Abstract
Acid sphingomyelinase (ASMase) is a key enzyme in sphingolipid metabolism, which can be activated by various cellular stress mechanisms including bacterial pathogens. Activation of ASMase generates ceramide, which is important for innate immune response to eliminate infected pathogens. The current study reveals a defective ASMase pathway after Pseudomonas aeruginosa infection in both a cystic fibrosis (CF) bronchial epithelial cell line (IB3-1 cell) and in the lungs of CF transmembrane conductance regulator (CFTR) knockout (KO) mice as compared with S9 cells and wild-type C57BL/6 mice. ASMase activity and total ceramide levels significantly increased in S9 cells and C57BL/6 mice with P. aeruginosa infection, but not in IB3-1 cells and CFTR KO mice. The silencing of CFTR by CFTR RNAi in S9 cells significantly decreased ASMase activity after bacterial infection as compared with controls. This study also demonstrates that induction of ASMase is responsible for modulating the immune response to bacterial infection. Blocking ASMase activity with specific ASMase RNAi, an ASMase inhibitor, or an ASMase antibody in S9 cells significantly increased IL-8 levels with P. aeruginosa infection compared with controls. Reciprocally, adding exogenous bacterial sphingomyelinase to IB3-1 cells significantly decreased IL-8 levels compared with untreated cells. In addition, silencing of ASMase in S9 cells also significantly decreased bacterial internalization. Adding exogenous bacterial sphingomyelinase to IB3-1 cells reconstituted the cell death response to P. aeruginosa infection. This study demonstrates that the defective ASMase pathway in CF is a key contributor to the unabated IL-8 response with P. aeruginosa infection and to the compromised host response failing to eradicate bacteria.
Keywords: cystic fibrosis, acid sphingomyelinase, P. aeruginosa, ceramide, immune response
CLINICAL RELEVANCE
This article reveals a defective acid sphingomyelinase response to Pseudomonas aeruginosa infection in cystic fibrosis (CF), which is a key factor contributing to the unabated IL-8 response, reduced bacterial uptake, and decreased apoptotic response to P. aeruginosa infection in CF.
Cystic fibrosis (CF), the most common lethal genetic disease in whites, affects 30,000 people in the United States and 250,000 people worldwide. The disease arises from mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (1) that lead to electrolyte imbalance in epithelial cells and mucous plugging in affected organs. CF is characterized by pancreatic insufficiency, intestinal obstruction, male infertility, and most importantly, chronic lung bacterial infection. According to the CF registry, over 80% of patients with CF suffer from chronic lung infection with Pseudomonas aeruginosa, which contributes to death in over three-quarters of patients with CF (2).
Patients with CF infected with P. aeruginosa have an abnormal immune response, which includes massive levels of proinflammatory cytokines (such as TNF-α, IL-1β, IL-6, and IL-8) in their airways, for prolonged periods of time (3, 4). In contrast, the level of an anti-inflammatory cytokine, IL-10, is much lower in airways of patients with CF than in those of patients without CF (3, 5). Despite exaggerated neutrophil influx, patients with CF fail to eradicate bacteria in their airways efficiently. CF cells internalize less bacteria (6) and have a delayed apoptotic response compared with cells expressing the wild-type (WT) CFTR (7). The intense neutrophil-dominated inflammatory response in CF is the major key in promoting progressive obstruction and deterioration in lung function (8).
The mechanisms linking a defective CFTR to the compromised immune response to P. aeruginosa infection are not fully elucidated. Currently, there are several hypotheses, which include the ideas that (1) there are decreased salt-sensitive bactericidal peptides in CF airway surface liquid due to high salt content (9, 10); (2) there is increased adherence of P. aeruginosa to respiratory epithelial cells in CF airway due to alterations in glycolipid sialylation (11); (3) there is decreased bacterial clearance from the periciliary layer above the epithelial cells in CF airway due to dehydration (12); and (4) CFTR serves as a specific receptor for P. aeruginosa (6). Although these hypotheses explain to some extent why patients with CF are highly susceptible to P. aeruginosa infection, currently no hypothesis describes why patients with CF exhibit an overwhelming proinflammatory cytokine response in their airways while still failing to eradicate P. aeruginosa.
Recent studies highlight the importance of sphingolipids in cell growth, polarization, cell differentiation, senescence, apoptosis, and immune regulation (13–17), and some have begun to implicate sphingolipids in regulation of bacterial infections (16, 18). Acid sphingomyelinase (ASMase), an important enzyme in sphingolipids metabolism, can be activated by various stress or stimulus signals (17). When ASMase is activated, it is translocated from intracellular vesicles to the outer leaflet of the plasma membrane (17, 19), where it hydrolyzes membrane spingomyelin and generates ceramide. Grassme and coworkers (18) demonstrated that P. aeruginosa infection triggers very acute activation of ASMase in non-CF epithelial cells, which is involved in bacterial internalization, IL-1β regulation, and induction of apoptotic response after P. aeruginosa infection. However, the regulation of ASMase in CF in response to P. aeruginosa infection has not been studied. Moreover, the specific roles of ASMase in the interplay of P. aeruginosa infection and CFTR have not been defined.
In our previous microarray studies (20), an up-regulation of ASMase mRNA after P. aeruginosa infection was discovered in human bronchial epithelial cells with WT CFTR (S9 cells), but not in the epithelial cells with mutant CFTR (IB3-1 cells). Therefore, we hypothesized that there was a defective ASMase pathway in CF that contributed to the compromised immune response to P. aeruginosa infection in CF. The present study verifies our microarray data and investigates the roles of ASMase in the immune response to P. aeruginosa infection.
MATERIALS AND METHODS
Cell Lines
The IB3-1 cell line, the CFTR-defective (ΔF508/W1282X) immortalized bronchial epithelial cell line from a patient with CF (21), and S9 cell line, a stable IB3-1 subclone transduced with the recombinant AAV-CFTR vector, which expresses a functional Cl- channel (22–24), were used in this study. IB3-1 cells and S9 cells were obtained from the University of Florida and cultured in LHC-8 medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS) at 37°C with 5% CO2.
Preparation of Bacteria
A clinical mucoid strain of P. aeruginosa was isolated from the sputum culture of an unidentified patient. A nonmucoid strain, PAO1, was also used for this study. P. aeruginosa were grown into mid-logarithmic phase before infection of cells. The bacteria were centrifuged and diluted in cell medium to an optical density at 600 nm (OD600) of 1.0 (∼ 7 × 108 colony-forming units [cfu]/ml for mucoid strain of P. aeruginosa, ∼ 3 × 109 cfu/ml for PAO1 strain of P. aeruginosa).
Bacterial Infection of Cells
IB3-1 and S9 cells were either uninfected or infected with a mucoid or nonmucoid strain of P. aeruginosa (25 cfu/cell) for different time periods (10, 30, 60, 180, 300 min). The cells were collected for determination of ASMase mRNA levels, enzyme activity, and the levels of ceramide production.
Reverse Transcription PCR and Real-Time PCR
RNA was isolated from IB3-1/S9 cells, or the lung homogenates of mice using the RNeasy Mini kit (Qiagen, Valencia, CA). Complementary DNA was synthesized from 2 μg of total RNA using the Omniscript reverse transcriptase kit (Qiagen). The real-time PCR was performed on an iCycler system using an iQ SYBR Green Supermix kit (Bio-Rad Laboratories, Hercules, CA). The data were normalized to levels of an internal control gene, β-actin. For the regular PCR reaction, the mixture was denatured at 94°C for 2 minutes and the target genes were amplified by 35 cycles of reaction (94°C 30 s, 55°C 30 s, and 72°C 1 min). For real-time PCR reaction, the mixture was denatured at 95°C for 3 minutes and the target genes were amplified by 40 cycles of reaction (95°C 10 s, 55°C 45 s). The primers used were as follows: 5′-ATTGGCAATGAGCGGTTCC-3′ and 5′-GGTAGTTTCGTGGATGCCACA-3′ for human β-actin; 5′-TCCTTCTTGGGTATGGAATCCTGTGG-3′ and 5′-CGCTCAGGAGGAGCAATGATCTTG-3′ for mouse β-actin; 5′-TGGCTCTATGAAGCGATGGC-3′ and 5′-TTGAGAGAGATGAGGCGGAGAC-3′ for ASMase, 5′-CTCTTCCGGCAAGCCATCAG-3′ and 5′-GCACCTCTTCTTCTGTCTCCTC-3′ for human CFTR.
Western Blotting Assays
IB3-1 and S9 cells were lysed in RIPA lysis buffer (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The lungs of mice were homogenized and lysed in 2 ml of cell lysis buffer (Bio-Rad). Thirty micrograms of total proteins from each lysate were loaded onto 4 to 20% gradient SDS polyacrylamide gels, subjected to electrophoresis and then transferred to polyvinylidene difluoride membranes. The blots were incubated overnight at 4°C with rabbit anti-PARP (1:1,000; Cell Signaling Technology, Inc., Danvers, MA), or rabbit anti–caspase-3 (1:1000; Cell Signaling Technology). Later, the blots were incubated with goat anti-rabbit IgG-horseradish peroxidase secondary antibody (1:5,000, Santa Cruz Biotechnology) and then developed using ECL chemiluminescence reagents (Pierce Biotechnology, Rockford, IL). Protein quantification was performed using NIH image software.
Assay of ASMase Activity
IB31 and S9 cells were lysed in 50 mM Tris, 0.2% Triton-X100 buffer (pH 7.4). Fifty micrograms of protein were adjusted to a total volume of 100 μl of reaction mixture, which contains 1 mM of EDTA, 250 mM sodium acetate (pH 5.0), 100 mM (choline-methy-14c) sphingomyelin, and 0.1% Triton X-100. After incubation for 1 hour at 37°C, the reaction was stopped by adding 1.5 ml of chloroform/methanol (2:1), followed by adding 200 μl of water. Phases were separated by centrifugation at 2,000 × g for 5 minutes. ASMase activity was determined by quantification of the amount of released radioactive phosphocholine by scintillation counting.
Mass Spectrometry for Ceramide
Sphingolipids were extracted from the samples by the Bligh Dyer technique. Sphingolipid analysis was performed using Electrospray Ionization/Tandem Mass Spectrometry (ESI-MS/MS) on a Thermo Finnigan TSQ 7000 triple quadruple mass spectrometer (Thermo Finnigan Inc., Austin, TX), operating in a multiple reaction monitoring positive ionization mode. This technique has been previously described by Bielawski and coworkers (25).
Cytokine Assays
IB3-1 and S9 cells were lysed in cell lysis buffer (Bio-Rad Laboratories). Cytokines in cell lysates were measured by the Bio-Plex system using a human Bio-Plex cytokine kit (Bio-Rad Laboratories).
ASMase Inhibitor, Sphingomyelinase, and Anti-Human ASMase Antibody Treatment Experiments
IB3-1 cells were either untreated or treated with various concentrations (0.02 or 0.03 U/ml) of bacterial sphingomyelinase from Staphylococcus aureus (Sigma Aldrich, St. Louis, MO) for 1 hour before P. aeruginosa infection. S9 cells were either untreated or treated with 32 μM of an ASMase inhibitor desipramine (26) (Sigma Aldrich) 1 hour before P. aeruginosa infection; alternatively, the S9 cells were either treated with 10 μg/ml of rabbit anti-ASMase antibody (27) or 10 μg/ml of normal rabbit IgG (Santa Cruz Biotechnology) for 1 hour before P. aeruginosa infection. The IB3-1 and S9 cells were harvested 5 hours after P. aeruginosa infection.
Apoptosis Assay
Apoptosis of IB3-1 /or S9 cells was determined 5 hours after infection with P. aeruginosa using fluorescein isothiocyanate (FITC)-labeled Annexin V and propidium iodide (BD Biosciences, San Diego, CA) staining. The cells were sorted by a Becton Dickinson FACSCalibur flow cytometer (BD Biosciences).
RNA Interference Assays
The pre-designed ON-TARGETplus SMARTpool RNA interference (RNAi) specific for human ASMase (Dharmacon, Lafayette, CO) consists of four duplex RNAi (target sequence 5′-GCACUGGGAUCAUGACUACUU-3′; 5′-GCACACCUGUCAAUAGCUUUU-3′; 5′-CAGGUUACAUCGCAUAGUGUU-3′; 5′-GGCACAACCUGGUAUAUCGUU-3′). The pre-designed ON-TARGETplus SMARTpool RNAi specific for human CFTR (Dharmacon) consists of four duplex RNAi (target sequence 5′-GAACACAUACCUUCGAUAU-3′; 5′-GUACAAACAUGGUAUGACU-3′; 5′-GUGAAAGACUUGUGAUUAC-3′; 5′-GCAGGUGGGAUUCUUAAUA-3′). The control ON-TARGETplus siCONTROL Non-targeting Pool (Dharmacon) consists of four pairs of scrambled RNAi (target sequence 5′-UGGUUUACAUGUCGACUAA-3′; 5′-UGGUUUACAUGUUGUGUGA-3′; 5′-UGGUUUACAUGUUUUCUGA-3′; 5′-UGGUUUACAUGUUUUCCUA-3′). To target expression, 2 × 105 S9 cells were transfected with either 100 nM or 50 nM of ASMase RNAi, CFTR RNAi, or scrambled RNAi by DharmaFECT1 Transfection Reagent (Dharmacon) for 48 hours before P. aeruginosa infection and ASMase enzyme activity or RT-PCR analysis.
Bacterial Internalization Assays
IB3-1 and S9 cells were infected with P. aeruginosa (25 cfu/cell) for 2 hours. Internalization of bacteria was quantified by the well-established gentamicin survival assay (28, 29). Briefly, cells were washed with PBS and incubated for 2 hours in LHC-8 medium containing cell-impermeable antibiotic gentamicin (300 μg/ml) to kill extracellular bacteria. The cells were washed with PBS and lysed in PBS containing 5 mg/ml saponin for 20 minutes at room temperature. The number of viable bacteria was quantified by serial dilution of cell lysates and spread on tryptic soy agar plates. cfu were determined 20 h after plating.
Animal Experiments
The CFTR KO mice are Cftrtm1Unc-TgN(FABPCFTR) (fatty acid–binding protein [FABP]-CFTR) mice that have a stop codon in the murine CFTR gene (S489X) but also express human CFTR in the gut epithelium due to transgenic introduction of CFTR under the control of the FABP promoter (30). Breeding pairs of these CFTR KO mice were obtained from the University of Florida and further bred in our facility. Wild-type C57BL/6 mice were purchased from Charles River Laboratory (Wilmington, MA). The mice were caged in Micro-Isolator Top Flow Ventilated cages and studied under Institutional Animal Care and Use Committee–approved protocols in the animal facilities of Medical University of South Carolina. Eight-week-old C57BL/6 mice or CFTR KO mice were either untreated or given various doses (2 × 106, 1 × 107, or 5 × 107 cfu) of PAO1 strain of P. aeruginosa by oropharyngeal instillation. There were eight mice for each dose group. The mouse lungs were collected 6 hours after bacterial administration. Among each group, the lungs of four mice were harvested for ASMase mRNA assay and the lungs of the other four mice were used for ceramide assays.
Oropharyngeal Instillation of P. aeruginosa in Mice
Oropharyngeal instillation with surfactant phospholipids is a reliable alternative to intratracheal injection for establishing lung infection in mice (31). To prepare for the inoculum, P. aeruginosa were grown into mid-logarithmic phase. The bacteria were centrifuged and diluted in PBS to an optical density at 600 nm (OD600) of 1.0 (3 × 109 cfu/ml for PAO1). The bacteria were further diluted to the desired dose with PBS and mixed with surfactant phospholipids liposomes (4 mg/ml). Forty microliters of mixture of bacteria with surfactant phospholipids was instilled at the back of oral cavity above the tracheal opening.
Statistics
Data are expressed as the mean ± SD. Data were analyzed by paired or unpaired two-tailed Student's t test. All statistical tests were performed using GraphPad InStat version 3.05 for Window 95 (GraphPad Software, San Diego, CA).
RESULTS
Defective ASMase Activity in CF Bronchial Epithelial Cells Infected with P. aeruginosa
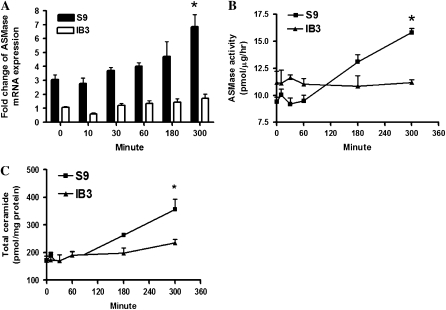
To evaluate ASMase activity, we performed a kinetic study to measure ASMase mRNA, enzyme activity, as well as ceramide levels at different time points (0, 10, 30, 60, 180, and 300 min) after P. aeruginosa infection. Both a mucoid strain and a nonmucoid PAO1 strain were used in this study. The in vitro study demonstrated that infection with both PAO1 strain and a mucoid strain (data not shown) of P. aeruginosa induced a sustained activation of the ASMase/ceramide pathway in wild-type S9 cells (cells with corrected CFTR genes). The ASMase mRNA levels (Figure 1A, n = 3, *P < 0.01), ASMase enzyme activity (Figure 1B, n = 4, *P < 0.01), and total ceramide levels (Figure 1C, n = 4, *P < 0.01) significantly increased in S9 cells 300 minutes after P. aeruginosa infection compared with uninfected controls. In contrast, infection with P. aeruginosa did not elicit significant increase of ASMase mRNA, and enzyme activity in IB3-1 cells (cells with mutant CFTR genes). At 300 minutes after P. aeruginosa infection, there was only a mild (20%) increase of total ceramide level in IB3-1 cells compared with a significant increase (78%) in infected S9 cells (n = 4, *P < 0.01). These results demonstrate that the induction of ASMase in response to P. aeruginosa infection is dependent on normal CFTR expression, and there is a clear defect in induction of ASMase in CF bronchial epithelial cells after P. aeruginosa infection.
Figure 1.
Defective acid sphingomyelinase (ASMase) response to Pseudomonas aeruginosa (PA) infection in cystic fibrosis (CF) bronchial epithelial cells. IB3-1 and S9 cells were infected with PAO1 (25 cfu/cell) for 0, 10, 30, 60, 180, and 300 minutes. (A) ASMase mRNA expression in IB3-1 versus S9 cells with PAO1 infection (n = 3, *P < 0.01). The data were normalized to levels of an internal control gene, β-actin. (B) ASMase activity in IB3-1 versus S9 cells with PA infection (n = 4, *P < 0.01). (C) Total ceramide in IB3-1 versus S9 cells with PA infection (n = 4, *P < 0.01).
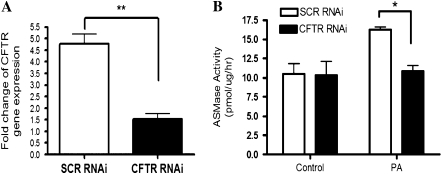
To further confirm that ASMase induction after P. aeruginosa infection is dependent on functional CFTR expression, S9 cells were transfected with either human CFTR RNAi or scrambled RNAi. The effect of knocking down CFTR mRNA expression was confirmed by real-time PCR 48 hours after RNAi transfection in S9 cells treated with CFTR RNAi compared with cells treated with scrambled RNAi (Figure 2A, n = 4, **P < 0.01). Importantly, S9 cells treated with CFTR RNAi lost ASMase induction after P. aeruginosa infection compared with S9 cells treated with scrambled RNAi (Figure 2B, n = 4, *P < 0.05). These results demonstrated that activation of ASMase in response to P. aeruginosa infection was dependent on wild-type CFTR expression in bronchial epithelial cells.
Figure 2.
Induction of ASMase in response to P. aeruginosa (PA) infection is dependent on normal CF transmembrane conductance regulator (CFTR) expression in bronchial epithelial cells. S9 cells were transfected with 100 nM of human CFTR RNA interference (RNAi) or scrambled (SCR) RNAi for 48 hours. (A) Human CFTR mRNA expression in S9 cells treated with human CFTR RNAi versus scrambled RNAi (n = 4, **P < 0.01). The data were normalized to the levels of an internal control gene, β-actin. (B) ASMase activity in S9 cells treated with human CFTR RNAi versus scrambled RNAi with PA infection for 5 hours (n = 4, *P < 0.05).
Defective ASMase Activity in CFTR KO Mice with P. aeruginosa Infection
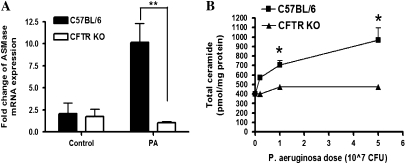
To extend the in vitro experiments, in vivo experiments in CFTR KO mice and WT C57BL/6 mice were conducted. The nonmucoid PAO1 strain was used for the in vivo study. Eight-week-old WT C57BL/6 mice and CFTR KO mice were either uninfected or infected with various doses (2 × 106, 1 × 107, or 5 × 107 cfu) of P. aeruginosa for 6 hours. RNA was extracted from lung homogenates of half of the mice for ASMase mRNA analysis. The lungs of the other half group of mice were used for ceramide assay. The result of real-time PCR revealed that infection with P. aeruginosa (1 × 107 cfu) elicited a significant increase of ASMase mRNA in WT C57BL/6 mice compared with uninfected WT mice, while infection with P. aeruginosa failed to induce a significant increase of ASMase mRNA levels in CFTR KO mice after bacterial infection (Figure 3A, n = 4, **P < 0.0001). A dose-dependent increase of total ceramide levels was detected in the lung homogenates of infected WT C57BL/6 mice with increasing dose of P. aeruginosa (Figure 3B). Total ceramide levels increased in a dose-dependent manner ranging from 43% (for 2 × 106 cfu dose) to 140% (for 5 × 107 cfu dose). In contrast, the total ceramide levels in the lung homogenates of infected CFTR KO mice remained close to the basal level after infection for 2 × 106 cfu dose, and there was only a marginal 17% increase for the maximal 5 × 107 cfu dose. A paired t test demonstrated very significant difference between WT C57BL/6 mice and CFTR KO mice after P. aeruginosa infection (n = 4, *P < 0.01). A replicate animal experiment confirmed the above results. These in vivo results demonstrate significant induction of ASMase in WT mice, but not in CFTR KO mice. Thus, together with the tissue culture results, these findings reveal acute up-regulation of ASMase after P. aeruginosa infection, leading to formation of ceramide in WT cells or animals. This effect is clearly defective in cells with mutant CFTR and CFTR KO mice.
Figure 3.
Defective ASMase response to P. aeruginosa (PA) infection in CFTR KO mice. C57BL/6 mice and CFTR KO mice (Cftrtm1Unc-TgN (FABPCFTR)) were administered 0, 2 × 106, 1 × 107, or 5 × 107 cfu of PAO1 strain of P. aeruginosa by oral-pharyngeal instillation (eight mice in each group). The mice were killed 6 hours after PA administration. The lungs of half of each group of mice were used for ASMase mRNA analysis and the lungs of the other half of each group of mice were used for ceramide assays. (A) ASMase mRNA expression in lung homogenates of CFTR KO mice versus C57BL/6 mice. The mice were either uninfected (control group) or infected with 1 × 107 cfu of PA for 6 hours (n = 4, **P < 0.0001). (B) Total ceramide in lung homogenates of CFTR KO mice versus C57BL/6 mice (n = 4, *P < 0.01).
Exaggerated IL-8 Cytokine Response after P. aeruginosa Infection in CF and the Role of ASMase
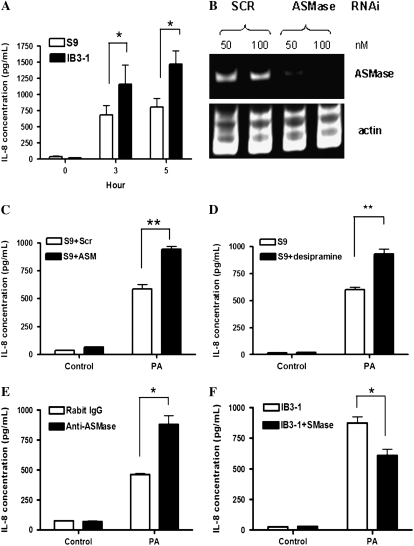
Patients with CF infected with P. aeruginosa have a prolonged and exaggerated IL-8 response (3, 4). The exaggerated IL-8 response was further confirmed by CFTR KO mice experiments (32–34) and the current in vitro study. As shown in Figure 4A, IL-8 levels were much higher in infected IB3-1 cells than in infected S9 cells in all five separate studies (n = 5, *P < 0.05). Since sphingolipids are increasingly implicated in inflammatory pathways (15, 16, 18), we hypothesized that the loss of induction of ASMase in CF bronchial epithelial cells might account, at least in part, for the exaggerated IL-8 cytokine expression after P. aeruginosa infection.
Figure 4.
Exaggerated IL-8 cytokine response with P. aeruginosa infection in CF is associated with defective ASMase activity. (A) Exaggerated IL-8 response in CF bronchial epithelial cells with PA infection. IB3-1 and S9 cells were infected with PAO1 (25 cfu/cell) for 0, 3, and 5 h (n = 5, *P < 0.05). (B) RT-PCR result 48 hours after RNAi transfection. S9 cells were transfected with either 50 nM or 100 nM of ASMase RNAi or SCR RNAi for 48 hours. (C) Effect of ASMase RNAi on IL-8 expression. S9 cells were transfected with either 50 nM of ASMase RNAi or SCR RNAi 48 hours before PA infection. The cells were either uninfected (control group) or infected with PAO1 (25 cfu/cell) for 5 hours (n = 4, **P = 0.001). (D) Effect of ASMase inhibitor on IL-8 expression. Desipramine 32 μM) was added to S9 cells 1 hour before PA infection. The cells were either uninfected (control group) or infected with PAO1 (25 cfu/cell) for 5 hours (n = 5, **P < 0.001). (E) Effect of ASMase antibody on IL-8 expression. S9 cells were treated with a polyclonal rabbit anti-human ASMase antibody or a control normal rabbit IgG 10 μg/ml 1 hour before PA infection. The cells were either uninfected (control group) or infected with PAO1 (25 cfu/cell) for 5 hours (n = 4, *P < 0.01). (F) Effect of exogenous sphingomyelinase (SMase) on IL-8 expression. Exogenous SMase from Staphylococcus aureus (0.02 U/ml) was added to IB3-1 cells 1 hour before PA infection. The cells were either uninfected (control group) or infected with PAO1 (25 cfu/cell) for 5 hours (n = 4, *P < 0.01).
To test this hypothesis, first RNAi technology was employed to knock down ASMase gene expression in S9 cells. To ensure the specificity of RNA interference, the pre-designed ON-TARGETplus SMARTpool RNAi and control Nontargeting Pool (each pool consists of four duplex RNAi; Dharmacon) were used in this study. The silencing of ASMase mRNA expression (> 70%) by ASMase RNAi was confirmed by RT-PCR, as shown in Figure 4B. Forty-eight hours after RNAi transfection, S9 cells were infected with P. aeruginosa for 5 hours. Silencing ASMase by ASMase RNAi significantly increased IL-8 levels in S9 cells with P. aeruginosa infection compared with scrambled (SCR) RNAi group (Figure 4C, n = 4, **P = 0.001). To corroborate the RNAi results, a commonly used ASMase inhibitor, desipramine (26), was added to S9 cells 1 h before P. aeruginosa infection. IL-8 levels significantly increased in S9 cells treated with desipramine after P. aeruginosa infection compared with infected untreated cells (Figure 4D, n = 5, **P < 0.001). Since ASMase is proposed to hydrolyze sphingomyelin to ceramide on the outer leaflet of the plasma membrane, we also evaluated the effect of a specific antibody to ASMase (27) on IL-8 production. A specific rabbit anti-ASMase antibody was added to S9 cells 1 hour before P. aeruginosa infection. The control S9 cells were treated with a control rabbit IgG. IL-8 levels significantly increased after P. aeruginosa infection in S9 cells treated with the ASMase antibody compared with S9 cells treated with the normal rabbit IgG (Figure 4E, n = 4, *P < 0.01). In summary, these results suggest a role for the ASMase/ceramide pathway in regulating IL-8 response to P. aeruginosa infection.
Reciprocally, since IB3-1 cells have a defective ASMase response, a gain-of-function approach was used in this cell line to determine if “reconstituting” sphingomyelinase (SMase) activity would influence the IL-8 response. Bacterial SMase (0.02 U/ml) from Staphylococcus aureus was added 1 hour before P. aeruginosa infection. IL-8 levels were significantly reduced in IB3-1 cells treated with SMase after P. aeruginosa infection compared with control untreated cells (Figure 4F, n = 4, *P < 0.01). These results demonstrate that the loss of ASMase induction after bacterial infection in IB3-1 cells is responsible for the exaggerated IL-8 response in CF. Together with the results in S9 cells, these results reveal a critical role for ASMase in regulating the IL-8 response to P. aeruginosa infection in a CFTR-dependent manner.
Bacterial Internalization in CF Bronchial Epithelial Cells and the Role of ASMase
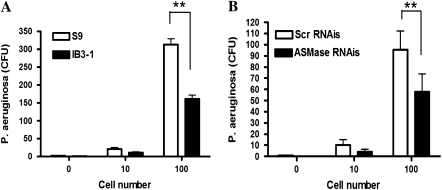
Activation of ASMase leads to the formation of ceramide-enriched lipid rafts, which have been implicated in bacterial internalization (18). Since CF bronchial epithelial cells have defective ASMase activity after P. aeruginosa infection, we hypothesized that this would affect bacterial internalization in CF. To test the hypothesis, the gentamicin survival assay was used to evaluate bacterial internalization in IB3-1 and S9 cells. This is a well-established assay and has been previously used by other investigators (28, 29) to quantify internalized bacteria after P. aeruginosa infection. As shown in Figure 5A, S9 cells engulfed approximately twice the number of bacteria after bacterial infection compared with infected IB3-1 cells (n = 6, **P < 0.0001). Importantly, the number of internalized bacteria decreased approximately 2-fold in S9 cells treated with ASMase RNAi compared with S9 cells treated with scrambled RNAi (Figure 5B, n = 6, **P = 0.0001). This result supports the critical role of ASMase in bacterial internalization.
Figure 5.
Decreased bacteria internalization with P. aeruginosa (PA) infection in CF is associated with defective ASMase activity. (A) CF bronchial epithelial cells internalize less number of P. aeruginosa compared with cells with corrected CFTR gene. IB3-1 and S9 cells were infected with PAO1 for 2 hours. Internalization of bacteria was quantified by gentamicin survival assay (n = 6, **P < 0.0001). (B) Knockdown of ASMase in S9 cells attenuates PA internalization. S9 cells were transfected with either ASMase RNAi (50 nM) or control SCR RNAi for 48 hours before PAO1 infection (n = 6, **P = 0.0001).
Apoptotic Response to P. aeruginosa Infection in CF and the Role of ASMase
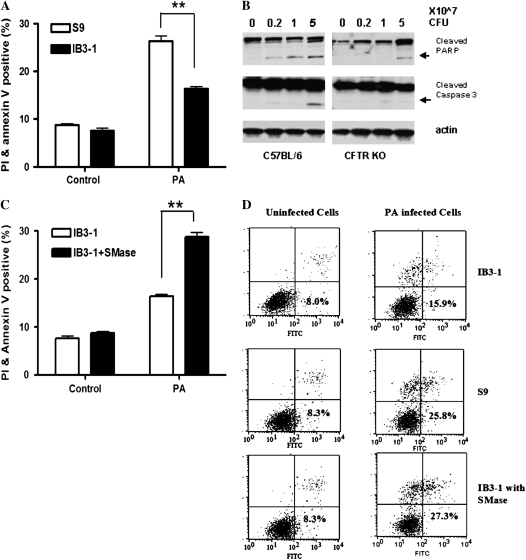
Apoptosis plays an important role in host defense against bacterial infection by killing infected cells, and sphingolipids have been implicated in several apoptotic responses (14, 15). To investigate the apoptotic response in IB3-1 and S9 cells after P. aeruginosa infection, Annexin V–FITC and propidium iodide (PI)-labeled flow cytometry assay was used in this study. S9 and IB3-1 cells were infected with PAO1 (50 cfu/cell) for 5 hours. As shown in Figures 6A and 6D, 26% of S9 cells underwent apoptosis or necrosis (positive staining with Annexin V and/or PI) as compared with 16% of the IB3-1 cells (n = 4, **P < 0.0001). There was 1.6-fold difference between S9 and IB3-1 cells. A lactate dehydrogenase cytotoxicity study in infected IB3-1 cells and S9 cells (data not shown) indicated that cytotoxicity of infected S9 cells and IB3-1 cells was inversely correlated with the dose of P. aeruginosa. With lower dose of bacteria (20 cfu/cell), there was more than a 7-fold difference in cytotoxicity between S9 cells and IB3-1 cells. However, this difference decreased with increasing dose of bacteria, such that a 2.1-fold difference was seen with 40 cfu/cell, and a 1.7-fold difference was seen with 80 cfu/cell.
Figure 6.
Decreased apoptotic response to P. aeruginosa (PA) infection in CF is associated with defective ASMase activity. (A) Defective apoptotic/necrotic response in CF bronchial epithelial cells. IB3-1 and S9 cells were infected with PAO1 (50 cfu/cell) for 5 hours. Apoptotic/necrotic responses were analyzed by fluorescein isothiocyanate–labeled Annexin V and propidium iodide (PI) staining flow cytometry assay (n = 4, **P < 0.0001). (B) Decreased apoptotic response to PA infection in CFTR KO mice. CFTR KO (Cftrtm1Unc-TgN(FABPCFTR)) mice and C57BL/6 mice were either uninfected or infected with various doses (2 × 106, 1 × 107, and 5 × 107 cfu) of PAO1 for 6 hours. The lung homogenates were collected to analyze Poly (ADP-ribose) polymerase (PARP) and caspase 3 protein levels by Western blot. (C) Exogenous bacteria SMase increased apoptotic/necrotic responses with PAO1 infection in CF bronchial epithelial cells. Exogenous bacteria SMase (0.03 U/ml) from Staphylococcus aureus was added to IB3-1 cells 1 hour before PA infection. Apoptotic/necrotic responses were analyzed by Annexin V and PI staining flow cytometry assay (n = 4, **P < 0.0001). (D) Annexin V and PI staining flow cytometry assay results. Treatments of IB3-1 cells and S9 cells were mentioned as above. Annexin V and PI values for each treatment were plotted on the x axis and y axis, respectively. Shown are % cells of each treatment group in top left, top right, and bottom right quadrants (Annexin V+ and/or PI +). Data represent four independent experiments.
Importantly, a decrease in apoptotic response was also observed in vivo in CFTR KO mice infected with P. aeruginosa as compared with WT C57BL/6 mice. The CFTR KO mice and WT C57BL/6 mice were either uninfected or infected with various doses (2 × 106, 1 × 107, or 5 × 107 cfu) of PAO1 for 6 hours. The lung homogenates were collected to analyze poly-ADP-ribose-polymerase (PARP) and caspase-3 protein levels. As shown in Figure 6B, PARP protein degradation occurred at the lower dose (2 × 106 cfu) of P. aeruginosa infection in C57BL/6 mice, and exhibited a dose-dependent increase with increasing dose of infected P. aeruginosa. In contrast, PARP protein degradation only occurred at a very high dose (5 × 107 cfu) of P. aeruginosa infection in CFTR KO mice. Caspase-3 degradation also occurred only at a high dose (5 × 107 cfu) of P. aeruginosa infection in C57BL/6 mice. In contrast, there was no significant caspase-3 degradation in CFTR KO mice at any of the various doses of P. aeruginosa. These in vivo results demonstrate a defective apoptotic pathway in CFTR KO mice.
To determine the role of the ASMase/ceramide pathway in the above defective apoptotic response, exogenous bacterial SMase from S. aureus (0.03 U/ml) was added to IB3-1 cells 1 hour before P. aeruginosa infection to “reconstitute/overcome” the defect in induction of endogenous ASMase. The percentage of cells that underwent necrosis or apoptosis increased to 28% in the infected IB3-1group treated with bacterial SMase as compared with 16% in infected untreated IB3-1 cells (Figures 6C and 6D, n = 4, **P < 0.0001). These results provide strong evidence that the ASMase/ceramide pathway plays an important role in inducing bactericidal activity after infection. Defects in the ASMase/ceramide pathway in CF cells or CFTR KO mice contribute to compromised apoptotic response after bacterial infection.
DISCUSSION
Lung infection with P. aeruginosa is the major cause of morbidity and mortality in patients with CF. Patients with CF infected with P. aeruginosa have a dysfunctional immune response, which fails to remove the infected pathogens from the airway, but also causes more sustained inflammatory responses, resulting in airway obstruction and extensive lung damage. Currently, the mechanisms underlying this dysfunctional immune response are unclear. For the first time, the results from this study demonstrate that CF bronchial epithelium exhibits a defective ASMase pathway as revealed by in vitro and in vivo experiments. First, the results show a significant induction of ASMase in response to infection with P. aeruginosa in WT cells and animals. Second, the results reveal a clear defect in induction of ASMase after P. aeruginosa infection in CF bronchial epithelial cells and CFTR KO mice. Functionally, the results provide evidence that this defective ASMase induction plays a key role in the overwhelming IL-8 response to P. aeruginosa infection, in bacterial internalization, and in the apoptotic response to bacterial infection.
In a previous study conducted by Grassme and colleagues (18) in WI-38 cells (normal human fetal lung fibroblast cells), the activation of ASMase after P. aeruginosa infection was detected very acutely 5 to 10 minutes after infection. ASMase activity and the generated ceramide reached peak levels 10 to 20 minutes after infection, and gradually decreased to basal levels over the next 30 minutes. The authors suggested that these effects are caused by acute activation and translocation of ASMase from intracellular vesicles to the extracellular leaflet of the membrane. In contrast, our results show a prolonged time frame (5–6 h) and a mechanism involving induction of protein levels. The ASMase activity and produced ceramide levels kept increasing until 5 or 6 hours after bacterial infection (Figures 1 and 3). Whether ASMase activation could last longer than 6 hours and whether ASMase/ceramide pathway plays a role in chronic bacterial infection need further study.
The reasons for the compromised ASMase pathway in CF have not been fully elucidated. It is now known that ASMase exists as a lysosomal form, a secretory form, or a surface form. When ASMase is activated, it is translocated from intracellular vesicles to the outer leaflet of the plasma membrane (17, 19), where it hydrolyzes spingomyelin and generates ceramide. Studies by Di and coworkers (35) and by Barasch and colleagues (36) revealed that CFTR regulates the pH value of endosomes and lysosomes due to the counterion effect of Cl- in promoting luminal H+ accumulation. Normally acidic compartments (such as the Golgi network, endosomes, and lysosomes) were more alkaline in cells with mutant CFTR compared with cells with WT CFTR (35, 36). Treatment of WT alveolar macrophages with reagents that inhibited CFTR-dependent chloride transport or the vacuolar proton ATPase led to alkalinization of lysosomes (35). Since ASMase is primarily an endosomal/lysosomal enzyme, which needs an acidic environment to function normally, the increasing pH value in endosome/lysosomes might affect ASMase activity and processing from the lysosomal from to the surface form.
Studies of alternate chloride channels in endosomes/lysosomes also confirm that defects in chloride channels affect the acidification of these membrane organelles. Specifically, defects of the chloride channels CIC-3 (37, 38); CIC-4 (39); and CIC-5 (40) impair the acidification of endosomes/lysosomes. Loss of the chloride channel CIC-7 leads to lysosomal storage disease (41). The low luminal pH in these membrane organelles is important for several processes, such as receptor–ligand interactions, trafficking along the endosomal pathway, and luminal enzymatic activity (42). How CFTR protein interacts with lipid metabolism and ASMase activity needs further investigation. Of note, recent studies suggest that sphingolipids interact with CFTR at multiple stages. Lipid rafts have been reported to play a role in recruiting CFTR (18, 28), and the SMase reaction also affects CFTR ion channel activity (43, 44).
In CF with abnormal pH value in lysosomes, there might be less ASMase translocated from lysosomes to the outer leaflet of the plasma membrane, resulting in less ceramide production. A alternative mechanisms for ceramide generation still exist in CF cells and CFTR KO mice. There was a 20% increase in total ceramide in infected IB3-1 cells 300 minutes after bacterial infection and 17% increase of total ceramide in infected CFTR KO mice after 5 × 107cfu PAO1 infection. Our results are consistent with the finding of Guilbault and coworkers (45) that patients with CF have significantly lower plasma ceramide levels compared with healthy control subjects. Guilbault and colleagues (45) also showed that CFTR KO mice displayed diminished ceramide levels in CF-related organs (lungs, pancreas, and ileum) compared with their littermate controls. These findings support a defective ASMase/ceramide pathway in CF. A recent study by Teichgraber and coworkers (46) revealed that the defect in acidification of endosomes/lysosomes in CF also affects acid ceramidase activity. They reported a constitutive accumulation of ceramide in old (16- to 32-wk-old) CFTR KO mice, but not in 8-week-old CFTR KO mice without bacterial infection. These results are distinct from those of Guilbault and colleagues (45), who showed that patients with CF (aged 20–59 yr) had a significantly lower plasma levels of ceramide compared with healthy control groups. No correlation was found between the ceramide levels in the lungs and the age of the mice (45). In the current study, which was conducted in CF bronchial epithelial cells and 8-week-old CFTR KO mice, there was no significant difference of basal levels of ceramide between CF groups and WT controls. The levels of ceramide only exhibited significant difference between CF groups and WT groups after bacterial infection.
Using a combination of approaches, the current study reveals an important role for ASMase in attenuating IL-8 responses to P. aeruginosa infection in CF, and it suggests a role for ceramide in a feedback mechanism that regulates the IL-8 response. These results are consistent with those of Vilela and coworkers (47), who revealed that generation of ceramide by fenretinide inhibited TNF-α–induced IL-8 production in CF tracheal epithelial cells. Since induction of IL-8 appears to be a major detriment in patients with CF, a better understanding of the mechanisms behind the IL-8 response, including the failure to up-regulate ASMase in infected CF cells, is critical to the development of new therapies for airway inflammation. Our results suggest that the ASMase/ceramide pathway is one of multiple pathways that contribute to cytokine regulation. CF cells have been reported to have higher levels of nuclear factor (NF)-κB, higher level of activator protein-1 (AP-1) activity, and higher extracellular signal–regulated kinase (ERK) phosphorylation (48). How the ASMase/ceramide pathway interacts with those transcriptional factors and protein kinases needs further study.
In addition to the effects of ASMase on airway inflammation in CF, our results support a critical role for ASMase in mediating/enhancing bacterial uptake in infected cells. CF bronchial epithelial cells (IB3-1 cells) had decreased bacterial uptake with defective ASMase activity as compared with control S9 cells (Figure 5A). This observation extends earlier findings by Pier and colleagues (6, 49) that cells with wild-type CFTR ingested more infected P. aeruginosa than cells with mutant CFTR gene. Although there is only an approximate 2-fold difference of bacterial uptake between IB3-1 cells and S9 cells after P. aeruginosa infection, when we take into consideration the fact that twice as many S9 cells underwent apoptosis as IB3-1 cells, the difference of ingested bacteria between cell lines is approximately 4-fold. This is consistent with the finding of Schroeder and coworkers (49) that WT C57BL/6 mice ingested 3.9 times more bacteria than did the CF mice. This study demonstrates that the decreased bacterial ingestion can be fully attributed to the defective ASMase activity in CF. Silencing ASMase activity by ASMase RNAi in S9 cells (with normal ASMase activity) significantly decreased the amount of internalized P. aeruginosa, down to the levels seen in IB3-1 cells (Figure 5B).
Once the bacteria are ingested, the infected cells normally undergo apoptosis as a means of clearing the bacteria. In addition to its role in bacterial uptake, ASMase is involved in regulating the apoptotic response to infection with P. aeruginosa. We demonstrated a reduced apoptotic response to P. aeruginosa infection in IB3-1 cells compared with the S9 cells (Figure 6A). This observation is in line with the findings of Cannon and colleagues (7) that cells expressing mutant CFTR had an approximately 2-fold less apoptotic rate with P. aeruginosa infection than cells with the WT CFTR. Furthermore, the current study also shows a decreased apoptotic response after P. aeruginosa infection in CF mice in vivo (Figure 6B). The addition of exogenous bacterial SMase to IB3-1 cells greatly increased the necrotic/apoptotic response after P. aeruginosa infection (Figure 6C). In CF, with decreased bacterial uptake and decreased bacterial killing, there is increasing bacterial burden. Schroeder and coworkers (49) revealed that mice with at least one WT CFTR allele had a 1.1- to 3.4-fold increase in cfu in the lungs over the infecting inoculums after 4.5 hours of P. aeruginosa infection, whereas homozygous ΔF508 CFTR mice had a 7.5-fold increase in the cfu of P. aeruginosa in the lungs. The increased bacterial burden in CF in turn could stimulate immune response and finally result in an overwhelming chronic infection.
In conclusion, the current study demonstrates a defective ASMase/ceramide pathway with P. aeruginosa infection in CF bronchial epithelial cells as well as in CFTR KO mice. This defective ASMase pathway is associated with the overwhelming IL-8 cytokine response, decreased bacterial uptake and reduced apoptotic response in CF with P. aeruginosa infection. This compromised ASMase response might permit P. aeruginosa to escape from killing and in turn cause unabated proinflammatory cytokine response in CF. Understanding the mechanisms involved in the predilection for P. aeruginosa infection in CF and the emergence of a chronic infection/inflammation is critical to the development of novel therapeutics aimed at preventing or clearing infection and decreasing inflammation in CF airways.
Acknowledgments
The Bio-Plex Multiplex System was provided by funds from the South Carolina Physicians Charity. The authors thank the Lipidomics Core Facility (Drs. Jacek Bielawski and Alicja Bielawska) at the Medical University of South Carolina. The work at the Lipidomics Core is supported by National Institutes of Health grant C06 RR018823.
This study was supported by grants from the NIH/NHLBI P01-HL51811 and from CFRI New Horizons in CF Therapeutics, and by NIH grant CA87584.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0295OC on January 23, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066–1073. [DOI] [PubMed] [Google Scholar]
- 2.Pier GB. CFTR mutations and host susceptibility to Pseudomonas aeruginosa lung infection. Curr Opin Microbiol 2002;5:81–86. [DOI] [PubMed] [Google Scholar]
- 3.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med 1995;152:2111–2118. [DOI] [PubMed] [Google Scholar]
- 4.Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med 1999;160:186–191. [DOI] [PubMed] [Google Scholar]
- 5.Bonfield TL, Konstan MW, Burfeind P, Panuska JR, Hilliard JB, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol 1995;13:257–261. [DOI] [PubMed] [Google Scholar]
- 6.Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA 1997;94:12088–12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon CL, Kowalski MP, Stopak KS, Pier GB. Pseudomonas aeruginosa-induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. Am J Respir Cell Mol Biol 2003;29:188–197. [DOI] [PubMed] [Google Scholar]
- 8.Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev 1999;79(1, Suppl)S215–S255. [DOI] [PubMed] [Google Scholar]
- 9.Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997;88:553–560. [DOI] [PubMed] [Google Scholar]
- 10.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 1996;85:229–236. [DOI] [PubMed] [Google Scholar]
- 11.Bryan R, Kube D, Perez A, Davis P, Prince A. Overproduction of the CFTR r domain leads to increased levels of asialogm1 and increased Pseudomonas aeruginosa binding by epithelial cells. Am J Respir Cell Mol Biol 1998;19:269–277. [DOI] [PubMed] [Google Scholar]
- 12.Matsui H, Verghese MW, Kesimer M, Schwab UE, Randell SH, Sheehan JK, Grubb BR, Boucher RC. Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces. J Immunol 2005;175:1090–1099. [DOI] [PubMed] [Google Scholar]
- 13.Levade T, Jaffrezou JP. Signalling sphingomyelinases: which, where, how and why? Biochim Biophys Acta 1999;1438:1–17. [DOI] [PubMed] [Google Scholar]
- 14.Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry 2001;40:4893–4903. [DOI] [PubMed] [Google Scholar]
- 15.Matko J, Szollosi J. Landing of immune receptors and signal proteins on lipid rafts: a safe way to be spatio-temporally coordinated? Immunol Lett 2002;82:3–15. [DOI] [PubMed] [Google Scholar]
- 16.Gulbins E, Dreschers S, Wilker B, Grassme H. Ceramide, membrane rafts and infections. J Mol Med 2004;82:357–363. [DOI] [PubMed] [Google Scholar]
- 17.Bollinger CR, Teichgräber V, Gulbins E. Ceramide-enriched membrane domains. Biochim Biophys Acta 2005;1746:284–294. [DOI] [PubMed] [Google Scholar]
- 18.Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med 2003;9:322–330. [DOI] [PubMed] [Google Scholar]
- 19.Rotolo JA, Zhang J, Donepudi M, Lee H, Fuks Z, Kolesnick R. Caspase-dependent and -independent activation of acid sphingomyelinase signaling. J Biol Chem 2005;280:26425–26434. [DOI] [PubMed] [Google Scholar]
- 20.Virella-Lowell I, Herlihy JD, Liu B, Lopez C, Cruz P, Muller C, Baker HV, Flotte TR. Effects of CFTR, interleukin-10, and Pseudomonas aeruginosa on gene expression profiles in a CF bronchial epithelial cell line. Mol Ther 2004;10:562–573. [DOI] [PubMed] [Google Scholar]
- 21.Zeitlin PL, Lu L, Rhim J, Cutting G, Stetten G, Kieffer KA, Craig R, Guggino WB. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-sv40 infection. Am J Respir Cell Mol Biol 1991;4:313–319. [DOI] [PubMed] [Google Scholar]
- 22.Egan M, Flotte T, Afione S, Solow R, Zeitlin PL, Carter BJ, Guggino WB. Defective regulation of outwardly rectifying Cl–channels by protein kinase a corrected by insertion of CFTR. Nature 1992;358:581–584. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Hill WG, Pilewski JM, Weisz OA. Glycosylation differences between a cystic fibrosis and rescued airway cell line are not CFTR dependent. Am J Physiol 1997;273:L913–L920. [DOI] [PubMed] [Google Scholar]
- 24.Schneider SW, Egan ME, Jena BP, Guggino WB, Oberleithner H, Geibel JP. Continuous detection of extracellular ATP on living cells by using atomic force microscopy. Proc Natl Acad Sci USA 1999;96:12180–12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 2006;39:82–91. [DOI] [PubMed] [Google Scholar]
- 26.Zeidan YH, Pettus BJ, Elojeimy S, Taha T, Obeid LM, Kawamori T, Norris JS, Hannun YA. Acid ceramidase but not acid sphingomyelinase is required for tumor necrosis factor-{alpha}-induced PGE2 production. J Biol Chem 2006;281:24695–24703. [DOI] [PubMed] [Google Scholar]
- 27.Zeidan YH, Hannun YA. Activation of acid sphingomyelinase by protein kinase Cdelta-mediated phosphorylation. J Biol Chem 2007;282:11549–11561. [DOI] [PubMed] [Google Scholar]
- 28.Kowalski MP, Pier GB. Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for Pseudomonas aeruginosa-induced cellular activation. J Immunol 2004;172:418–425. [DOI] [PubMed] [Google Scholar]
- 29.Darling KE, Dewar A, Evans TJ. Role of the cystic fibrosis transmembrane conductance regulator in internalization of Pseudomonas aeruginosa by polarized respiratory epithelial cells. Cell Microbiol 2004;6:521–533. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA, Whitsett JA. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science 1994;266:1705–1708. [DOI] [PubMed] [Google Scholar]
- 31.Yu H, Buff SM, Baatz JE, Virella-Lowell I. Oral instillation with surfactant phospholipid: a reliable alternative to intratracheal injection in mouse studies. Lab Anim 2008;42:294–304. [DOI] [PubMed] [Google Scholar]
- 32.Gosselin D, Stevenson MM, Cowley EA, Griesenbach U, Eidelman DH, Boule M, Tam MF, Kent G, Skamene E, Tsui LC, et al. Impaired ability of CFTR knockout mice to control lung infection with Pseudomonas aeruginosa. Am J Respir Crit Care Med 1998;157:1253–1262. [DOI] [PubMed] [Google Scholar]
- 33.Kent G, Iles R, Bear CE, Huan LJ, Griesenbach U, McKerlie C, Frndova H, Ackerley C, Gosselin D, Radzioch D, et al. Lung disease in mice with cystic fibrosis. J Clin Invest 1997;100:3060–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Heeckeren AM, Schluchter MD, Xue W, Davis PB. Response to acute lung infection with mucoid Pseudomonas aeruginosa in cystic fibrosis mice. Am J Respir Crit Care Med 2006;173:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol 2006;8:933–944. [DOI] [PubMed] [Google Scholar]
- 36.Barasch J, Kiss B, Prince A, Saiman L, Gruenert D, al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature 1991;352:70–73. [DOI] [PubMed] [Google Scholar]
- 37.Weylandt KH, Nebrig M, Jansen-Rosseck N, Amey JS, Carmena D, Wiedenmann B, Higgins CF, Sardini A. Clc-3 expression enhances etoposide resistance by increasing acidification of the late endocytic compartment. Mol Cancer Ther 2007;6:979–986. [DOI] [PubMed] [Google Scholar]
- 38.Hara-Chikuma M, Yang B, Sonawane ND, Sasaki S, Uchida S, Verkman AS. Clc-3 chloride channels facilitate endosomal acidification and chloride accumulation. J Biol Chem 2005;280:1241–1247. [DOI] [PubMed] [Google Scholar]
- 39.Mohammad-Panah R, Harrison R, Dhani S, Ackerley C, Huan LJ, Wang Y, Bear CE. The chloride channel clc-4 contributes to endosomal acidification and trafficking. J Biol Chem 2003;278:29267–29277. [DOI] [PubMed] [Google Scholar]
- 40.Hara-Chikuma M, Wang Y, Guggino SE, Guggino WB, Verkman AS. Impaired acidification in early endosomes of clc-5 deficient proximal tubule. Biochem Biophys Res Commun 2005;329:941–946. [DOI] [PubMed] [Google Scholar]
- 41.Kasper D, Planells-Cases R, Fuhrmann JC, Scheel O, Zeitz O, Ruether K, Schmitt A, Poet M, Steinfeld R, Schweizer M, et al. Loss of the chloride channel clc-7 leads to lysosomal storage disease and neurodegeneration. EMBO J 2005;24:1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jentsch TJ. Chloride and the endosomal-lysosomal pathway: emerging roles of clc chloride transporters. J Physiol 2007;578:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito Y, Sato S, Ohashi T, Nakayama S, Shimokata K, Kume H. Reduction of airway anion secretion via CFTR in sphingomyelin pathway. Biochem Biophys Res Commun 2004;324:901–908. [DOI] [PubMed] [Google Scholar]
- 44.Ramu Y, Xu Y, Lu Z. Inhibition of CFTR Cl–channel function caused by enzymatic hydrolysis of sphingomyelin. Proc Natl Acad Sci USA 2007;104:6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guilbault C, De Sanctis JB, Wojewodka G, Saeed Z, Lachance C, Skinner TA, Vilela RM, Kubow S, Lands LC, Hajduch M, et al. Fenretinide corrects newly found ceramide deficiency in cystic fibrosis. Am J Respir Cell Mol Biol 2008;38:47–56. [DOI] [PubMed] [Google Scholar]
- 46.Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 2008;14:382–391. [DOI] [PubMed] [Google Scholar]
- 47.Vilela RM, Lands LC, Meehan B, Kubow S. Inhibition of IL-8 release from CFTR-deficient lung epithelial cells following pre-treatment with fenretinide. Int Immunopharmacol 2006;6:1651–1664. [DOI] [PubMed] [Google Scholar]
- 48.Verhaeghe C, Remouchamps C, Hennuy B, Vanderplasschen A, Chariot A, Tabruyn SP, Oury C, Bours V. Role of IKK and ERK pathways in intrinsic inflammation of cystic fibrosis airways. Biochem Pharmacol 2007;73:1982–1994. [DOI] [PubMed] [Google Scholar]
- 49.Schroeder TH, Reiniger N, Meluleni G, Grout M, Coleman FT, Pier GB. Transgenic cystic fibrosis mice exhibit reduced early clearance of Pseudomonas aeruginosa from the respiratory tract. J Immunol 2001;166:7410–7418. [DOI] [PubMed] [Google Scholar]