Abstract
Background
The aquaporin-4-specific serum autoantibody, NMO-IgG, is a validated biomarker distinguishing neuromyelitis optica spectrum disorders (NMOSD) from multiple sclerosis (MS). Because fulminant attacks are more common in NMOSD than MS, some suggest that NMO-IgG may be a marker of destructive demyelination rather than a disease-specific biomarker. This study is the first to compare NMO-IgG serostatus among patients with fulminant CNS-inflammatory demyelinating disease (CNS-IDD).
Objective
To determine if NMO-IgG distinguishes NMOSD from other fulminant, steroid-refractory CNS-IDDs.
Design
Descriptive historical cohort
Setting
Neuroimmunology Laboratory and Neurology Practice, Mayo Clinic.
Patients and Methods
Sera from 74 patients plasmapheresed between 1993 and 2007 for a steroid-refractory CNS-IDD were tested for NMO-IgG (by indirect immunofluorescence assay). Two blinded observers scored sera (tested at 1:120 dilution). Clinical data were obtained by medical record review.
Results
Pre-plasmapheresis sera were available from 74 patients (F:M =2.5 ); mean time from blood draw to plasmapheresis was 13 days. At plasmapheresis, mean age was 46 years (range, 7 to 8 0); mean EDSS was 7 (range, 3.5 to 9.5) and diagnoses were NMO (14), longitudinally extensive transverse myelitis (LETM; ≥ 3 vertebral segments; 20), transverse myelitis (TM; < 3 vertebral segments; 8), acute disseminated encephalomyelitis (ADEM; 1), MS (definite, 18; probable, 11), and optic neuritis (ON; 2). NMO-IgG was detected in 20 patients (27%): 9 NMO, 10 LETM and one recurrent ON, and in no patient with fulminant ADEM, MS, TM or monophasic ON.
Conclusions
NMO-IgG is a specific biomarker for NMOSD and is not simply a marker of destructive CNS-IDD.
INTRODUCTION
Neuromyelitis optica (NMO) is a severe demyelinating disease of the central nervous system (CNS), conventionally recognized for its propensity to preferentially affect the optic nerves and spinal cord. Typically, NMO has a worse clinical outcome than multiple sclerosis (MS), with more frequent and early relapses.1 Prototypic MS is not usually fulminant in nature and MS lesions are not typically destructive. This is in contrast to NMO. It tends to be more fulminant and is characterized immunopathologically by destructive lesions affecting both grey and white matter, with eosinophilic and neutrophilic inflammatory infiltrates, necrosis, vascular hyalinization, and extensive vasculocentric deposits of immunoglobulins and complement.2 Early distinction of NMO from MS is prognostically and therapeutically important because their optimal treatments differ (immunosuppression for NMO and immunomodulation for MS).1 Although the aquaporin-4-specific autoantibody, NMO-IgG, has been convincingly shown to be a biomarker that reliably distinguishes NMOSDs from classical MS, no previous study has investigated the specificity of NMO-IgG expressly in the setting of fulminant NMO as compared to fulminant inflammatory CNS demyelinating disorders (CNS-IDDs).3–13 Fulminant attacks are more common in NMO than MS, therefore some suggest that NMO-IgG may simply be a marker of fulminant inflammatory CNS demyelinating disease rather than specific for NMO. Plasmapheresis is an effective treatment for steroid-refractory fulminant attacks of CNS-IDD.14 In this study, we analyze NMO-IgG serostatus among a cohort of CNS-IDD patients who received plasmapheresis for a fulminant attack, in order to determine whether NMO-IgG is merely a marker of a destructive CNS demyelinating process or is indeed a specific biomarker for NMOSDs.
Methods
Mayo Clinic patients treated between 1993 and 2007 by plasmapheresis for a fulminant, steroid-refractory attack of CNS-IDD were identified from a larger plasmapheresis cohort (n=212). Inclusion criteria were: 1) CNS inflammatory demyelinating disease, 2) plasmapheresis for a fulminant, steroid-refractory CNS-IDD attack, 3) archival sera collected 0–3 months prior to plasmapheresis. Sera were tested for NMO-IgG by indirect immunofluorescence and the typical immunostaining pattern was scored, by two independent observers blinded to diagnosis, as positive (titer 120 or higher) or negative (tested at 1:120 dilution). Clinical data were obtained by medical record review. Diagnoses were based on published criteria: NMO and NMO spectrum disorders (LETM; ≥ 3 vertebral segments; and recurrent ON),1 monophasic ON, MS (probable or definite),15, 16 ADEM,17 and short TM (TM; < 3 vertebral segments).18 Prior to serum sampling, all patients had received immunosuppressant or immunomodulatory therapies.
Results
Pre-plasmapheresis serum was available from 74 patients (F:M = 2.5); mean interval between blood draw and plasmapheresis was 13 days (range 0 to 95); mean age at time of plasmapheresis was 46 years (range 7 to 80) and mean Expanded Disability Status Score (EDSS) at plasmapheresis was 7 (range 3.5 to 9.5 [10 is death]). Diagnoses at attack onset were: NMO (14; monophasic, 1; recurrent, 13), LETM (20; monophasic, 7; recurrent, 13), optic neuritis (2; monophasic,1; recurrent, 1), short TM (8; monophasic, 6; recurrent, 2), ADEM (1), and MS (29; definite, 18; probable, 11). NMO-IgG was detected in 20 patients (27%): nine had NMO, 10 LETM, and 1 recurrent ON. NMO-IgG was notably absent in any patient with definite or probable MS, ADEM, monophasic ON, and short TM. Titer values ranged from 240 to 61,140. Mean disease duration at last follow-up was 4.8 years (range 48 days to 38 years). At last follow-up, 10 (53%) of the NMO seropositive patients had subsequent relapses and 5 LETM patients (3 NMO-IgG positive) had progressed to meet diagnostic criteria for definite NMO. Diagnoses at last follow-up were recurrent NMO,19; LETM, 14 (monophasic, 4; recurrent, 10); recurrent ON, 1; TM, 7 (monophasic, 5; recurrent, 2); MS, 27 (definite, 22; probable, 5); clinically isolated demyelinating syndrome, 1; ADEM, 1; and monophasic ON, 1. Diagnoses were unknown for 3 patients.
Discussion
Neuromyelitis optica is often misdiagnosed as multiple sclerosis and is still considered by some to be a variant of MS. Recently reported clinical, radiographic, pathologic, and immunologic characteristics of NMO have led to recognition of NMO as a distinct entity from prototypic MS.1 Discovery of the aquaporin 4-specific autoantibody, NMO-IgG, defined a specific humoral immune response associated with NMO, and supported the existence of distinct underlying immunopathogenic mechanisms in NMO and MS.3–13, 19 However, all these previous studies examined the specificity of NMO-IgG in the setting of classic, non-fulminant MS. Since fulminant attacks are more frequent in NMOSD and atypical in MS, the question of whether or not NMO-IgG is simply a marker of fulminant, CNS-IDDs remains unaddressed. The present study confirms our previous reports that NMO-IgG is a specific biomarker for NMO and its spectrum disorders, and establishes that it is not an accompaniment of other severe inflammatory CNS demyelinating disorders, even in fulminant cases. NMO-IgG was restricted to patients with NMO or an NMO spectrum disorder. It was not detected in any patient with fulminant MS, or other CNS-IDD. Our observations further support NMO being a distinct entity from MS and emphasize the necessity for early diagnosis to ensure early initiation of appropriate immunosuppressive therapies.
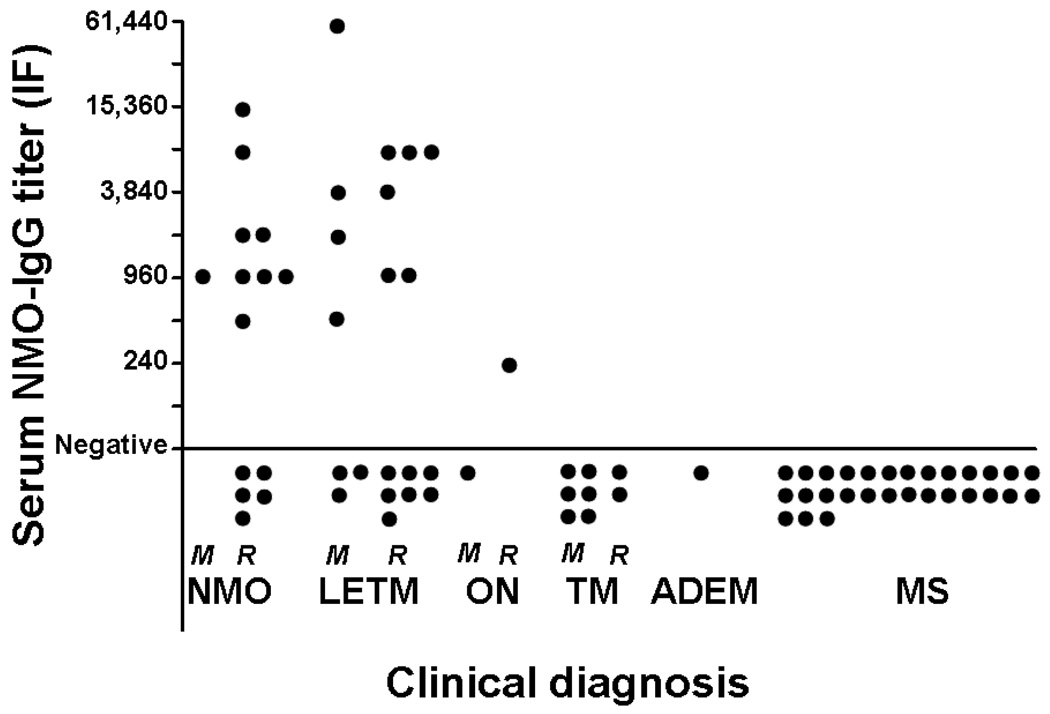
Figure 1.
NMO-IgG titers by clinical diagnosis at attack onset. NMO-IgG was detected exclusively in NMO spectrum disorders and was notably absent in any patient with monophasic ON, short TM, ADEM, or MS.
M=monophasic; R=recurrent
Acknowledgments
Funding/Support: This work was supported by Clinical and Translational Science Awards grant TL1 RR024152-01 from the National Institutes of Health (Ms Magaña) and by the Guthy-Jackson Charitable Foundation (Drs. Lennon, Weinshenker, and Lucchinetti).
Footnotes
Financial Disclosures: In accordance with the Bayh–Dole Act of 1980 and Mayo Foundation policy, Drs. Lennon, Lucchinetti, and Weinshenker stand to receive royalties for intellectual property related to the AQP4 autoantigen. To date, the authors have received less than $10,000 in royalties. Dr. Weinshenker served as a consultant to Genentech Inc for development of a clinical trial for NMO, which does not relate directly to the subject of this article. The other authors report no conflicts of interest.
References
- 1.Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 2.Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain. 2002;125:1450–1461. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakashima I, Fujihara K, Miyazawa I, et al. Clinical and MRI features of Japanese patients with multiple sclerosis positive for NMO-IgG. J Neurol Neurosurg Psychiatry. 2006;77:1073–1075. doi: 10.1136/jnnp.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 5.Scott TF, Kassab SL, Pittock SJ. Neuromyelitis optica IgG status in acute partial transverse myelitis. Arch Neurol. 2006;63:1398–1400. doi: 10.1001/archneur.63.10.1398. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka K, Tani T, Tanaka M, et al. Anti-aquaporin 4 antibody in selected Japanese multiple sclerosis patients with long spinal cord lesions. Mult Scler. 2007;13:850–855. doi: 10.1177/1352458507076976. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130:1235–1243. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka T, Matsushita T, Kawano Y, et al. Heterogeneity of aquaporin-4 autoimmunity and spinal cord lesions in multiple sclerosis in Japanese. Brain. 2007;130:1206–1223. doi: 10.1093/brain/awm027. [DOI] [PubMed] [Google Scholar]
- 9.Paul F, Jarius S, Aktas O, et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med. 2007;4:e133. doi: 10.1371/journal.pmed.0040133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saiz A, Zuliani L, Blanco Y, et al. Revised diagnostic criteria for neuromyelitis optica (NMO). Application in a series of suspected patients. J Neurol. 2007;254:1233–1237. doi: 10.1007/s00415-007-0509-8. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Tanaka K, Komori M, Saida T. Anti-aquaporin 4 antibody in Japanese multiple sclerosis: the presence of optic spinal multiple sclerosis without long spinal cord lesions and anti-aquaporin 4 antibody. J Neurol Neurosurg Psychiatry. 2007;78:990–992. doi: 10.1136/jnnp.2006.114165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banwell B, Tenembaum S, Lennon VA, et al. Neuromyelitis optica-IgG in childhood inflammatory demyelinating CNS disorders. Neurology. 2008;70:344–352. doi: 10.1212/01.wnl.0000284600.80782.d5. [DOI] [PubMed] [Google Scholar]
- 13.Marignier R, De Seze J, Vukusic S, et al. NMO-IgG and Devic's neuromyelitis optica: a French experience. Mult Scler. 2008;14:440–445. doi: 10.1177/1352458507084595. [DOI] [PubMed] [Google Scholar]
- 14.Keegan M, Pineda AA, McClelland RL, et al. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58:143–146. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- 15.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 16.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 17.Wingerchuk DM, Lucchinetti CF. Comparative immunopathogenesis of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis. Curr Opin Neurol. 2007;20:343–350. doi: 10.1097/WCO.0b013e3280be58d8. [DOI] [PubMed] [Google Scholar]
- 18.Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499–505. doi: 10.1212/wnl.59.4.499. [DOI] [PubMed] [Google Scholar]
- 19.Roemer SF, Parisi JE, Lennon VA, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]