Summary
Recent studies have indicated that regulatory T cells contribute to the human immunodeficiency virus type 1 (HIV-1)-related immune pathogenesis. However, it is not clear whether T cells with suppressive properties reside within the HIV-1-specific T-cell population. Here, peripheral blood mononuclear cells from HIV-1-infected individuals were stimulated with a 15-mer Gag peptide pool, and HIV-1-specific T cells were enriched by virtue of their secretion of interleukin (IL)-10 or interferon (IFN)-γ using immunomagnetic cell-sorting. Neither the IL-10-secreting cells nor the IFN-γ-secreting cells expressed the regulatory T-cell marker forkhead box P3 (FOXP3), yet the IL-10-secreting cells potently suppressed anti-CD3/CD28-induced CD4+ as well as CD8+ T-cell proliferative responses. As shown by intracellular cytokine staining, IL-10- and IFN-γ-producing T cells represent distinct subsets of the HIV-1-specific T cells. Our data collectively suggest that functionally defined HIV-1-specific T-cell subsets harbor potent immunoregulatory properties that may contribute to HIV-1-associated T-cell dysfunction.
Keywords: HIV-1, HIV Infections, Interleukin-10, T-Lymphocytes, Regulatory, HIV Antigens
Introduction
Chronic HIV-1 infection is characterized by loss of CD4+ T cells, immune activation and a progressive immune dysfunction[1-4]. The HIV-1-associated T-cell dysfunction involves impaired cellular immunity and T-cell proliferation, aberrant T-cell signaling and a sequential loss of in vitro responsiveness to antigen-specific, allogeneic, and mitogenic stimulation[5-9]. The immune dysfunction contributes both to the inability to control HIV-1 replication in vivo and to a generalized state of immunodeficiency underlying the susceptibility to opportunistic infections and neoplasms[6;10;11]. T-cell subsets with regulatory properties suppress HIV-1-specific immune responses and contribute to reduced viral control[12-17]. It is not clear, however, whether suppressive T-cell subsets are found within the HIV-1-specific T-cell population.
The thymus-derived CD4+CD25+ natural regulatory T (Treg) cells expressing the transcription factor forkhead box P3 (FOXP3) remain the best characterized suppressive T-cell subset[18;19]. These cells are critical for the maintenance of self-tolerance and play an important role in a wide range of clinical conditions such as autoimmune diseases, transplantation rejection reactions, and cancer, as well as infectious diseases[19-22]. However, T cells with regulatory properties include both CD4+ and CD8+ T cells, which can be both thymus-derived or induced from naive T cells in the periphery[21;23;24]. Whereas thymus-derived CD4+CD25+FOXP3+ Treg cells constitute a stable T-cell lineage, peripherally induced Treg cells represent an adaptive means of limiting tissue inflammation[24]. Here, we have analyzed the suppressive ability of IL-10- and IFN-γ-secreting HIV-1-specific T cells. We demonstrate that T cells secreting IL-10 in response to stimulation with HIV-1 Gag peptides potently suppress polyclonal CD4+ and CD8+ T-cell proliferation, whereas T cells that secrete IFN-γ do not. The HIV-1-specific IL-10-producing T-cell population contains both CD4+ and CD8+ T cells, and represents a small, yet distinct, subset of HIV-1-specific T cells that may contribute to both the specific and the generalized immunodeficiency associated with chronic HIV-1 infection.
Results
HIV-1-specific IL-10-secreting T cells suppress T-cell proliferation
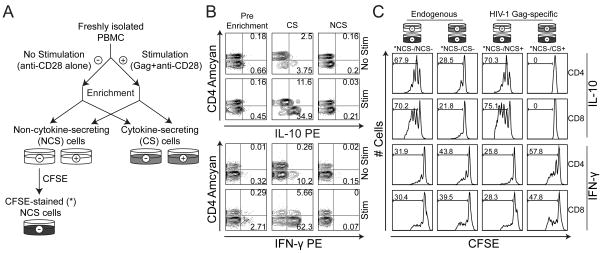
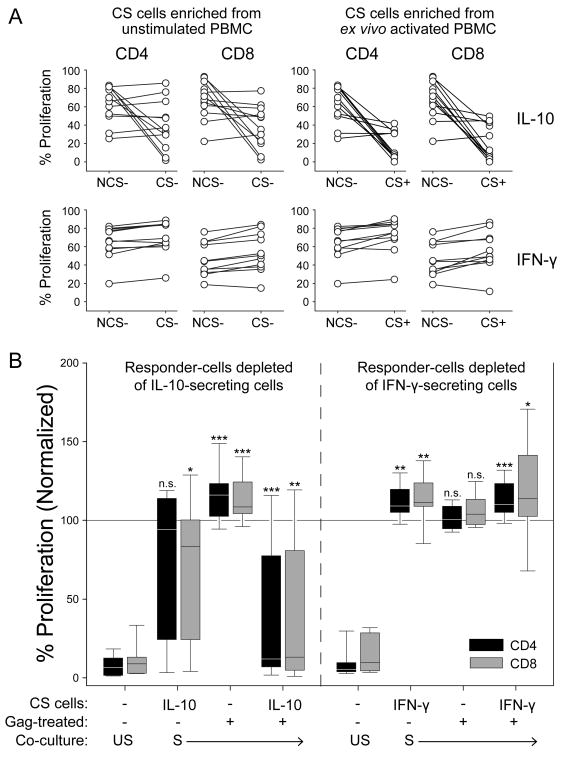
PBMC from twelve HIV-1-infected donors were enriched for IL-10-secreting T cells using immunomagnetic cell-sorting after stimulation with a 15-mer Gag peptide pool and anti-CD28, or anti-CD28 alone (Fig. 1a and b). The effect of enriched IL-10-secreting T cells on anti-CD2/CD3/CD28-induced T-cell proliferation was assessed by CFSE dilution after co-culture for 3-4 days and compared to the effect of the corresponding non-IL-10-secreting cells (PBMC depleted of IL-10-secreting cells; NCS; Fig. 1c). The Gag-stimulated IL-10-secreting T cells potently suppressed both CD4+ and CD8+ T-cell proliferation (P < 0.005 and P < 0.01, respectively), whereas the NCS cells did not (Fig. 2a and b; see also Fig. 1c). Interestingly, in five out of twelve donors, the IL-10-secreting T cells obtained from PBMC in the absence of ex vivo stimulation with Gag peptides also suppressed T-cell proliferation (Fig. 2b; left panel), although the summarized data did not reach the level of statistical significance (P = 0.06). We observed an inverse correlation between the level of suppression caused by these endogenously activated IL-10-secreting T cells and the CD4:CD8 ratio (P < 0.05 for the suppression of CD8+ T cells; data not shown), which indicates that these cells could be of relevance to the clinical progression of HIV-1 disease.
Figure 1. Experimental setup.
A) Overview of the enrichment procedure used to isolate IL-10- and IFN-γ-secreting T cells. B) Characterization of PBMC prior to (Pre Enrichment) and after (CS; NCS) the enrichment procedure, following stimulation with anti-CD28 in the presence (Stim) or absence (No Stim) of Gag peptides. Representative data from enrichment of IL-10-secreting cells (upper panel) and IFN-γ-secreting cells (lower panel) are shown. Scatter-plots are gated on live T cells; numbers represent percentage of parent population. C) Proliferation in co-culture experiments with PBMC in the absence or presence of IL-10- or IFN-γ-secreting T cells (upper and lower panels, respectively). Histograms are gated on live, CFSE-stained CD4+ and CD8+ T cells, respectively; numbers represent percentage proliferating cells. The presented data are representative of n = 12 (IL-10) and n = 11 (IFN-γ) experiments.
Figure 2. IL-10-secreting HIV-1-specific T cells suppress the proliferation of both CD4+ and CD8+ T cells in co-culture experiments.
A) Individual paired data for suppression of CD4+ and CD8+ T-cell proliferation following co-culture with IL-10-secreting (top) or IFN-γ-secreting (bottom) cells isolated after stimulation ex vivo with Gag peptides and anti-CD28 (right panels) or with anti-CD28 alone (left panels). B) Proliferation of CD4+ and CD8+ T cells in co-culture experiments with PBMC depleted of, or enriched with, IL-10-secreting (left; n = 12) or IFN-γ-secreting (right; n =11) T cells in the presence (S) or absence (US) of anti-CD2/CD3/CD28-coated MicroBeads. Boxes indicate the 25th and 75th percentiles; the line within marks the median. Whiskers indicate the 10th and 90th percentiles. The horizontal line at 100 % indicates the proliferation of NCS- cells (not Gag-treated) alone, to which the other co-cultures are compared. CS, cytokine-secreting; NCS, non-cytokine-secreting. Statistical analysis was performed using the Wilcoxon signed ranks test.*P < 0.05, **P < 0.01, *** P < 0.005.
In some donors (n = 11), IFN-γ-secretion in response to HIV-1 Gag peptides was exploited to isolate a subset of HIV-1-specific effector T cells that were not expected to be suppressive. The enrichment of IFN-γ-secreting T cells was facilitated by the relative abundance of these cells and generally resulted in enriched populations of more than 50 % purity, as determined by FACS analysis (gated on live T cells; Fig. 1b, bottom panel). In contrast to co-culture experiments with enriched IL-10-secreting T cells, add-back of the same number of enriched IFN-γ-secreting T cells led to an increase in T-cell proliferation (P < 0.005 and 0.05 for CD4+ and CD8+ T cells, respectively; Fig. 2a and b; see also Fig. 1c). Consequently, our data show that the enrichment of HIV-1-specific IL-10-secreting T cells coincides with the isolation of a suppressive entity that is not found among the IFN-γ-secreting T cells. Importantly, this distinction was made strictly on the basis of cytokine secretion, thus providing an effective means of separating functionally distinct T-cell subsets upon antigenic stimulation.
IL-10- and IFN-γ-secreting T cells represent distinct HIV-1-specific subsets
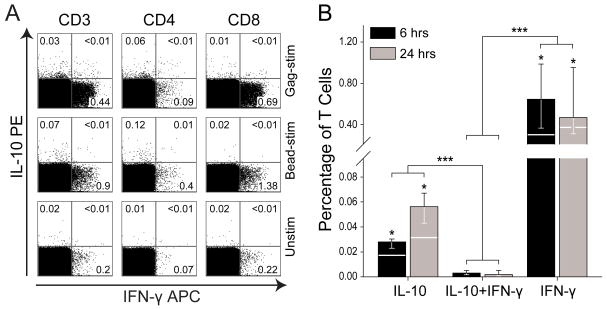
Having demonstrated that T cells secreting IL-10 suppressed T-cell proliferation, whereas IFN-γ-secreting T cells did not, we assessed whether these subsets were distinct T-cell populations, or if there existed a subset of T cells producing both IL-10 and IFN-γ. Intracellular cytokine staining and FACS analysis were performed in order to explore the production of IL-10 and IFN-γ upon activation for 6 or 24 hours with HIV-1 Gag peptides and anti-CD28 (Fig. 3a). Cytokine production was also examined after stimulation with anti-CD2/CD3/CD28-coated MicroBeads or anti-CD28 alone. Consistently, PBMC from HIV-1-positive donors presented with low-level spontaneous production of both IL-10 and IFN-γ when cultured ex vivo, which may reflect the chronic immune activation in these donors. However, upon ex vivo antigen stimulation, the level of cytokine expression was significantly increased (P < 0.05; Fig. 3b). Stimulated PBMC were stained simultaneously for IL-10 and IFN-γ, and the resulting FACS analysis revealed distinctly separated populations of IL-10- and IFN-γ-producing T cells, respectively, whereas double-positive T cells – producing both IL-10 and IFN-γ – were virtually absent (Fig. 3a and b).
Figure 3. Interleukin-10-producing T cells represent an HIV-1-specific T-cell subset distinct from the HIV-1-specific IFN-γ-secreting T cells.
A) Analysis of IL-10- and IFN-γ-production in T cells in response to a 15-mer Gag peptide pool and anti-CD28 (top row; Gag-stim); anti-CD2/CD3/CD28-coated MicroBeads (middle row; Bead-stim); or only anti-CD28 (bottom row; Unstim) using intracellular cytokine staining and FACS analysis. Cytokine-production was assessed at 6 hours and 24 hours, respectively, with n = 6 donors at each time-point. One representative donor is shown; values signify percentage of parent. B) Frequency of T cells producing IL-10, IFN-γ, or both, following stimulation of PBMC with a 15-mer Gag peptide pool and anti-CD28 for 6 (black bars) or 24 (grey bars) hours. The three groups of cytokine-producing cells were compared using the Wilcoxon signed ranks test (comparisons within each time-point; n = 6 – significant differences from the IL-10+IFN-γ-group indicated above each bar) and Mann-Whitney U test (comparing the groups irrespective of time-point; n = 12). Bars represent the median; 25th and 75th percentiles are indicated. The white horizontal lines traversing the bars signify the median value of the corresponding unstimulated samples. * P < 0.05; *** P < 0.001.
Suppressive HIV-1-specific IL-10-secreting T cells do not express FOXP3
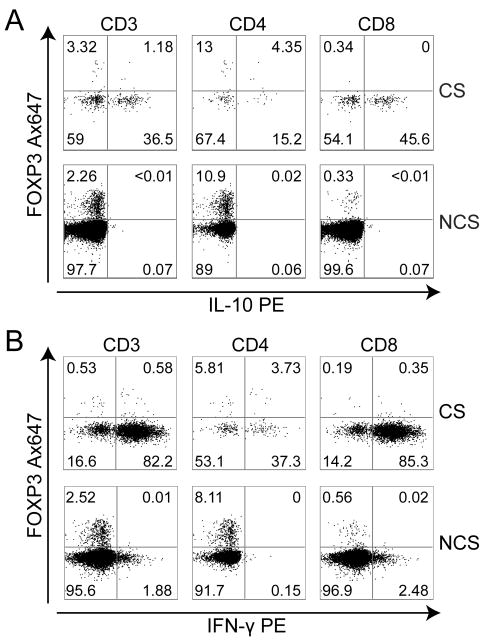
To assess whether the suppressive capacity of IL-10-secreting T cells could be attributed to the expression of FOXP3, T cells secreting IL-10 or IFN-γ upon ex vivo stimulation of PBMC with HIV-1 antigens and anti-CD28 were isolated by cytokine capture and immunomagnetic enrichment and evaluated for expression of FOXP3 by flow cytometry. As demonstrated (Fig. 4a and b), neither the IL-10-nor the IFN-γ-secreting T cells expressed FOXP3. The HIV-1-specific IL-10-secreting T cells described in the present study appear therefore to be similar to the type 1 regulatory T (Tr1) cells[25;26].
Figure 4. HIV-1-specific IL-10-producing T cells do not express the transcription factor FOXP3.
Expression of FOXP3 in A) IL-10-secreting T cells (representative of n = 3) and B) IFN-γ-secreting T cells (representative of n = 3). FACS plots are gated on CD3+, CD3+CD4+, or CD3+CD8+ T cells as indicated. Numbers represent percentage of parent.
Discussion
Loss of CD4+ T cells and impaired CD4+ and CD8+ T-cell function are the most prominent clinical and immunological hallmarks of HIV-1 disease[3;27;28]. The T-cell dysfunction affects HIV-1-specific immunity as well as T-cell responsiveness to recall antigens and polyclonal stimuli[5-7;29;30]. As such, the T-cell impairment is antigen non-specific and ultimately leads to susceptibility to opportunistic infections and neoplasms[31]. In parallel, the failure of the immune system to mount an efficient HIV-1-specific immune response paves the way for persistent viral replication and chronic immune activation. HIV-1-specific Treg cells that emerge as a result of continuous exposure to viral antigens may establish a circulus vitiosus where the viral antigens induce immunosuppressive T cells that impair viral control and foster viral replication.
Interleukin-10 is produced by a number of different immune cells, including T cells, NK cells, B cells, monocytes, macrophages, and dendritic cells, in addition to both natural and adaptive Treg cells[25;32;33]. An increase in the systemic production of this predominantly immunosuppressive cytokine has been reported in a variety of human viral infections, including infections with hepatitis C virus, HIV, and Epstein–Barr virus[34-37]. A number of viruses, including the Epstein–Barr virus, are capable of expressing IL-10 homologues as a method of undermining the antiviral defense mechanisms [38]. Two recent reports by Brooks et al.[39] and Ejrnaes et al.[40] suggest that the IL-10/IL-10R pathway plays a key role in determining whether an infection follows an acute or a chronic course of disease. By blocking the IL-10/IL-10R pathway immediately upon lymphocytic choriomeningitis virus (LCMV) infection in mice, the authors were able to restore T-cell function and prevent viral persistence. A persevering viral infection, on the other hand, could eventually lead to exhaustion of virus-specific CD8+ T cells[41].
In the present study, we have demonstrated the ability of a small subset of HIV-1-specific T-cells that secrete IL-10 to inhibit the proliferation of other T cells in an antigen non-specific manner. The primary site of action for these IL-10-secreting HIV-1-specific T cells in vivo is likely to be in the secondary lymphoid tissues, in the context of antigen presentation, although this remains to be elucidated. The low frequencies available in venous blood pose limitations to the mechanistic study of these cells, yet a number of putative mechanisms exist: The secretion of IL-10 may in itself cause immunosuppression, as IL-10 has been described to have potent immunoregulatory effects and to drastically impact the outcome of an infection, at least in part by inhibiting the cytokine production and proliferation of effector T cells[32;33;41]. Another possible mechanism of IL-10-mediated immune suppression is through the induction of anergic or regulatory T cells, either directly or through effects on antigen-presenting cells. The clonal expansion and suppressive activity of T-cell subsets with regulatory function have been ascribed to IL-10 in several studies[25;42-45]. However, other mechanisms than the direct effects of IL-10 may also contribute to the suppression observed in this experimental context. We and others have demonstrated that both CD4+ and CD8+ T cells can acquire suppressive activity when subjected to prolonged in vitro and in vivo antigen stimulation, with or without the concomitant expression of IL-10[15;24;34;46]. These adaptive Treg-cell subsets may have a shorter half-life than thymus-derived CD4+CD25+FOXP3+ Treg cells and do not consistently express FOXP3[24;25;47], but may be of considerable importance in clinical conditions with persistent antigenic challenge and chronic immune activation.
Experimental procedures for induction of IL-10-secreting Treg cells in vitro commonly involve long-term or reiterated stimulation that may influence the properties of the resulting Treg cells. Here, we were able to isolate IL-10-secreting cells from freshly isolated PBMC, preceded only by overnight incubation and stimulation with antigenic peptides. The enriched HIV-1-specific IL-10-secreting T cells were shown to potently inhibit the proliferation of effector T cells. Correspondingly, Elrefaei et al. recently reported that the removal of HIV-1-specific IL-10-secreting T cells from PBMC in vitro resulted in increased CD8+ T-cell effector functions, as determined by IL-2 production and expression of CD107 as a marker of cytotoxic T-lymphocyte degranulation[48]. It seems, from our present data, that the short-term effects observed by Elrefaei et al. are followed by profound effects on T-cell proliferation that affects both CD4+ and CD8+ T cells.
The effects mediated by IL-10 or IL-10-secreting Treg cells may also be related in some degree to the phenomenon of T-cell exhaustion. The PD-1/PD-L1 pathway has been introduced as a major determinant of T-cell exhaustion, and it has been suggested that IL-10 works in synergy with the PD-1/PD-L1 pathway to mediate immune dysfunction[33;49]. Blocking this pathway has been shown to restore T-cell function and alleviate immune pathology in chronic HIV infection[50]. Furthermore, two recent reports have suggested the involvement of this pathway in the impairment of HIV-1-specific CD8+ T-cell effector functions in HIV-1 infection[51;52]. The combined blocking of IL-10 and the PD-1/PD-L1 pathway may thus have synergistic effects in the treatment of HIV-1 infection.
Interleukin-10 has been linked to the HIV-1-associated immune dysfunction in several studies[53;54]. Moreover, gene polymorphisms leading to reduced expression of IL-10 have been shown to delay progression to AIDS in HIV-infected individuals. Thus, controlling HIV-1-specific IL-10-secreting T cells that confer suppressive rather than anti-viral responses in infected individuals may be of value to an immune stimulatory treatment strategy in HIV-1 infection and in the management of HIV-1-associated immune dysfunction. Furthermore, the isolation of functionally distinct subpopulations of HIV-1-specific T cells based on their cytokine profiles may be instrumental in delineating the mechanisms underlying immune regulation in acute as well as chronic infection. At present, however, the relative scarcity of HIV-1-specific IL-10-secreting T cells remains a major obstacle to resolving their mechanism of action, and a more detailed characterization of these cells will be the objective of future studies.
Materials and methods
Study subjects and samples
HIV-1-infected volunteers off antiviral treatment (n = 23) were recruited from Ullevål University Hospital upon written informed consent. None of the study participants had received antiviral treatment for the last 6 months prior to enrolment and n = 11 were treatment naive. A summary of the clinical parameters is presented in Table 1. The study was approved by the Regional Ethics Review Board. Peripheral-blood CD4+ and CD8+ T-cell counts were monitored using the TriTEST™ reagent kit (BD Biosciences), whereas HIV-1 RNA was quantified by the COBAS™ Amplicor HIV-1 monitor test (Roche), with a detection limit of 50 copies per ml. PBMC were isolated by Isopaque-Ficoll (Lymphoprep™; Nycomed) density gradient centrifugation within 2-4 hours after blood sampling. In a few cases isolated PBMC were cryopreserved in liquid nitrogen prior to use.
Table 1. Clinical and experimental characteristics of the study participantsa).
| Procedureb) | Donor | HIV RNA, copies/ml | CD4+:CD8+ ratio | CD4+ cells/μl | CD8+ cells/μl |
|---|---|---|---|---|---|
| IL-10 | A | 21000 | 0.20 | 300 | 1530 |
| B | 5600 | 0.23 | 380 | 1670 | |
| C | 9500 | 0.23 | 540 | 2400 | |
| D | 5500 | 0.32 | 270 | 840 | |
| E | 200 | 0.14 | 190 | 1380 | |
| F | 1100 | 0.41 | 340 | 830 | |
| G | 7600 | 0.35 | 1040 | 2960 | |
| H | 500 | 0.98 | 580 | 590 | |
| I | 3300 | 0.47 | 770 | 1630 | |
| J | 97000 | 0.23 | 300 | 1310 | |
| K | 17000 | 0.57 | 1210 | 2140 | |
| L | 190000 | 0.45 | 620 | 1370 | |
| Median | 6600 | 0.34 | 460 | 1455 | |
| Range | 200 - 190000 | 0.14 - 0.98 | 190 - 1210 | 590 - 2960 | |
| IFN-γ | M | 600 | 0.66 | 270 | 410 |
| N | 170000 | 0.28 | 280 | 1010 | |
| O | 2600 | 1.58 | 840 | 530 | |
| P | 19000 | 0.36 | 400 | 1110 | |
| Q | 110000 | 0.16 | 180 | 1100 | |
| R | 87000 | 0.65 | 350 | 540 | |
| S | 12000 | 0.83 | 571 | 686 | |
| T | 250000 | 0.19 | 428 | 2222 | |
| U | 160000 | 0.14 | 198 | 1370 | |
| V | 9500 | 0.33 | 363 | 1091 | |
| W | 91000 | 0.26 | 335 | 1282 | |
| Median | 87000 | 0.33 | 350 | 1091 | |
| Range | 600 - 250000 | 0.14 - 1.58 | 180 - 840 | 410 - 2222 | |
Presented are the donors included in co-culture experiments.
Refers to whether the PBMC from the respective donor were subjected to IL-10- or IFN-γ enrichment.
Enrichment of cytokine-secreting T cells
Cells secreting IL-10 or IFN-γ were enriched using the respective Cytokine Secretion Assay kits from Miltenyi Biotec according to the manufacturer's protocol. Briefly, PBMC were stimulated for 3-6 hours or overnight at 5 × 106 cells/ml with a 15-mer Gag peptide pool (2 μg/ml/peptide; NIH) and anti-CD28 (1 μg/ml; BD Biosciences), or anti-CD28 alone. Following stimulation, all leukocytes were labeled with bi-functional IL-10- or IFN-γ capture antibodies and incubated for 45 min at 37 °C to allow for cytokine secretion. The cells were then stained with PE-conjugated anti-IL-10 or anti-IFN-γ and magnetically labeled with anti-PE MicroBeads. Cytokine-secreting (CS) cells were enriched on magnetic columns and separated from the depleted flow-through consisting of non-cytokine-secreting (NCS) cells.
Co-culture experiments
In order to assess the influence of antigen-specific CS cells on T-cell proliferation, a series of co-culture experiments were performed (see Fig. 1), in which enriched CS or NCS cells were incubated at a 1:2 ratio with NCS responder cells stained with CFSE (Sigma-Aldrich), as described previously[55], in the presence or absence of anti-CD2/CD3/CD28-coated MicroBeads (Miltenyi Biotec; bead-to-cell ratio of 1:5), for 3-4 days. Propidium Iodide (BD Biosciences) was added at 0.25 μg/ml immediately prior to acquisition on a FACSCalibur™ or FACSCantoII™ flow cytometer (BD Biosciences) for the exclusion of dead cells. The level of proliferation was determined by CFSE-dilution as analyzed with FlowJo™ v8.5.2 (Tree Star).
Cytokine flow cytometry
Antigen-specific T cells were analyzed for the expression of IL-10 and IFN-γ using intracellular cytokine staining and FACS analysis, as previously described[56] and, in a second setup, following a protocol optimized for IL-10. In brief, freshly isolated PBMC were stimulated with a 15-mer Gag peptide pool (2 μg/ml/peptide; NIH) and anti-CD28 (1 μg/ml; BD Biosciences) or anti-CD2/CD3/CD28-coated MicroBeads (bead-to-cell ratio of 1:5; Miltenyi Biotec) for 6 or 24 hours at 1 × 106 cells/ml. Anti-CD28 alone was used as negative control. Brefeldin A or monensin was added after 2 or 18 hours, respectively (both at 10 μg/ml; Sigma-Aldrich), for intracellular cytokine accumulation. Stained cells were acquired on a FACSCantoII™ flow cytometer (BD Biosciences) and analyzed with FlowJo™ v8.5.2 (Tree Star). Cell clusters and dead cells were excluded by virtue of scatter parameters and, in the 24-hour setup, according to staining with 7-AAD (10 μg/ml; BD Biosciences). The two setups yielded comparable results.
Flow-based analysis of FOXP3-expression
Enriched populations of IL-10- or IFN-γ-secreting T cells were assessed with respect to expression of the transcription factor FOXP3 using anti-FOXP3 (BD Biosciences) in accordance with the manufacturer's instructions. CS and NCS cells stained for CD3, CD4, and FOXP3 in addition to secreted IL-10 or IFN-γ were acquired on a FACSCantoII™ flow cytometer (BD Biosciences), and the data were analyzed in FlowJo v8.5.2 (Tree Star).
Antibodies and stimulating agents
Anti-CD3 Alexa 700 and -Pacific Blue (UCHT1); anti-CD4 Amcyan and -PE-Cy7 (SK3); anti-CD8 APC-H7 (SK1); anti-IL-10 PE (JES3-9D7); anti-IFN-γ APC (25723.11); anti-FOXP3 Alexa 647 (259D/C7) were purchased from BD Biosciences. Anti-CD4 APC (Edu-2) and anti-CD8 APC (MCD8) were supplied by IQ Products. The antibodies used for cytokine capture and enrichment were all provided in the Cytokine Secretion Assays from Miltenyi Biotec.
HIV-1 Gag group B and group M consensus sequence peptide sets (123 peptides each; 15-mers with 11 amino-acid overlaps) were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH (Bethesda, MD). The lyophilized peptides were reconstituted according to the manufacturer's instructions and combined to obtain a group B and a group M peptide pool, respectively, which were used at a final concentration of 2 μg/ml per peptide. Only one of the pools was employed per sample. Anti-CD28 (CD28.2) was purchased from BD Biosciences. Anti-biotin MicroBeads were obtained from Miltenyi Biotec and coated with biotinylated anti-CD2, -CD3, and -CD28 (Miltenyi Biotec) according to the manufacturer's instructions at a final concentration of 5 μg/ml of each of the antibodies.
Statistical analysis
Paired statistical analyses were performed using the Wilcoxon signed rank test. Unpaired data were compared using the Mann-Whitney U test. Correlations were assessed using the Spearman rank test. All statistical analyses were conducted in SPSS™ v15.0 (SPSS Inc.). Graphs were made in SigmaPlot v8.0 (Systat Software Inc.). The two-sided P-values were considered statistically significant at P < 0.05.
Acknowledgments
E.A.T., L.C.N., E.M.A., K.T., F.O.P., and D.K. designed the research; F.O.P. and D.K. enrolled and managed the patients; E.A.T. performed most of the experiments, analyzed and interpreted the results, and made the figures; L.C.N., T.L.L., and A.R.J. performed some of the co-culture experiments with E.A.T.; T.L.L. also did some of the phenotype experiments; E.M.A., K.T., L.C.N., D.F.N., and K.M.T. contributed to data analysis and participated in the interpretation of data; K.T. provided most of the financial support, to which D.K., D.F.N., and L.C.N. contributed; E.A.T. and E.M.A. wrote the paper; and all authors critically revised the manuscript and approved of the final version.
We are grateful for the technical assistance of Mette Sannes at Ullevål University Hospital.
This work was supported by grants from the Clinical Research Program, the Global Health Program, and the Norwegian Functional Genomics Program, Research Council of Norway (K.T. and D.K.); the UCSF AIDS Biology Program of the AIDS Research Institute (ARI), NIH grant (AI068498) (D.N.); and an Irvington Institute Fellowship Program of the Cancer Research Institute (L.C.N.).
Abbreviations
- CS
cytokine-secreting
- FOXP3
forkhead box P3
- NCS
non-cytokine-secreting
Footnotes
Conflict of interest: The authors declare no financial conflict or commercial of interest.
References
- 1.Miedema F. Immunological abnormalities in the natural history of HIV infection: mechanisms and clinical relevance. Immunodefic Rev. 1992;3:173–193. [PubMed] [Google Scholar]
- 2.Sheppard HW, Ascher MS. The natural history and pathogenesis of HIV infection. Annu Rev Microbiol. 1992;46:533–564. doi: 10.1146/annurev.mi.46.100192.002533. [DOI] [PubMed] [Google Scholar]
- 3.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 4.Greene WC. A history of AIDS: looking back to see ahead. Eur J Immunol. 2007;37 1:S94–102. doi: 10.1002/eji.200737441. [DOI] [PubMed] [Google Scholar]
- 5.Dolan MJ, Clerici M, Blatt SP, Hendrix CW, Melcher GP, Boswell RN, Freeman TM, et al. In vitro T cell function, delayed-type hypersensitivity skin testing, and CD4+ T cell subset phenotyping independently predict survival time in patients infected with human immunodeficiency virus. J Infect Dis. 1995;172:79–87. doi: 10.1093/infdis/172.1.79. [DOI] [PubMed] [Google Scholar]
- 6.Roos MT, Miedema F, Koot M, Tersmette M, Schaasberg WP, Coutinho RA, Schellekens PT. T cell function in vitro is an independent progression marker for AIDS in human immunodeficiency virus-infected asymptomatic subjects. J Infect Dis. 1995;171:531–536. doi: 10.1093/infdis/171.3.531. [DOI] [PubMed] [Google Scholar]
- 7.Aandahl EM, Aukrust P, Skalhegg BS, Muller F, Froland SS, Hansson V, Tasken K. Protein kinase A type I antagonist restores immune responses of T cells from HIV-infected patients. FASEB J. 1998;12:855–862. doi: 10.1096/fasebj.12.10.855. [DOI] [PubMed] [Google Scholar]
- 8.Kvale D, Ormaasen V, Kran AM, Johansson CC, Aukrust P, Aandahl EM, Froland SS, Tasken K. Immune modulatory effects of cyclooxygenase type 2 inhibitors in HIV patients on combination antiretroviral treatment. AIDS. 2006;20:813–820. doi: 10.1097/01.aids.0000218544.54586.f1. [DOI] [PubMed] [Google Scholar]
- 9.Clerici M, Sarin A, Berzofsky JA, Landay AL, Kessler HA, Hashemi F, Hendrix CW, et al. Antigen-stimulated apoptotic T-cell death in HIV infection is selective for CD4+ T cells, modulated by cytokines and effected by lymphotoxin. AIDS. 1996;10:603–611. doi: 10.1097/00002030-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman J, Shankar P, Manjunath N, Andersson J. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood. 2001;98:1667–1677. doi: 10.1182/blood.v98.6.1667. [DOI] [PubMed] [Google Scholar]
- 12.Kinter AL, Hennessey M, Bell A, Kern S, Lin Y, Daucher M, Planta M, et al. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 15.Garba ML, Pilcher CD, Bingham AL, Eron J, Frelinger JA. HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J Immunol. 2002;168:2247–2254. doi: 10.4049/jimmunol.168.5.2247. [DOI] [PubMed] [Google Scholar]
- 16.Andersson J, Boasso A, Nilsson J, Zhang R, Shire NJ, Lindback S, Shearer GM, Chougnet CA. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174:3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 17.Bi X, Suzuki Y, Gatanaga H, Oka S. High frequency and proliferation of CD4+ FOXP3+ Treg in HIV-1-infected patients with low CD4 counts. Eur J Immunol. 2009;39:301–309. doi: 10.1002/eji.200838667. [DOI] [PubMed] [Google Scholar]
- 18.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 20.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 21.Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunological Reviews. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 22.Baker CA, Clark R, Ventura F, Jones NG, Guzman D, Bangsberg DR, Cao H. Peripheral CD4 loss of regulatory T cells is associated with persistent viraemia in chronic HIV infection. Clin Exp Immunol. 2007;147:533–539. doi: 10.1111/j.1365-2249.2006.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. 2006;177:246–254. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- 24.Mahic M, Yaqub S, Bryn T, Henjum K, Eide DM, Torgersen KM, Aandahl EM, Tasken K. Differentiation of naive CD4+ T cells into CD4+CD25+FOXP3+ regulatory T cells by continuous antigen stimulation. J Leukoc Biol. 2008 doi: 10.1189/jlb.0507329. [DOI] [PubMed] [Google Scholar]
- 25.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunological Reviews. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 26.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 27.Baker CA, Clark R, Ventura F, Jones NG, Guzman D, Bangsberg DR, Cao H. Peripheral CD4 loss of regulatory T cells is associated with persistent viraemia in chronic HIV infection. Clin Exp Immunol. 2007;147:533–539. doi: 10.1111/j.1365-2249.2006.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon V, Ho DD. HIV-1 dynamics in vivo: implications for therapy. Nat Rev Microbiol. 2003;1:181–190. doi: 10.1038/nrmicro772. [DOI] [PubMed] [Google Scholar]
- 29.Clerici M, Sarin A, Berzofsky JA, Landay AL, Kessler HA, Hashemi F, Hendrix CW, et al. Antigen-stimulated apoptotic T-cell death in HIV infection is selective for CD4+ T cells, modulated by cytokines and effected by lymphotoxin. AIDS. 1996;10:603–611. doi: 10.1097/00002030-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Musey LK, Krieger JN, Hughes JP, Schacker TW, Corey L, McElrath MJ. Early and persistent human immunodeficiency virus type 1 (HIV-1)-specific T helper dysfunction in blood and lymph nodes following acute HIV-1 infection. J Infect Dis. 1999;180:278–284. doi: 10.1086/314868. [DOI] [PubMed] [Google Scholar]
- 31.Sereti I, Lane HC. Immunopathogenesis of human immunodeficiency virus: implications for immune-based therapies. Clin Infect Dis. 2001;32:1738–1755. doi: 10.1086/320758. [DOI] [PubMed] [Google Scholar]
- 32.Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 33.Filippi CM, von Herrath MG. IL-10 and the resolution of infections. J Pathol. 2008;214:224–230. doi: 10.1002/path.2272. [DOI] [PubMed] [Google Scholar]
- 34.Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, Abrignani S, et al. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–972. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ameglio F, Cordiali FP, Solmone M, Bonifati C, Prignano G, Giglio A, Caprilli F, et al. Serum IL-10 levels in HIV-positive subjects: correlation with CDC stages. J Biol Regul Homeost Agents. 1994;8:48–52. [PubMed] [Google Scholar]
- 36.Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, Coffman RL, Shearer GM. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994;93:768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swaminathan S. Molecular biology of Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus. Semin Hematol. 2003;40:107–115. doi: 10.1053/shem.2003.50011. [DOI] [PubMed] [Google Scholar]
- 38.Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;248:1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 39.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 44.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mills KH, McGuirk P. Antigen-specific regulatory T cells--their induction and role in infection. Semin Immunol. 2004;16:107–117. doi: 10.1016/j.smim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 48.Elrefaei M, Ventura FL, Baker CA, Clark R, Bangsberg DR, Cao H. HIV-specific IL-10-positive CD8+ T cells suppress cytolysis and IL-2 production by CD8+ T cells. J Immunol. 2007;178:3265–3271. doi: 10.4049/jimmunol.178.5.3265. [DOI] [PubMed] [Google Scholar]
- 49.Green KA, Okazaki T, Honjo T, Cook WJ, Green WR. The programmed death-1 and interleukin-10 pathways play a down-modulatory role in LP-BM5 retrovirus-induced murine immunodeficiency syndrome. J Virol. 2008;82:2456–2469. doi: 10.1128/JVI.01665-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 51.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 52.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, et al. Upregulation of PD-1 expression on HIV-specific CD8(+) T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 53.Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, Coffman RL, Shearer GM. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994;93:768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ostrowski MA, Gu JX, Kovacs C, Freedman J, Luscher MA, MacDonald KS. Quantitative and qualitative assessment of human immunodeficiency virus type 1 (HIV-1)-specific CD4+ T cell immunity to gag in HIV-1-infected individuals with differential disease progression: reciprocal interferon-gamma and interleukin-10 responses. J Infect Dis. 2001;184:1268–1278. doi: 10.1086/324005. [DOI] [PubMed] [Google Scholar]
- 55.Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. 2006;177:246–254. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- 56.Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjornbeth BA, Tasken K. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2007 doi: 10.1007/s00262-007-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]