Abstract
Heme oxygenase-1 (HO-1), a ubiquitous inducible stress-response protein, serves a major metabolic function in heme turnover. HO activity cleaves heme to form biliverdin-IXα, carbon monoxide (CO), and iron. Genetic experiments have revealed a central role for HO-1 in tissue homeostasis, protection against oxidative stress, and in the pathogenesis of disease. Four decades of research have witnessed not only progress in elucidating the molecular mechanisms underlying the regulation and function of this illustrious enzyme, but also have opened remarkable translational applications for HO-1 and its reaction products. CO, once regarded as a metabolic waste, can act as an endogenous mediator of cellular signaling and vascular function. Exogenous application of CO by inhalation or pharmacologic delivery can confer cytoprotection in preclinical models of lung/vascular injury and disease, based on anti-apoptotic, anti-inflammatory, and anti-proliferative properties. The bile pigments, biliverdin and bilirubin, end products of heme degradation, have also shown potential as therapeutics in vascular disease based on anti-inflammatory and anti-proliferative activities. Further translational and clinical trials research will unveil whether the HO-1 system or any of its reaction products can be successfully applied as molecular medicine in human disease.
Keywords: carbon monoxide, bilirubin, heme oxygenase-1, lung injury
Since its discovery four decades ago (1, 2), the heme oxygenase (HO) enzyme system continues to fascinate researchers across many areas of basic and clinical sciences, interconnecting basic science fields (e.g., molecular cell biology and genetics, pharmacology) with modern medical disciplines such as critical care (3–5), pulmonology (6, 7), cardiology (8, 9), exercise physiology (10), and transplant immunology (11–13). This diversity of perspectives on a single enzyme is a reflection of the ubiquitous nature of heme oxygenase-1 (HO-1) as an inducible stress response and critical mediator of cellular homeostasis (14, 15). The initial discovery (ca. 1968–1969) of the HO enzyme (heme, hydrogen-donor:oxygen oxidoreductase [α-methene-oxidizing, hydroxylating], EC: 1:14:99:3) revealed the precise molecular mechanism for the rate-limiting step in the enzymatic conversion of heme to the bile pigments, thus solving a major problem in biochemistry of that era (1, 2). This discovery placed HO in a central role of basic metabolism, replacing previous theories that heme degradation required cytochrome p-450, the major mechanism for xenobiotic and steroid metabolism (1, 2, 16).
In parallel avenues of investigation, the last two decades have also witnessed the emergence of the stress protein field, which began with the observations that cells subjected to elevated temperatures beyond the normal physiologic range responded with the elevated synthesis of distinct proteins, termed the heat shock proteins (HSPs) (17). The inducible HSPs were in turn associated with acquired thermal tolerance or cytoprotection (18). In contrast, the elevated synthesis of a low-molecular-weight stress protein (p32) was observed in human cells in response to a wider range of stimuli, including arsenic, oxidants, ultraviolet-A radiation, and heavy metals (14, 19). The identity of p32 was resolved when research teams, including Keyse and Tyrrell (14) and Kageyama and coworkers (20), independently cloned p32 from human and mouse cells, respectively, and established its molecular sequence identity as HO-1 (14, 20). Although HO-1 was initially classified as a heat shock protein in the rat, further study revealed that the thermal response of HO-1 was generally restricted to rodents but not humans (21–23).
Beyond its intrinsic role as a metabolic enzyme, HO-1 is now recognized as a ubiquitous inducible cellular stress protein, which, like its analogous HSPs, exerts a major role in cellular defense mechanisms (24). Since the discovery of the stress proteins, including HO-1, intensive research has focused on the possible manipulation of these systems for therapeutic benefit in organ injury and disease. The hypothesis that the cytoprotective effects of HO-1 are dependent on the generation of its enzymatic reaction products (i.e., carbon monoxide, biliverdin), has led to much research aimed at developing the therapeutic delivery of these substances (25–29). In this review, we will highlight the steps in the transformation of the HO-1 field from its roots as an intriguing area of basic biochemistry to its current status as a candidate for molecular medicine.
HEME OXYGENASES: ENZYMOLOGY AND REACTION PRODUCTS
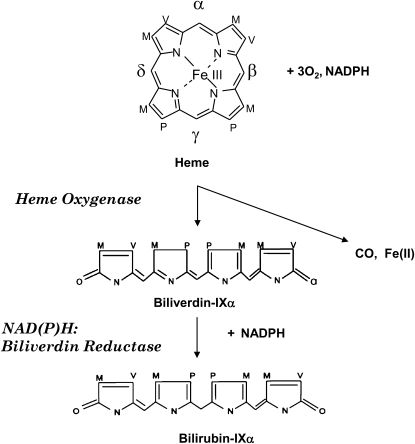
Heme oxygenase activity serves an essential metabolic function as the rate-determining step in heme degradation, by cleaving heme-b at the α-methene bridge carbon, to generate carbon monoxide (CO), biliverdin-IXα (BV), and ferrous iron (Scheme 1) (1). The reaction requires molecular oxygen as well as reducing equivalents from NADPH cytochrome p450 reductase. The BV generated in the HO reaction is reduced to bilirubin-IXα (BR) by NADPH biliverdin reductase (BVR) (1). Two major isoforms of HO (HO-1, HO-2) with heme catalytic activity have been described (30, 31). The expression of HO-1 occurs at low levels in most tissues under physiologic conditions, with the exception of the spleen, the site of erythrocyte hemoglobin turnover. In contrast, HO-2 is constitutively expressed under basal conditions in most human tissues, including testes, spleen, liver, kidney, cardiovascular and nervous systems. HO-1 and HO-2, the products of two genes, differ in primary amino acid sequence and biochemical/biophysical properties (32, 33). HO-1 and HO-2 share a highly conserved heme catalytic domain as well as hydrophobic regions at the carboxyl-terminus (34, 35). Though initially characterized as a major component of the smooth endoplasmic reticulum, recent studies have opened up the intriguing possibility that HO-1 can localize to distinct subcellular compartments, including plasma membrane caveolae (36), mitochondria (37), and nucleus (38). The nuclear form of HO-1 potentially functions as a transcriptional regulator (38). The functional significance of HO-1 in these specialized compartments remains unclear, but early studies strongly suggest that subcellular compartmentalization of HO-1 in the mitochondria and caveolae confer important biological and physiologic functions.
Scheme 1.
Heme oxygenase (HO) reaction. The HO reaction catalyzes the oxidative degradation of the heme molecule, to generate biliverdin, CO, and ferrous iron. The HO reaction proceeds through three serial monooxygenation cycles in which three molecules of O2 are consumed per heme molecule oxidized. NADPH cytochrome p450 reductase provides electrons for the reduction of the heme iron. The biliverdin released from the HO reaction, which is specific for the α isomer, is enzymatically reduced by NAD(P)H:biliverdin reductase, to form bilirubin.
HEME OXYGENASE-1: GENE REGULATION
The transcriptional induction of the gene encoding HO-1 (Hmox1 in mice, HMOX1 in humans) and subsequent synthesis of the corresponding HO-1 protein occurs as a general response to cellular stress (14, 15). In addition to the substrate heme, a broad spectrum of stimuli can induce HO-1 expression. Such agents include nitric oxide (NO), cytokines, heavy metals, hormones, growth factors, thiol-reactive substances, oxidants, extreme oxygen environments, ischemia/reperfusion (I/R) injury, and ultraviolet-A radiation (24). Since many of these inducing conditions are associated with the stimulation of pro-oxidant states, HO-1 is considered an inducible defense mechanism against oxidative cellular stress (14, 15). A subclass of inducing agents includes electrophilic antioxidant compounds, many of which are plant-derived polyphenols, which generally trigger the expression of several detoxification associated genes (including Hmox1, glutathione S-transferase A2 and NADPH: quinone oxidoreductase) through common activation of transcription factor nuclear factor erythroid 2–related factor-2 (Nrf2) (39).
The multiplicity of inducing conditions has presented a challenge in determining the molecular mechanisms of Hmox1 gene regulation. The major discoveries in this area include the identification of distinct DNA sequence-dependent enhancer regions in the upstream regulatory regions of the mouse Hmox1 gene. The two major enhancer elements localize to −4 kb and −10 kb relative to the mouse Hmox1 transcriptional start site and account for the majority of the Hmox1 transcriptional response to such agents as bacterial lipopolysaccharide (LPS), phorbol ester, and heavy metals (40–42). The active cis-acting DNA sequence element of the enhancers, termed the stress-responsive element (StRE), contain sequences homologous to antioxidant response element (ARE). While activator protein-1 (AP-1) has been previously shown to bind to these sequences (42), the major positive transcriptional regulator of Hmox1 acting on the StRE/ARE is represented by Nrf2, a Cap‘n’collar/basic-leucine zipper transcription factor (43). Nrf2 is negatively regulated by a cytoplasmic protein Kelch-like ECH-associated protein (Keap1). Keap1 facilitates the targeting of Nrf2 by Cullin 3-based E3 ubiquitin ligase complex, which marks Nrf2 for proteasomal degradation (44–46). Under basal conditions, Keap1 complexes Nrf2 and prevents its nuclear translocation. Due to the reversible oxidation state of critical cysteine residues, Keap1 has been proposed to act as a molecular sensor of oxidative stress (47). When cells are exposed to inducing stimuli, such as heme, electrophiles, and oxidants, Nrf2 dissociates from Keap1 and translocates to the nucleus (43–45). Nrf2 transcriptional activity depends on heterodimerization with its binding partners, the small Maf proteins (i.e., MafF, MafG), which are b-zip proteins originally identified as cellular homologs of the v-maf oncoprotein. A negative transcriptional regulator of Hmox1 (Bach1) forms complexes with the small Maf proteins and competes against Nrf2 for binding at the StRE. Heme can form a complex with Bach1, which impairs its DNA-binding activity and promotes its nuclear export (48, 49).
The Nrf2/StRE system has been characterized extensively in the mouse as the major stress-inducible operator of the Hmox1 gene. However, the rodent Hmox1 or human HMOX1 gene promoters are known to contain additional functional cis-acting elements that may contribute to transcriptional regulation in a cell type– and inducer-specific fashion. Among these, those that have been associated with functional significance in inducer-specific responses include heat shock– and hypoxia-responsive elements (in rodent Hmox1 promoters) (reviewed in Ref. 24). While the human HMOX1 promoter contains regions analogous to the upstream enhancer regions described in the mouse, additional complexities include specific sequence variation in heavy metal responsive elements at −4 kb (50), as well as a metalloporphyrin-responsive element at −9.5 kb corresponding to a binding site for early growth response-1 (Egr-1) (51). The presence of numerous additional candidate regulatory sequences and element-rich regions in the Hmox1 or HMOX1 genes of rodents or humans suggest the possibility of complex cooperative interactions between transcriptional factors in mediating responses to specific agents, though the functional significance of many these elements remain unclear at present (24, 52–54).
More recent advances in HO-1 genetics include the identification of microsatellite (GT)n dinucleotide length polymorphisms in the regulatory regions of the human HMOX1 gene, which can result in the impaired transcriptional regulation and decreased expression of HO-1 in individuals that carry the long (L) allele (i.e., (GT)n ≥ 30) of this polymorphism (55, 56). Association between HMOX1 polymorphisms and several disease conditions have been proposed, including chronic obstructive pulmonary disease, vascular restenosis, and rheumatoid arthritis (56–60). A recent study has described an association between HMOX1 promoter length polymorphisms and renal function after renal transplantation (61), though similar studies report no associations in the outcome of cardiac or renal transplantation (62, 63). Though additional studies are required before a consensus can be reached, these observations suggest that a genetically dependent down-regulation of HO-1 expression may arise in subpopulations, possibly linked to increased susceptibility to oxidative stress and associated disease conditions.
HO-1 IN HOMEOSTASIS AND TISSUE PROTECTION
The central role of HO-1 in vascular and tissue homeostasis is exemplified by a unique case of human HO-1 deficiency, the subject of which died before reaching adulthood (64). The subject suffered from persistent hemolytic anemia characterized by marked erythrocyte fragmentation and intravascular hemolysis, with increased serum haptoglobin and low serum bilirubin. An abnormal coagulation/fibrinolysis system, associated with elevated thrombomodulin and von Willebrand factor, indicated the presence of severe, persistent endothelial damage (64). Analysis of HO-1 gene deleted (Hmox1−/−) mice also support the notion that HO-1 is an important molecule in systemic responses to stress, as well as iron homeostasis (65, 66). Both the Hmox1−/− mice and the HO-1–deficient child displayed hepatic and renal iron deposition and anemia (64, 65). Furthermore, endothelial cells derived from Hmox1−/− were more susceptible to cytotoxicity induced by pro-oxidant stimuli, such as heme or hydrogen peroxide, and produced more intracellular reactive oxygen species (ROS) when challenged with such stimuli (66). The largely protective role of HO-1 in injury and disease is evident from phenotypic studies of Hmox1−/− mice in preclinical disease models. For example, Hmox1−/− mice subjected to arterial injury displayed increased vascular cell proliferation and developed hyperplastic lesions in comparison to wild-type mice (67). The Hmox1−/− mice also displayed increased mortality during pulmonary I/R injury (68). During chronic hypoxia exposure, Hmox1−/− mice suffered right ventricular dilation and right myocardial infarction relative to wild-type mice that sustained no cardiac defect (69).
A number of studies using HO-1 transgenic mice are also supportive of HO-1–mediated protection. For example, cardiac-specific overexpression of human HO-1 protected against cardiac I/R injury in the transgenic mice (70), as well as improved functional recovery after reperfusion, and limited cardiomyocyte apoptosis (9). Lung-specific overexpression of human HO-1 in transgenic mice protected against the development of pulmonary hypertension and vascular hypertrophy induced by chronic hypoxia, and conferred anti-inflammatory protection by down-regulating proinflammatory cytokine and chemokine expression (7). Lung-specific HO-1 expression also conferred anti-inflammatory protection against acute hypoxic and LPS challenge (71). Recent studies using keratinocyte-specific human HO-1 transgenic mice indicate that targeted overexpression of HO-1 in the skin promoted cutaneous wound healing and neovascularization (72). These studies, taken together, have implicated HO-1 as a critical defense against tissue injury.
HO-1 GENE DELIVERY AND GENE THERAPY
The protective phenotypes observed with HO-1 expression in transgenic mice in models of tissue injury suggest potential translational application through “gene therapy” approaches. Gene therapy refers to the targeted delivery of a gene for therapeutic gain by restoring an inherent deficiency or enhancing the natural expression of the gene product (73). The safety and efficacy of gene delivery systems (i.e., vectors) for human application remains an important general consideration (73). Adenovirus and retroviral-based vectors have been the most widely tested for HO-1 delivery in vivo. HO-1 expression by adenovirus-mediated gene transfer (Ad-HO-1) has been shown to confer cytoprotection in a number of studies. Ad-HO-1 (human) infection protected against heme-mediated cellular injury in rabbit corneal endothelial cells (74). Microinjection of the human HO-1 transgene to rabbit eyes resulted in site-specific expression of human HO-1 protein in rabbit ocular tissue (75). Ad-HO-1 reduced lung cell injury in response to hyperoxia in vivo after intratracheal administration, and protected epithelial cells against hyperoxa-mediated cell killing in vitro (76, 77). Delivery of the human HO-1 cDNA using a replication-deficient retroviral vector to spontaneously hypertensive rats reversed the hypertensive phenotype (78).
Further progress in gene therapy, as it may apply to HO-1 delivery, has required the development of site- or organ-specific gene delivery systems. Progress in this area has included the identification of promoter sequences that can express the target gene specifically in heart, lung, kidney, smooth muscle cells, endothelial cells, keratinocytes, or neurons (73). Recent studies have also explored the possibility of using the HMOX-1 promoter in lentiviral vectors as a means of conferring stress-inducible regulation to any transgene for gene therapy applications (79).
HO-1: MECHANISMS OF CYTOPROTECTION
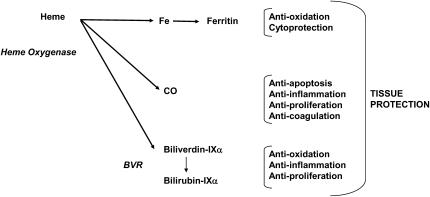
The protective phenotypes observed in association with the expression of HO-1 have prompted intensive investigation on the general mechanism of acquired cytoprotection. The precise molecular mechanisms by which HO-1 confers tissue protection remain incompletely understood. HO-1 activity provides a possible antioxidative function by accelerating the removal of heme to limit oxidative stress sustained through heme-iron dependent mechanisms (14, 80). Elevations of cellular heme bioavailability, however, have not necessarily been documented under all conditions that induce this enzyme. Thus, the source and abundance of the substrate heme for conversion into HO catalytic by-products remain unclearly defined. Nevertheless, the current view of HO-dependent protection is that the reaction products of HO activity (i.e., biliverdin, CO, iron), each contribute, alone or in concert, to the restoration of cellular homeostasis under inducing conditions (Scheme 2) (24, 81). Stocker and colleagues suggested that antioxidative function of HO-1 could be transduced through the elevated production of the bile pigments (82). This group showed that biliverdin and bilirubin, the end products of heme degradation, can act as chain-breaking antioxidants in vitro (82). Pharmacologic application of these pigments can protect cultured cells against injury by oxidative or nitrosative stress (83).
Scheme 2.
Multimodal effects of HO end-products on tissue protection. The three end-products of HO activity can contribute to cytoprotective mechanisms. CO has been implicated in anti-inflammatory, anti-apoptotic, and anti-proliferative pathways. Biliverdin-IXα and bilirubin-IXα, potent antioxidants, can exert anti-inflammatory and anti-proliferative effects. Iron released from HO activity stimulates a cytoprotective pathway involving the synthesis of ferritin.
Iron released from the HO-1 reaction may potentially act as a catalyst of deleterious pro-oxidant reactions, such as the metal-catalyzed Haber-Weiss cycle, or iron-dependent lipid peroxidation (79). Potential pro-oxidant effects of HO-1 or HO-2 activity, related to transient accumulation of heme-derived reactive iron, have been described (84, 85). These effects, which likely occur under conditions of excessive HO-1 expression, suggest that there is a toxic threshold for HO-1 overexpression, and raise a potentially important consideration for gene therapy applications. On the other hand, HO-derived iron, despite its inherent toxicity, can potentially contribute to cytoprotection through indirect mechanisms, such as by the stimulation of ferritin synthesis, which sequesters reactive iron (80). Vile and coworkers demonstrated an association between iron released from HO activity and the synthesis of ferritin, which protected cells from ultraviolet A–induced oxidative stress by iron sequestration (86, 87).
CO, the elimination product of the heme degradation, initially attracted little attention as an endogenous physiologic mediator. The discoveries surrounding NO, a similar low-molecular-weight gas, as an endogenous regulator of vascular function, rekindled interest in biological CO. In 1993, Verma and colleagues proposed a neurotransmitter function for HO-derived CO in olfactory neurotransmission (88). Subsequently, Suematsu and coworkers demonstrated a vascular role for endogenous CO in regulating hepatic perfusion (89). Otterbein and colleagues demonstrated that exogenous CO conferred anti-inflammatory effects in cultured macrophages challenged with lipopolysaccharide (LPS) (25). Application of low concentration CO (e.g., 250 ppm) inhibited the LPS-dependent production of macrophage-derived proinflammatory cytokines (i.e., TNF-α, IL-1β, and macrophage inflammatory protein-1β) whereas it increased the LPS-induced expression of the anti-inflammatory cytokine IL-10 (25). Brouard and coworkers (90) and subsequent studies as reviewed in Ref. 91 have described an anti-apoptotic effect for CO in cultured cells stimulated with cytokines, or pro-oxidant stimuli. An anti-proliferative effect of exogenous or HO-derived CO, was first described by Morita and coworkers in cultured vascular smooth muscle cells (92). These reports and many subsequent studies have now pointed to physiologic roles of endogenous CO, as well as pleiotropic pharmacologic effects of applied CO, despite the known toxicity of this gas at elevated concentrations.
CO SIGNALING
The findings that CO can confer protective (i.e., anti-inflammatory, anti-apoptotic, anti-proliferative) effects at low concentration in cells led to much intensive investigation as to the underlying molecular mechanisms involved. The primary known molecular target of CO is the heme iron center of hemoproteins. By this mechanism, high CO concentrations cause hypoxemia by competitive binding to the oxygen-binding sites of hemoglobin to form carboxyhemoglobin (CO-Hb), with an affinity approximately 245 times that of oxygen (93). In humans, prolonged or elevated CO exposures can cause a number of acute clinical effects, including nausea, dizziness, and loss of consciousness. Symptoms of CO poisoning begin to appear at 20% CO-Hb, while death occurs between 50 and 80% CO-Hb (93). In contrast, CO exposures associated with cytoprotective effects in rodents (i.e., against the injurious effects of mechanical ventilation) resulted in attained CO-Hb levels typically within the 15 to 20% range (4, 94).
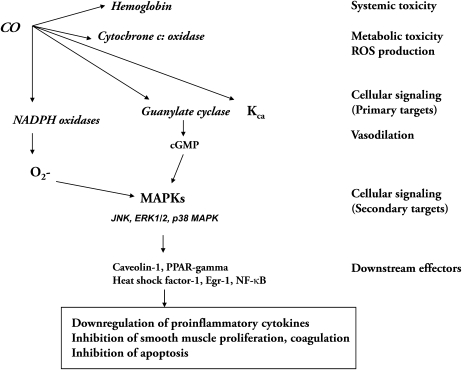
CO, when applied at low concentrations, can influence a number of signaling pathways in cultured cells, including those regulated by soluble guanylate cyclase (sGC) and/or mitogen-activated protein kinases (MAPK) (Scheme 3) (95–99). At a cellular level, CO can stimulate sGC to increase the production of guanosine 3′,5′-monophosphate (cGMP), which has been demonstrated in vascular smooth muscle cells (SMC) (98). HO-derived CO and its effects on cGMP formation have been implicated in several neuronal signaling processes, as well as in the regulation of vascular functions (i.e., vessel tone, smooth muscle proliferation and platelet aggregation) (24, 97). CO treatment can modulate the activation of MAPK pathways that are critical for cellular signal transduction in response to stress and inflammation. In particular, the p38 MAPK signaling pathway has been implicated in the anti-inflammatory, anti-apoptotic, and anti-proliferative effects of CO (25, 26, 90, 99). The integration of these pathways appears to vary in a cell type–specific fashion (24). In one example, CO inhibits smooth muscle cell proliferation by activation of sGC, downstream activation of p38β MAPK, leading to increased expression of the cyclin-dependent kinase inhibitor p21Waf1/Cip1 (26). On the other hand, the activation of p38 MAPK by CO has been reported to be independent of sGC with respect to macrophages, indicating that the proximal target, presumably a heme protein, remains incompletely understood (25).
Scheme 3.
Molecular targets of CO, and proposed signaling pathways. CO modulates intracellular signaling pathways involving hemoproteins, as well as downstream effectors. The primary targets include guanylate cyclase and calcium-dependent potassium channels (KCa) involved in vasoregulation. NADPH oxidase, a putative target of CO, regulates intracellular ROS production. Downstream pathways include mitogen-activated protein kinases (MAPK), caveolin-1, and transcription factors such as heat shock factor-1 (HSF-1), NF-κB, and Egr-1.
The modulation of cellular ROS production from membrane or mitochondria-dependent sources has been implicated in CO-dependent signaling. Inhibition of membrane NADPH: oxidase by CO and subsequent down-regulation of O2− production, has been implicated in the antiproliferative and anti-inflammatory effects of CO (100, 101). Recent observations from this laboratory and others reveal additional candidate molecules that function as downstream effector molecules of CO signaling, including the 70-kD heat shock protein (Hsp-70), peroxysome proliferator-activated receptor-γ (PPAR-γ), an anti-inflammatory nuclear regulator, Egr-1, and caveolin-1 (102–105). Our recent studies have also identified novel mechanisms by which CO may exert anti-inflammatory effects involving the down-regulation of Toll-like receptor (TLR) trafficking and activation (100, 105).
CO AND BV/BR: TISSUE PROTECTION IN PRECLINICAL MODELS
Experimental evidence has mounted over recent years that application of HO-1 reaction products (ie., CO or BV/BR) can confer tissue protection in preclinical animal models of injury and disease (Table 1). These now include various models of acute lung injury, vascular injury, pulmonary hypertension, liver injury, organ I/R injury, organ transplantation, and numerous others (reviewed in Refs. 24, 81, 91). Enthusiasm and clinical potential for CO-based inhalation therapies initially arose from several in vitro and preclinical observations. CO conferred anti-inflammatory effects in a murine model of endotoxemia, induced by systemic injection of LPS in male C57BL/6 mice (25). Exposure to CO at low ambient concentration (e.g., 250 ppm) inhibited the LPS-inducible expression of pro-inflammatory cytokines in serum, whereas it increased the LPS-induced expression of the anti-inflammatory cytokine IL-10, similar to those effects observed in vitro with cultured macrophages (25). Furthermore, low concentrations of CO prevented histologic and biochemical manifestations of tissue injury in rodents subjected to high oxygen stress (hyperoxia) and prolonged survival under these conditions (106). CO application reduced lung cell apoptosis in vivo during lung I/R injury in C57BL/6J mice (107).
TABLE 1.
THERAPEUTIC APPLICATIONS OF HO-1 END-PRODUCTS
| Molecule | Injury/Disease Model | Protection Phenotype | References |
|---|---|---|---|
| Carbon monoxide (gas) | Systemic endotoxemia (mice) | Decreased proinflammatory cytokine production | 25 |
| Hyperoxia-induced acute lung injury (mice) | Decreased proinflammatory cytokine production, increased survival | 99, 106 | |
| Lung ischemia/reperfusion injury (mice) | Reduced apoptosis | 107 | |
| Ventilator-induced lung injury (rats and mice) | Decreased proinflammatory cytokine production | 4, 94, 108 | |
| Vascular injury (rats and mice) | Reduced inflammation, inhibition of vascular smooth muscle proliferation | 26 | |
| Pulmonary hypertension (rat) | Inhibition of smooth muscle proliferation, increased smooth muscle apoptosis | 109 | |
| Cytokine-induced liver injury (mice) | Organ preservation, reduced apoptosis | 120 | |
| Organ transplantation (lung, heart, liver, kidney, small intestinal) (rat) | Decreased inflammation, apoptosis, reduced graft rejection | 12, 110–114, 121 | |
| Systemic endotoxemia (pigs) | Improved organ function, decreased proinflammatory cytokine production | 122 | |
| Carbon monoxide (CORM) | Systemic endotoxemia (mice) | Decreased inflammation | 123 |
| Organ transplantation | Improved organ function | 124 | |
| Biliverdin/Bilirubin | Vascular Injury (rats) | Inhibition of smooth muscle proliferation | 115 |
| Transplantation (rats) | Decreased inflammation, apoptosis, reduced graft rejection | 113, 27–29 |
In more specialized applications, CO inhibited the tissue injury during mechanical ventilation in mice, by reducing inflammation and preventing alveolo-capillary barrier dysfunction (4, 94, 108). Low concentration CO can reverse established pulmonary hypertension in rats, induced by chronic hypoxia or monocrotaline administration (109). Observations of CO-mediated tissue protection have also been described in transplantation of heart, lung, kidney, liver, and intestinal allografts or xenografts, whereby application of CO to the recipient or to the graft reduced inflammation and apoptosis associated with I/R injury, thus reducing the probability of graft rejection (12, 110–114). More recent advances include observations that pharmacologic application of BV/BR can also confer protection in vascular injury and transplantation models (27–29, 113, 115). These studies collectively have provided a rationale for pursuing the translational applications of CO and BR/BV as potential therapies for human disease.
CO: CLINICAL TRIALS
The successful demonstration of CO-dependent protection in numerous animal models of disease has evoked the intriguing proposition that CO may be applicable as a molecular medicine in corresponding human disease states. While progress in this area has been slow due to regulatory and safety concerns associated with human experimentation with inhalation gases, we are now closer to the prospect of directly examining the therapeutic potential of inhaled CO at low concentration in human disease. In an initial study, inhalation of CO for 1 hour at 500 ppm, which raised CO-Hb levels to 7%, had no demonstratable anti-inflammatory effect, with respect to modulation of cytokines production, against an LPS infusion (116). An ongoing phase II trial has addressed the safety of inhaled CO during renal transplantation (Clinicaltrials.gov #NCT00531856). A report on a recently completed clinical trial demonstrates the feasibility of administering a low dose of inhaled CO to humans with chronic obstructive pulmonary disease (COPD) (Clinicaltrials.gov #NCT00122694) (117). In this study, ex-smoking patients with stable COPD were subjected to CO inhalation (100–125 ppm for 2 h/d on 4 consecutive days), which produced a maximal individual CO-Hb level of 4.5%. Inhalation of CO by patients with stable COPD led to trends in reduction of sputum eosinophils and improvement of methacholine responsiveness (117). Further studies are required to confirm the safety and efficacy of CO inhalation as a treatment for inflammatory lung diseases.
FUTURE DIRECTIONS
The characterization of the complex HO reaction still currently attracts the attention of structural biologists and biochemists and may eventually lead to the rational design of pharmaceuticals or chemical inhibitors of HO for therapeutic gain in cases in which down-regulation of HO-1 is required (118). The analysis of the transcriptional regulation of HO-1 continues today with major progress in elucidating the complexities of human gene regulation. New exciting areas are emerging in mechanisms of post-translational HO-1 regulation, including functional subcellular compartmentalization and trafficking of HO-1 (24). These new areas will further the understanding of how HO-1 is regulated and delivered to cellular sites of action.
The functional significance of HO-1 continues to open new perplexing questions as more pathways and mechanisms are proposed. Unanswered questions include the distribution and concentration of the molecular substrate heme, which drives the production of HO end-products. While the protective effects of HO-1 have been documented in multiple animal models of tissue injury, a frontier for development continues in the search for optimal delivery systems for harnessing the therapeutic actions of HO-1 in human disease. Pharmaceuticals, in particular those with low inherent toxicity, may be used to induce HO-1 therapeutically. Optimization and organ targeting of vectors for gene therapy approaches involving HO-1 represents another avenue of active development (73). A preventive medicine approach may involve the screening of individuals for HMOX1 promoter polymorphisms to identify those individuals who may have an increased risk of disease.
With respect to the direct delivery of HO end-products, the use of bile pigments as pharmaceuticals has gained recent momentum from preclinical studies showing protective effects in vascular disease (115). CO, which mimics the protective effects of HO-1, is currently under development as an inhalation therapeutic. The success of this line of research will depend on the initiation and completion of ongoing and/or future carefully conducted and controlled human clinical trials. As an alternative to inhalation of CO, pharmacologic application of CO with the transition-metal carbonyl CO-releasing molecules (CORMs) may provide an additional therapeutic avenue (119). Whether direct application of CO by either pharmacologic administration or inhalation will provide a safe and effective modality for the treatment of human disease requires further research directed at understanding the pharmacokinetics and toxicology of CO application in humans.
CONCLUSIONS
Heme oxygenase-1 serves a dual function as a major metabolic enzyme and inducible stress protein in mammalian cells.
Cytoprotection conferred by HO-1 is related to heme catalytic activity and generation of end-products: biliverdin, CO, and iron.
Manipulation of HO-1 expression through pharmacologic or “gene-therapy” approaches may be used for therapeutic gain in specific applications (e.g., organ transplantation).
Pharmacologic application of bile pigments show potential as therapies for vascular disorders.
Inhalation or pharmacologic application of CO may potentially be used as a clinical therapy for inflammatory lung injury/sepsis pending the outcome of additional clinical trials.
Acknowledgments
The authors thank Dr. Seon-Jin Lee for designing Figure 1.
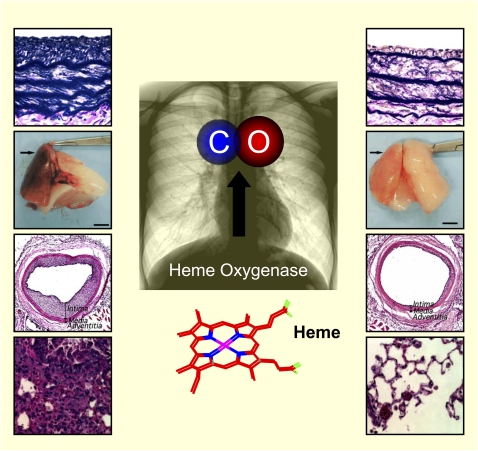
Figure 1.
Carbon monoxide (CO) is produced endogenously in the body as a by-product of heme degradation catalyzed by the action of heme oxygenase (HO) enzymes. Induction of HO-1 occurs as a general cellular and tissue response to stress, and can confer cytoprotection in animal models of tissue injury. Recent studies have described that application of CO at low concentration in tissue injury models can also confer protection. This review highlights the evolution of the HO-1/CO field from basic biochemistry to translational applications. (Images adapted from Refs. 26, 104, 111, and 125.)
This work was supported by National Institutes of Health grants P01-HL70807, R01-HL60234, R01-HL55330, and R01-HL079904 (to A.M.K.C.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0170TR on July 17, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase: characterization of the enzyme. J Biol Chem 1969;244:6388–6394. [PubMed] [Google Scholar]
- 2.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 1968;61:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mumby S, Upton RL, Chen Y, Stanford SJ, Quinlan GJ, Nicholson AG, Gutteridge JM, Lamb NJ, Evans TW. Lung heme oxygenase-1 is elevated in acute respiratory distress syndrome. Crit Care Med 2004;32:1130–1135. [DOI] [PubMed] [Google Scholar]
- 4.Hoetzel A, Dolinay T, Vallbracht S, Zhang Y, Kim HP, Ifedigbo E, Alber S, Kaynar AM, Schmidt R, Ryter SW, et al. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am J Respir Crit Care Med 2008;177:1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoetzel A, Leitz D, Schmidt R, Tritschler E, Bauer I, Loop T, Humar M, Geiger KK, Pannen BH. Mechanism of hepatic heme oxygenase-1 induction by isoflurane. Anesthesiology 2006;104:101–109. [DOI] [PubMed] [Google Scholar]
- 6.Lee PJ, Alam J, Sylvester SL, Inamdar N, Otterbein L, Choi AM. Regulation of heme oxygenase-1 expression in vivo and in vitro in hyperoxic lung injury. Am J Respir Cell Mol Biol 1996;14:556–568. [DOI] [PubMed] [Google Scholar]
- 7.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA 2001;98:8798–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Pachori AS, Ward CA, Davis JP, Gnecchi M, Kong D, Zhang L, Murduck J, Yet SF, Perrella MA, et al. Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. FASEB J 2006;20:207–216. [DOI] [PubMed] [Google Scholar]
- 9.Vulapalli SR, Chen Z, Chua BH, Wang T, Liang CS. Cardioselective overexpression of HO-1 prevents I/R-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol 2002;283:H688–H694. [DOI] [PubMed] [Google Scholar]
- 10.Thompson D, Basu-Modak S, Gordon M, Poore S, Markovitch D, Tyrrell RM. Exercise-induced expression of heme oxygenase-1 in human lymphocytes. Free Radic Res 2005;39:63–69. [DOI] [PubMed] [Google Scholar]
- 11.Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RB, Choi AM, Poss KD, et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med 1998;4:1073–1077. [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, et al. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol 2001;166:4185–4194. [DOI] [PubMed] [Google Scholar]
- 13.Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graça-Souza AV, Ollinger R, Czismadia E, May GA, Ifedigbo E, Otterbein LE, et al. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J 2004;18:771–772. [DOI] [PubMed] [Google Scholar]
- 14.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA 1989;86:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res 1991;51:974–978. [PubMed] [Google Scholar]
- 16.Maines MD, Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci USA 1974;71:4293–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welch WJ, Feramisco JR. Purification of the major mammalian heat shock proteins. J Biol Chem 1982;257:14949–14959. [PubMed] [Google Scholar]
- 18.Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci USA 1982;79:3218–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keyse SM, Tyrrell RM. Both near ultraviolet radiation and the oxidizing agent hydrogen peroxide induce a 32-kDa stress protein in normal human skin fibroblasts. J Biol Chem 1987;262:14821–14825. [PubMed] [Google Scholar]
- 20.Kageyama H, Hiwasa T, Tokunaga K, Sakiyama S. Isolation and characterization of a complementary DNA clone for a Mr 32,000 protein which is induced with tumor promoters in BALB/c 3T3 cells. Cancer Res 1988;48:4795–4798. [PubMed] [Google Scholar]
- 21.Shibahara S, Sato M, Muller RM, Yoshida T. Structural organization of the human heme oxygenase gene and the function of its promoter. Eur J Biochem 1989;179:557–563. [DOI] [PubMed] [Google Scholar]
- 22.Shibahara S, Müller RM, Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem 1987;262:12889–12892. [PubMed] [Google Scholar]
- 23.Yoshida T, Biro P, Cohen T, Müller RM, Shibahara S. Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem 1988;171:457–461. [DOI] [PubMed] [Google Scholar]
- 24.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 2006;86:583–650. [DOI] [PubMed] [Google Scholar]
- 25.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 2000;6:422–428. [DOI] [PubMed] [Google Scholar]
- 26.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med 2003;9:183–190. [DOI] [PubMed] [Google Scholar]
- 27.Fondevila C, Shen XD, Tsuchiyashi S, Yamashita K, Csizmadia E, Lassman C, Busuttil RW, Kupiec-Weglinski JW, Bach FH. Biliverdin therapy protects rat livers from ischemia and reperfusion injury. Hepatology 2004;40:1333–1341. [DOI] [PubMed] [Google Scholar]
- 28.Adin CA, Croker BP, Agarwal A. Protective effects of exogenous bilirubin on ischemia–reperfusion injury in the isolated, perfused rat kidney. Am J Physiol Renal Physiol 2005;288:F778–F784. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, Csizmadia E, Smith RN, Soares MP, Bach FH. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. FASEB J 2004;18:765–767. [DOI] [PubMed] [Google Scholar]
- 30.Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem 1986;261:411–419. [PubMed] [Google Scholar]
- 31.Trakshel GM, Kutty RK, Maines MD. Purification and characterization of the major constitutive form of testicular heme oxygenase. J Biol Chem 1986;261:11131–11137. [PubMed] [Google Scholar]
- 32.Cruse I, Maines MD. Evidence suggesting that the two forms of heme oxygenase are products of different genes. J Biol Chem 1998;263:3348–3353. [PubMed] [Google Scholar]
- 33.Rotenberg MO, Maines MD. Isolation, characterization, and expression in Escherichia coli of a cDNA encoding rat heme oxygenase-2. J Biol Chem 1990;265:7501–7506. [PubMed] [Google Scholar]
- 34.Rotenberg MO, Maines MD. Characterization of a cDNA-encoding rabbit brain heme oxygenase-2 and identification of a conserved domain among mammalian heme oxygenase isozymes: possible heme-binding site? Arch Biochem Biophys 1991;290:336–344. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa K, Sato M, Yoshida T. Expression of rat heme oxygenase in Esherichia coli as a catalytically active, full length form that binds to bacterial membranes. Eur J Biochem 1991;202:161–165. [DOI] [PubMed] [Google Scholar]
- 36.Kim HP, Wang X, Galbiati F, Ryter SW. Choi AMK. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J 2004;18:1080–1089. [DOI] [PubMed] [Google Scholar]
- 37.Slebos DJ, Ryter SW, van der Toorn M, Liu F, Guo F, Baty CJ, Karlsson JM, Watkins SC, Kim HP, Wang X, et al. Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death. Am J Respir Cell Mol Biol 2007;36:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem 2007;282:20621–20633. [DOI] [PubMed] [Google Scholar]
- 39.Pickett CB, Nguyen T, Nioi P. The Nrf2-ARE signaling pathway and its activation by oxidative stress. J Biol Chem 2009;284:13291–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alam J, Cai J, Smith A. Isolation and characterization of the mouse heme oxygenase-1 gene. Distal 5′ sequences are required for induction by heme or heavy metals. J Biol Chem 1994;269:1001–1009. [PubMed] [Google Scholar]
- 41.Alam J, Camhi S, Choi AM. Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer-dependent transcription enhancer. J Biol Chem 1995;270:11977–11984. [DOI] [PubMed] [Google Scholar]
- 42.Alam J, Den Z. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J Biol Chem 1992;267:21894–21900. [PubMed] [Google Scholar]
- 43.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap‘n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 1999;274:26071–26078. [DOI] [PubMed] [Google Scholar]
- 44.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor T, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 2003;8:379–391. [DOI] [PubMed] [Google Scholar]
- 45.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 1999;13:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 2004;24:7130–7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA 2002;99:11908–11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J 2002;21:5216–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci USA 2004;101:1461–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeda K, Ishizawa S, Sato M, Yoshida T, Shibahara S. Identification of a cis-acting element that is responsible for cadmium-mediated induction of the human heme oxygenase gene. J Biol Chem 1994;269:22858–22867. [PubMed] [Google Scholar]
- 51.Yang G, Nguyen X, Ou J, Rekulapelli P, Stevenson DK, Dennery PA. Unique effects of zinc protoporphyrin on HO-1 induction and apoptosis. Blood 2001;97:1306–1313. [DOI] [PubMed] [Google Scholar]
- 52.Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol Renal Physiol 2004;286:F425–F441. [DOI] [PubMed] [Google Scholar]
- 53.Choi AM, Alam J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol 1996;15:9–19. [DOI] [PubMed] [Google Scholar]
- 54.Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol 2007;36:166–174. [DOI] [PubMed] [Google Scholar]
- 55.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med 2004;37:1097–1104. [DOI] [PubMed] [Google Scholar]
- 56.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet 2000;66:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Exner M, Schillinger M, Minar E, Mlekusch W, Schlerka G, Haumer M, Mannhalter C, Wagner O. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J Endovasc Ther 2001;8:433–440. [DOI] [PubMed] [Google Scholar]
- 58.Chen YH, Chau LY, Lin MW, Chen LC, Yo MH, Chen JW, Lin S. Heme oxygenase-1 gene promotor microsatellite polymorphism is associated with angiographic restenosis after coronary stenting. Eur Heart J 2004;25:39–47. [DOI] [PubMed] [Google Scholar]
- 59.Schillinger M, Exner M, Minar E, Mlekusch W, Müllner M, Mannhalter C, Bach FH, Wagner O. Heme oxygenase-1 genotype and restenosis after balloon angioplasty: a novel vascular protective factor. J Am Coll Cardiol 2004;43:950–957. [DOI] [PubMed] [Google Scholar]
- 60.Rueda B, Oliver J, Robledo G, López-Nevot MA, Balsa A, Pascual-Salcedo D, González-Gay MA, González-Escribano MF, Martín J. HO-1 promoter polymorphism associated with rheumatoid arthritis. Arthritis Rheum 2007;56:3953–3958. [DOI] [PubMed] [Google Scholar]
- 61.Exner M, Böhmig GA, Schillinger M, Regele H, Watschinger B, Hörl WH, Raith M, Mannhalter C, Wagner OF. Donor heme oxygenase-1 genotype is associated with renal allograft function. Transplantation 2004;77:538–542. [DOI] [PubMed] [Google Scholar]
- 62.Ullrich R, Exner M, Schillinger M, Zuckermann A, Raith M, Dunkler D, Horvat R, Grimm M, Wagner O. Microsatellite polymorphism in the heme oxygenase-1 gene promoter and cardiac allograft vasculopathy. J Heart Lung Transplant 2005;24:1600–1605. [DOI] [PubMed] [Google Scholar]
- 63.Courtney AE, McNamee PT, Middleton D, Heggarty S, Patterson CC, Maxwell AP. Association of functional heme oxygenase-1 gene promoter polymorphism with renal transplantation outcomes. Am J Transplant 2007;7:908–913. [DOI] [PubMed] [Google Scholar]
- 64.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 1999;103:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poss KD, Tonegawa S. Heme oxygenase-1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA 1997;94:10919–10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase-1 deficient cells. Proc Natl Acad Sci USA 1997;94:10925–10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Webb RC, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med 2001;7:693–698. [DOI] [PubMed] [Google Scholar]
- 68.Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med 2001;7:598–604. [DOI] [PubMed] [Google Scholar]
- 69.Yet SF, Perrella MA, Layne MD, Hsieh CM, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee ME. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest 1999;103:R23–R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, et al. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res 2001;89:168–173. [DOI] [PubMed] [Google Scholar]
- 71.Zampetaki A, Minamino T, Mitsialis SA, Kourembanas S. Effect of heme oxygenase-1 overexpression in two models of lung inflammation. Exp Biol Med (Maywood) 2003;228:442–446. [DOI] [PubMed] [Google Scholar]
- 72.Grochot-Przeczek A, Lach R, Mis J, Skrzypek K, Gozdecka M, Sroczynska P, Dubiel M, Rutkowski A, Kozakowska M, Zagorska A, et al. Heme oxygenase-1 accelerates cutaneous wound healing in mice. PLoS One 2009;4:e5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abraham NG, Asija A, Drummond G, Peterson S. Heme oxygenase-1 gene therapy: recent advances and therapeutic applications. Curr Gene Ther 2007;7:89–108. [DOI] [PubMed] [Google Scholar]
- 74.Abraham NG, Lavrovsky Y, Schwartzman ML, Stoltz RA, Levere RD, Gerritsen ME, Shibahara S, Kappas A. Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: protective effect against heme and hemoglobin toxicity. Proc Natl Acad Sci USA 1995;92:6798–6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abraham NG, da Silva JL, Lavrovsky Y, Stoltz RA, Kappas A, Dunn MW, Schwartzman ML. Adenovirus-mediated heme oxygenase-1 gene transfer into rabbit ocular tissues. Invest Ophthalmol Vis Sci 1995;36:2202–2210. [PubMed] [Google Scholar]
- 76.Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxic lung injury. J Clin Invest 1999;103:1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee PJ, Alam J, Wiegand GW, Choi AM. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci USA 1996;93:10393–10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sabaawy HE, Zhang F, Nguyen X, ElHosseiny A, Nasjletti A, Schwartzman M, Dennery P, Kappas A, Abraham NG. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension 2001;38:210–215. [DOI] [PubMed] [Google Scholar]
- 79.Hurttila H, Koponen JK, Kansanen E, Jyrkkänen HK, Kivelä A, Kylätie R, Ylä-Herttuala S, Levonen AL. Oxidative stress-inducible lentiviral vectors for gene therapy. Gene Ther 2008;15:1271–1279. [DOI] [PubMed] [Google Scholar]
- 80.Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity: heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 2000;28:289–309. [DOI] [PubMed] [Google Scholar]
- 81.Ryter SW, Morse D, Choi AM. Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am J Respir Cell Mol Biol 2007;36:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stocker R, Yamamoto Y, McDonagh A, Glazer A, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043–1045. [DOI] [PubMed] [Google Scholar]
- 83.Foresti R, Green CJ, Motterlini R. Generation of bile pigments by haem oxygenase: a refined cellular strategy in response to stressful insults. Biochem Soc Symp 2004;71:177–192. [DOI] [PubMed] [Google Scholar]
- 84.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J 1999;13:1800–1809. [DOI] [PubMed] [Google Scholar]
- 85.Kvam E, Hejmadi V, Ryter S, Pourzand C, Tyrrell RM. Heme oxygenase activity causes transient hypersensitivity to oxidative ultraviolet A radiation that depends on release of iron from heme. Free Radic Biol Med 2000;28:1191–1196. [DOI] [PubMed] [Google Scholar]
- 86.Vile GF, Tyrrell RM. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J Biol Chem 1993;268:14678–14681. [PubMed] [Google Scholar]
- 87.Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci USA 1994;91:2607–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science 1993;259:381–384. [DOI] [PubMed] [Google Scholar]
- 89.Suematsu M, Kashiwagi S, Sano T, Goda N, Shinoda Y, Ishimura Y. Carbon monoxide as an endogenous modulator of hepatic vascular perfusion. Biochem Biophys Res Commun 1994;205:1333–1337. [DOI] [PubMed] [Google Scholar]
- 90.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses endothelial cell apoptosis. J Exp Med 2000;192:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morse D, Lin L, Choi AM, Ryter SW. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med 2009;47:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morita T, Mitsialis SA, Koike H, Liu Y, Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J Biol Chem 1997;272:32804–32809. [DOI] [PubMed] [Google Scholar]
- 93.Von Burg R. Toxicology update: carbon monoxide. J Appl Toxicol 1999;19:379–386. [DOI] [PubMed] [Google Scholar]
- 94.Dolinay T, Szilasi M, Liu M, Choi AM. Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med 2004;170:613–620. [DOI] [PubMed] [Google Scholar]
- 95.Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol 2006;46:411–449. [DOI] [PubMed] [Google Scholar]
- 96.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 1997;37:517–554. [DOI] [PubMed] [Google Scholar]
- 97.Ryter SW, Morse D, Choi AMK. Carbon monoxide: to boldly go where NO has gone before. Sci STKE 2004;230:re6. [DOI] [PubMed] [Google Scholar]
- 98.Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA 1995;92:1475–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Otterbein LE, Otterbein SL, Ifedigbo E, Liu F, Morse DE, Fearns C, Ulevitch RJ, Knicklebein R, Flavell RA, Choi AM. MKK3 mitogen activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant induced lung injury. Am J Pathol 2003;163:2555–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Wang X, Sasidhar M, Nabel EG, et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med 2006;203:2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taillé C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem 2005;280:25350–25360. [DOI] [PubMed] [Google Scholar]
- 102.Kim HP, Wang Y, Suh GY, Zhang J, Ryter SW, Choi AMK. Heat shock factor-1 mediates the cytoprotective effect of carbon monoxide. J Immunol 2005;175:2622–2629. [DOI] [PubMed] [Google Scholar]
- 103.Bilban M, Bach FH, Otterbein SL, Ifedigbo E, d'Avila JC, Esterbauer H, Chin BY, Usheva A, Robson SC, Wagner O, et al. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity 2006;24:601–610. [DOI] [PubMed] [Google Scholar]
- 104.Kim HP, Wang X, Nakao A, Kim SI, Murase N, Choi ME, Ryter SW, Choi AM. Caveolin-1 expression by means of p38beta mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. Proc Natl Acad Sci USA 2005;102:11319–11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol 2009;182:3809–3818. [DOI] [PubMed] [Google Scholar]
- 106.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol 1999;276:L688–L694. [DOI] [PubMed] [Google Scholar]
- 107.Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, Lee PJ. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem 2003;278:1248–1258. [DOI] [PubMed] [Google Scholar]
- 108.Hoetzel A, Schmidt R, Vallbracht S, Goebel U, Dolinay T, Kim HP, Ifedigbo E, Ryter SW, Choi AM. Carbon monoxide prevents ventilator-induced lung injury via caveolin-1. Crit Care Med 2009;37:1708–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zuckerbraun BS, Chin BY, Wegiel B, Billiar TR, Czsimadia E, Rao J, Shimoda L, Ifedigbo E, Kanno S, Otterbein LE. Carbon monoxide reverses established pulmonary hypertension. J Exp Med 2006;203:2109–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakao A, Kimizuka K, Stolz DB, Seda Neto J, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Bauer AJ, Nalesnik MA, et al. Protective effect of carbon monoxide inhalation for cold-preserved small intestinal grafts. Surgery 2003;134:285–292. [DOI] [PubMed] [Google Scholar]
- 111.Song R, Kubo M, Morse D, Zhou Z, Zhang X, Dauber JH, Fabisiak J, Alber SM, Watkins SC, Zuckerbraun BS, et al. Carbon monoxide induces cytoprotection in rat orthotopic lung transplantation via anti-inflammatory and anti-apoptotic effects. Am J Pathol 2003;163:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakao A, Toyokawa H, Tsung A, Nalesnik MA, Stolz DB, Kohmoto J, Ikeda A, Tomiyama K, Harada T, Takahashi T, et al. Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am J Transplant 2006;6:2243–2255. [DOI] [PubMed] [Google Scholar]
- 113.Nakao A, Neto JS, Kanno S, Stolz DB, Kimizuka K, Liu F, Bach FH, Billiar TR, Choi AM, Otterbein LE, et al. Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am J Transplant 2000;5:282–291. [DOI] [PubMed] [Google Scholar]
- 114.Kohmoto J, Nakao A, Kaizu T, Tsung A, Ikeda A, Tomiyama K, Billiar TR, Choi AM, Murase N, McCurry KR. Low-dose carbon monoxide inhalation prevents ischemia/reperfusion injury of transplanted rat lung grafts. Surgery 2006;140:179–185. [DOI] [PubMed] [Google Scholar]
- 115.Ollinger R, Bilban M, Erat A, Froio A, McDaid J, Tyagi S, Csizmadia E, Graca-Souza AV, Liloia A, Soares MP, et al. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation 2005;112:1030–1039. [DOI] [PubMed] [Google Scholar]
- 116.Mayr FB, Spiel A, Leitner J, Marsik C, Germann P, Ullrich R, Wagner O, Jilma B. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med 2005;171:354–360. [DOI] [PubMed] [Google Scholar]
- 117.Bathoorn E, Slebos DJ, Postma DS, Koeter GH, van Oosterhout AJ, van der Toorn M, Boezen HM, Kerstjens HA. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Respir J 2007;30:1131–1137. [DOI] [PubMed] [Google Scholar]
- 118.Kinobe RT, Dercho RA, Nakatsu K. Inhibitors of the heme oxygenase - carbon monoxide system: on the doorstep of the clinic? Can J Physiol Pharmacol 2008;86:577–599. [DOI] [PubMed] [Google Scholar]
- 119.Motterlini R, Mann BE, Foresti R. Therapeutic applications of carbon monoxide–releasing molecules. Expert Opin Investig Drugs 2005;14:1305–1318. [DOI] [PubMed] [Google Scholar]
- 120.Zuckerbraun BS, Billiar TR, Otterbein SL, Kim PK, Liu F, Choi AM, Bach FH, Otterbein LE. Carbon monoxide protects against liver failure through nitric oxide-induced heme oxygenase 1. J Exp Med 2003;198:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kaizu T, Nakao A, Tsung A, Toyokawa H, Sahai R, Geller DA, Murase N. Carbon monoxide inhalation ameliorates cold ischemia/reperfusion injury after rat liver transplantation. Surgery 2005;138:229–235. [DOI] [PubMed] [Google Scholar]
- 122.Mazzola S, Forni M, Albertini M, Bacci ML, Zannoni A, Gentilini F, Lavitrano M, Bach FH, Otterbein LE, Clement MG. Carbon monoxide pretreatment prevents respiratory derangement and ameliorates hyperacute endotoxic shock in pigs. FASEB J 2005;19:2045–2047. [DOI] [PubMed] [Google Scholar]
- 123.Cepinskas G, Katada K, Bihari A, Potter RF. Carbon monoxide liberated from carbon monoxide-releasing molecule CORM-2 attenuates inflammation in the liver of septic mice. Am J Physiol Gastrointest Liver Physiol 2008;294:G184–G191. [DOI] [PubMed] [Google Scholar]
- 124.Musameh MD, Green CJ, Mann BE, Fuller BJ, Motterlini R. Improved myocardial function after cold storage with preservation solution supplemented with a carbon monoxide-releasing molecule (CORM-3). J Heart Lung Transplant 2007;26:1192–1198. [DOI] [PubMed] [Google Scholar]
- 125.Zhou Z, Song R, Fattman CL, Greenhill S, Alber S, Oury TD, Choi AM, Morse D. Carbon monoxide suppresses bleomycin-induced lung fibrosis.Am J Pathol 2005;166:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]