Abstract
Oxidative stress plays an important role in the pathogenesis of lung inflammation. Respiratory syncytial virus (RSV) infection induces reactive oxygen species (ROS) production in vitro and oxidative injury in lungs in vivo; however, the mechanism of RSV-induced cellular oxidative stress has not been investigated. Therefore, we determined whether RSV infection of airway epithelial cells modified the expression and/or activities of antioxidant enzymes (AOE). A549 cells, a human alveolar type II–like epithelial cell line, and small airway epithelial (SAE) cells, normal human cells derived from terminal bronchioli, were infected with RSV and harvested at various time points to measure F2-8 isoprostanes by enzyme-linked immunosorbent assay and total and reduced glutathione (GSH and GSSG) by colorimetric assay. Superoxide dismutase (SOD) 1, 2, and 3, catalase, glutathione peroxidase (GPx), and glutathione S-transferase (GST) expression was determined by quantitative real-time PCR and Western blot, and their activity was measured by colorimetric assays. RSV infection induced a significant increase of lipid peroxidation products as well as a significant decrease in the GSH/GSSG ratio. There was a significant decrease in SOD 1, SOD 3, catalase, and GST expression with a concomitant increase of SOD 2 in RSV-infected cells, compared with uninfected cells. Total SOD activity was increased, but catalase, GPx, and GST activities were decreased, after RSV infection. Our findings suggest that RSV-induced cellular oxidative damage is the result of an imbalance between ROS production and antioxidant cellular defenses. Modulation of oxidative stress represents a potential novel pharmacologic approach to ameliorate RSV-induced acute lung inflammation.
Keywords: RSV, airway epithelial cells, antioxidant enzymes, oxidative stress
Respiratory syncytial virus (RSV) is the one of the most important causes of viral upper and lower respiratory tract infections in infants and young children. RSV is so ubiquitous in nature that it will infect 100% of children before the age of 3. The number of children hospitalized each year in the United States with viral lower respiratory tract infections (LRTI) has recently been estimated at over 200,000, and 500 deaths occur per year in children under 5 years of age (1). Although the mechanisms of RSV-induced airway disease and associated long-term consequences remain incompletely defined, the lung inflammatory response is thought to play a fundamental role. Oxidative stress has been shown to contribute to the pathogenesis of both acute and chronic lung inflammatory diseases (reviewed in Refs. 2–4). Reactive oxygen species (ROS) are highly unstable molecules produced from the pulmonary epithelial and endothelial cells involved in many forms of tissue damage, including the damage caused to cellular components such as lipids, proteins, and DNA (reviewed in Refs. 5, 6). We have previously shown that RSV infection of airway epithelial cells induces ROS production, which is involved in transcription factor activation and chemokine gene expression (7, 8). We have also shown that RSV induces oxidative stress in lungs in vivo, using a mouse model of RSV infection, and that antioxidant treatment significantly ameliorates RSV-induced clinical disease and pulmonary inflammation (9).
Cells are protected against oxidative damage by well-developed enzymatic and nonenzymatic antioxidant systems, including superoxide dismutase (SOD), catalase, glutathione-dependent enzymes, thioredoxin, and peroxiredoxins, which protect cells against ROS and cytotoxic products of lipid peroxidation. Antioxidant enzymes (AOE) can either directly decompose ROS (e.g., SOD and catalase) or facilitate these antioxidant reactions (e.g., peroxidase using glutathione as a reducing agent). The molecular mechanism(s) responsible for RSV-induced oxidative damage is not known; therefore, the aim of this study was to investigate whether RSV infection modified AOE expression and/or activity in airway epithelial cells. Our results show that RSV induced significant oxidative stress in infected airway epithelial cells, as indicated by the increase of F2-8 isoprostanes, a marker of lipid peroxidation, and by a significant decrease of the total versus reduced glutathione (GSH/GSSG) ratio. Expression of SOD 1, SOD 3, catalase, and glutathione S-transferase (GST) was significantly reduced after RSV-infection, with a concomitant increase only of SOD 2 expression. Enzymatic assay results showed that total SOD activity was increased but that catalase, glutathione peroxidase (GPx), and GST activities were decreased, suggesting that RSV-induced cellular oxidative damage is the result of an imbalance between ROS production and antioxidant cellular defenses.
MATERIALS AND METHODS
RSV Preparation
The RSV A2 strain was grown in Hep-2 cells and purified by centrifugation on discontinuous sucrose gradients as described elsewhere (10). The virus titer of the purified RSV pools was 8 to 9 log10 plaque-forming units (PFU)/ml using a methylcellulose plaque assay. No contaminating cytokines were found in these sucrose-purified viral preparations (11). LPS, assayed using the limulus hemocyanin agglutination assay, was not detected. Virus pools were aliquoted, quick-frozen on dry ice/alcohol, and stored at −70°C until used.
Cell Culture and Infection of Epithelial Cells with RSV
A549 cells, a human alveolar type II–like epithelial cell line (American Type Culture Collection, Manassas, VA) and small alveolar epithelial (SAE) cells (from Clonetics, San Diego, CA), normal human airway epithelial cells derived from terminal bronchioli, were grown according to the manufacturer's instructions. A549 and SAE were maintained in F12K and small airway epithelial cell (SAEC) growth medium, respectively, containing 10% (vol/vol) FBS, 10 mM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin for F12K medium; and 7.5 mg/ml bovine pituitary extract (BPE), 0.5 mg/ml hydrocortisone, 0.5 μg/ml hEGF, 0.5 mg/ml epinephrine, 10 mg/ml transferrin, 5 mg/ml insulin, 0.1 μg/ml retinoic acid, 0.5 μg/ml triiodothyronine, 50 mg/ml gentamicin, and 50 mg/ml bovine serum albumin (BSA) for SAEC medium. When SAE were used for RSV infection, they were changed to basal medium, not supplemented with growth factors, 6 hours before and throughout the length of the experiment. At around 80 to 90% confluence, cell monolayers were infected with RSV at multiplicity of infection (MOI) of 1 (unless otherwise stated), as previously described (12). An equivalent amount of a 30% sucrose solution was added to uninfected A549 and SAE cells, as a control.
For the catalytic scavenger experiment, cells were pretreated with EUK-163 or EUK-134 (Eukarion, Inc., Bedford, MA) for 1 hour and then infected in the presence of the compound. Since EUKs were diluted in ethanol, equal amounts of ethanol were added to untreated cells, as a control. Total number of viable cells and viral replication after antioxidant treatment were measured by trypan blue exclusion and by plaque assay, respectively. There was no significant change in either cell viability or viral replication with both compounds.
Measurement of Lipid Peroxidation Products
Measurements of F2 8-isoprostane were performed using a competitive enzyme immunoassay from Cayman Chemical (Ann Arbor, MI), according to manufacturer's instructions. Measurement of lipid peroxidation markers malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) was performed using a lipid peroxidation kit from Calbiochem (San Diego, CA).
Determination of Glutathione
Total or reduced glutathione concentration in the total cell lysates was measured spectrophotometrically by using a glutathione assay kit (Cayman Chemical). Briefly, total cell lysates from control and RSV-treated A549 and SAE cells were deproteinated by adding an equal volume of metaphosphoric acid (MPA) reagent (5 g of MPA in 50 ml of water). After incubation at room temperature for 5 minutes and centrifugation at 2,000 × g for 2 minutes, supernatant was collected and, after addition of triethanolamine (TEAM) reagent (4 M), it was used for GSH measurement. For GSSG measurement, 2-vinylpyridine (1 M) was added to the sample solution with TEAM reagent, which was incubated at room temperature for 1 hour and assayed for GSSG concentration, according to the manufacturer's instructions. GSH and GSSG amounts were calculated in micromoles.
Western Blotting
Nuclear extracts were prepared from A549 cells uninfected or infected with RSV for various lengths of time using the hypotonic/nonionic detergent lysis as previously described (13). Total cell lysates were prepared from control and infected A549 cells by adding ice-cold lysis buffer (50 mM Tric-HCl, pH 7.4, 150 mM NaCl, 1 mM EGTA, 0.25% sodium deoxycholate, 1 mM Na3VO4, 1 mM NaF, 1% Triton X-100, and 1 μg/ml of aprotinin, leupeptin, and pepstatin). After incubation on ice for 10 minutes, the lysates were collected and detergent insoluble materials were removed by centrifugation at 4°C at 14,000 × g. Proteins (10–20 μg per sample) were then boiled in 2× Laemmli buffer and resolved on SDS-PAGE. Proteins were transferred onto Hybond-polyvinylidene difluoride membrane (Amersham, Piscataway, NJ) and nonspecific binding sites were blocked by immersing the membrane in Tris-buffered saline-Tween (TBST) blocking solution (10 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.05% Tween-20 [vol/vol]) containing 5% skim milk powder or 5% bovine serum albumin for 30 minutes. After a short wash in TBST, the membranes were incubated with the primary antibody overnight at 4°C, followed by an anti-rabbit peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1:10,000 in TBST for 30 minutes at room temperature. After washing, the proteins were detected using ECL (Amersham) according to manufacturer's protocol. The primary antibodies used for Western blots were anti SOD 1, 2, and 3 rabbit polyclonal antibodies from Stressgen Bioreagents (Ann Arbor, MI), anti-catalase rabbit polyclonal antibody from Calbiochem, anti–nuclear factor (NF)-E2–related transcription factors (Nrf)-2 from Santa Cruz Biotechnology, and anti–β-actin monoclonal antibody from Sigma-Aldrich (St. Louis, MO).
Densitometric Analyses of AOE
Densitometric analysis of Western blot band intensities was performed using Alpha Ease software, version 2200 (2.2 d) (Alpha Innotech Co., San Leandro, CA). Bands in RSV-infected samples were normalized to uninfected control sample background.
Quantitative Real-Time PCR
Total RNA was extracted from control and RSV-infected A549 cells and SAE cells by RNeasy mini kit (Qiagen, Germantown, MD). For SOD 1, 2, 3, and catalase amplification by quantitative real-time PCR (Q-RT-PCR), Applied Biosystems assays-on-demand 20× mix of primers and TaqMan MGB probes (FAM-dye labeled) for target genes and 18S rRNA (VIC dye–labeled probe) TaqMan assay reagent (P/N 4319413E) for controls were used. Separate tubes (singleplex) one-step RT-PCR was performed with 80 ng RNA for both target genes and endogenous control. The cycling parameters for one-step RT-PCR were the following: reverse transcription 48°C for 30 minutes, AmpliTaq activation 95°C for 10 minutes, denaturation 95°C for 15 seconds, and annealing/extension 60°C for 1 minute (repeat 30 times) on ABI7000. Duplicate CT values were analyzed in Microsoft Excel using the comparative CT (ΔΔCT) method as described by the manufacturer (Applied Biosystems). The amount of target (2−ΔΔCT) was obtained by normalizing to endogenous reference (18S) sample.
Biochemical Assays
Catalase, GST, GPx, and SOD activities were determined using specific kits (Cayman Chemical; Catalog No. 707002, 703302, 703102 and 706002, respectively, for catalase, GST, GPx, and SOD), according to the manufacturer's instructions. Total cell lysates for the activity assays were prepared from uninfected and infected cells at different time points after infection according to the protocol provided by the assay kits for the respective enzymes.
The quantification of catalase activity in the total cell lysates was based on the reaction of the enzyme with methanol in the presence of an optimal concentration of H2O2. The formaldehyde produced is measured spectrophotometrically with 4-amino-3-hydrazino-5-mercapto-124-triazole (purpald) as the chromogen. The catalase activity was expressed as nmol/min/mg of protein in the sample.
The total GST activity was quantified by measuring the conjugation of 1-chloro-2,4-dinitrobenzene (CDNB) with reduced glutathione. The conjugation is accompanied by an increase in absorbance at 340 nm. The rate of increase is directly proportional to the GST activity in the sample. The GST activity was expressed as nmol/min/mg of protein in the sample.
The GPx activity was determined spectrophotometrically in the total cell lysates through an indirect coupled reaction with glutathione reductase (GR). Oxidized glutathione (GSSG), produced upon reduction of hydroperoxide by GPx, is recycled to its reduced state by GR and NADPH. The oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm. Under conditions in which the GPx activity is rate limiting, the rate of decrease in the A340 is directly proportional to the GPx activity in the sample.
SOD activity was determined by using tetrazolium salt for the detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical.
ELISA
Immunoreactive RANTES and IL-8 were quantified by a double antibody ELISA kit (DuoSet; R&D Systems, Minneapolis, MN) following the manufacturer's protocol. The sensitivity of the assay was 7.8 to 2,000 pg/ml.
Statistics
A two tailed Student's t test using a 95% confidence level was performed on all experiments. Significance is designated by the following: *P < 0.05.
RESULTS
RSV Induces Oxidative Stress in Airway Epithelial Cells
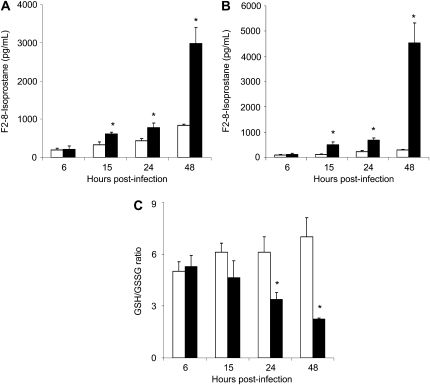
We have previously shown that RSV is a potent inducer of ROS in airway epithelial cells in vitro (7) and that RSV causes significant oxidative stress lung damage in vivo, as demonstrated by the increase of lipid peroxidation markers such as F2 8-isoprostane, MDA, and 4-HNE in an animal model of infection (9). To determine whether RSV induced oxidative stress directly in airway epithelial cells, the major target of infection, we investigated F2-isoprostane production and changes in GSH/GSSG ratio in A549 cells either uninfected or infected with RSV at various time points after infection. There was a progressive increase in F2-isoprostane levels in RSV-infected cells at all time points, with a 7-, 25-, 80-, and 154-fold increase at 6, 15, 24, and 48 hours after infection, respectively, when compared with control cells (Figure 1A). Similarly, there was a significant increase in other lipid peroxidation products, such as MDA-5 and 4-HNE, at various time points of RSV infection (data not shown). To confirm our findings in A549 cells, a similar experiment was performed using normal human SAE, which we have previously shown to behave very similarly to A549 cells in terms of chemokine/cytokine gene expression, transcription factor, and signaling pathway activation, as well as in sensitivity to antioxidant treatment, after RSV infection (7, 12, 14–16). Similar to A549 cells, there was a time-dependent increase in F2-isoprostane levels in RSV-infected cells, when compared with control cells (Figure 1B).
Figure 1.
Evidence of oxidative stress in A549 cells. (A) A549 or (B) small airway epithelial (SAE) cells were infected with respiratory syncytial virus (RSV) (solid bars) and harvested at 6, 15, 24, and 48 hours after infection to measure F2-isoprostanes and GSH/GSSG ratio (C). Open bars, control. *P < 0.05 relative to uninfected cells. The figure is representative of two different experiments run in triplicate.
On the other hand, we observed a progressive decrease of the GSH/GSSG ratio in A549 cells after RSV infection, with a 20, 40, and 60% reduction of the ratio at 15, 24, and 48 hours after infection, respectively, compared with uninfected cells (Figure 1C). A significant decrease in the GSH/GSSG ratio was also observed in SAE cells, going from approximately 2.4 in uninfected cells to approximately 1.8 (a 25% reduction) in cells infected with RSV at 48 hours after infection (data not shown). The increase in F2 8-isoprostane levels significantly correlated with the decreased levels of GSH to GSSG ratio, both indicating that RSV infection induces significant oxidative stress in airway epithelial cells.
RSV Infection Modifies the Expression of Antioxidant Enzymes
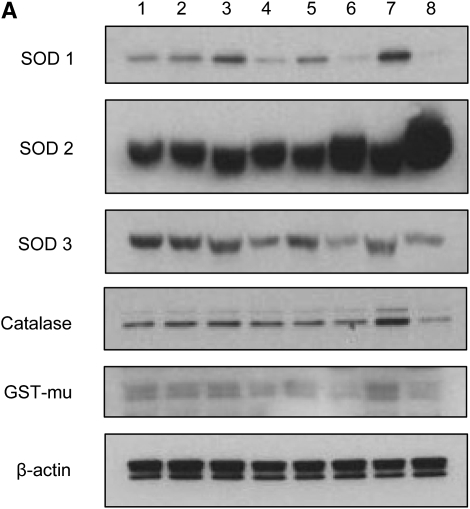
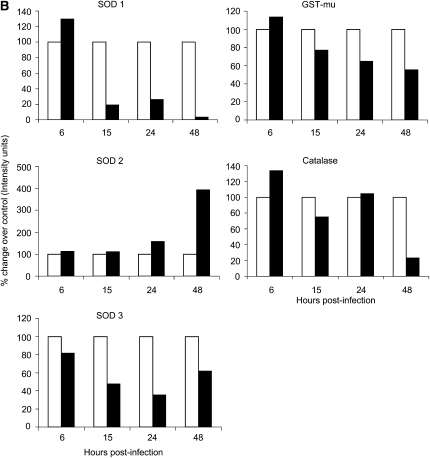
We have recently shown that the membrane-bound NAD(P)H oxidase system plays a fundamental role in initiating ROS-dependent signaling in airway epithelial cells infected by RSV, as treatment with a variety of NAD(P)H oxidase inhibitors blocks RSV-induced IRF-3 and STAT transcription factor activation (8, 17). The first ROS produced in the reduction pathway of oxygen is the superoxide anion (O2−), which is metabolized to hydrogen peroxide (H2O2) by superoxide dismutases, SOD 1, 2, or 3, depending on the primary site of O2−production. Catalase and GPx then detoxify H2O2 by generating water and oxygen, while GST is important for detoxification of a variety of nonradical reactive metabolites. To investigate the effect of RSV infection on the expression of AOE in A549 cells, as a protective mechanism against oxidative damage, SOD 1, 2, and 3, catalase, and GST protein expression was evaluated by Western blot analysis. A549 cells were infected with RSV at MOI 1 and harvested at 6, 15, 24, and 48 hours after infection. The results showed an initial increase of SOD 1, GST, and catalase protein expression and a subsequent decrease of all AOE in infected A549 cells, compared with uninfected cells, with the exception of SOD 2, whose level continued to increase with the progression of viral infection (Figure 2A). Densitometric analysis of the Western blot results showed that there was an initial increase of 29.41% (1.3-fold) of SOD 1 protein expression at 6 hours after infection, which gradually decreased by 80% (5.25-fold), 74% (3.8-fold), and 96% (29-fold), respectively, at 15, 24, and 48 hours after RSV infection (Figure 2B), compared with uninfected cells. SOD 2 protein expression progressively increased throughout the course of RSV infection, compared with uninfected cells, with an increase of 58% (1.6-fold) and 293% (3.94-fold) at 24 and 48 hours after infection, respectively (Figure 2B). SOD 3 protein levels in RSV-infected A549 cells decreased significantly throughout the course of infection, with approximately 20, 50, 65, and 40% reduction at 6, 15, 24, and 48 hours after infection, compared with uninfected cells (Figure 2B). Catalase protein level showed an initial increase at 6 hours after infection (33.33%), with a significant decrease only at the latest time point of infection (80% at 48 h after infection) (Figure 2B). Similarly, there was an initial increase in the GST protein level at 6 hours after infection of 14% and later a progressive decrease of 23, 35, and 45% at 15, 24, and 48 hours after infection (Figure 2B), compared with uninfected cells.
Figure 2.
RSV infection modifies the expression of AOE in A549 cells. Total cell lysates, prepared from A549 cells uninfected or infected with RSV for 6, 15, 24, and 48 hours, were resolved on 10% SDS-PAGE, and Western blot was performed using antibodies against superoxide dismutase (SOD) 1, 2, 3, catalase, and glutathione-S-transferase (GST) proteins. Membranes were stripped and reprobed for β-actin as an internal control for protein integrity and loading. (A) Lanes 1, 3, 5, and 7: uninfected control cells for 6, 15, 24, and 48 hours after infection; lanes 2, 4, 6, and 8: RSV-infected cells for corresponding time points of infection. (B) Densitometric analysis of Western blot bands. Open bars, control; solid bars, RSV. The figure is representative of two independent experiments.
RSV Infection Down-Regulates Antioxidant Gene Expression in Airway Epithelial Cells
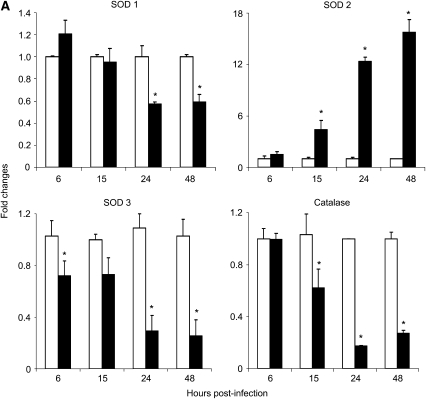
To investigate the mechanism responsible for the observed changes in AOE protein levels in response to RSV infection, we investigated AOE mRNA levels. Total RNA was isolated from control and RSV-infected A549 cells at 6, 15, 24, and 48 hours after infection and SOD 1, 2, 3, and catalase genes were amplified by Q-RT-PCR. Similar to what we observed for the AOE protein levels, there was a significant decrease of SOD 1, 3, and catalase mRNA expression during the course of RSV infection, in particular at the later time points (24 and 48 h after infection), while there was an increase in SOD 2 mRNA level, starting at 15 hours after infection (Figure 3A). SOD1 expression decreased by approximately 30 and 40% at 24 and 48 hours after infection, while SOD 3 mRNA expression was lower in RSV cells throughout the course of infection, with a decrease of 30, 70, and 75% of the levels of expression at 6, 15, 24, and 48 hours after infection, compared with uninfected cells. Similarly, catalase mRNA expression also decreased by approximately 40, 80, and 75% at 15, 24, and 48 hours of RSV infection. On the other hand, SOD 2 mRNA expression was significantly higher in RSV-infected A549 cells, with a 4.5-, 12-, and 16-fold increase at 15, 24, and 48 hours after infection, compared with uninfected cells.
Figure 3.
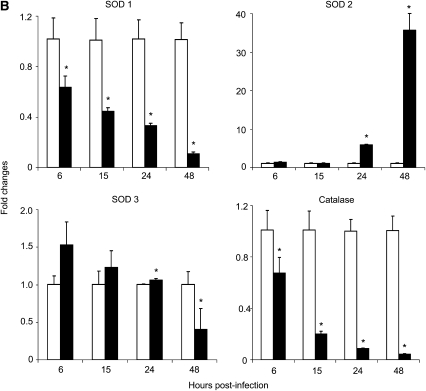
RSV infection down-regulates AOE gene expression in A549 and SAE cells. (A) A549 or (B) SAE cells were infected with RSV (solid bars) and harvested to prepare total RNA at various time points after infection. SOD 1, 2, 3 and catalase gene expression was measured by Q-RT-PCR. Open bars, control. *P < 0.05 relative to uninfected cells. The figure represents three independent experiments.
To confirm our findings in A549 cells, a similar experiment was performed using normal SAE cells. The pattern of AOE mRNA expression in SAE cells infected with RSV was similar to the one found in infected A549 cells (Figure 3B). SOD 1 mRNA expression in SAE-infected cells decreased by approximately 40, 55, 67, and 90% at 6, 15, 24, and 48 hours after infection, respectively, compared with uninfected cells. SOD 2 mRNA expression was significantly higher in RSV-infected SAE cells both at 24 hours (∼ 6-fold) and 48 hours (∼ 35-fold) after infection, compared with uninfected cells. SOD 3 mRNA expression was slightly higher at the earlier time points of RSV infection, 1.5-fold at 6 hours after infection and 1.3-fold at 15 hours after infection, but significantly decreased at 48 hours after infection (60% of uninfected cell level). On the other hand, catalase mRNA expression significantly decreased in SAE-infected cells throughout the course of infection, with a 33, 80, 90, and 95% reduction at 6, 15, 24, and 48 hours after infection.
RSV Infection Modifies AOE Activities in Airway Epithelial Cells
To determine whether changes in AOE gene and protein expression resulted in changes in their activity in response to RSV infection, total cell lysates were prepared from A549 and SAE cells either uninfected or infected for 6, 15, 24, and 48 hours to measure SOD, catalase, GST, and GPx enzymatic activity. There was a significant reduction of all AOE activities in both cell types with the progression of RSV infection, in particular of GPx and GST, with the exception of total SOD, which increased at the later time points of infection (Figure 4). In RSV-infected A549 cells, total SOD activity initially decreased at the earlier time points of infection (∼ 50% reduction both at 6 and 15 h after infection), compared with uninfected cells, with a subsequent increase starting at 24 hours (25% increase) and in particular at 48 hours after infection (1,397.8%) (Figure 4A). Similar results were obtained in SAE cells infected with RSV (Figure 4B), in which there was an initial decrease in the SOD activity at 6 hours after infection (∼ 60%), compared with uninfected cells, and then a progressive increase at 24 (66%) and 48 hours after infection (3,500%).
Figure 4.
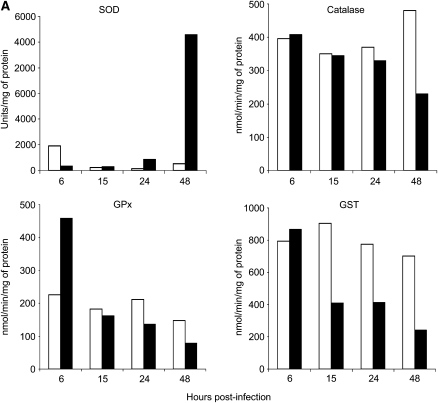
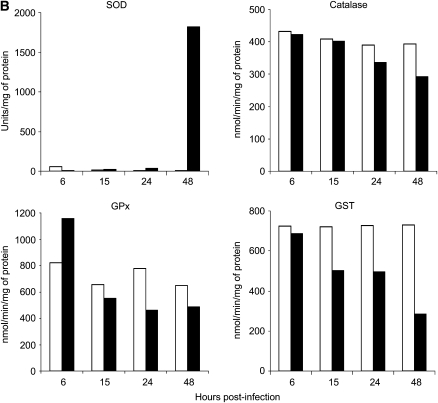
Effect of RSV infection on AOE activities in A549 and SAE cells. Total cell lysates were prepared from uninfected (open bars) and RSV-infected (solid bars) (A) A549 cells or (B) SAE cells for 6, 15, 24, and 48 hours to measure total SOD, catalase, GPx, and GST enzyme activities. The figure is representative of two independent experiments.
There was no change in catalase activity at 6 hours after infection in RSV-infected A549 cells, compared with uninfected cells, but we observed a progressive decrease in activity with the progression of infection, with a reduction of 13.5% at 15 hours, 17.5% at 24 hours, and 42% at 48 hours after infection (Figure 4A). A similar trend was present in infected SAE cells, with no changes in catalase activity at 6 and 15 hours after infection and a decrease of approximately 20% at 24 hours and 30% at 48 hours after infection (Figure 4B).
Because the glutathione redox cycle is complementary to catalase in scavenging H2O2, we also investigated GPx activity in response to RSV infection. There was a significant increase (138.5%) in the GPx activity initially at 6 hours in RSV-infected A549 cells compared with control cells; however, GPx activity progressively decreased at 15 hours (15.9%), 24 hours (30%), and 48 hours (60%) after infection (Figure 4A). GPx activity in SAE cells also showed an initial increase at 6 hours after infection (78.2%), compared with uninfected cells, with a 15% decrease at 15 hours, 30% at 24 hours, and 25% decrease at 48 hours after infection (Figure 4B).
Finally, GST activity initially increased at 6 hours after infection, compared with uninfected cells, but then decreased at all subsequent time points of infection, with a reduction of 50% at 15 and 24 hours after infection and 75% at 48 hours after infection (Figure 4A). GST activity in SAE cells also showed a progressive decrease in response to RSV infection, with a reduction of 6% at 6 hours, 30% at 15 and 24 hours, and 60% at 48 hours after infection, compared with uninfected cells (Figure 4B).
In summary, the finding of increased SOD activity and decreased catalase, GPx, and GST activity in airway epithelial cells indicates that RSV infection likely results in enhanced intracellular H2O2 production, which is not detoxified by AOE, leading to the generation of highly reactive oxygen species, such as the hydroxyl radical (OH-) and significant cellular damage.
Effect of Catalytic Scavengers on RSV-Induced RANTES and IL-8 Secretion
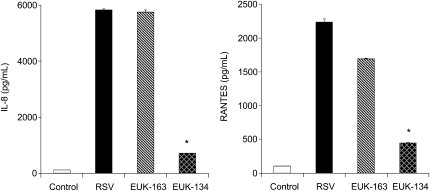
We have previously shown that RSV-induced ROS production regulates transcription factor activation and chemokine gene expression, as antioxidant treatment significantly reduces RSV-induced IL-8 and RANTES secretion (7). EUK-163 and EUK-134 are a novel class of synthetic superoxide dismutase/catalase mimetics, with EUK-163 being mainly a SOD mimetic and EUK-134 having significant catalase/peroxidase activity (18). To determine the effect of increased SOD activity alone or in combination with other antioxidant activities on RSV-induced chemokine production, A549 cells were pretreated with 400 μM EUK-163 or EUK-134 (Eukarion, Inc./Proteome System, Woburn, MA) and then infected with RSV. Cell culture supernantants from uninfected or infected cells were harvested at 24 hours after infection and tested for IL-8 and RANTES secretion by ELISA. Treatment of A549 cells with EUK-134, but not EUK-163, significantly inhibited RSV-induced chemokine secretion (Figure 5), suggesting that enhancement of SOD activity in infected cells cannot reduce ROS-mediated signaling and subsequent viral-induced gene expression, while increasing the levels of catalase and/or peroxidase activity can.
Figure 5.
Effect of catalytic scavengers on RSV-induced chemokine secretion. A549 cells were infected with RSV in the absence or presence of 400 μM EUK-134 or EUK-163. Culture supernatants, from uninfected and infected cells, were assayed 24 hours later for RANTES and IL-8 production by ELISA. *P < 0.05 relative to untreated, RSV-infected cells. The figure is representative of two independent experiments run in triplicate.
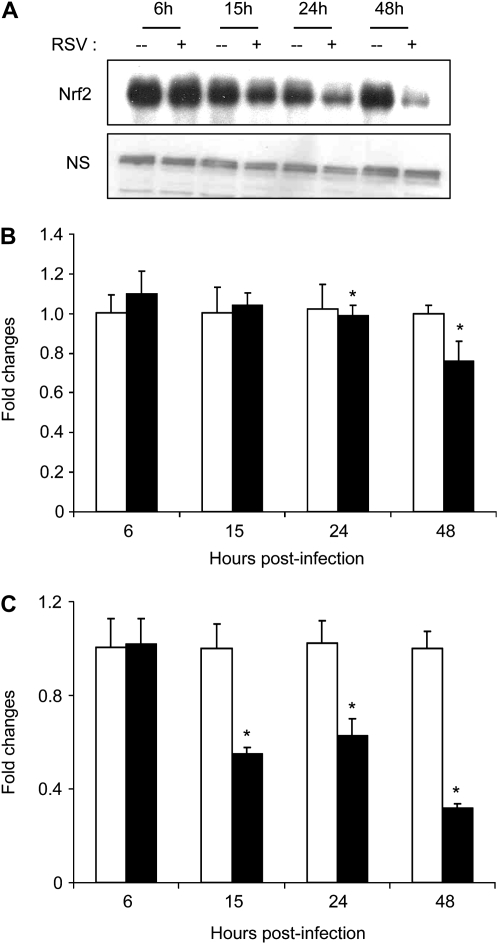
RSV Infection Down-Regulates Nrf-2 Expression in Airway Epithelial Cells
Transcription of many oxidative stress–inducible genes is regulated in part through cis-acting sequences known as antioxidant response elements (AREs). These elements have been identified in the regulatory regions of many genes encoding phase-2 detoxification enzymes and various other cytoprotective proteins such as NQO1 (NAD(P)H:quinone oxidoreductase) that catalyze reduction of a variety of quinones and quinoid compounds, GST, and other antioxidant enzymes (reviewed in Ref. 19). Nrfs are basic-leucine-zipper (bZIP) proteins belonging to the cap-n-collar (CNC) family of transcription factors, which bind to the ARE and coordinate the expression of antioxidant and phase 2 metabolizing enzymes in response to oxidative stress. The CNC family consists of four closely related members including Nrf1, Nrf2, Nrf3, and p45NFE2, as well as two distantly related factors, Bach1 and Bach2. To establish the base for a potential mechanism involved in the down-regulation of AOE expression in response to RSV infection, we conducted preliminary experiments to determine whether the expression of the key transcription factor Nrf2, which control basal and inducible expression of several AOE, was affected by viral infection. We found that RSV-infected A549 cells had decreased nuclear levels of Nrf2, compared with uninfected cells, starting as early as 15 hours after infection, with minimal levels observed by 48 hours after infection (Figure 6A). A similar trend was observed in Nrf2 mRNA levels in A549 cells infected with RSV (Figure 6B), with a more pronounced decrease in Nrf2 mRNA expression in SAE cells (Figure 6C), suggesting that the decrease level in AOE gene expression observed in response to RSV infection could be due to the progressive reduction in Nrf2 nuclear levels.
Figure 6.
RSV infection down-regulates Nrf2 expression. A549 cells were infected with RSV for various lengths of time and harvested to prepare nuclear extracts. (A) Nuclear amounts of Nrf2 protein were determined by Western blot. Nrf2 gene expression after RSV infection was quantified in (B) A549 cells or (C) SAE cells by real-time PCR. Open bars, control; solid bars, RSV. Data are presented as fold changes and are representative of two independent experiments. *P < 0.05 relative to uninfected cells.
DISCUSSION
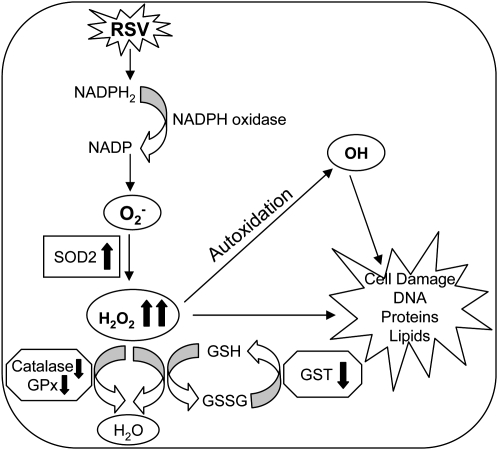
Free radicals and reactive oxygen species have been shown to function as cellular signaling molecules influencing a variety of molecular and biochemical processes, including expression of proinflammatory mediators, such as cytokines and chemokines (reviewed in Ref. 5). However, excessive ROS formation can lead to a condition of oxidative stress, which has been implicated in the pathogenesis of several acute and chronic airway diseases, such as asthma and chronic obstructive pulmonary disease (COPD) (reviewed in Ref. 20). Inducible ROS generation has been shown after stimulation with a variety of molecules and infection with certain viruses like HIV, Hepatitis B, influenza, and rhinovirus (reviewed in Ref. 21). We have recently shown that RSV infection of airway epithelial cells induces ROS production, in part through an NAD(P)H oxidase–dependent mechanism, and that antioxidant treatment blocks transcription factor activation and chemokine gene expression in vitro (7, 8, 17) and ameliorates RSV-induced clinical illness in vivo (9), indicating a central role of ROS in RSV-induced cellular signaling and lung disease. In the present study, we investigated whether RSV infection of airway epithelial cells led to a condition of cellular oxidative stress, defined as a disruption of the pro-oxidant–antioxidant balance in favor of the former, due to an impairment of antioxidant defense systems (Figure 7). Our findings of a progressive increase in lipid peroxidation products, such as 8-isoprostanes, MDA and 4-HNE, and a progressive reduction of GSH/GSSG ratio, provide a strong evidence of increased oxidative stress in RSV-infected airway epithelial cells. Interestingly, the protective mechanism of up-regulating antioxidant defenses in response to RSV occurred only at the very beginning of infection, with an increase in SOD 1, SOD 2, catalase and GST expression, and GPx activity at 6 hours after infection. While SOD 2 expression and total SOD activity continued to increase during the time course of RSV infection, there was a progressive decrease in the expression and activity of all the other tested AOEs. SOD enzymes convert superoxide anion to H2O2, and catalase and GPx convert H2O2 to water and oxygen. The increase in total SOD activity, together with the progressive decrease in catalase, GPx, and GST expression and activity, suggests that RSV infection likely results in enhanced intracellular H2O2 production, which is not detoxified by AOEs, leading to the generation of free radicals, such as the hydroxyl radical (OH), which react with lipids, proteins, and DNA, causing structural cellular damage (Figure 7).
Figure 7.
Schematic representation of the proposed mechanism(s) of oxidative cell damage in RSV infection.
Although there is increasing evidence that generation of oxidative stress is linked to the pathogenesis of a variety of acute and chronic inflammatory lung diseases, little is known regarding the role of ROS in respiratory virus–induced lung diseases and the effect of respiratory viruses on AOE expression and/or activity. In a recent study investigating global gene expression in airway epithelial cells infected with human metapneumovirus (hMPV), a recently identified paramyxovirus, we found that hMPV induces a progressive decrease of SOD 3, catalase, GST, and peroxiredoxin expression levels in infected airway epithelial cells, with a concomitant increase in SOD 2 and no change in SOD 1 expression (22), similar to what we have observed with RSV. Rhinovirus infection of bronchial epithelial cells has been shown to induce ROS formation (23) and to increase SOD 1 expression and total SOD activity at early time points of infection, with no changes in SOD 2, catalase, and GPx (24). However, AOE expression/activity was investigated only at 6 hours after infection but not at later time points. Similar to RSV, influenza virus induces SOD 2 gene expression in airway epithelial cells, with a concomitant decrease in catalase gene expression (25). Similar findings were reported in a mouse model of influenza infection, with increased expression of SOD 2 (26); however, total lung SOD and catalase activity, as well as the ratio of GSH/GSSG, have been shown to be reduced in mice after influenza infection (27), supporting the knowledge that oxidative stress plays a significant role in the pathogenesis of influenza-induced pneumonia (28, 29). In preliminary studies, using a mouse model of RSV infection, we have also observed a progressive decrease in SOD, catalase, and GST expression and activity in bronchoalveolar lavage of infected animals (Y. Hosakote, personal communication), which could explain our previous finding that RSV infection induces significant oxidative stress in vivo and that antioxidant treatment improves clinical disease and lung inflammation (9).
Although an increase in antioxidant defenses has been shown to occur in certain pulmonary diseases with increased oxidant burden such as exposure to hyperoxia (30), ozone (31), and cigarette smoke (32), decreased antioxidant expression and/or activity has been reported in other respiratory acute and chronic inflammatory disease such as asthma and COPD (33–36). Reduced SOD and catalase activity has been shown in airway epithelial cells and/or bronchoalveolar lavage obtained from patients with asthma (33–35). Similarly, decreased catalase and GST expression has been found in the lungs of patients with COPD in association with chronic smoke exposure (36).
The SOD 2 gene promoter contains binding sites for several transcription factors such as NF-κB and activator protein (AP)-1 (reviewed in Ref. 37), with NF-κB being necessary for SOD 2 gene expression in response to cytokine stimulation (38, 39). We have previously shown that RSV is a potent activator of NF-κB in airway epithelial cells (12) and it is likely that its induction is responsible for the observed increase in SOD 2 expression and activity level observed in the course of RSV infection, as well as in the course of infection with other respiratory viruses. On the other hand, the mechanism leading to decreased expression/activity of AOE is not clear. SOD 3 and catalase expression have been shown to be negatively regulated in response to cytokine stimulation such as IL-1, TNF-α, and IFN-γ (40). Furthermore, oxidative stress can lead to SOD and catalase inactivation, as it has been shown in the BALs of patients with asthma (35). Nrf2 is a central transcription factor regulating the expression of a variety of cytoprotective genes involved in detoxification of xenobiotics and in counteracting cellular oxidative stress, including inducible AOE genes (reviewed in Ref. 41). In preliminary studies, we have observed that RSV infection leads to a progressive decrease in the nuclear protein levels of Nrf2, suggesting a potential mechanism for down-regulation of AOE gene expression. A recent study has shown that the Nrf2-ARE pathway plays a protective role in the murine airways against RSV-induced injury and oxidative stress (42). More severe RSV disease, including higher viral titers, augmented inflammation, and enhanced mucus production and epithelial injury were found in Nrf2−/− mice compared with Nrf2+/+ mice.
In the past few years several classes of synthetic antioxidant mimetics have been generated and tested as a potential therapeutic approach to oxidant-related lung damage. The salen class of AOE mimetics includes compounds that have mainly SOD activity as well as compounds that in addition exhibit catalase and peroxidase activity. These molecules have been shown to be effective in preventing lung injury in animal models of oxidative stress, as well as to protect against damage of other organs, such as heart, kidney, and liver (reviewed in Ref. 37). Our findings that treatment of epithelial cells with EUK-134, but not EUK-163, significantly inhibited RSV-induced chemokine secretion suggest that enhancement of cellular SOD activity alone in response to RSV infection cannot modulate ROS-mediated signaling and subsequent viral-induced gene expression, while increasing the levels of catalase and/or peroxidase activity is beneficial in reducing proinflammatory gene expression. These results, together with our previous finding that antioxidant treatment attenuates symptoms and pathology in RSV infection (9), warrant further investigation of AOE mimetics as a novel therapeutic approach to modulate virus-induced pulmonary disease.
Acknowledgments
The authors thank Dr. Yogesh Awasthi for the generous gift of the anti-GST antibody and Cynthia Tribble for her assistance in manuscript editing and submission.
This work was supported by grants NIEHS 06676, NIAID P01 062885, and N01 HV28184, and Flight Attendant Medical Research Institute Clinical innovative Awards to A.C. and R.P.G.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0330OC on January 16, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J 2002;21:629–632. [DOI] [PubMed] [Google Scholar]
- 2.MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol 2001;429:195–207. [DOI] [PubMed] [Google Scholar]
- 3.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 1996;154:1055–1060. [DOI] [PubMed] [Google Scholar]
- 4.Morcillo EJ, Estrela J, Cortijo J. Oxidative stress and pulmonary inflammation: pharmacological intervention with antioxidants. Pharmacol Res 1999;40:393–404. [DOI] [PubMed] [Google Scholar]
- 5.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med 2000;28:463–499. [DOI] [PubMed] [Google Scholar]
- 6.Gabbita SP, Robinson KA, Stewart CA, Floyd RA, Hensley K. Redox regulatory mechanisms of cellular signal transduction. Arch Biochem Biophys 2000;376:1–13. [DOI] [PubMed] [Google Scholar]
- 7.Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofal RP. Oxidant tone regulates RANTES gene transcription in airway epithelial cells infected with Respiratory Syncytial Virus: role in viral-induced Interferon Regulatory Factor activation. J Biol Chem 2001;276:19715–19722. [DOI] [PubMed] [Google Scholar]
- 8.Liu T, Castro S, Brasier AR, Jamaluddin M, Garofalo RP, Casola A. Reactive oxygen species mediate virus-induced STAT activation: role of tyrosine phosphatases. J Biol Chem 2004;279:2461–2469. [DOI] [PubMed] [Google Scholar]
- 9.Castro SM, Guerrero-Plata A, Suarez-Real G, Adegboyega PA, Colasurdo GN, Khan AM, Garofalo RP, Casola A. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am J Respir Crit Care Med 2006;174:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueba O. Respiratory syncytial virus: I. concentration and purification of the infectious virus. Acta Med Okayama 1978;32:265–272. [PubMed] [Google Scholar]
- 11.Patel JA, Kunimoto M, Sim TC, Garofalo R, Eliott T, Baron S, Ruuskanen O, Chonmaitree T, Ogra PL, Schmalstieg F. Interleukin-1 alpha mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am J Respir Cell Mol Biol 1995;13:602–609. [DOI] [PubMed] [Google Scholar]
- 12.Garofalo RP, Sabry M, Jamaluddin M, Yu RK, Casola A, Ogra PL, Brasier AR. Transcriptional activation of the interleukin-8 gene by respiratory syncytial virus infection in alveolar epithelial cells: nuclear translocation of the RelA transcription factor as a mechanism producing airway mucosal inflammation. J Virol 1996;70:8773–8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao X, Liu T, Spetch L, Kolli D, Garofalo RP, Casola A. Airway epithelial cell response to human metapneumovirus infection. Virology 2007;368:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe SE, Mei F, Ogra PL, Garofalo RP. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol 1998;72:4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Luxon B, Casola A, Garofalo RP, Jamaluddin M, Brasier AR. Expression of RSV-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J Virol 2001;75:9044–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pazdrak K, Olszewska-Pazdrak B, Liu B, Takizawa R, Brasier AR, Garofalo RP, Casola A. MAP-kinase activation is involved in post-transcriptional regulation of RSV-induced RANTES gene expression. Am J Physiol 2002;283:L364–L372. [DOI] [PubMed] [Google Scholar]
- 17.Indukuri H, Castro SM, Liao SM, Feeney LA, Dorsch M, Coyle AJ, Garofalo RP, Brasier AR, Casola A. Ikkepsilon regulates viral-induced interferon regulatory factor-3 activation via a redox-sensitive pathway. Virology 2006;353:155–165. [DOI] [PubMed] [Google Scholar]
- 18.Limoli CL, Giedzinski E, Baure J, Doctrow SR, Rola R, Fike JR. Using superoxide dismutase/catalase mimetics to manipulate the redox environment of neural precursor cells. Radiat Prot Dosimetry 2006;122:228–236. [DOI] [PubMed] [Google Scholar]
- 19.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 2004;36:1199–1207. [DOI] [PubMed] [Google Scholar]
- 20.Folkerts G, Kloek J, Muijsers RB, Nijkamp FP. Reactive nitrogen and oxygen species in airway inflammation. Eur J Pharmacol 2001;429:251–262. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz KB. Oxidative stress during viral infection: a review. Free Radic Biol Med 1996;21:641–649. [DOI] [PubMed] [Google Scholar]
- 22.Bao X, Sinha M, Liu T, Hong C, Luxon BA, Garofalo RP, Casola A. Identification of human metapneumovirus-induced gene networks in airway epithelial cells by microarray analysis. Virology 2008;374:114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biagioli MC, Kaul P, Singh I, Turner RB. The role of oxidative stress in rhinovirus induced elaboration of IL-8 by respiratory epithelial cells. Free Radic Biol Med 1999;26:454–462. [DOI] [PubMed] [Google Scholar]
- 24.Kaul P, Singh I, Turner RB. Effect of rhinovirus challenge on antioxidant enzemes in respiratory epithelial cells. Free Radic Res 2002;36:1085–1089. [DOI] [PubMed] [Google Scholar]
- 25.Jacoby DB, Choi AM. Influenza virus induces expression of antioxidant genes in human epithelial cells. Free Radic Biol Med 1994;16:821–824. [DOI] [PubMed] [Google Scholar]
- 26.Choi AM, Knobil K, Otterbein SL, Eastman DA, Jacoby DB. Oxidant stress responses in influenza virus pneumonia: gene expression and transcription factor activation. Am J Physiol 1996;271:L383–L391. [DOI] [PubMed] [Google Scholar]
- 27.Kumar P, Khanna M, Srivastava V, Tyagi YK, Raj HG, Ravi K. Effect of quercetin supplementation on lung antioxidants after experimental influenza virus infection. Exp Lung Res 2005;31:449–459. [DOI] [PubMed] [Google Scholar]
- 28.Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng YM, Dietzschold B, Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA 1996;93:2448–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suliman HB, Ryan LK, Bishop L, Folz RJ. Prevention of influenza-induced lung injury in mice overexpressing extracellular superoxide dismutase. Am J Physiol Lung Cell Mol Physiol 2001;280:L69–L78. [DOI] [PubMed] [Google Scholar]
- 30.Erzurum SC, Danel C, Gillissen A, Chu CS, Trapnell BC, Crystal RG. In vivo antioxidant gene expression in human airway epithelium of normal individuals exposed to 100% O2. J Appl Physiol 1993;75:1256–1262. [DOI] [PubMed] [Google Scholar]
- 31.Boehme DS, Hotchkiss JA, Henderson RF. Glutathione and GSH-dependent enzymes in bronchoalveolar lavage fluid cells in response to ozone. Exp Mol Pathol 1992;56:37–48. [DOI] [PubMed] [Google Scholar]
- 32.Gilks CB, Price K, Wright JL, Churg A. Antioxidant gene expression in rat lung after exposure to cigarette smoke. Am J Pathol 1998;152:269–278. [PMC free article] [PubMed] [Google Scholar]
- 33.Smith LJ, Shamsuddin M, Sporn PH, Denenberg M, Anderson J. Reduced superoxide dismutase in lung cells of patients with asthma. Free Radic Biol Med 1997;22:1301–1307. [DOI] [PubMed] [Google Scholar]
- 34.De Raeve HR, Thunnissen FB, Kaneko FT, Guo FH, Lewis M, Kavuru MS, Secic M, Thomassen MJ, Erzurum SC. Decreased Cu,Zn-SOD activity in asthmatic airway epithelium: correction by inhaled corticosteroid in vivo. Am J Physiol 1997;272:L148–L154. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh S, Janocha AJ, Aronica MA, Swaidani S, Comhair SA, Xu W, Zheng L, Kaveti S, Kinter M, Hazen SL, et al. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J Immunol 2006;176:5587–5597. [DOI] [PubMed] [Google Scholar]
- 36.Tomaki M, Sugiura H, Koarai A, Komaki Y, Akita T, Matsumoto T, Nakanishi A, Ogawa H, Hattori T, Ichinose M. Decreased expression of antioxidant enzymes and increased expression of chemokines in COPD lung. Pulm Pharmacol Ther 2007;20:596–605. [DOI] [PubMed] [Google Scholar]
- 37.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 2003;167:1600–1619. [DOI] [PubMed] [Google Scholar]
- 38.Das KC, Lewis-Molock Y, White CW. Activation of NF-kappaB and elevation of MnSOD gene expression by thiol reducing agents in lung adenocarcinoma. Lung Cell Mol Physiol 1995;13:L588–L602. [DOI] [PubMed] [Google Scholar]
- 39.Das KC, Lewis-Molock Y, White CW. Thiol modulation of TNF alpha and IL-1 induced MnSOD gene expression and activation of NF-kappa B. Mol Cell Biochem 1995;148:45–57. [DOI] [PubMed] [Google Scholar]
- 40.Chung-man HJ, Zheng S, Comhair SA, Farver C, Erzurum SC. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res 2001;61:8578–8585. [PubMed] [Google Scholar]
- 41.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 2007;47:89–116. [DOI] [PubMed] [Google Scholar]
- 42.Cho HY, Imani F, Miller-Degraff L, Walters D, Melendi GA, Yamamoto M, Polack FP, Kleeberger SR. 2008. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus (RSV) disease. Am J Respir Crit Care Med 2009;179:138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]