2008. (Accessed 2009 Jan 4). Available from: http://www.cdc.gov/vaccines/recs/acip/downloads/agenda-oct08.pdf
- 4.Fedson DS, Musher DM. 2003. Pneumococcal polysaccharide vaccine. In: Plotkin SA and Orenstein WA, editors. Vaccines, 4th ed. Philadelphia: Saunders. pp. 529–588.
- 5.Nichol KL, Baken L, Wuorenma J, Nelson A. The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease. Arch Intern Med 1999;159:2437–2442. [DOI] [PubMed] [Google Scholar]
- 6.Ochoa-Gondar O, Vila-Corcoles A, Ansa X, Rodriguez-Blanco T, Salsench E, de Diego C, Raga X, Gomez F, Valdivieso E, Fuentes C, et al. Effectiveness of pneumococcal vaccination in older adults with chronic respiratory diseases: results of the EVAN-65 study. Vaccine 2008;26:1955–1962. [DOI] [PubMed] [Google Scholar]
- 7.Alfageme I, Vazquez R, Reyes N, Munoz J, Fernandez A, Hernandez M, Merino M, Perez J, Lima J. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax 2006;61:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis AL, Aranda CP, Schiffman G, Christianson LC. Pneumococcal infection and immunologic response to pneumococcal vaccine in chronic obstructive pulmonary disease. A pilot study. Chest 1987;92:204–212. [DOI] [PubMed] [Google Scholar]
- 9.Leech JA, Gervais A, Ruben FL. Efficacy of pneumococcal vaccine in severe chronic obstructive pulmonary disease. CMAJ 1987;136:361–365. [PMC free article] [PubMed] [Google Scholar]
- 10.Steentoft J, Konradsen HB, Hilskov J, Gislason G, Andersen JR. Response to pneumococcal vaccine in chronic obstructive lung disease–the effect of ongoing, systemic steroid treatment. Vaccine 2006;24:1408–1412. [DOI] [PubMed] [Google Scholar]
- 11.Schenkein JG, Nahm MH, Dransfield MT. Pneumococcal vaccination for patients with COPD: current practice and future directions. Chest 2008;133:767–774. [DOI] [PubMed] [Google Scholar]
- 12.Abraham-Van Parijs B. Review of pneumococcal conjugate vaccine in adults: implications on clinical development. Vaccine 2004;22:1362–1371. [DOI] [PubMed] [Google Scholar]
- 13.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000;19:187–195. [DOI] [PubMed] [Google Scholar]
- 14.de Roux A, Schmole-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N, Baker SA, Razmpour A, Emini EA, Fernsten PD, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008;46:1015–1023. [DOI] [PubMed] [Google Scholar]
- 15.Jackson LA, Neuzil KM, Nahm MH, Whitney CG, Yu O, Nelson JC, Starkovich PT, Dunstan M, Carste B, Shay DK, et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2007;25:4029–4037. [DOI] [PubMed] [Google Scholar]
- 16.Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol 2006;13:1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubins JB, Puri AK, Loch J, Charboneau D, MacDonald R, Opstad N, Janoff EN. Magnitude, duration, quality, and function of pneumococcal vaccine responses in elderly adults. J Infect Dis 1998;178:431–440. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson S, Vidarsson G, Valdimarsson H, Schiffman G, Schneerson R, Jonsdottir I. Vaccination of COPD patients with a pneumococcus type 6B tetanus toxoid conjugate vaccine. Eur Respir J 2002;20:813–818. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Steiner S, Musher DM, Cetron MS, Pais LB, Groover JE, Fiore AE, Plikaytis BD, Carlone GM. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis 1999;29:281–288. [DOI] [PubMed] [Google Scholar]
- 20.Granoff DM, Gupta RK, Belshe RB, Anderson EL. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J Infect Dis 1998;178:870–874. [DOI] [PubMed] [Google Scholar]
- 21.Musher DM, Rueda AM, Nahm MH, Graviss EA, Rodriguez-Barradas MC. Initial and subsequent response to pneumococcal polysaccharide and protein-conjugate vaccines administered sequentially to adults who have recovered from pneumococcal pneumonia. J Infect Dis 2008;198:1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson LA, Benson P, Sneller VP, Butler JC, Thompson RS, Chen RT, Lewis LS, Carlone G, DeStefano F, Holder P, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 1999;281:243–248. [DOI] [PubMed] [Google Scholar]
- 23.Johnson SE, Rubin L, Romero-Steiner S, Dykes JK, Pais LB, Rizvi A, Ades E, Carlone GM. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. J Infect Dis 1999;180:133–140. [DOI] [PubMed] [Google Scholar]
- 24.Musher DM, Phan HM, Watson DA, Baughn RE. Antibody to capsular polysaccharide of Streptococcus pneumoniae at the time of hospital admission for Pneumococcal pneumonia. J Infect Dis 2000;182:158–167. [DOI] [PubMed] [Google Scholar]
- 25.Malley R, Lipsitch M, Bogaert D, Thompson CM, Hermans P, Watkins AC, Sethi S, Murphy TF. Serum antipneumococcal antibodies and pneumococcal colonization in adults with chronic obstructive pulmonary disease. J Infect Dis 2007;196:928–935. [DOI] [PubMed] [Google Scholar]
- 26.Nieminen T, Eskola J, Kayhty H. Pneumococcal conjugate vaccination in adults: circulating antibody secreting cell response and humoral antibody responses in saliva and in serum. Vaccine 1998;16:630–636. [DOI] [PubMed] [Google Scholar]
- 27.Bogaert D, van der Valk P, Ramdin R, Sluijter M, Monninkhof E, Hendrix R, de Groot R, Hermans PW. Host-pathogen interaction during pneumococcal infection in patients with chronic obstructive pulmonary disease. Infect Immun 2004;72:818–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, Jackson D, Thomas A, Beall B, Lynfield R, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis 2007;196:1346–1354. [DOI] [PubMed] [Google Scholar]
- 29.Scott DA, Komjathy SF, Hu BT, Baker S, Supan LA, Monahan CA, Gruber W, Siber GR, Lockhart SP. Phase 1 trial of a 13-valent pneumococcal conjugate vaccine in healthy adults. Vaccine 2007;25:6164–6166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.