Abstract
Transforming growth factor-β (TGF-β) is a cytokine implicated in wound healing and in the pathogenesis of pulmonary fibrosis. TGF-β stimulates myofibroblast differentiation characterized by expression of contractile smooth muscle (SM)-specific proteins such as SM–α-actin. In the present study, we examined the role of serum response factor (SRF) in the mechanism of TGF-β–induced pulmonary myofibroblast differentiation of human lung fibroblasts (HLF). TGF-β stimulated SM–α-actin expression in HLF, which paralleled with a profound induction of SRF expression and activity. Inhibition of SRF by the pharmacologic SRF inhibitor (CCG-1423), or via adenovirus-mediated transduction of SRF short hairpin RNA (shSRF), blocked the expression of both SRF and SM–α-actin in response to TGF-β without affecting Smad-mediated signaling of TGF-β. However, forced expression of SRF on its own did not promote SM–α-actin expression, whereas expression of the constitutively transactivated SRF fusion protein (SRF-VP16) was sufficient to induce SM–α-actin expression, suggesting that both expression and transactivation of SRF are important. Activation of protein kinase A (PKA) by forskolin or iloprost resulted in a significant inhibition of SM–α-actin expression induced by TGF-β, and this was associated with inhibition of both SRF expression and activity, but not of Smad-mediated gene transcription. In summary, this is the first direct demonstration that TGF-β–induced pulmonary myofibroblast differentiation is mediated by SRF, and that inhibition of myofibroblast differentiation by PKA occurs through down-regulation of SRF expression levels and SRF activity, independent of Smad signaling.
Keywords: transforming growth factor-β, serum response factor, myofibroblast, protein kinase A, Smad
CLINICAL RELEVANCE
This study demonstrates the important role of serum response factor in mediating pulmonary myofibroblast differentiation by transforming growth factor-β. Inhibition of serum response factor may be a useful strategy for treating pulmonary fibrosis.
Idiopathic pulmonary fibrosis (IPF) is a progressive, fatal disease characterized by parenchymal fibrosis and structural distortion of the lungs. There is currently no effective treatment, and novel targets of pharmacologic intervention need to be identified. Alveolar fibroblasts, under stimulation by transforming growth factor (TGF)-β, respond by altering their gene expression profile, with de novo expression of cytoskeletal and contractile proteins normally found within smooth muscle cells (1, 2), resulting in a phenotype that is in an intermediary state between fibroblasts and smooth muscle cells, termed the myofibroblast (3, 4). Smooth muscle (SM)–α-actin, a contractile protein incorporated into stress filaments, is an accepted marker for myofibroblast differentiation.
TGF-β, a cytokine implicated in the pathogenesis of both experimental pulmonary fibrosis and human disease (5–8), is a well-established inducer of myofibroblast differentiation. At the cellular level, TGF-β is known to signal through transmembrane receptor serine/threonine kinases, phosphorylating and activating its canonical signaling intermediates, receptor-activated Smad proteins (Smad2/3), leading to their heterotrimerization with common-mediator Co-Smad (Smad4), nuclear accumulation of the complex, and activation of Smad-binding elements (SBEs), which modulate the transcription of target genes (9, 10). Previous studies in cultured lung fibroblasts have implicated SBEs in mediating TGF-β–induced SM–α-actin promoter activation (11–13), while others have implicated a TGF-β control element (TCE) activated by Sp1/3 transcription factors and regulated by Kruppel-like factors (14–16). In addition, it was shown that the cAMP-elevating agonist, prostaglandin E2, attenuates TGF-β–induced pulmonary myofibroblast differentiation without affecting Smad signaling (17, 18).
In vascular smooth muscle cells (VSMC), SM-gene transcription is controlled primarily through the binding of serum response factor (SRF) to its target cis-elements, the CArG boxes (CC(AT)6GG) within the promoter regions of most SM genes (19). SRF activation is commonly induced by G protein–coupled receptor agonists such as endothelin-1 (20), which, as we showed previously, is a powerful stimulator of SM gene expression in VSMC (21). Furthermore, we also showed that SRF activity is highly sensitive to regulation by protein kinase A (PKA) in VSMC (21). However, the role of SRF in TGF-β–induced myofibroblast differentiation remains obscure. Previous studies employing CArG mutagenesis of mouse SM–α-actin promoter (16), or decoy CArG oligonucleotides (22), did not suggest an absolute requirement of CArGs for TGF-β–induced SM-actin transcription in lung fibroblasts. These data contrast with the evidence for a CArG requirement for activation of SM–α-actin expression by TGF-β in an rat fibroblast cell line (23).
Given these conflicting observations, we sought to (1) examine the role of SRF in TGF-β–induced pulmonary myofibroblast differentiation by using pharmacologic and siRNA approaches, and (2) test whether SRF regulation by PKA may explain the inhibition of TGF-β–induced myofibroblast differentiation by cAMP-elevating agents.
MATERIALS AND METHODS
Isolation and Primary Culture of Human Pulmonary Fibroblasts
Tissue samples from explanted lungs from patients undergoing lung transplantation for pulmonary fibrosis were obtained and placed in Dulbecco's modified Eagle's medium (DMEM). Alveolated lung was minced, washed in PBS, and plated onto 10-cm plates in growth media containing DMEM supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/ml streptomycin, 250 ng/ml amphotericin B, and 100 U/ml penicillin. Expanded populations of fibroblasts were subsequently subcultured after 4 to 5 days, resulting in the development of a homogenous fibroblast population. All primary cultures were used from passage 5–10, and grown on plastic. In all experiments, cells were serum-deprived overnight in DMEM containing 0.1% bovine serum albumin (BSA) and 2 mM L-glutamine before stimulation with agonists.
Transient DNA Transfection and Adenovirus-Mediated Gene Transduction
Transient DNA transfections were performed using LipofectAMINE-PLUS reagent (Invitrogen) following the standard manufacturer's protocol. Adenovirus-mediated gene transduction was performed by incubating cells with desired adenoviruses (100 plaque-forming units [pfu] per cell) in the medium containing 0.1% BSA, as described previously (24).
DNA and Other Reagents
The plasmid for wild-type SRF was provided by Dr. Julian Solway (University of Chicago, Chicago, IL). The plasmid for a constitutively active SRF-VP16 chimeric protein, in which the C-terminal trans-activation domain of SRF was replaced by the trans-activation domain of the viral co-activator VP16 (25), and the plasmid for the chimeric protein GAL4-VP16, were kind gifts from Dr. Joseph Miano (University of Rochester, Rochester, NY). The firefly luciferase reporter for the rat −764 base pairs SM–α-actin promoter was a kind gift from Dr. Sem Phan (University of Michigan, Ann Arbor, MI) and was described previously (13). The firefly luciferase reporter driven by two copies of CArG elements (SRF-Luc) was used previously (21, 26–28). The firefly luciferase reporter driven by four copies of Smad-binding elements (SBE-Luc) was provided by Dr. Bert Vogelstein and was used previously (24). The plasmid for the thymidine kinase promoter (TK)-driven renilla luciferase was from Promega (Madison, WI). Recombination-deficient adenovirus expressing short hairpin RNA against SRF (Ad-shSRF), or the control adenovirus expressing shRNA against GFP (Ad-shGFP), were kindly provided by Dr. Joseph Miano (University of Rochester) and were described previously (29). CCG-1423 was obtained from Cayman Chemical (Ann Arbor, MI). Anti-VASP antibodies and TGF-β were from EMD Biosciences (Gibbstown, NJ). Forskolin (FSK) was from Fisher Bioreagents (Fair Lawn, NJ). Antibodies against β-actin and SM–α-actin were from Sigma (St. Louis, MO). SRF and Smad4 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-Smad2 and Smad2 antibodies were from Cell Signaling (Danvers, MA).
Western Blotting
After stimulation of quiescent cells with desired agonists, cells were lysed in RIPA buffer containing 25 mM HEPES (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 2 mM EGTA, 10% glycerol, 1 mM NaF, 200 μM Na-orthovanadate, and protease inhibitors (1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM PMSF). The lysates were cleared from insoluble material by centrifugation at 20,000 × g for 10 minutes, boiled in Laemmli buffer, subjected to polyacrylamide gel electrophoresis, and analyzed by Western blotting with 0.5 μg/ml primary desired primary antibodies, followed by 1:3,000 dilution of HRP-conjugated secondary antibodies (EMD), and developed by an enhanced chemilumeniscence (ECL) reaction (Pierce, Rockford, IL).
Luciferase Reporter Assay
Subconfluent cells were grown in 24-well plates were co-transfected with desired firefly luciferase reporter plasmid (500 ng/ml), and 20 ng/ml TK-Renilla luciferase plasmid. Cells were placed in growth media overnight, and then serum-starved for 24 hours, followed by stimulation with the desired agonists for the relevant times. Cells were then washed with phosphate-buffered saline and lysed in protein extraction reagent. The lysates are assayed for firefly and Renilla luciferase activity using the Promega Dual luciferase assay kit (Promega). To account for differences in transfection efficiency, firefly luciferase activity of each sample is normalized to Renilla luciferase activity.
Statistical Analysis
All the data represents the results of at least three independent experiments. Quantitative data were analyzed by the Student t test, and values of P < 0.05 were considered as statistically significant.
RESULTS
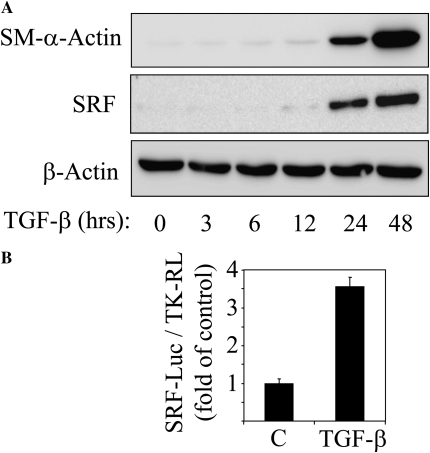
Figure 1A shows a profound induction of SM–α-actin expression by TGF-β in serum-starved human pulmonary fibroblasts, with no change in housekeeping β-actin, indicative of myofibroblast differentiation. Interestingly, SRF, which is thought to be ubiquitously expressed, has extremely low-level expression in resting pulmonary fibroblasts, but its expression is profoundly induced by TGF-β with a time course similar to that of SM–α-actin induction. In this respect, quiescent pulmonary fibroblasts differ dramatically from vascular smooth muscle cells, which express SRF abundantly in the basal state (data not shown). Importantly, TGF-β–induced SRF expression was associated with an increase of SRF activity, as assessed by SRF-luciferase reporter driven by two CArG elements (Figure 1B). This suggests that TGF-β may stimulate SRF-dependent gene transcription by (1) up-regulating SRF expression and (2) stimulating SRF trans-activation.
Figure 1.
Transforming growth factor (TGF)-β–induced expression of smooth muscle (SM)–α-actin is accompanied by expression and activation of serum response factor (SRF). (A) Human lung fibroblasts were grown to subconfluence, serum-starved overnight, and stimulated with 2 ng/ml TGF-β for the indicated times. The cell extracts were analyzed by Western blotting with antibodies against SM–α-actin, SRF, or β-actin as indicated. (B) Subconfluent human lung fibroblasts cells were transfected with SRF-firefly luciferase reporter (SRF-luc) along with thymidine kinase-driven renilla luciferase (TK-RL) control reporter and serum-starved overnight, followed by stimulation with vehicle (C) or 2 ng/ml TGF-β for 24 hours. The activity of firefly luciferase (SRF-Luc) was then measured in cell lysates and normalized to the activity of renilla luciferase (TK-RL). Data represent the results of three experiments performed in triplicate.
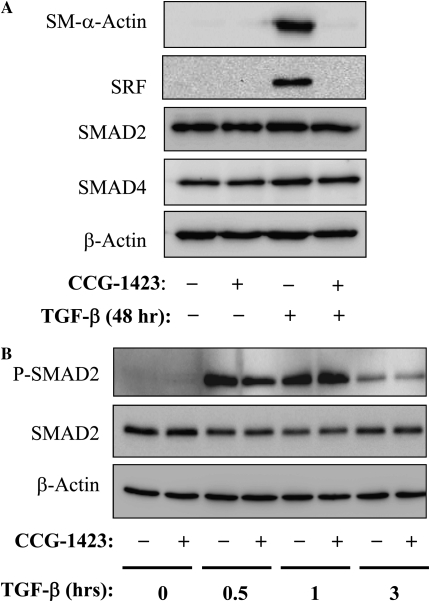
To examine if SRF was required for SM–α-actin expression in response to TGF-β, we first used the recently described pharmacologic inhibitor of SRF, CCG-1423 (30). As shown in Figure 2A, CCG-1423 pre-treatment resulted in a complete inhibition of TGF-β–induced SM-α-actin expression. CCG-1423 also inhibited SRF protein expression in response to TGF-β, which is consistent with the previously reported role of SRF in the induction of its own gene transcription (31, 32). In contrast, CCG-1423 did not affect the expression of Smad2, Smad4, or β-actin (Figure 2A), nor of TGF-β–induced Smad2 phosphophorylation (Figure 2B), suggesting that regulation of TGF-β–induced SM–α-actin expression by CCG-1423 is independent of a canonical Smad signaling.
Figure 2.
Down-regulation of SM–α-actin and SRF expression by pharmacologic SRF inhibitor, CCG-1423. (A) Serum-starved human lung fibroblasts were pretreated with 10 μM CCG-1423 for 1 hour, followed by stimulation with 2 ng/ml TGF-β for 48 hours. The cell extracts were analyzed by Western blotting with antibodies against SM–α-actin, SRF, Smad2, Smad4, or β-actin as indicated. (B) Serum-starved human lung fibroblasts were pretreated with 10 μM CCG-1423 for 1 hour, followed by stimulation with 2 ng/ml TGF-β for indicated times. The cell extracts were analyzed by Western blotting with antibodies against phospho-Smad2 (Ser465/Ser467), total Smad2 or β-actin as indicated.
To further investigate the potential requirement of SRF for SM–α-actin expression, we used an SRF knockdown approach by adenovirus-mediated expression of short hairpin RNA against SRF (Ad-shSRF). As shown in Figure 3, transduction of human pulmonary fibroblasts with Ad-shSRF, but not with control Ad-shGFP, resulted in an efficient knockdown of SRF expression without affecting the expression of Smad2, Smad4, or β-actin. Importantly, the knockdown of SRF was associated with a complete inhibition of SM–α-actin expression in response to TGF-β. Together, the results shown in Figures 2 and 3 strongly suggest that SRF expression and/or activity is required for TGF-β–induced myofibroblast differentiation.
Figure 3.
Down-regulation of SM–α-actin expression by SRF knockdown. Subconfluent human lung fibroblasts were transduced with recombination-deficient adenovirus expressing short hairpin RNA against SRF (Ad-shSRF), or with control adenovirus expressing shRNA against GFP (Ad-shGFP) in 0.1% bovine serum albumin for 48 hours, followed by stimulation of cells with 2 ng/ml TGF-β for an additional 48 hours. The cell extracts were analyzed by Western blotting with antibodies against SRF, SM–α-actin, Smad2, Smad4, or β-actin as indicated.
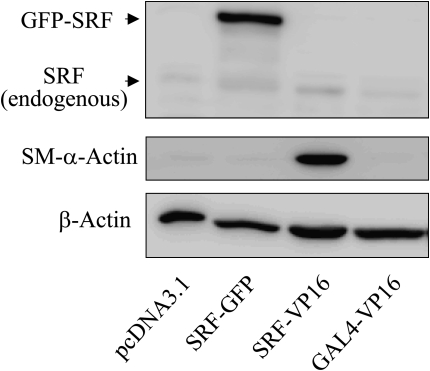
Given the parallel induction of SRF and SM–α-actin by TGF-β (Figure 1) and the dependence of SM–α-actin expression on SRF in response to TGF-β (Figures 2 and 3), we sought to determine whether forced overexpression of SRF was sufficient to drive SM–α-actin expression. Due to limitations in DNA transfection into primary cultured human pulmonary fibroblasts, we used the HT-1080 fibrosarcoma cell line, which permits a high efficiency of transient transfection and also expresses low levels of SRF under the basal state. To discriminate between endogenous and the ectopic SRF, we used GFP-tagged SRF cDNA. As shown in Figure 4, ectopic expression of GFP-SRF failed to increase SM–α-actin protein levels in HT-1080 cells. In contrast, expression of the constitutively active chimeric protein SRF-VP16, in which the C-terminal transcription activation domain of SRF was replaced by the transcription activation domain of the viral co-activator VP16 (25), induced SM–α-actin expression (Figure 4). This effect of SRF-VP16 was specific to SRF, as expression of the GAL4-VP16 chimeric protein (containing the transcription activation domain of VP16, but not the GAL4 DNA-binding domain, which does not interact with the SRF target cis-element CArG box) did not induce SM–α-actin expression (Figure 4). These data suggest that SRF expression on its own is not sufficient for SM–α-actin expression, but that trans-activation of SRF is also required. Interestingly, while SRF-VP16 potently promoted SM–α-actin expression, it did not significantly induce the expression of endogenous SRF (Figure 4), suggesting that additional mechanisms may be required for SRF gene transcription.
Figure 4.
SRF overexpression is not sufficient on its own to drive SM–α-actin expression. HT-1080 cells were transfected with empty vector or with cDNAs for control vector (pcDNA3.1), GFP-tagged wild-type SRF, constitutively active SRF-VP16 fusion protein, or the GAL4-VP16 fusion protein for 48 hours. The cell extracts were analyzed by Western blotting with antibodies against SRF, SM–α-actin, or β-actin as indicated. Note that SRF antibodies against C-terminus of SRF do not recognize SRF-VP16, because the C-terminal trans-activation domain of SRF was replaced by the trans-activation domain of the viral co-activator VP16 (25).
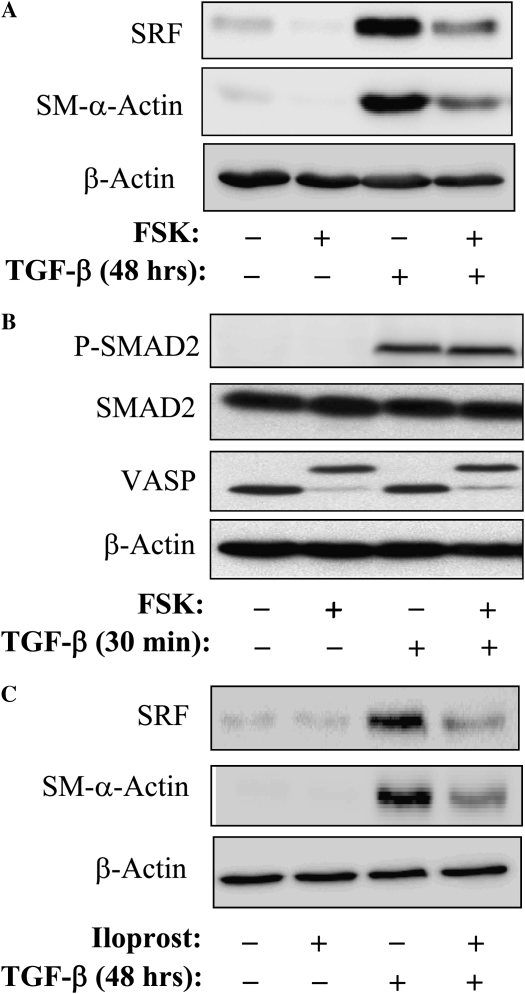
We have previously reported that SRF activity and SM–α-actin expression induced by G protein–coupled agonists, such as endothelin-1 or extracellular ATP, are highly sensitive to regulation by protein kinase A (PKA) in vascular smooth muscle cells (21, 26). Therefore, we examined whether PKA regulates TGF-β–induced expression of SRF and SM–α-actin in human pulmonary fibroblasts. Figure 5A shows that stimulation of PKA by an activator of adenylyl cyclase, FSK, resulted in a profound inhibition of TGF-β–induced expression of SRF and SM–α-actin in human pulmonary fibroblasts, without having an effect on β-actin expression. FSK had no effect on TGF-β–induced Smad2 phosphorylation (Figure 5B), whereas its effectiveness in PKA activation was confirmed by phosphorylation of the PKA substrate, vasodilator-stimulated phosphoprotein VASP (33), as determined by electrophoretic mobility shift of phosphorylated VASP (Figure 5B), which is mediated by PKA (21). Furthermore, the stable prostacyclin analog, iloprost, which signals via the prostanoid receptors to activate PKA (34), had a similar inhibitory effect on TGF-β–induced SM–α-actin and SRF expression (Figure 5C).
Figure 5.
Inhibition of TGF-β–induced SM–α-actin and SRF expression by cAMP-elevating agents. (A) Serum-starved human lung fibroblasts were pretreated with 10 μM forskolin (FSK) for 15 minutes, followed by stimulation with 2 ng/ml TGF-β for 48 hours. The cell extracts were analyzed by Western blotting with antibodies against SRF, SM–α-actin, or β-actin as indicated. (B) Serum-starved human lung fibroblasts were pretreated with 10 μM FSK for 15 minutes, followed by stimulation with 2 ng/ml TGF-β for 30 minutes. The cell extracts were analyzed by Western blotting with antibodies against phospho-Smad2 (Ser465/Ser467), total Smad2, vasodilator stimulated phosphoprotein (VASP), or β-actin as indicated. Electorphorectic mobility shift of VASP occurs with its phosphorylation by PKA (21). (C) Serum-starved human lung fibroblasts were pretreated with 10 μM Iloprost (Ilo) for 15 minutes, followed by stimulation with 2 ng/ml TGF-β for 48 hours. The cell extracts were analyzed by Western blotting with antibodies against SRF, SM–α-actin, or β-actin as indicated.
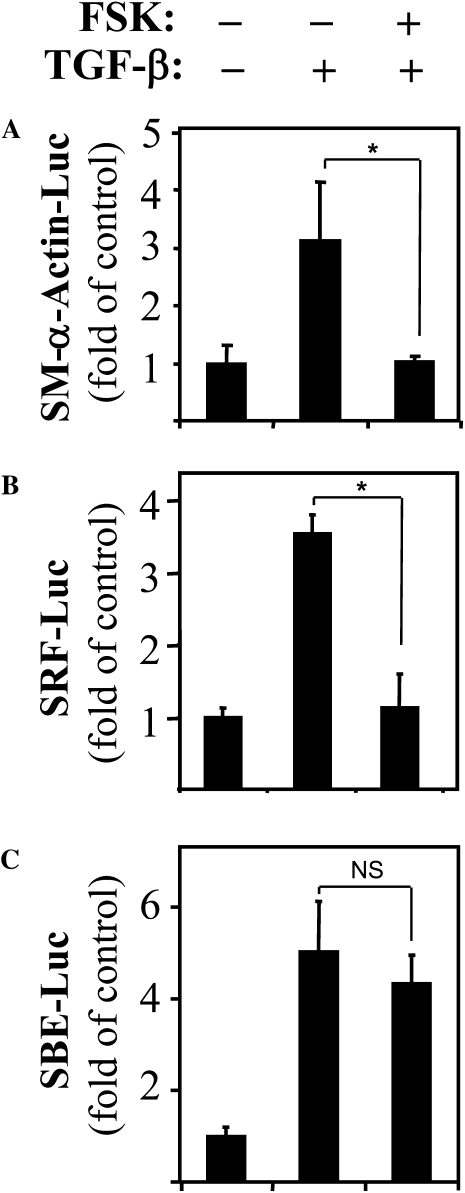
To further define the transcriptional control of SM–α-actin expression by PKA, we used the SM–α-actin promoter (−764 base pair) luciferase reporter that contains both SRF-binding sites (CArG boxes) and SBE (13). As shown in Figure 6A, TGF-β induced a 3-fold induction in luciferase activity, which was abolished by FSK treatment. This suggests that regulation of SM–α-actin expression by PKA occurs at the level of gene transcription. Furthermore, FSK blocked TGF-β–induced activation of the SRF-luciferase reporter driven by two CArG elements (Figure 6B), without affecting activation of SBE-luciferase reporter by TGF-β (Figure 6C). Together, these data strongly suggest that inhibition of TGF-β–induced SM–α-actin expression by PKA occurs via suppression of SRF expression and activity, but not of Smad-dependent gene transcription.
Figure 6.
PKA regulates TGF-β–induced SM–α-actin promoter activation through inhibition of SRF, but not of Smad-binding elements (SBE). Human lung fibroblasts were transfected with luciferase reporters for −764 base pairs (A) SM–α-actin promoter, (B) SRF-luciferase reporter, or (C) SBE-luciferase reporter, along with thymidine kinase-driven renilla (TK-RL) control reporter. Serum-starved cells were pretreated with 10 μM FSK for 15 minutes, followed by stimulation with 2 ng/ml TGF-β for 24 hours. The activity of luciferase was then measured in cell lysates and normalized to the activity of renilla. Data represent the results of at least three experiments performed in triplicate (*P < 0.05).
DISCUSSION
The first important observation from our studies is that stimulation of pulmonary fibroblasts with TGF-β leads to a profound induction of SRF expression (Figure 1). Relatively low levels of SRF are present under serum-starved conditions, but after 24 to 48 hours of treatment with TGF-β, SRF expression is induced to levels that are more typical of quiescent smooth muscle cells. This up-regulation temporally correlates with the induction of SM–α-actin expression, suggesting a link between these two events. Our observations are consistent with previous in vivo studies using rat model of bleomycin-induced pulmonary fibrosis, in which SRF expression was increased in parallel with induction of SM–α-actin–positive myofibroblasts (35).
Several possibilities exist for the mechanism of TGF-β–induced SRF expression, including transcriptional control. The SRF promoter mutagenesis studies by Spencer and Misra (31) established the CArG dependency of SRF induction by serum. Our data show that in HT1080 cells, the isolated transactivation of SRF via overexpression of SRF-VP16 has a negligible effect on the expression of endogenous SRF, while inducing a profound induction of SM–α-actin expression (Figure 4). These data, and the results of other studies, suggest that additional mechanisms may be required for transcription of SRF, including Sp1, Egr-1, and CCAATT-binding elements (32). Given that TGF-β can recruit Sp1 (16), it is possible that the initial transcription of SRF by TGF-β could occur through Sp1. On the other hand, analysis of the 5′ untranslated region of SRF gene predicts with high probability the presence of putative SBE upstream of CArG boxes (our unpublished observation), suggesting that TGF-β can also recruit canonical Smad signaling for SRF promoter activation. Therefore, it is possible that an initial induction of SRF expression by TGF-β (not necessarily through CArGs) may then subsequently drive the SRF promoter by newly synthesized (and transactivated) SRF in a positive feedback manner. Finally, post-translational regulation may also play a role, as in our additional experiments removal of TGF-β after 48 hours of exposure resulted in much faster disappearance of SRF protein than of SM–α-actin, suggesting that SRF is less stable. Similarly, Misra and colleagues have observed extensive phosphorylation of SRF (36), which may affect its stability. These possibilities are currently under investigation in our laboratory.
The second important conclusion of this study is that TGF-β–induced differentiation of pulmonary myofibroblasts is dependent on inducible SRF expression (Figures 2 and 3). However, using the HT-1080 fibrosarcoma cell line, we also show that SRF expression is not sufficient on its own for SM–α-actin induction, and SRF trans-activation is required (Figure 4). While it is prudent to be cautious when extrapolating the results from cancer cell lines to those obtained from primary cultures, we chose to use the HT1080 cell line to facilitate the transfection of SRF and SRF-VP16 into a sufficient percentage of cells to evaluate their functional effect. Second, while it does not recapitulate certain phenotypic characteristics of differentiated myofibroblasts (low proliferative capacity), it does serve as a useful model to study inducible contractile gene expression and SRF expression, as it maintains low levels of these proteins in the basal state, similar to the primary cultures of human lung fibroblasts.
The accepted mechanism of SRF activation by serum or G protein–coupled receptors involves RhoA activation and modulation of actin dynamics (37–39). Published data suggest a role for RhoA (40) in mediating TGF-β–induced myofibroblast differentiation as well, which we have confirmed using a specific Rho kinase inhibitor (data not shown). Given the delayed effect of TGF-β–induced activation of SRF and SM–α-actin (Figure 1A), the presence of an intermediate step requiring the synthesis and release of other mediators may be also possible. Sphingosine 1-phosphate, which is produced in response to TGF-β (40) and known to stimulate RhoA and SRF (41), could be one such mediator. Additional potential mechanisms may include TGF-β–induced Smad3/SRF interaction (12), or decreased interaction between inhibitory Smad7 and SRF (42).
Finally, our data suggests that SRF may be an important target for a negative regulation of pulmonary fibroblast differentiation, which could be of potential therapeutic importance. Other investigators have shown that PGE2 elicits its antifibrotic effect via elevation of cAMP, and this effect is independent of Smad signaling (17, 18). Our observations also show Smad-independent regulation of SM-α-actin expression by agents which signal via elevation of cAMP (FSK and iloprost), and more importantly, suggest that this occurs through inhibition of SRF (Figures 5, 6). The mechanism by which cAMP inhibits SRF activity is not completely understood. Our previous studies have shown that cAMP inhibits SRF activity in VSMC through activation of protein PKA (21, 26). It is known that PKA may phosphorylate RhoA (43), suggesting potential mechanism for SRF regulation through inhibition of RhoA. Second, our previous results (21) and present data (Figure 5B) show phosphorylation of vasodilator-stimulated phosphoprotein (VASP) by PKA. Given that VASP promotes actin polymerization and SRF activation (44), and given that phosphorylated VASP is unable to bind actin filaments (45), VASP could provide an additional mechanism for regulation of SRF by PKA. Third, SRF itself can be phosphorylated by PKA (46), potentially suggesting a direct regulation of SRF by PKA. Finally, cAMP has been shown more recently to stimulate cAMP-activated exchange protein Epac (47) that may also regulate the profibrotic features of myofibroblasts such as collagen synthesis (48), and proliferation (49, 50), suggesting the potential for PKA-independent regulation of SRF and/or SM–α-actin expression by cAMP. These possibilities are being examined in our laboratory.
Acknowledgments
The authors thank Dr. Julian Solway (University of Chicago), Dr. Joseph Miano (University of Rochester), Dr. Sem Phan (University of Michigan), and Dr. Bert Vogelstein (The Johns Hopkins University) for providing the DNA and adenovirus reagents.
This study was supported by National Institutes of Health grant HL071755 (to N.O.D.) and American Heart Association post-doctoral fellowship award AHA 0520109Z (to N.S.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0288OC on January 16, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 2004;18:816–827. [DOI] [PubMed] [Google Scholar]
- 2.Phan SH. Fibroblast phenotypes in pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:S87–S92. [PubMed] [Google Scholar]
- 3.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 2007;170:1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts: I. Paracrine cells important in health and disease. Am J Physiol 1999;277:C1–C9. [DOI] [PubMed] [Google Scholar]
- 5.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA 1991;88:6642–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang HR, Cho SJ, Lee CG, Homer RJ, Elias JA. Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J Biol Chem 2007;282:7723–7732. [DOI] [PubMed] [Google Scholar]
- 7.Santana A, Saxena B, Noble NA, Gold LI, Marshall BC. Increased expression of transforming growth factor beta isoforms (beta 1, beta 2, beta 3) in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 1995;13:34–44. [DOI] [PubMed] [Google Scholar]
- 8.Phan SH, Kunkel SL. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res 1992;18:29–43. [DOI] [PubMed] [Google Scholar]
- 9.Feng XH, Derynck R. Specificity and versatility in TGF-beta signaling through Smads. Annu Rev Cell Dev Biol 2005;21:659–693. [DOI] [PubMed] [Google Scholar]
- 10.Derynck R, Zhang YE. Smad-dependent and smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577–584. [DOI] [PubMed] [Google Scholar]
- 11.Subramanian SV, Polikandriotis JA, Kelm RJ Jr, David JJ, Orosz CG, Strauch AR. Induction of vascular smooth muscle alpha-actin gene transcription in transforming growth factor beta1-activated myofibroblasts mediated by dynamic interplay between the pur repressor proteins and sp1/smad coactivators. Mol Biol Cell 2004;15:4532–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu P, Feng XH, Li L. Interaction of smad3 and srf-associated complex mediates TGF-beta1 signals to regulate sm22 transcription during myofibroblast differentiation. J Mol Cell Cardiol 2003;35:1407–1420. [DOI] [PubMed] [Google Scholar]
- 13.Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol 2003;29:397–404. [DOI] [PubMed] [Google Scholar]
- 14.Adam PJ, Regan CP, Hautmann MB, Owens GK. Positive- and negative-acting kruppel-like transcription factors bind a transforming growth factor beta control element required for expression of the smooth muscle cell differentiation marker sm22alpha in vivo. J Biol Chem 2000;275:37798–37806. [DOI] [PubMed] [Google Scholar]
- 15.Hu B, Wu Z, Liu T, Ullenbruch MR, Jin H, Phan SH. Gut-enriched kruppel-like factor interaction with smad3 inhibits myofibroblast differentiation. Am J Respir Cell Mol Biol 2007;36:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cogan JG, Subramanian SV, Polikandriotis JA, Kelm RJ Jr, Strauch AR. Vascular smooth muscle alpha-actin gene transcription during myofibroblast differentiation requires sp1/3 protein binding proximal to the mcat enhancer. J Biol Chem 2002;277:36433–36442. [DOI] [PubMed] [Google Scholar]
- 17.Thomas PE, Peters-Golden M, White ES, Thannickal VJ, Moore BB. PGE(2) inhibition of TGF-beta1-induced myofibroblast differentiation is smad-independent but involves cell shape and adhesion-dependent signaling. Am J Physiol Lung Cell Mol Physiol 2007;293:L417–L428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via e. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol 2003;29:537–544. [DOI] [PubMed] [Google Scholar]
- 19.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol 2003;35:577–593. [DOI] [PubMed] [Google Scholar]
- 20.Mao J, Yuan H, Xie W, Simon MI, Wu D. Specific involvement of G proteins in regulation of serum response factor-mediated gene transcription by different receptors. J Biol Chem 1998;273:27118–27123. [DOI] [PubMed] [Google Scholar]
- 21.Davis A, Hogarth K, Fernandes D, Solway J, Niu J, Kolenko V, Browning D, Miano JM, Orlov SN, Dulin NO. Functional significance of protein kinase a activation by endothelin-1 and ATP: negative regulation of srf-dependent gene expression by PKA. Cell Signal 2003;15:597–604. [DOI] [PubMed] [Google Scholar]
- 22.Roy SG, Nozaki Y, Phan SH. Regulation of alpha-smooth muscle actin gene expression in myofibroblast differentiation from rat lung fibroblasts. Int J Biochem Cell Biol 2001;33:723–734. [DOI] [PubMed] [Google Scholar]
- 23.Hautmann MB, Adam PJ, Owens GK. Similarities and differences in smooth muscle alpha-actin induction by TGF-beta in smooth muscle versus non-smooth muscle cells. Arterioscler Thromb Vasc Biol 1999;19:2049–2058. [DOI] [PubMed] [Google Scholar]
- 24.Yau DM, Sethakorn N, Taurin S, Kregel S, Sandbo N, Camoretti-Mercado B, Sperling AI, Dulin NO. Regulation of smad-mediated gene transcription by rgs3. Mol Pharmacol 2008;73:1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grueneberg DA, Natesan S, Alexandre C, Gilman MZ. Human and drosophila homeodomain proteins that enhance the DNA-binding activity of serum response factor. Science 1992;257:1089–1095. [DOI] [PubMed] [Google Scholar]
- 26.Hogarth DK, Sandbo N, Taurin S, Kolenko V, Miano JM, Dulin NO. Dual role of PKA in phenotypic modulation of vascular smooth muscle cells by extracellular ATP. Am J Physiol Cell Physiol 2004;287:C449–C456. [DOI] [PubMed] [Google Scholar]
- 27.Sandbo N, Qin Y, Taurin S, Hogarth DK, Kreutz B, Dulin NO. Regulation of serum response factor-dependent gene expression by proteasome inhibitors. Mol Pharmacol 2005;67:789–797. [DOI] [PubMed] [Google Scholar]
- 28.Sandbo N, Taurin S, Yau DM, Kregel S, Mitchell R, Dulin NO. Downregulation of smooth muscle alpha-actin expression by bacterial lipopolysaccharide. Cardiovasc Res 2007;74:262–269. [DOI] [PubMed] [Google Scholar]
- 29.Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van Nostrand W, Miano JM, Zlokovic BV. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer's phenotype. Proc Natl Acad Sci USA 2007;104:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evelyn CR, Wade SM, Wang Q, Wu M, Iniguez-Lluhi JA, Merajver SD, Neubig RR. Ccg-1423: a small-molecule inhibitor of rhoa transcriptional signaling. Mol Cancer Ther 2007;6:2249–2260. [DOI] [PubMed] [Google Scholar]
- 31.Spencer JA, Misra RP. Expression of the serum response factor gene is regulated by serum response factor binding sites. J Biol Chem 1996;271:16535–16543. [DOI] [PubMed] [Google Scholar]
- 32.Spencer JA, Misra RP. Expression of the srf gene occurs through a ras/sp/srf-mediated-mechanism in response to serum growth signals. Oncogene 1999;18:7319–7327. [DOI] [PubMed] [Google Scholar]
- 33.Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. CAMP- and CGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem 1994;269:14509–14517. [PubMed] [Google Scholar]
- 34.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol 2004;36:1187–1205. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Zhe X, Phan SH, Ullenbruch M, Schuger L. Involvement of serum response factor isoforms in myofibroblast differentiation during bleomycin-induced lung injury. Am J Respir Cell Mol Biol 2003;29:583–590. [DOI] [PubMed] [Google Scholar]
- 36.Misra RP, Rivera VM, Wang JM, Fan PD, Greenberg ME. The serum response factor is extensively modified by phosphorylation following its synthesis in serum-stimulated fibroblasts. Mol Cell Biol 1991;11:4545–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control srf activity by regulation of its coactivator mal. Cell 2003;113:329–342. [DOI] [PubMed] [Google Scholar]
- 38.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell 1999;98:159–169. [DOI] [PubMed] [Google Scholar]
- 39.Hill CS, Wynne J, Treisman R. The rho family GTPases rhoa, rac1, and cdc42hs regulate transcriptional activation by srf. Cell 1995;81:1159–1170. [DOI] [PubMed] [Google Scholar]
- 40.Kono Y, Nishiuma T, Nishimura Y, Kotani Y, Okada T, Nakamura S, Yokoyama M. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. Am J Respir Cell Mol Biol 2007;37:395–404. [DOI] [PubMed] [Google Scholar]
- 41.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem 2004;279:42422–42430. [DOI] [PubMed] [Google Scholar]
- 42.Camoretti-Mercado B, Fernandes DJ, Dewundara S, Churchill J, Ma L, Kogut PC, McConville JF, Parmacek MS, Solway J. Inhibition of transforming growth factor beta-enhanced serum response factor-dependent transcription by smad7. J Biol Chem 2006;281:20383–20392. [DOI] [PubMed] [Google Scholar]
- 43.Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of rhoa mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J 1996;15:510–519. [PMC free article] [PubMed] [Google Scholar]
- 44.Walders-Harbeck B, Khaitlina SY, Hinssen H, Jockusch BM, Illenberger S. The vasodilator-stimulated phosphoprotein promotes actin polymerisation through direct binding to monomeric actin. FEBS Lett 2002;529:275–280. [DOI] [PubMed] [Google Scholar]
- 45.Harbeck B, Huttelmaier S, Schluter K, Jockusch BM, Illenberger S. Phosphorylation of the vasodilator-stimulated phosphoprotein regulates its interaction with actin. J Biol Chem 2000;275:30817–30825. [DOI] [PubMed] [Google Scholar]
- 46.Gauthier-Rouviere C, Vandromme M, Lautredou N, Cai QQ, Girard F, Fernandez A, Lamb N. The serum response factor nuclear localization signal: general implications for cyclic AMP-dependent protein kinase activity in control of nuclear translocation. Mol Cell Biol 1995;15:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998;396:474–477. [DOI] [PubMed] [Google Scholar]
- 48.Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA. The cyclic AMP effector epac integrates pro- and anti-fibrotic signals. Proc Natl Acad Sci USA 2008;105:6386–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via e prostanoid 2 receptor and camp signaling. Am J Physiol Lung Cell Mol Physiol 2007;292:L405–L413. [DOI] [PubMed] [Google Scholar]
- 50.Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and EPAC-1. Am J Respir Cell Mol Biol 2008;39:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]