Abstract
Dendritic cells (DCs) are considered to be the most efficient antigen-presenting cells. Intratracheal administration of allergen-pulsed bone marrow–derived dendritic cells (BMDCs) before allergen challenge induces airway hyperresponsiveness (AHR) and inflammation. Ovalbumin (OVA)-pulsed BMDCs from wild-type (WT) mice were transferred into naive WT, CD4−/−, CD8−/−, or IL-13−/− mice. Two days (short protocol) or 10 days (long protocol) after BMDC transfer, mice were challenged with 1% OVA for 3 days and assayed 2 days later. Transfer of OVA-primed BMDCs into BALB/c or C57BL/6 mice elicited AHR in both protocols. Airway eosinophilia, Th2 cytokines, or goblet cell metaplasia were increased in the long but not short protocol. Lung T cells from both protocols produced Th2 cytokines in response to OVA in vitro. Carboxyfluorescein diacetate succinimidylester–labeled BMDCs were observed in bronchoalveolar lavage (BAL) fluid and lung parenchyma at early time points, and were detected in draining lymph nodes 48 hours after transfer. CD8−/− mice developed AHR comparable to WT mice in the short protocol, but decreased levels of AHR, airway eosinophilia, Th2 cytokines in BAL fluid, and goblet cell metaplasia compared with WT mice in the long protocol. CD4−/− or IL-13−/− mice did not develop AHR or airway inflammation in either protocol. These data suggest that allergen-pulsed BMDCs initiate development of AHR that is dependent initially on CD4+ T cells, and at later time periods on CD8+ T cells and IL-13. Thus, the timing of allergen challenge after transfer of allergen-pulsed BMDC affects the development of AHR and airway inflammation.
Keywords: dendritic cells, CD8+ T cells, airway hyperresponsiveness
Eosinophilic airway inflammation, Th2 cytokine production, and airway hyperresponsiveness (AHR) are characteristic features of allergic bronchial asthma (1). The immunologic basis of this disease has been a topic of intense investigation. T cell responses to allergens are critical in the pathogenesis of asthma. In addition, a large number of cytokines, chemokines, adhesion molecules, and costimulatory molecules have been shown to be involved (2).
A critical step in the induction of a T cell immune response is the uptake, processing, and presentation of antigen by antigen-presenting cells (APCs). Among the different types of APCs, dendritic cells (DCs) are the most predominant APCs in the airways (3, 4). In rats, allergen-primed lung DCs induce allergen-specific Th2-mediated IgG1 with little Th1-directed allergen-specific IgG2b generation (3). Lung DCs are potent regulators of Th2-biased responses to inhalant allergens (4).
Intratracheally administered allergen-pulsed DCs migrate to draining lymph nodes and initiate priming of T cells (5). After allergen challenge they elicit AHR and allergic airway inflammation (6–9). These studies showed that maturation, migration to draining lymph nodes, and expression of costimulatory molecules on DCs were all essential for the priming of T cells and for the development of allergic airway inflammation. Moreover, Fcγ receptors and Toll-like receptors, Fas ligand, Syk kinase activity, or epithelial-derived cytokines such as thymic stromal lymphopoietin (TSLP), have all been shown to regulate lung allergic responses through interactions with DCs (10–13). However, the interactions between DCs and specific T cell subsets in the development of AHR and airway allergic inflammation have not been defined.
In the present study, we investigated the role of locally administered DCs in the priming of CD4+ or CD8+ T cells in the development of allergen-induced AHR and airway inflammation. In this model, it appeared that the interval between lung allergen exposure and intratracheal administration of allergen-pulsed DCs dictated the outcomes and the requirements for specific T cell subsets.
MATERIALS AND METHODS
Animals
Female BALB/c and C57BL/6 (B6) mice were purchased at 8 to 12 weeks of age from Jackson Laboratories (Bar Harbor, ME). Age-matched CD4−/− mice and CD8−/− mice, generated by targeting the CD4 or CD8α chain gene in B6 mice, respectively (14, 15), were obtained from Dr. P. Marrack (National Jewish Health, Denver, CO). IL-13−/− mice (BALB/c background) initially provided by Dr. D. Umetsu (Stanford University, Stanford, CA) (16), were housed under specific pathogen–free conditions and maintained on an ovalbumin (OVA)-free diet. Experiments were conducted under a protocol approved by Institutional Animal Care and Use Committee of the National Jewish Health.
Local Administration of DCs for the Induction of Airway Allergic Inflammation
DCs were generated from bone marrow cells of naive BALB/c or B6 mice according to the procedure described previously (17). Briefly, bone marrow cells were obtained from femurs and iliac bones of mice and placed in DC culture medium (DC-CM; RPMI 1640 containing 10% heat-inactivated fetal calf serum [FCS], 50 μM 2-ME, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) (GIBCO, Carlsbad, CA), 10 ng/ml recombinant mouse GM-CSF, and 10 ng/ml recombinant mouse IL-4 (R&D Systems, Minneapolis, MN).
On Day 8, nonadherent cells were used as DCs. These cells were more than 95% CD11c+CD11b+. The remaining cells were CD11c−B220+ or CD11c−CD11b+ cells, but no CD3+ cells were detected. DCs were pulsed with OVA (200 μg/ml) for 24 hours and washed three times with PBS. As control, DCs were cultured without OVA. After incubation with OVA, the expression of costimulatory factors such as CD80 or CD86 on DCs were not significantly different compared with DCs not pulsed with OVA.
OVA-pulsed or nonpulsed DCs (1 × 106 cells) were instilled intratracheally into naive mice. In the short protocol, animals were challenged with nebulized OVA (1% in saline) for 20 minutes for 3 consecutive days beginning 2 days after DC instillation. In the long protocol, animals were challenged with OVA on 3 consecutive days beginning 10 days after DC instillation. Forty-eight hours after the last OVA challenge in both protocols, assays were performed.
Determination of Airway Responsiveness and Airway Inflammation
Airway responsiveness was assessed as previously described by measuring changes in lung resistance (Rl) in response to increasing doses of inhaled methacholine (MCh) (18). Many studies have demonstrated strain differences in airway responsiveness to inhaled MCh challenge (19, 20). C57BL/6 mice showing comparable changes in airway resistance as BALB/c mice but at higher MCh concentrations. Data are expressed as the percent change from baseline Rl values obtained after inhalation of saline. Baseline levels of Rl were not significantly different among the groups.
Immediately after measurement of AHR, lungs were lavaged via the tracheal tube as previously described (21). Briefly, bronchoalveolar lavage (BAL) fluid was collected with 1 ml of Hanks' balanced salt solution (HBSS) via the tracheal tube and slides were prepared with Cytospin III (Shandon Scientific, Pittsburgh, PA) and stained with May-Giemsa for differential cell counts. BAL was obtained after MCh challenge. Cell composition and cytokine levels in BAL fluid were not altered by MCh challenge.
Lungs were fixed in 10% formalin and processed into paraffin. Mucus-containing goblet cells were detected by staining of paraffin sections (5 μm thick) with periodic acid-Schiff (PAS). Histologic analyses were done as previously described (21).
Cell Preparations of Lung T Cells and Cultures with OVA
Lung cells were isolated as previously described (17) using collagenase digestion. Cells were resuspended in HBSS, and mononuclear cells (MNCs) were purified over 35% Percoll (Sigma-Aldrich, St. Louis, MO). Lung T cells were purified from lung using the Mouse T Cell Recovery Column Kit (Cederlane, Hornby, ON, Canada) (purity > 95%). Cells were washed, counted, and suspended at 4 × 106 cells/ml in RPMI 1640 (Life Technologies, Gaithersburg, MD) tissue culture medium, containing heat-inactivated 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 5 mM glutamine, and 50 μM 2-mercaptoethanol. As APCs, spleen MNCs were irradiated (30 Gy). Lung T cells were cultured with irradiated spleen MNCs and OVA (10 μg/ml) for 72 hours, at which time supernates were recovered for cytokine assays.
Measurement of Cytokines in Supernatants from Cell Culture or BAL Fluid
Supernatants from cell cultures or BAL fluid were stored at −80°C until used for cytokine measurements. Levels of cytokines were determined using commercially available enzyme-linked immunosorbent assays (ELISAs) following the manufacturers' instructions. ELISA kits for detection of IL-4, IL-5, and IFN-γ in supernatants were obtained from BD PharMingen (San Diego, CA). The IL-13 ELISA kit was purchased from R&D Systems. The limits of detection for each assay were as follows: 4 pg/ml for IL-4, IL-5, and IFN-γ; and 1.5 pg/ml for IL-13.
Tracking of Transferred DCs
DCs from C57BL/6 mice were labeled with carboxyfluorescein diacetate succinimidylester (CFSE) for 12 hours before intratracheal transfer. Twenty-four hours, 48 h, 96 h, and 7 d after bone marrow–derived DC (BMDC) transfer in the absence of challenge, BAL fluid, lungs, peribronchial lymph nodes, and spleens were taken. Cells were isolated from each organ and analyzed by flow cytometry for CFSE+ CD11c+ staining.
Statistical Analysis
The ANOVA t test was used to determine differences between two groups, and the Tukey-Kramer test was used for comparisons between multiple groups. Mann-Whitney U tests were also used to determine if the levels of difference between all groups remained significant, even if the underlying distribution was uncertain. The data were pooled from three independent experiments with four mice/group in each experiment (n = 12). Comparisons for all pairs were performed by Kruskal-Wallis test. Significance was assumed at P values of < 0.05 for all tests. Values for all measurements are expressed as means ± SEM.
RESULTS
Effect of Locally Administered Allergen-Pulsed DCs followed by Allergen Challenge
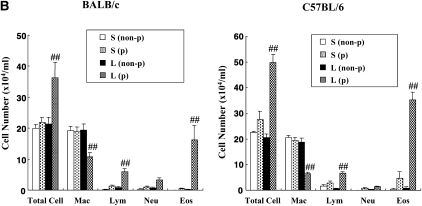
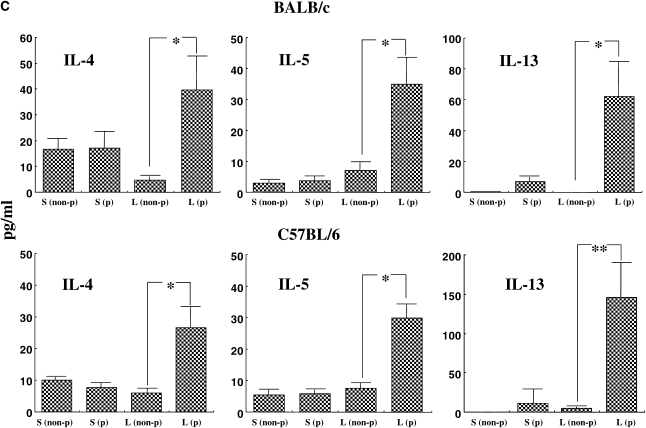
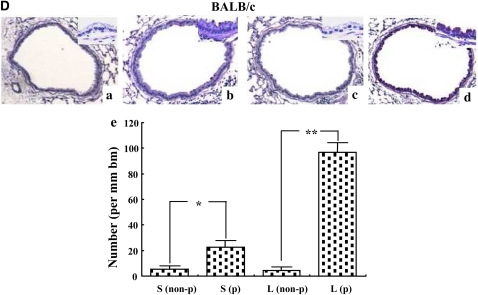
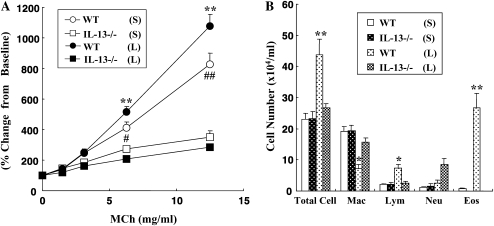
We initially examined the effects of DC transfer in recipients that were challenged at two time-points after transfer. Naive mice received OVA-pulsed BMDCs intratracheally on Day 0, then were challenged with OVA via the airways on Days 2 to 4 (short) or from Days 10 to 12 (long). As shown in Figure 1A, AHR developed under both the short and long protocols in both BALB/c and C57BL/6 mice after transfer of OVA-pulsed DCs but not after transfer of nonpulsed DCs. In C57BL/6 mice, the degree of AHR in the long protocol was greater than in the short protocol (Figure 1A). As reported previously, the responsiveness to MCh was different between these strain of mice (19, 20); the values of Rl in C57BL/6 mice were comparable to those in BALB/c mice at higher concentrations of MCh after adoptive transfer of OVA-pulsed DCs and OVA challenge. The numbers of eosinophils and lymphocytes in BAL fluid were also significantly increased under the long but not short protocol in both strains (Figure 1B). Assays of levels of Th2 cytokines in BAL fluid showed that intratracheal transfer of OVA-pulsed DCs induced significant increases in both strains compared with transfer of nonpulsed DCs, but these increases were only seen under the long protocol (Figure 1C). PAS staining of lung sections revealed that intratracheal administration of OVA-pulsed DCs induced goblet cell metaplasia under the long protocol; PAS+ cells were also increased under the short protocol, but to a lesser degree (Figure 1D).
Figure 1.
Effect of dendritic cell (DC) transfer on lung allergic responses. (A) Changes in airway resistance (Rl) after the short and long protocols. Increasing concentrations of nebulized MCh were administered through the tracheal cannula 48 hours after the last ovalbumin (OVA) challenge in BALB/c and C57BL/6 mice. (B) Bronchoalveolar lavage (BAL) cellular composition in BALB/c and C57BL/6 mice. (C) Cytokine levels in BAL fluid. (D) Sections of lung tissues obtained 48 hours after the last challenge were stained with periodic acid-Schiff (PAS). Representative histologic sections (BALB/c [a–d] and C57BL/6 mice [e–h]) after the short protocol with OVA nonpulsed DCs (a, f), short protocol with OVA-pulsed DCs (b, g), long protocol with OVA nonpulsed DCs (c, h), and long protocol with OVA-pulsed DCs (d, i). Quantitative analysis of PAS+ cells in BALB/c mice (e) and C57BL/6 mice (j). Data represent the means ± SEM from three independent experiments consisting of four mice/group (n = 12). *P < 0.05 or **P < 0.01 compared with recipients of OVA non-pulsed DCs in short protocol or as indicated. ##P < 0.01 compared with recipients of OVA non-pulsed DCs in long protocol. S: short protocol, L: long protocol, non-p: DCs were not pulsed with OVA, p: DCs were pulsed with OVA. Mac: macrophages, Lym: lymphocytes, Neu: neutrophils, Eos: eosinophils, bm: basement membrane.
Allergen-Specific Responses in Lung T Cells after DC Transfer
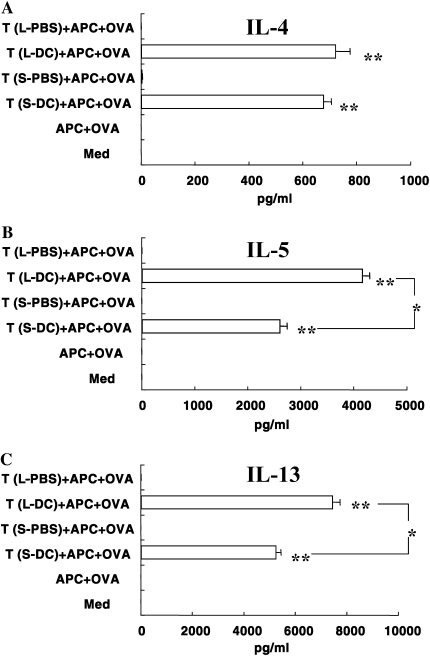
To investigate T cell responsiveness to allergen after DC transfer, lung T cells from C57BL/6 mice were purified, cultured in vitro, and supernates assayed for Th2-type cytokine production. Lung T cells from mice that received nonpulsed DC followed by allergen challenge did not show any production of cytokines, whereas lung T cells from recipients of allergen-pulsed DCs produced Th2-type cytokines in response to allergen (Figure 2). The levels of IL-5 and IL-13 from lung T cells (long protocol) were significantly higher than those obtained under the short protocol (Figure 2). The results were similar when lung T cells from BALB/c mice were assayed (data not shown).
Figure 2.
In vitro cytokine levels. Cytokine levels were determined in supernates from co-cultures of lung T cells together with irradiated spleen MNCs and OVA. Lung T cells from mice that received DCs were isolated as described in Materials and Methods. The lung T cells (2 × 105/well) were cultured with irradiated spleen MNCs (2 × 105/well) and OVA (10 μg/ml) in 96-well culture plates for 72 hours. T (L-PBS): Lung T cells from mice that received PBS after the long protocol. T (L-DC): Lung T cells from mice that received OVA-pulsed DC after the long protocol. T (S-PBS): Lung T cells from mice that received PBS after the short protocol. T (S-DC): Lung T cells from mice that received OVA-pulsed DC transfer after the short protocol. Data represent means ± SEM from three independent experiments performed in triplicate. *P < 0.05 or **P < 0.01 comparing PBS groups to DC groups, or between the groups indicated.
Trafficking of Intratracheally Transfered DCs
To investigate the fate of transferred DCs, DCs from C57BL/6 mice were labeled with CFSE and tissues were examined by flow cytometry gating on CFSE+CD11c+ cells. Transferred DCs were observed by 24 hours in BAL fluid and lung parenchyma. After 48 hours, transferred DCs were detected in draining lymph nodes. Few transferred DCs were found in the spleen (Figure 3). Experiments performed in the same manner in BALB/c mice revealed similar results (data not shown).
Figure 3.
Localization of carboxyfluorescein diacetate succinimidylester (CFSE)-labeled DCs after intratracheal administration. CFSE-labeled DCs from B6 mice (1 × 106) were transferred via the trachea, then were detected in BAL fluid, lungs, peribronchial lymph nodes, and spleen by flow cytometry. Transferred DCs could be discriminated from endogenous DCs by the green fluorescence of CFSE. (A) Representative scattergram of CFSE-labeled DCs detected in the peribronchial lymph node cells at different time-points. (B) Kinetics of CFSE-labeled DCs in BAL fluid (open circles), lungs (solid circles), peribronchial lymph nodes (solid squares), and spleen (open squares). Each time point represents four mice/group in two independent experiments (n = 8).
The Role of IL-13 in DC Transfer-Induced AHR and Allergic Airway Inflammation
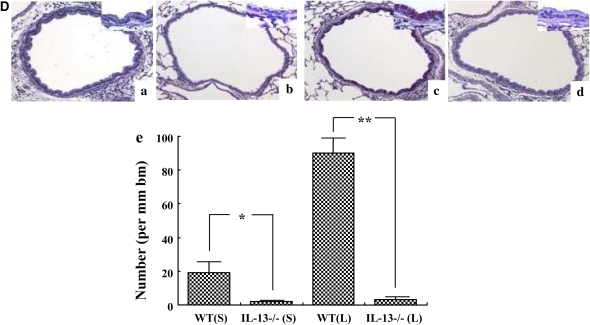
To determine the role of IL-13 in the development of lung allergic responses after DC transfer, IL-13–deficient or WT recipients were studied after transfer of WT DCs. After intratracheal administration of OVA-pulsed DCs and allergen challenge, IL-13–deficient mice failed to develop AHR or airway eosinophilia under both the short and long protocols (Figures 4A and 4B). Levels of IL-4, IL-5, and IL-13 in BAL fluid in the IL-13–deficient mice that received WT DCs were significantly lower compared with WT recipient mice (Figure 4C), whereas the levels of IFN-γ were not significantly different (data not shown). IL-13–deficient mice also failed to develop goblet cell metaplasia under both protocols (Figure 4D).
Figure 4.
Comparison of the response of wild-type (WT) and IL-13–deficient mice to DC transfer. (A) Changes in Rl in WT or IL-13–deficient (IL-13−/−) mice that received OVA-pulsed DCs after the short or long protocol. (B) BAL cellular composition. (C) Cytokine levels in BAL fluid. (D) Representative PAS-stained histologic sections of lung tissues obtained 48 hours after the last challenge in (a) WT recipients of OVA-pulsed DCs after the short protocol, (b) IL-13–deficient recipients of OVA-pulsed DCs after the short protocol, (c) WT recipients of OVA-pulsed DCs after the long protocol, (d) IL-13–deficient recipients of OVA-pulsed DCs after the long protocol, (e) Quantitative analysis of PAS+ cells. WT (S): WT mice following the short protocol. IL-13−/− (S): IL-13–deficient mice after the short protocol. WT (L): WT mice in long protocol. IL-13−/− (L): IL-13–deficient mice after the long protocol. Data represent means ± SEM from three independent experiments (n = 12). *P < 0.05 or **P < 0.01 compared with IL-13–deficient recipients of OVA-pulsed DCs after the long protocol. #P < 0.05 or ##P < 0.01 compared with IL-13–deficient recipients of OVA-pulsed DCs in short protocol. Mac: macrophages, Lym: lymphocytes, Neu: neutrophils, Eos: eosinophils, bm: basement membrane.
The Role of CD4+ and CD8+ T Cells
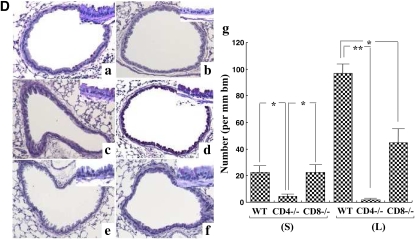
To determine the role of CD4+ or CD8+ T cells in the development of lung allergic responses after DC transfer and allergen challenge, CD4-deficient and CD8-deficient recipient mice were compared with WT recipients. Intratracheal administration of OVA-pulsed DCs into CD4-deficient mice induced little AHR or airway eosinophilia in both protocols (Figures 5A and 5B). Transfer of DCs to CD8-deficient mice did lead to some AHR, comparable to that in WT recipients, under the short protocol. However, under the long protocol, CD8-deficient mice showed significantly lower AHR and airway eosinophilia compared with WT mice (Figures 5A and 5B). Levels of IL-4, IL-5, and IL-13 in BAL fluid from CD4- or CD8-deficient mice receiving OVA-pulsed WT DCs were also significantly lower compared with WT recipients under the long protocol (Figure 5C), whereas the levels of IFN-γ were not significantly different (data not shown). Similarly, goblet cell metaplasia in CD4- or CD8-deficient mice was also significantly lower than in WT mice recipients (long protocol), while CD8-deficient mice but not CD4-deficient mice did develop an increase in goblet cell numbers in the short protocol, similar to WT recipients (Figure 5D).
Figure 5.
Effects of CD4 and CD8 T cell depletion on the response to DC transfer. (A) Changes in Rl in WT, CD4-deficient, or CD8-deficient recipients of OVA-pulsed DCs after the short or long protocols. CD4−/− (N); CD4-deficient mice in the absence of DC transfer but after OVA challenge. CD8−/− (N); CD8-deficient mice in the absence of DC transfer but after OVA challenge. (B) BAL cell composition. (C) Cytokine levels in BAL fluid (left, IL-4; middle, IL-5; right, IL-13). (D) Representative PAS-stained histologic sections of lung tissues obtained 48 hours after the last challenge: (a) WT mice that received OVA-pulsed DCs after the short protocol, (b) CD4-deficient mice that received OVA-pulsed DCs after the short protocol, (c) CD8-deficient mice that received OVA-pulsed DCs after the short protocol, (d) WT mice that received OVA-pulsed DCs after the long protocol, (e) CD4-deficient mice that received OVA-pulsed DCs after the long protocol, (f) CD8-deficient mice that received OVA-pulsed DCs after the long protocol, and (g) quantitative analysis of PAS+ cells. Data represent means ± SEM from three independent experiments (n = 12). *P < 0.05 or **P < 0.01 compared with WT mice that received OVA-pulsed DCs in long protocol. ##P < 0.01 compared with WT mice that received OVA-pulsed DCs in short protocol. Mac: macrophages, Lym: lymphocytes, Neu: neutrophils, Eos: eosinophils, bm: basement membrane.
DISCUSSION
DCs play a critical role in the innate immune response as well as in the initiation of adaptive immune responses through the priming of naive T cells. To function efficiently as APCs, DCs need to have matured after receiving a signal from a pathogen, often referred as a danger signal. BMDCs are differentiated, mature myeloid type DCs (22). In contrast, resident lung DCs are a mixed population of myeloid, plasmacytoid, and a recently identified CD103+ subset of DCs (23). Interestingly, among these DCs, only BMDCs have been shown to be potent inducers of AHR and allergic airway inflammation. This may explain some of the controversy of the failure of resident lung DCs to induce airway allergic inflammation in some reports (11, 24). Stampfli and coworkers showed that the overexpression of GM-CSF in bronchial mucosa induced the maturation of lung resident DCs and, after allergen challenge, resulted in airway allergic inflammation (25). In addition, diesel exhaust particles were shown to lead to the maturation of lung DCs, which primarily induced Th2 airway inflammation (26). Based on these findings, it appears that the maturation level of airway DCs is a pivotal component in the development of Th2-type airway inflammation in conjunction with AHR.
In the present study, intratracheally administered OVA-pulsed DCs were initially detected in BAL fluid and lung tissue, and later in draining lymph nodes before allergen challenge. After the two protocols, which varied the interval between DC transfer and allergen challenge, these DCs were capable of priming the recipients to allergen, as detected by the development of AHR in vivo and cytokine production in response to in vitro culture of lung T cells with OVA. However, after allergen challenge at the two different time-points after DC transfer, the responses could be distinguished when airway allergic inflammation, including eosinophilia, levels of Th2-type cytokines in BAL fluid, and goblet cell metaplasia were monitored. The differences observed after the two protocols were likely attributable to differences in the localization of transferred DCs before allergen challenge. In the short protocol, transferred DCs were not detected in the draining lymph nodes before allergen challenge, whereas allergen challenge was only begun after DCs had trafficked to the draining lymph nodes in the long protocol. Previous work has suggested that DCs migrate to draining lymph nodes to prime T cells after uptake of antigen (27). The results from this study indicated that transferred DCs also remained in the lung and were capable of priming lung T cells to allergen.
In the lungs, there are small populations of many other cell types besides αβ+ T cells, which may play a role in the development of AHR. For example, γδ T cells in the lung, especially in the peribronchial regions, play an important role in the induction of mucosal immune responses (28, 29). Thus, locally transferred DCs could interact with resident γδ T cells, resulting in AHR. However, the present results identify another pathway, since CD4-deficient mice were unable to develop AHR and most of the γδ T cells express CD8+ or are double-negative T cells (30). Based on the report that γδ T cells have the potential to present antigen to conventional αβ T cells (31), resident γδ T cells might enhance the transfer of information from DCs to resident CD4+ T cells. Further, locally administered DCs may interact with other resident cell types in the airways, such as B cells, mast cells, airway epithelial cells, or smooth muscle cells. A recent study showed that airway epithelial cells are a potent source of cytokines and chemokines, such as GM-CSF, IL-1β, or TNF-α (32), which are all known to activate DCs. Thymic stromal lymphopoietin (TSLP), which was recently demonstrated to induce Th2-type inflammation by modulating the activity of DCs (33), is also produced from epithelial cells and activates mast cells to produce Th2 cytokines (34). Therefore, transferred DCs might interact with airway epithelial cells to produce TSLP, which stimulate mast cells to secrete a variety of chemical mediators or cytokines, such as histamine or IL-13, contributing to the development of AHR. DCs also express receptors for neuropeptides and they are capable of releasing substance P and neurokinin A (NKA), and these neurokinins modulate smooth muscle cells through neurokinin receptor interactions (35). Potential autocrine or paracrine mechanisms may modulate airway function, but in the absence of inflammatory cell accumulation in the airways, as shown in the short protocol (36). Thus, the local instillation of allergen-pulsed DCs may activate distinct pathways that are associated with the development of AHR in the presence or absence of allergic airway inflammation.
In the present study, IL-13 was shown to play a role in the development of AHR and airway inflammation in both protocols using IL-13–deficient mice. Recent studies also suggest that IL-13 is produced not only by T cells, including Th2-polarized CD4 or CD8 T cells, but also non–T cell populations such as mast cells, basophils, and eosinophils (37, 38). Bellinghausen and colleagues reported that BMDCs can produce IL-13 in vitro (39). Nevertheless, IL-13 production from DCs was not likely essential, as there was no significant effect of administration of IL-13–sufficient DCs into IL-13–deficient recipients when AHR, airway inflammation, and induction of Th2-cytokine responses were examined, even in the long protocol. It is possible that IL-13 was released from another cell type. Temann and coworkers reported that IL-9 can induce the production of IL-13 from airway epithelium in the absence of inflammatory cells such as T cells or eosinophils (40). Padilla and colleagues demonstrated that endogenous IL-13 in the sensitization phase induces DC maturation in the lung and elicits strong immune responses to inhaled antigen (41), suggesting that IL-13 could play some role in the initial development of responsiveness in this DC transfer model. However, the failure to restore AHR after adoptive transfer of allergen-pulsed, IL-13–sufficient DCs into IL-13–deficient recipients suggested a critical role for IL-13 in the allergen challenge phase (effector phase) rather than in the sensitization phase (initial phase). Other studies have also indicated that IL-13 is an essential cytokine in the effector phase response, whereas IL-4 may be more critical for the initial development and expansion of antigen-specific Th2 polarized cells (41–43).
To determine the role of T cells in the response to transfer of allergen-pulsed DCs, CD4- or CD8-deficient mice were investigated. CD4+ T cells, similar to IL-13, were shown to be essential for the development of AHR and airway inflammation under both protocols (Figure 5). In the long protocol, the deficiency of CD8+ T cells was seen to result in a marked decrease in AHR and allergic inflammation compared with WT recipients, whereas AHR still developed in these mice under the short protocol. We recently reported that the functional activation of CD8+ T cells in a similar allergic airway model required CD4+ T cells from IL-4–sufficient mice in the sensitization phase but not in the challenge phase (44). Therefore, after longer periods as in the long protocol, the functional activation of CD8+ T cells induced after initial DC–CD4+ T cell interactions may play a more critical role in the development of AHR and airway inflammation. This is supported by other studies, which showed that the development of memory CD8+ T cells to bacterial or viral challenges are impaired in CD4+ T cell–deficient animals (45, 46). APC–CD4+ T cell interactions are also critical for induction of effective CD8+ CTL responses (47). Taken together, and as illustrated in Figure 6, transferred DCs may initiate contact with CD4+ T cells in the local environment and prime and activate them, as observed under the short protocol. With additional time and after DC migration into the draining lymph nodes, in cooperation with primed CD4+ T cells, priming and activation of CD8+ T cells occurs as suggested in the long protocol, resulting in the full and enhanced development of altered airway function and airway inflammation. Allergen-specific immunoglobulin, IgE and IgG1, have all been demonstrated to play a role in the development of allergic inflammation under different conditions (48). However, the serum levels of OVA-specific IgE and IgG1 were very low in the DC transfer model, far lower than seen after systemic sensitization and challenge (data not shown). Thus, these results are similar to those seen when B cell–deficient mice were sensitized (systemically) and challenged, but nonetheless developed comparable levels of AHR to WT mice (49).
Figure 6.
Schematic illustration of the potential mechanisms underlying responses after the short and long DC transfer protocol. Transferred DCs initially make contact and prime CD4+ T cells in the airways and later migrate to draining lymph nodes, where they further prime naive T cells. Subsequent antigen challenge induces AHR and goblet cell metaplasia mediated by CD4+ T cells in the short protocol. After the long protocol, there is further interaction, activation, and priming of both CD4+ and CD8+ T cells in the draining lymph nodes, and allergen exposure then induces a marked eosinophilia and Th2 cytokine production as well as AHR and goblet cell metaplasia.
A significant feature of DC transfer under the short protocol was the ability to elicit AHR after allergen challenge in the apparent absence of an accompanying and robust BAL (and tissue) eosinophilia or elevation of Th2 cytokine levels; only a modest increase in goblet cell numbers was detected. This dissociation of AHR and airway eosinophilia has been previously reported under varying experimental conditions (50–53) and was obviously different from the long protocol, in which AHR was associated with a marked airway eosinophilia, Th2 cytokine elevations, and goblet cell metaplasia. Further, the dissociation of AHR and eosinophilia was recently attributed to strain-specific differences (54), but here, after the short protocol, both BALB/c and C57BL/6 mice developed AHR (albeit to a lower degree in the latter). The development of AHR after the short protocol was dependent on antigen pulsing of DCs before transfer as well as CD4+ but not CD8+ T cells. IL-13 was also required as IL-13–deficient mice failed to develop AHR. Whether this induction of AHR represents a different mechanism than that observed in association with airway eosinophilia is unclear at present. At a minimum, the dissociation may only indicate that the pathways activated under both conditions differ quantitatively and not qualitatively, and that the requirements for AHR development are less than required for airway eosinophilia. The requirement for development of increases in goblet cell numbers may lie between, and all three responses are absolutely IL-13 dependent.
In summary, we have investigated the interactions between DCs and CD4+ and CD8+ T cells. When allergen-pulsed DCs were given intratracheally followed by allergen challenge after a short interval, in both BALB/c and C57BL6 mice AHR and a modest but significant goblet metaplasia developed. These findings were not observed in CD4- or IL-13–deficient mice, but were observed in CD8-deficient animals. When the interval between DC transfer and allergen challenge was extended, the mice fully developed AHR and airway allergic inflammation, but these responses were restricted to WT mice and not seen to the same degree in any of the deficient mice; these responses were significantly lower in CD8-deficient mice, and almost completely eliminated in the CD4- or IL-13–deficient animals. These results identify a complex series of interactions between DCs on one hand, and T cell subsets on the other hand, governed to some extent by the location where these interactions take place.
Acknowledgments
The authors are grateful for the expert help of Diana Nabighian in preparing the manuscript and to Lynn Cunningham for performing the immunolabeling studies.
This work was supported by grants HL-36577, HL-61005, and AI-77609 from National Institutes of Health, and grant R825702 from the Environmental Protection Agency (all to E.W.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0256OC on January 16, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Busse WW, Lemanske RF Jr. Asthma. N Engl J Med 2001;344:350–362. [DOI] [PubMed] [Google Scholar]
- 2.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 1999;17:255–281. [DOI] [PubMed] [Google Scholar]
- 3.Stumbles PA, Thomas JA, Pimm CL, Lee PT, Venaille TJ, Proksch S, Holt PG. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J Exp Med 1998;188:2019–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McWilliam AS, Nelson DJ, Holt PG. The biology of airway dendritic cells. Immunol Cell Biol 1995;73:405–413. [DOI] [PubMed] [Google Scholar]
- 5.Lambrecht BN, Pauwels RA, Fazekas De St Groth B. Induction of rapid T cell activation, division, and recirculation by intratracheal injection of dendritic cells in a TCR transgenic model. J Immunol 2000;164:2937–2946. [DOI] [PubMed] [Google Scholar]
- 6.Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest 2000;106:551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung S, Rose CE, Fu SM. Intratracheal priming with ovalbumin- and ovalbumin323–339 peptide-pulsed dendritic cells induces airway hyperresponsiveness, lung eosinophilia, goblet cell hyperplasia, and inflammation. J Immunol 2001;166:1261–1271. [DOI] [PubMed] [Google Scholar]
- 8.van Rijt LS, Vos N, Willart M, Kleinjan A, Coyle AJ, Hoogsteden HC, Lambrecht BN. Essential role of dendritic cell CD80/CD86 costimulation in the induction, but not reactivation, of Th2 effector responses in a mouse model of asthma. J Allergy Clin Immunol 2004;114:166–173. [DOI] [PubMed] [Google Scholar]
- 9.Hammad H, de Heer HJ, Soullie T, Angeli V, Trottein F, Hoogsteden HC, Lambrecht BN. Activation of peroxisome proliferator-activated receptor-gamma in dendritic cells inhibits the development of eosinophilic airway inflammation in a mouse model of asthma. Am J Pathol 2004;164:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitamura K, Takeda K, Koya T, Miyahara N, Kodama T, Dakhama A, Takai T, Hirano A, Tanimoto M, Harada M, et al. Critical role of the Fc receptor γ-chain on APCs in the development of allergen-induced airway hyperresponsiveness and inflammation. J Immunol 2007;178:480–488. [DOI] [PubMed] [Google Scholar]
- 11.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 2002;196:1645–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang YH, Suen JL, Chiang BL. Fas-ligand-expressing adenovirus-transfected dendritic cells decrease allergen-specific T cells and airway inflammation in a murine model of asthma. J Mol Med 2006;84:595–603. [DOI] [PubMed] [Google Scholar]
- 13.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med 2005;202:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature 1991;353:180–184. [DOI] [PubMed] [Google Scholar]
- 15.Fung-Leung WP, Schilham MW, Rahemtulla A, Kundig TM, Vollenweider M, Potter J, van Ewijk W, Mak TW. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell 1991;65:443–449. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie AN. Impaired development of Th2 cells in IL-13-deficient mice. Immunity 1998;9:423–432. [DOI] [PubMed] [Google Scholar]
- 17.Koya T, Kodama T, Takeda K, Miyahara N, Yang ES, Taube C, Joetham A, Park JW, Dakhama A, Gelfand EW. Importance of myeloid dendritic cells in persistent airway disease after repeated allergen exposure. Am J Respir Crit Care Med 2006;173:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med 1997;186:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ewart SL, Kuperman D, Schadt E, Tankersley C, Grupe A, Shubitowski DM, Peltz G, Wills-Karp M. Quantitative trait loci controlling allergen-induced airway hyperresponsiveness in inbred mice. Am J Respir Cell Mol Biol 2000;23:537–545. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol 2001;281:394–402. [DOI] [PubMed] [Google Scholar]
- 21.Koya T, Takeda K, Kodama T, Miyahara N, Matsubara S, Balhorn A, Joetham A, Dakhama A, Gelfand EW. Rantes (CCl5) regulates airway responsiveness after repeated allergen challenge. Am J Respir Cell Mol Biol 2006;35:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992;176:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung SS, Fu SM, Rose CE Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alpha)-beta7 integrin-positive epithelial dendritic cell population expressing langerin and tight junction proteins. J Immunol 2006;176:2161–2172. [DOI] [PubMed] [Google Scholar]
- 24.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol 2001;2:725–731. [DOI] [PubMed] [Google Scholar]
- 25.Stampfli MR, Wiley RE, Neigh GS, Gajewska BU, Lei XF, Snider DP, Xing Z, Jordana M. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J Clin Invest 1998;102:1704–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleck B, Tse DB, Jaspers I, Curotto de Lafaille MA, Reibman J. Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation. J Immunol 2006;176:7431–7437. [DOI] [PubMed] [Google Scholar]
- 27.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol 2000;18:767–811. [DOI] [PubMed] [Google Scholar]
- 28.Wands JM, Roark CL, Aydintug MK, Jin N, Hahn YS, Cook L, Yin X, Dal Porto J, Lahn M, Hyde DM, et al. Distribution and leukocyte contacts of γδ T cells in the lung. J Leukoc Biol 2005;78:1086–1096. [DOI] [PubMed] [Google Scholar]
- 29.Born WK, Reardon CL, O'Brien RL. The function of γδ T cells in innate immunity. Curr Opin Immunol 2006;18:31–38. [DOI] [PubMed] [Google Scholar]
- 30.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 2000;18:975–1026. [DOI] [PubMed] [Google Scholar]
- 31.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human γδ T cells. Science 2005;309:264–268. [DOI] [PubMed] [Google Scholar]
- 32.Stick SM, Holt PG. The airway epithelium as immune modulator: the LARC ascending. Am J Respir Cell Mol Biol 2003;28:641–644. [DOI] [PubMed] [Google Scholar]
- 33.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002;3:673–680. [DOI] [PubMed] [Google Scholar]
- 34.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med 2007;204:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joos GF, Germonpre PR, Pauwels RA. Role of tachykinins in asthma. Allergy 2000;55:321–337. [DOI] [PubMed] [Google Scholar]
- 36.Lambrecht BN. Immunologists getting nervous: neuropeptides, dendritic cells and T cell activation. Respir Res 2001;2:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev 2004;202:175–190. [DOI] [PubMed] [Google Scholar]
- 38.Schmid-Grendelmeier P, Altznauer F, Fischer B, Bizer C, Straumann A, Menz G, Blaser K, Wuthrich B, Simon HU. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol 2002;169:1021–1027. [DOI] [PubMed] [Google Scholar]
- 39.Bellinghausen I, Brand P, Bottcher I, Klostermann B, Knop J, Saloga J. Production of interleukin-13 by human dendritic cells after stimulation with protein allergens is a key factor for induction of T helper 2 cytokines and is associated with activation of signal transducer and activator of transcription-6. Immunology 2003;108:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Temann UA, Laouar Y, Eynon EE, Homer R, Flavell RA. IL9 leads to airway inflammation by inducing IL-13 expression in airway epithelial cells. Int Immunol 2007;19:1–10. [DOI] [PubMed] [Google Scholar]
- 41.Padilla J, Daley E, Chow A, Robinson K, Parthasarathi K, McKenzie AN, Tschernig T, Kurup VP, Donaldson DD, Grunig G. IL-13 regulates the immune response to inhaled antigens. J Immunol 2005;174:8097–8105. [DOI] [PubMed] [Google Scholar]
- 42.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 43.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koya T, Miyahara N, Takeda K, Matsubara S, Matsuda H, Swasey C, Balhorn A, Dakhama A, Gelfand EW. CD8+ T cell-mediated airway hyperresponsiveness and inflammation is dependent on CD4+IL-4+ T cells. J Immunol 2007;179:2787–2796. [DOI] [PubMed] [Google Scholar]
- 45.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 2003;300:339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003;421:852–856. [DOI] [PubMed] [Google Scholar]
- 47.Xiang J, Huang H, Liu Y. A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J Immunol 2005;174:7497–7505. [DOI] [PubMed] [Google Scholar]
- 48.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol 2008;8:205–217. [DOI] [PubMed] [Google Scholar]
- 49.Hamelmann E, Takeda K, Schwarze J, Vella AT, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness requires IL-5 but not IgE or B lymphocytes. Am J Respir Cell Mol Biol 1999;21:480–489. [DOI] [PubMed] [Google Scholar]
- 50.Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, Wang H, Biechelle TL, O'Neill KR, Ansay TL, et al. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol 2003;170:3296–3305. [DOI] [PubMed] [Google Scholar]
- 51.Joetham A, Takeda K, Taube C, Miyahara N, Kanehiro A, Dakhama A, Gelfand EW. Airway hyperresponsiveness in the absence of CD4+ T cells after primary but not secondary challenge. Am J Respir Cell Mol Biol 2005;33:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med 1996;183:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, et al. A critical role for eosinophils in allergic airways remodeling. Science 2004;305:1776–1779. [DOI] [PubMed] [Google Scholar]
- 54.Whitehead GS, Walker JK, Berman KG, Foster WM, Schwartz DA. Allergen-induced airway disease is mouse strain dependent. Am J Physiol 2003;285:L32–L42. [DOI] [PubMed] [Google Scholar]