Abstract
In budding yeast, the protein phosphatase Cdc14 is a key regulator of late mitotic events. Research over the last decade has revealed many of its functions and today we know that this protein phosphatase orchestrates several aspects of chromosome segregation and is the key trigger of exit from mitosis. The elucidation of the mechanisms controlling Cdc14’s activity through nucleolar sequestration now serves as a paradigm for how the regulation of the subcellular localization of proteins regulates protein function. Here I review these findings focusing on how discoveries in my lab helped elucidate the function and regulation of Cdc14.
Keywords: Cdc14, mitosis, Mitotic Exit Network, FEAR network, phosphatase
Discovery of Cdc14 as the key regulator of exit from mitosis in budding yeast
In 1995 my scientific life changed. I was given the unique opportunity to become a Whitehead Fellow. The Whitehead Fellow’s Program allows young scientists, mostly straight out of Graduate School, to pursue their independent research program. At the time I was a postdoctoral fellow in Ruth Lehman’s lab and Ruth had decided to move to NewYork. I could not come with her (and also realized that flies were not my thing) and decided to give this a try. But what should I work on?
As a graduate student in Kim Nasmyth’s lab I had studied the regulation of cyclin-dependent kinases (CDKs), which are composed of a catalytic kinase subunit and a regulatory cyclin subunit. A specific form of these CDKs, the mitotic CDKs (Clb1, 2, 3 and 4-CDKs in budding yeast), promote entry into mitosis (reviewed [1]). Previous work by Andrew Murray and Marc Kirschner had shown that degradation of the regulatory mitotic cyclin subunit was required for exit from mitosis in Xenopus [2]. I could show that in yeast this was the case too. Expression of a form of the mitotic Clb2 cyclin that was resistant to ubiquitin-mediated protein degradation arrests cells in late anaphase, after the completion of chromosome segregation but prior to spindle disassembly [3]. Subsequent work by the Kirschner, Nasmyth, Ruderman and Hershko labs showed that a ubiquitin ligase known as the Anaphase Promoting Complex or Cyclosome (APC/C) degraded mitotic cyclins at the end of mitosis [4], [5], [6]. How the ubiquitin ligase was activated at the end of mitosis was however not clear.
As a Whitehead fellow I decided to return to this question. In particular, I was interested in determining the role of CDKs in the regulation of mitotic Clb cyclin degradation, by examining the consequences of CDK inactivation on mitotic Clb cyclin degradation. I arrested cells in a stage of the cell cycle, when Clb cyclins are normally stable because the APC/C is inactive (in metaphase) and then examined the consequences of CDK inactivation on Clb protein levels. To inhibit Clb-CDKs, I overexpressed Sic1, a Clb-CDK inhibitor that inhibits Clb kinases by binding to them [1]. To inhibit Cln-CDKs I treated cells with pheromone. This leads to the production of Far1, an inhibitor of Cln-CDKs [1]. In this experiment, which we ended up calling the “sic-trick”, Clb cyclins were efficiently degraded [7]. Inactivation of the APC/C prevented Clb cyclin degradation in the “sic-trick” experiment, indicating that degradation of Clb cyclins was brought about by the same mechanisms responsible for degrading Clb cyclins at the end of mitosis [7]. This result was not very well received by the field because it was rather unexpected. Mitotic cyclin degradation is initiated in the presence of high CDK activity, and phosphorylation of the APC/C promoted cyclin degradation in vitro. Thus, everyone assumed that mitotic CDKs promoted rather than inhibited mitotic cyclin degradation. This is certainly true, though only in part. APC/C bound to its activating subunit Cdc20 degrades mitotic cyclins in the presence of high mitotic CDK activity. However, today we know that a second APC/C activator, Cdh1, is inhibited by CDK-dependent phosphorylation (reviewed [8]). In the sic-trick experiment it presumably is this form of APC that becomes active when CDKs are inhibited.
How do APC/C-Cdc20 and APC/C-Cdh1 regulate cyclin degradation? In most eukaryotes the bulk of mitotic cyclin degradation occurs at the metaphase-anaphase transition and is mediated by APC/C-Cdc20. APC/C-Cdh1 is needed during G1 to maintain mitotic CDK activity low. In budding yeast however a significant amount of mitotic CDK activity persists until late anaphase (reviewed [9]). This pool of mitotic CDK activity is targeted for degradation by APC/C-Cdh1, which is inhibited by CDK-dependent phosphorylation. (Figure 1; [10], [11], [12], reviewed [8]). Thus, for APC/C-Cdh1 to degrade Clb cyclins at the end of mitosis a phosphatase needs to dephosphorylate Cdh1. At the time we all assumed that it must be CDK down-regulation that initiates Cdh1 dephosphorylation allowing constitutively active phosphatases to win the upper hand. This idea was of course wrong. Today we know that a phosphatase – namely Cdc14 – is activated to bring about Cdh1 dephopshorylation. It is also clear now that regulation of phosphatases is important for many cell cycle events.
Figure 1. Cdc14 promotes Clb-CDK inactivation.
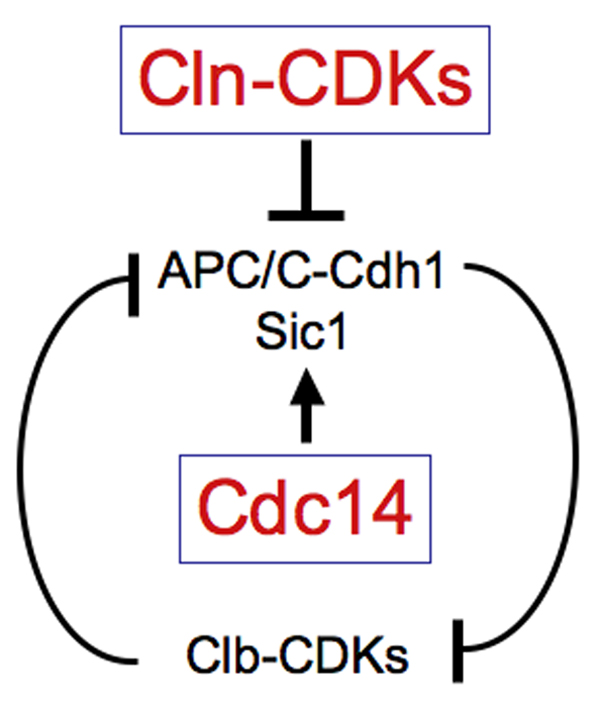
Clb-CDKs and the factors that inactivate it mutually inhibit each other. During S phase and early mitosis, Clb-CDKs inhibit APC/C-Cdh1 by preventing the association of Cdh1 with APC/C. Clb-CDKs also prevent the accumulation of Sic1. They prevent entry of the transcription factor Swi5 into the nucleus thereby inhibiting SIC1 transcription. They also phopshorylate Sic1, which targets it for degradation. Activation of Cdc14 during anaphase dephosphorylates Cdh1, which causes activation of the APC-Cdh1 and hence mitotic cyclin degradation. Cdc14 also dephosphorylates Swi5 and Sic1, causing SIC1 transcription and Sic1 stabilization, respectively. This leads to the Clb-CDK inhibitors to gain the upper hand, keeping Clb-CDKs in the inactive state. Cln-CDKs break this inhibition once they are active at the G1 – S phase transition. They target Sic1 for degradation and phosphorylate Cdh1, allowing Clb-CDKs to accumulate again.
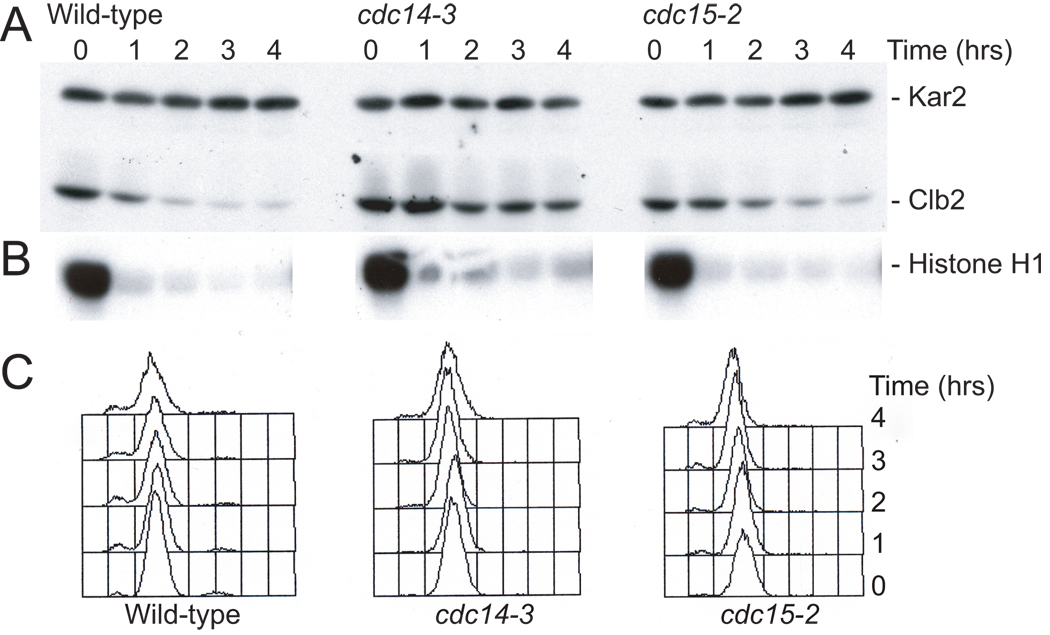
So how did we realize that Cdc14 was the key regulator of exit from mitosis? A number of screens, most notable among them the cell division cycle (cdc) screen by Lee Hartwell and colleagues [13], had identified several temperature sensitive mutants that, when shifted to the restrictive temperature, arrest with high levels of Clb cyclins in late anaphase, prior to exit from mitosis (reviewed [9]). However, we did not know whether this inability to degrade Clb cyclins was due to these mutants being defective in Clb cyclin degradation or due to the mutants arresting in a cell cycle stages when Clb degradation had not yet commenced. The ability to induce Clb cyclin degradation at will and in stages of the cell cycle when Clb cyclins are normally stable enabled me to distinguish between these possibilities. The cdc mutants, which arrested in anaphase with high levels of Clb CDK activity, were also defective in cyclin degradation in metaphase-arrested cells in which Clb degradation was induced using the sic-trick (Figure 2). Thus, the inability of these mutants to degrade Clb cyclins was not a mere consequence of the cell cycle arrest they caused.
Figure 2. CDC14 is required for Clb2 degradation brought about by ectopic CDK inactivation.
GAL-SIC1 (A810), cdc14-3 GAL-SIC1 (A831) and cdc15-2 GAL-SIC1 (A844) cells were arrested with nocodazole (15µg/ml) for 165 minutes in YEP medium containing 2% raffinose at 23°C. Then the 0 time point was taken and cells were shifted to 37°C for 30 minutes. Then 2% galactose and α–factor (5 µg/ml) were added to induce Sic1 production and to inhibit Cln-CDKs, respectively. 5µg/ml nocodazole was readded at the same time to ensure that microtubules remain depolymerized. Samples were taken at the indicated times after temperature shift to determine the amount of Clb2 protein (A), Clb2-associated histone H1 kinase activity (B) and DNA content (C). Kar2 was used as a loading control in western blots. Methods were as described in Amon (1997).
The sic-trick not only identified a number of genes as being important for cyclin degradation but also pointed towards the phosphatase Cdc14 as being critically important for Clb cyclin degradation. In cdc14-3 mutants inactivation of CDKs did not induce Clb2 degradation. Inactivation of other genes required for exit from mitosis such as CDC15 also delayed Clb2 cyclin degradation upon CDK inactivation, but the effects were not as dramatic (Figure 2). Among all the cdc mutants that were defective in exit from mitosis, cdc14 mutants exhibited the most severe defect in Clb2 degradation in the sic-trick experiment. We now understand why this is the case. Cdc14 is the phosphatase that dephosphorylates Cdh1. Its inactivation has the most dramatic effect on Clb cyclin degradation. CDC15 is a component of one of the two pathways that activate Cdc14. In cdc15 mutants, Cdc14 is thus not completely inactive. The observation that inactivation of CDC14 led to the most severe defect in Clb cyclin degradation led me to focus on understanding how this phosphatase regulates Clb-CDK inactivation and hence exit from mitosis and how it itself is controlled.
Cdc14 triggers CDK inactivation by multiple mechanisms
I was very lucky for Rosella Visintin to be the first person to have joined my lab. She came in March of 1997 and decided to first investigate how Cdc14 controls exit from mitosis. She found that cells lacking Cdc14 function arrest in late anaphase with high mitotic CDK activity [14]. Conversely, overexpression of CDC14 results in inappropriate mitotic CDK inactivation. Rosella could further show that Cdc14 promotes mitotic CDK inactivation by reversing CDK phosphorylation events. Cdc14 dephosphorylates Cdh1, which promotes its association with the APC/C thereby activating it [14], [15]. Cdc14 also triggers Sic1 accumulation by dephosphorylating Sic1 and its transcription factor Swi5, which lead to the stabilization of Sic1 and upregulation of SIC1 transcription, respectively [14]. It is now clear that Cdc14 has many substrates in the cell and it is likely that Cdc14 dephosphorylates many if not all Clb-CDK substrates and perhaps substrates of other mitotic kinases such as the polo kinase Cdc5 and the Aurora B kinase Ipl1. This general reversal of mitotic phosphorylation events rapidly triggers exit from mitosis.
Regulation of Cdc14 – the nucleolus moves into the limelight
The finding that CDC14 was unique among the genes required for mitotic exit in that it was necessary and sufficient to bring about mitotic CDK inactivation begged the question whether the activity of Cdc14 was regulated during the cell cycle. Insight into this question came from localization studies on Cdc14. Rosella found that Cdc14 was localized in the nucleolus from G1 until the onset of anaphase. During anaphase Cdc14 was present throughout the nucleus and cytoplasm. This result was very exciting. All our previous studies on Cdc14 predicted that the phosphatase ought to become active during anaphase to bring about mitotic CDK inactivation. The observation that the phosphatase changed its localization during the stage of the cell cycle when CDK inactivation commences raised the very interesting possibility that this change in subcellular localization reflects a change in Cdc14 activity.
We now know that this is the case. When Cdc14 is located in the nucleolus it is bound to a competitive inhibitor Cfi1/Net1 [16], [17]. During anaphase, the phosphatase is released from its inhibitor and spreads into the nucleus and cytoplasm, allowing it to dephosphorylate its substrates [16], [17]. We identified Cfi1 as a Cdc14-interacting protein in a two hybrid screen [17]. At the same time, Wenying Shou in Ray Deshaies’s lab isolated yeast mutants that were able to proliferate in the absence of CDC15, a gene required for exit from mitosis. The screen revealed mutations in the same gene, which Ray called NET1 [16]. This elegant screen not only identified loss of function alleles in NET1 but also gain-of-function alleles in CDC14 as suppressors of cdc15Δ mutants. The simplest interpretation of these findings was that NET1 functioned, directly or indirectly as an inhibitor of CDC14 and that CDC15 was needed to alleviate this inhibition. This idea turned out to be correct. Using biochemical approaches the Deshaies and Charbonneau labs showed that Cfi1/Net1 functions as an inhibitor of Cdc14 in vitro [16], [18]. Consistent with this idea, overexpression of CFI1/NET1 prevented exit from mitosis [17].
I would like to note here that at first, I was not very excited about the fact that Cdc14 resided in the nucleolus. A cool cell cycle protein like Cdc14 could not possibly be in a place as mundane as the nucleolus. Today, it is clear that nucleolar sequestration is a mechanism employed by cells to regulate many protein involved in numerous different cellular processes, from transcription factors such as Hand1 [19], checkpoint proteins such as Pch2 [20] to tumor suppressors such as p19-Arf [21].
MEN
If release of Cdc14 from its inhibitor was a key step in the regulation of exit from mitosis, mutants that fail to do so should also not be able to exit from mitosis. Indeed, Rosella in my lab and Wenying in Ray Deshaies’ lab both found that mutants that failed to exit from mitosis arrested in late anaphase with Cdc14 sequestered in the nucleolus. Furthermore, inactivation of the Cdc14 inhibitor Cfi1/Net1 suppressed the temperature sensitive lethality of these mutants, indicating that releasing Cdc14 from its inhibitor during exit from mitosis was the essential function of these genes [16], [17]. Earlier, David Morgan had studied the genes required for exit from mitosis and identified many genetic interactions between them, therefore, collectively calling them the Mitotic Exit Network, short MEN [22].
The MEN is essential for exit from mitosis and was the first signal transduction pathway shown to regulate Cdc14 localization. The pathway resembles a Ras-like GTPase signaling cascade (Figure 3)(reviewed in [9], [23]). The research aimed at understanding how the individual components within MEN functioned to promote Cdc14 release from the nucleolus was guided by work on the homologous pathway in S. pombe. Kathy Gould, Dan McCollum and Viesturs Simanis studied cytokinesis in fission yeast and identified a Ras-like signaling cascade essential for this process [24], [25]. Using genetic, cell biological and biochemical techniques they quickly ordered the genes into a pathway that they named the Septation Initiation Network (SIN, reviewed [26]). Similar analyses revealed that the MEN components function in an analogous order as their SIN counterparts (reviewed [27], [26]) (Figure 3). The GTPase Tem1 is positively regulated by the GTPase activating protein (GAP) complex Bub2-Bfa1. Lte1 resembles a Guanine nucleotide exchange factor (GEF) that positively regulates Tem1, though whether it actually functions as a GEF for Tem1 is not known [28], [29]. Activated Tem1 is thought to propagate a signal to the protein kinase Cdc15 [30], [31], [32], [33], [4], which in turn activates the protein kinase complex Dbf2 – Mob1 [35] [36], [37]. Spindle pole bodies (SPBs), the yeast equivalent of centrosomes, appear to be the signaling center of the MEN, with Nud1 functioning as a scaffold [38] – [43]. How localization of MEN components to SPBs is utilized to contribute to the integration of exit from mitosis with spindle position will be discussed below. How activation of the MEN ultimately leads to the release of Cdc14 from the nucleolus is still not understood. Presumably phosphorylation of Cdc14 and/or Cfi1/Net1 or as yet unknown factors by the MEN protein kinase Dbf2 brings about this event.
Figure 3. The FEAR network and the Mitotic Exit Network control Cdc14 localization.

The degradation of Pds1 and hence activation of Esp1 marks the onset of anaphase. Esp1 then not only cleaves cohesins to bring about sister chromatid segregation but together with Slk19 also promotes the down-regulation of the protein phosphatase PP2A. This allows Clb-CDKs to phosphorylate Cfi1/Net1 and Spo12, which brings about the dissociation of Cdc14 from its inhibitor. Phosphorylation of Cfi1/Net1 directly disrupts the Cdc14 – Cfi1/Net1 complex. Phosphorylation of Spo12 promotes the protein to inhibit Fob1, which inhibits Cdc14 – Cfi1/Net1 dissociation. Movement of the MEN-bearing SPB into the bud, where Lte1 is located, down-regulation of Bub2-Bfa1 activity by Cdc5, inactivation of Kin4 and other unknown signals promote MEN activation during anaphase. Tem1, presumably in its GTP bound form, then activates Cdc15, which activates Dbf2 in a Mob1-dependent manner. Dbf2-Mob1 promotes Cdc14 release from the nucleolus by an unknown mechanism.
…and FEAR
The discovery of the second signaling pathway that regulates Cdc14 was a great piece of detective work. When Rosella examined the localization of Cdc14 in MEN mutants progressing through the cell cycle in a synchronous manner she noticed that Cdc14 was briefly released from the nucleolus during early anaphase but was sequestered again when cells entered the late anaphase arrest. Having convinced ourselves that this transient release was not simply due to incomplete inactivation of the MEN in temperature sensitive MEN mutants we concluded that there must be a second pathway that controls Cdc14 localization during early anaphase [44]. Elmar Schiebel’s and Akio Toh-E’ groups came to the same conclusion [45], [29].
But what controlled Cdc14 localization during early anaphase? Insight into this question came from a publication by Orna Cohen-Fix in Doug Koshland’s lab and Rachel Tinker-Kulberg in David Morgan’s lab. They had previously shown that the key regulator of the metaphase – anaphase transition, Separase, a protease that triggers the separation of sister chromatids at the metaphase – anaphase transition (reviewed [46]) also regulates cyclin degradation and hence exit from mitosis [47], [48]. Frank Stegmeier, a graduate student in the lab, took Rosella’s observation and the result from the Koshland and Morgan labs and put them together. Could Separase (known as Esp1 in yeast) be required for releasing Cdc14 from the nucleolus during early anaphase in a MEN independent manner? The answer was yes. Frank had discovered another pathway controlling Cdc14 activity. He subsequently identified several other factors important for Cdc14 localization during early anaphase. Based on the observation that all these proteins regulated Cdc14 localization during early anaphase we collectively called them the FEAR network, short for Cdc Fourteen Early Anaphase Release network.
How does the FEAR network bring about Cdc14 release from the nucleolus? Work by Ray Deshaies and Frank Uhlmann provided insight into this question. CDK-dependent phosphorylation of Cfi1/Net1 on 6 CDK consensus sites brings about the transient release of Cdc14 from the nucleolus during early anaphase [49]. How are Clb-CDKs induced to phosphorylate the inhibitor? Interestingly, the FEAR network components Esp1 and Slk19 do not promote activation of the kinase but rather inactivate the protein phosphatase PP2A associated with the targeting subunit Cdc55 ([50], Figure 3). Esp1 and Slk19 also promote Spo12 phosphorylation by Clb-CDKs, which is essential for Cdc14 release during early anaphase (Brett Tomson, personal communications; [9]) (Figure 3).
The polo kinase Cdc5 is also required for the release of Cdc14 from the nucleolus during early anaphase. How Cdc5 functions in the FEAR network is not yet clear. Epistasis analyses place Cdc5 either downstream of Esp1-Slk19 or in parallel to the complex [51]. Furthermore, Cdc5 promotes phosphorylation of Cdc14 in vivo [52], [53], [51] and can directly bind to and phosphorylate the phosphatase in vitro ([52] – [54], R. Rahal and R. Visintin personal communications) suggesting that it functions in parallel to the Esp1-Slk19 branch to dissociate Cdc14 from its inhibitor. Identifying the Cdc5 phosphorylation sites in the Cdc14-Cfi1/Net1 complex and examining the consequences of mutating them to residues resistant to phosphorylation will be essential to elucidate Cdc5’s role in the MEN and the FEAR network.
Cellular events controlling Cdc14 activation
The discovery that two pathways control Cdc14 activity immediately raised the question as to which cellular events control Cdc14 and why cells employ two pathways to control the phosphatase. Two components of the FEAR network, the Separase Esp1 and the Polo kinase Cdc5, are also key regulators of sister chromatid separation. This dual employment certainly provides a means for the cell to ensure that exit from mitosis does not occur prior to the onset of sister-chromatid separation [44], [55] but it does not provide a mechanism for ensuring that sister chromatid separation occurs prior to exit from mitosis. Other regulatory signals such as the high-osmolarity MAP kinase pathway have also being implicated in regulation of FEAR network activity [56], but how these pathways help regulate Cdc14 activity through the FEAR network is not really understood and is an important question that we need to address in the future.
The function of the MEN in coordinating Cdc14 activation with other cellular events is better defined. Today we know that the MEN is employed to ensure that exit from mitosis only occurs when the nucleus is positioned correctly along the mother – bud axis. This connection was first made by my first graduate student Allison Bardin and at the same time by Gislene Pereira in Elmar Schiebel’s lab. Allison joined the lab in 1999 when it became clear that the MEN was an important regulator of Cdc14 activity. Given that components of the MEN resembled constituents of the RAS pathway, Allison wanted to identify potential signals controlling the MEN. Her approach was simple. She asked: Can we learn something about putative MEN signals by determining where in the cell MEN components that function at the top of the pathway are located? She found that Tem1 (and we now know all other MEN components) localized to the spindle pole body (SPB) that migrates into the bud during anaphase. The MEN activator Lte1 localizes to the bud cortex concomitant with bud formation [38], [40]. This localization pattern led us to hypothesize that MEN activation does not occur until the Tem1-bearing SPB migrates into the bud during anaphase [38], [40].
This hypothesis appeared to be correct. Allison as well as Schiebel and coworkers showed that restraining MEN activity was essential to prevent exit from mitosis in cells that failed to pull the nucleus into the bud during anaphase. In 1995, Kerry Bloom’s lab had shown that cells that fail to align the spindle correctly along the mother bud axis and hence elongate the spindle in the mother cell, arrest prior to cytokinesis until the spindle position defect was corrected [57]. He called the mechanisms responsible for this cell cycle delay the spindle position checkpoint. Allison and Schiebel’s lab showed that spindle mis-position restrained MEN activity, in part through sequestration of Lte1 in the bud and in part through regulating the activity of the Tem1 GAP, Bub2-Bfa1 (Figure 3). Several recent studies provided insight into how Bub2-Bfa1 activity is regulated by the spindle position checkpoint. The protein kinase Kin4 phosphorylates Bfa1. This precludes phosphorylation and hence inactivation of the GAP by Cdc5 [58]–[61], Figure 3).
Although the spindle position checkpoint restrains MEN activity when the spindle is not correctly aligned along the mother – bud axis, it is clear that during an unperturbed cell cycle this pathway alone is not solely responsible for controlling MEN activity. Identifying the pathways regulate MEN activity during an unperturbed cell cycle will be an important task in the future.
Turning off MEN and FEAR
The inactivation of Cdc14 after mitotic exit has been completed is as important for successful cell division as its activation during anaphase. Cells with unrestrained Cdc14 activity exhibit severe growth defects [16], [14], [17]. FEAR network activity appears to be restricted to a very brief time period during early anaphase, as Cdc14 becomes re-sequestered into the nucleolus during late anaphase in cells lacking a functional MEN [45], [44], [29]. How FEAR network activity is restricted to early anaphase is unknown. However how both the MEN and FEAR network are silenced once mitotic exit has been completed is understood. Cdc14 plants the seeds of its own inactivation. The protein phosphatase activates APC-Cdh1, which targets Cdc5 for degradation [54]. Other factors also contribute to the silencing of the MEN, such as dephopshorylation of Bfa1 and hence reactivation of the GAP [58], [45]. Lte1 activity may also be controlled by Cdc14 [28], [63], but Cdc5 degradation appears to be mainly responsible for silencing the machinery that promotes Cdc14 activation [54].
Cdc14 – more than just promoting CDK inactivation
A question that arises from the observation that at least two pathways control Cdc14 localization is why budding yeast utilizes two pathways to regulate Cdc14 rather than one. One possibility is that Cdc14 released by the FEAR network performs functions during mitosis that are different from that of Cdc14 released by the MEN. This appears to be the case. Cdc14 released by the FEAR network plays important roles in promoting MEN activation and in regulating chromosome segregation, mitotic spindle dynamics and chromosomal passenger proteins localization [64], [65], [66], [67], [68], [69], [70] (Figure 4). Cdc14 released by the MEN triggers CDK inactivation and hence exit from mitosis [16], [17]. How Cdc14 brings about these many different events is understood, at least in some instances in detail, and summarized in a review by D’Amours and Amon [71].
Figure 4. Cdc14 orchestrates anaphase events.
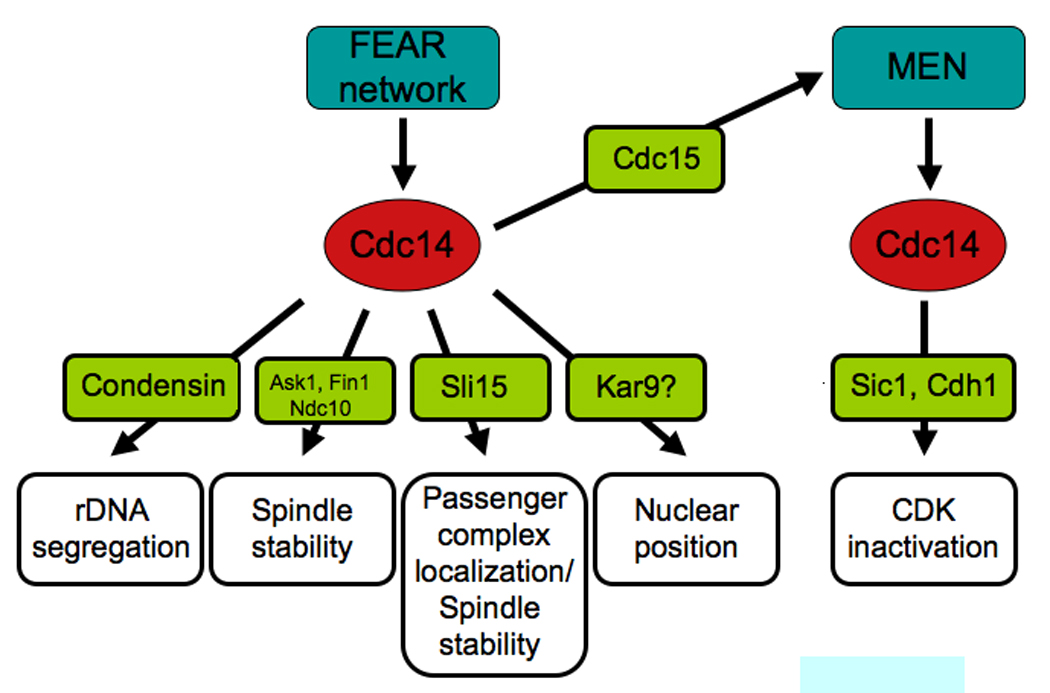
At anaphase onset, Cdc14 is activated by the FEAR network and controls many aspects of anaphase chromosome movement. The protein phosphatase promotes rDNA segregation by targeting condensins to the rDNA. It stabilizes the anaphase spindle by dephosphorylating kinetochore and spindle proteins such as Ask1, Ndc10, Fin1 and the chromosomal passenger complex. Cdc14 promotes the localization of the chromosomal passenger protein complex to the spindle midzone and controls nuclear position. Cdc14 also promotes MEN activity, which is necessary to maintain Cdc14 in the released active state during late stages of anaphase. Once activated the MEN further promotes Cdc14 activity. This sustained activation of Cdc14 then brings about exit from mitosis.
Here, rather than focusing on the details of Cdc14’s functions in chromosome segregation I want to ask the more fundamental question: Why are events necessary for the faithful segregation of chromosomes regulated by a transient FEAR network-dependent burst of Cdc14 activity, whereas exit from mitosis by a sustained level of MEN-dependent Cdc14 activity? I suspect that the gradual activation of Cdc14 is not unlike that of Clb-CDK activation earlier during mitosis. A recent study by Rami Rahal in my lab showed that entry into mitosis requires less Clb-CDK activity than progression through the metaphase – anaphase transition. This dependency of early mitotic events on increasing amounts of Clb-CDK activity may help establish the order of events during early mitosis [72]. Perhaps a gradual activation of Cdc14 and hence gradual decrease of Clb-CDK activity after entry into anaphase accomplishes a similar task. A low level transient activation of Cdc14 by the FEAR network helps orchestrate anaphase events by either locally inhibiting Clb-CDKs or dampening the effects of the kinases throughout the cell. Full activation of Cdc14 by the MEN eliminates Clb-CDKs and hence promotes exit from mitosis (Figure 5). Perhaps mitosis is all about fine-tuning mitotic CDK activity.
Figure 5. Different Clb-CDK and Cdc14 thresholds establish order in the progression through mitosis.

A certain amount of Clb-CDK activity is required for cells to enter mitosis (Threshold 1). The amount of Clb-CDK activity needed for entry into mitosis is not as high as that for anaphase initiation (Threshold 2). This is illustrated by the fact that inactivation of Clb1 – 4 leads to a G2 arrest. Inactivation of Clb2 and Clb1 or Clb2 and Clb3 results in a metaphase delay. The need for increasing amounts of Clb-CDK activity could help establish an order of events as cells progress from G2 into metaphase. Increasing levels of Cdc14 activity and hence decreasing levels of Clb-CDK activity could also help establish order to the progression from metaphase to G1. Once cells enter anaphase Clb-CDK activity needs to decline some for accurate anaphase chromosome movement (Threshold 3). Cdc14 released by the FEAR network brings about this event. For cells to exit from mitosis, Clb-CDK activity needs to be lowered even further (Threshold 4). Eventually all Clb-CDKs are inactivated resetting the cell cycle to a GAP phase state.
How do FEAR and MEN bring about the release of Cdc14 from the nucleolus – a model
So how does it all work? Once chromosomes have correctly aligned on the metaphase spindle, Securin is degraded leading to the activation of Separase (Figure 3). The protease then not only initiates anaphase chromosome movement but also, as a component of the FEAR network promotes the release of Cdc14 from the nucleolus. By down-regulating PP2A, Esp1 bound to Slk19 leads to high levels of Clb-CDK activity (perhaps especially in the nucleolus). Clb-CDKs in turn phosphorylate Cfi1/Net1 and Spo12. These Clb-CDK phosphorylation sites could then function as binding sites for Cdc5. Its phosphorylation of Cdc14 (and Cfi1/Net1) then promotes the release of Cdc14 from the nucleolus. The reverse could of course also be true. Cdc5 phosphorylation could be a prerequisite for Clb-CDKs to promote the dissociation of Cdc14 from its inhibitor. Spo12 phosphorylation could either aid in this process or through its interaction with Fob1 further destabilize the interaction between Cdc14 and its inhibitor. Once Cdc14 is released, the protein phosphatase orchestrates anaphase chromosome segregation and stimulates MEN activity thereby setting in motion exit from mitosis (Figure 4).
Exit from mitosis is triggered when the MEN is fully activated. FEAR network function, spindle position and perhaps other signals all converge on Tem1 to bring about activation of the MEN. In the face of declining Clb-CDK activity, brought about by APC/C-Cdc20 activity and a transient activation of Cdc14 that dephosphorylates Clb-CDK substrates, Dbf2-Mob1 could take over Clb-CDK function. Cdc14 then remains spread throughout the cell giving the phosphatase time to set in motion the events that lead to Clb-CDK inactivation.
What is left to do?
During the last decade we have made great strides towards understanding how Cdc14 controls anaphase progression and exit from mitosis and how the phosphatase is itself regulated. However, several important questions remain to be answered. How is the release of Cdc14 from the nucleolus regulated at the molecular level and how do the protein kinases implicated in the phosphatase’s regulation function together to bring about this event. In in vitro reconstitution experiments several kinases are able to disrupt the Cdc14-Cfi1/Net1 complex indicating that such reconstitution approaches are not likely to yield answers to this question. It will therefore be necessary to obtain a complete phosphorylation landscape of Cdc14, Cfi1/Net1 and perhaps other binding partners to fully understand how Cdc14 activity is controlled.
We are still lacking a thorough understanding of the signaling events in the FEAR network and the MEN. The MEN resembles a Ras-like signaling cascade, which is in many respects unusual. A thorough biochemical characterization, particularly of the GTPase and its regulators will be necessary to not only obtain a detailed understanding of the pathway but also to generate new tools to understand how the pathway is regulated in vivo. Is one essential pathway activating the MEN during anaphase or are many non-essential signals functioning as inputs to bring about activation of the pathway as the spindle pole moves into the bud during anaphase? Similarly, we are still lacking a thorough understanding of the FEAR network. How does Separase inhibit PP2A? How does the Spo12 – Fob1 branch of the pathway contribute to the disassembly of the inhibitory complex during anaphase and so forth.
How Cdc14 itself brings about the various anaphase events is understood in quite some detail, but several questions remain. Most pressing among them are Cdc14’s role in rDNA segregation and the relationship between Cdc14 and the MEN GAP complex Bub2-Bfa1. Furthermore, identifying additional functions of Cdc14 during anaphase will be an important task. The use of substrate trap alleles of Cdc14 in mass spectrometry or two-hybrid approaches will yield new putative substrates and roles for Cdc14. Finally, the most important question that remains to be addressed is whether or not Cdc14 is active in the nucleolus. Biochemical analyses indicate that Cfi1/Net1 is a competitive inhibitor with a high specific activity (Ki=3nM), which would argue that this is not the case [16], [18]. However, recent evidence indicates that Cfi/Net1 is not the only factor that binds Cdc14 in the nucleolus. Tof2 also binds Cdc14 and appears to be an activator rather than an inhibitor of Cdc14 [73]. It is thus possible that Cdc14 is also active in the nucleolus and there could regulate important aspects of rRNA biogenesis.
Finally, we must determine the broader significance of the findings in budding and fission yeast. Homologs of Cdc14 exist in mammals, with one isoform (Cdc14B) residing in the nucleolus during interphase but not during mitosis and one isoform (Cdc14A) located at centrosomes [74], [75], [76]. The mechanisms whereby Cdc14B is anchored in the nucleolus and Cdc14A at centrosomes are not understood. However, it appears that as in yeast, mammalian Cdc14 functions as antagonists of CDKs [74] and has been implicated in a broad range of cellular processes ranging from centrosome function to cytokinesis [75], [76]. It is however important to note that several of the described phenotypes of Cdc14-loss of function may be artifacts of the knock-down procedure. A recent analysis of human cells deleted for Cdc14B revealed no significant mitotic defects [77]. Thus, the two Cdc14 isoforms are either truly redundant (at least in a tissue culture setting) or Cdc14 is dispensable for cell proliferation in mammals. Clearly, a Cdc14A Cdc14B double knock-out will be needed to answer this question. Whether the signaling pathways that control Cdc14 in yeast exist in higher eukaryotes is also not clear. Some components of the FEAR network and the MEN exist but whether the same pathways control Cdc14 localization in higher eukaryotes is not known. Figuring this out will probably take another decade.
ACKNOWLEDGMENTS
I thank Brett Tomson, Rami Rahal and Rosella Visintin for communicating unpublished results. I am grateful to the members of the Amon lab, past and present for their hard work and dedication. I thank Jenny Cimino for help with the preparation of this manuscript and Leon Chan, Fernando Monje-Casas, Jeremy Rock, Frank Stegmeier and Rosella Visintin for comments on the manuscript. I would also like to apologize to the many colleagues whose work I could not discuss because of space constrains. Work in my lab was supported by a grant from the National Institutes of Health (GM056800). I am also an investigator of the Howard Hughes Medical Institute.
References
- 1.Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Bio. 2007;8(2):149–160. doi: 10.1038/nrm2105. Review. [DOI] [PubMed] [Google Scholar]
- 2.Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- 3.Surana U, Amon A, Dowzer C, Mcgrew J, Byers B, Nasmyth K. Destruction of the CDC28/CLB kinase is not required for metaphase/anaphase transition in yeast. EMBO J. 1993;12:1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irniger S, Piatti S, Michaelis C, Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995;81(2):269–278. doi: 10.1016/0092-8674(95)90337-2. Erratum in: Cell 93(3), 487. [DOI] [PubMed] [Google Scholar]
- 5.King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81(2):279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 6.Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca FC, Ruderman JV, Hershko A. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell. 1995;6(2):185–197. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amon A. Regulation of B-type cyclin proteolysis by Cdc28-associated kinases in budding yeast. EMBO J. 1997;16:2693–2702. doi: 10.1093/emboj/16.10.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell. 2002;9(5):931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 9.Stegmeier F, Huang J, Rahal R, Zmolik J, Moazed D, Amon A. The replication fork block protein Fob1 functions as a negative regulator of the FEAR network. Curr Biol. 2004;14:467–480. doi: 10.1016/j.cub.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Schwab M, Lutum AS, Seufert W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell. 1997;90(4):683–693. doi: 10.1016/s0092-8674(00)80529-2. [DOI] [PubMed] [Google Scholar]
- 11.Visintin R, Prinz S, Amon A. CDC20 and CDH1: A family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- 12.Zachariae W, Schwab M, Nasmyth K, Seufert W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science. 1998;282(5394):1721–1724. doi: 10.1126/science.282.5394.1721. [DOI] [PubMed] [Google Scholar]
- 13.Hartwell LH, Culotti J, Reid B. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc Natl Acad Sci U S A. 1970;66:352–359. doi: 10.1073/pnas.66.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visintin R, Craig K, Hwang ES, Prinz S, Tyers M, Amon A. The phosphataseCdc14 triggers mitotic exit by reversal of CDK-dependent phosphorylation. Mol Cell. 1998;2:709–718. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- 15.Jaspersen SL, Charles JF, Morgan DO. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr Biol. 1999;9:227–236. doi: 10.1016/s0960-9822(99)80111-0. [DOI] [PubMed] [Google Scholar]
- 16.Shou W, Seol JH, Shevchenko A, Baskerville C, Moazed D, Chen ZW, Jang J, Shevchenko A, Charbonneau H, Deshaies RJ. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell. 1999;97:233–244. doi: 10.1016/s0092-8674(00)80733-3. [DOI] [PubMed] [Google Scholar]
- 17.Visintin R, Hwang ES, Amon A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature. 1999;398:818–823. doi: 10.1038/19775. [DOI] [PubMed] [Google Scholar]
- 18.Traverso EE, Baskerville C, Liu Y, Shou W, James P, Deshaies RJ, Charbonneau H. Characterization of the Net1 cell cycle-dependent regulator of the Cdc14 phosphatase from budding yeast. J Biol Chem. 2001;276:21924–21931. doi: 10.1074/jbc.M011689200. [DOI] [PubMed] [Google Scholar]
- 19.Martindill DM, Risebro CA, Smart N, Franco-Viseras Mdel M, Rosario CO, Swallow CJ, Dennis JW, Riley PR. Nucleolar release of Hand1 acts as a molecular switch to determine cell fate. Nat Cell Biol. 2007;9(10):1131–1141. doi: 10.1038/ncb1633. [DOI] [PubMed] [Google Scholar]
- 20.San-Segundo PA, Roeder GS. Pch2 links chromatin silencing to meiotic checkpoint control. Cell. 1999;97(3):313–324. doi: 10.1016/s0092-8674(00)80741-2. [DOI] [PubMed] [Google Scholar]
- 21.Weber JD, Kuo ML, Bothner B, DiGiammarino EL, Kriwacki RW, Roussel MF, Sherr CJ. Cooperative signals governing ARF-mdm2 interaction and nucleolar localization of the complex. Mol Cell Biol. 2000;20(7):2517–2528. doi: 10.1128/mcb.20.7.2517-2528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaspersen SL, Charles JF, Tinker-Kulberg RL, Morgan DO. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:2803–2817. doi: 10.1091/mbc.9.10.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bardin AJ, Amon A. Men and sin: what's the difference? Nat. Rev. Mol. Cell Biol. 2001;11:815–826. doi: 10.1038/35099020. [DOI] [PubMed] [Google Scholar]
- 24.Krapp A, Gulli MP, Simanis V. SIN and the art of splitting the fission yeast cell. Curr Biol. 2004;14(17):R722–R730. doi: 10.1016/j.cub.2004.08.049. Review. [DOI] [PubMed] [Google Scholar]
- 25.McCollum D. Cytokinesis: breaking the ties that bind. Curr Biol. 2005;15(24):R998–R1000. doi: 10.1016/j.cub.2005.11.049. Review. [DOI] [PubMed] [Google Scholar]
- 26.McCollum D, Gould KL. Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 2001;11(2):89–95. doi: 10.1016/s0962-8924(00)01901-2. Review. [DOI] [PubMed] [Google Scholar]
- 27.Stegmeier F, Amon A. Regulation of Mitotic Exit. Ann Rev Gen. 2004;38:203–232. doi: 10.1146/annurev.genet.38.072902.093051. [DOI] [PubMed] [Google Scholar]
- 28.Jensen S, Geymonat M, Johnson AL, Segal M, Johnston LH. Spatial regulation of the guanine nucleotide exchange factor Lte1 in Saccharomyces cerevisiae. J Cell Sci. 2002;115:4977–4991. doi: 10.1242/jcs.00189. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S, Asakawa K, Toh-e A. Mitotic exit network controls the localization of Cdc14 to the spindle pole body in Saccharomyces cerevisiae. Curr Biol. 2002;12:944–950. doi: 10.1016/s0960-9822(02)00870-9. [DOI] [PubMed] [Google Scholar]
- 30.Asakawa K, Yoshida S, Otake F, Toh-e A. A novel functional domain of Cdc15 kinase is required for its interaction with Tem1 GTPase in Saccharomyces cerevisiae. Genetics. 2001;157:1437–1450. doi: 10.1093/genetics/157.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bardin AJ, Boselli MG, Amon A. Mitotic exit regulation through distinct domains within the protein kinase Cdc15. MCB. 2003;23:5018–5030. doi: 10.1128/MCB.23.14.5018-5030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SE, Jensen S, Frenz LM, Johnson AL, Fesquet D, Johnston LH. The Bub2-dependent mitotic pathway in yeast acts every cell cycle and regulates cytokinesis. Journal of Cell Science. 2001;114:2345–2354. doi: 10.1242/jcs.114.12.2345. [DOI] [PubMed] [Google Scholar]
- 33.Menssen R, Neutzner A, Seufert W. Asymmetric spindle pole localization of yeast Cdc15 kinase links mitotic exit and cytokinesis. Curr Biol. 2001;11:345–350. doi: 10.1016/s0960-9822(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 34.Visintin R, Amon A. Regulation of the mitotic exit protein kinases Cdc15 and Dbf2. Mol Biol Cell. 2001;12:2961–2974. doi: 10.1091/mbc.12.10.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mah AS, Jang J, Deshaies RJ. Protein kinase Cdc15 activates the Dbf2-Mob1 kinase complex. Proc Natl Acad Sci U S A. 2001;98:7325–7330. doi: 10.1073/pnas.141098998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komarnitsky SI, Chiang YC, Luca FC, Chen J, Toyn JH, Winey M, Johnston LH, Denis CL. DBF2 protein kinase binds to and acts through the cell cycle-regulated MOB1 protein. Mol Cell Biol. 1998;18:2100–2107. doi: 10.1128/mcb.18.4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luca FC, Winey M. MOB1, an essential yeast gene required for completion of mitosis and maintenance of ploidy. Mol Biol Cell. 1998;9:29–46. doi: 10.1091/mbc.9.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bardin AJ, Visintin R, Amon A. A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell. 2000;102:21–31. doi: 10.1016/s0092-8674(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 39.Gruneberg U, Campbell K, Simpson C, Grindlay J, Schiebel E. Nud1p links astral microtubule organization and the control of exit from mitosis. Embo J. 2000;19:6475–6488. doi: 10.1093/emboj/19.23.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereira G, Hofken T, Grindlay J, Manson C, Schiebel E. The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol Cell. 2000;6:1–10. [PubMed] [Google Scholar]
- 41.Xu S, Huang HK, Kaiser P, Latterich M, Hunter T. Phosphorylation and spindle pole body localization of the Cdc15p mitotic regulatory protein kinase in budding yeast. Curr Biol. 2000;10:329–332. doi: 10.1016/s0960-9822(00)00382-1. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida S, Toh-e A. Regulation of the localization of Dbf2 and mob1 during cell division of saccharomyces cerevisiae. Genes Genet Syst. 2001;76:141–147. doi: 10.1266/ggs.76.141. [DOI] [PubMed] [Google Scholar]
- 43.Pereira G, Tanaka TU, Nasmyth K, Schiebel E. Modes of spindle pole body inheritance and segregation of the Bfa1p-Bub2p checkpoint protein complex. Embo J. 2001;20:6359–6370. doi: 10.1093/emboj/20.22.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stegmeier F, Visintin R, Amon A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell. 2002;108:207–220. doi: 10.1016/s0092-8674(02)00618-9. [DOI] [PubMed] [Google Scholar]
- 45.Pereira G, Manson C, Grindlay J, Schiebel E. Regulation of the Bfa1p-Bub2p complex at spindle pole bodies by the cell cycle phosphatase Cdc14p. J Cell Biol. 2002;157:367–379. doi: 10.1083/jcb.200112085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nasmyth K. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu Rev Genet. 2001;35:673–745. doi: 10.1146/annurev.genet.35.102401.091334. Review. [DOI] [PubMed] [Google Scholar]
- 47.Cohen-Fix O, Koshland D. Pds1p of budding yeast has dual roles: inhibition of anaphase initiation and regulation of mitotic exit. Genes Dev. 1999;13(15):1950–1959. doi: 10.1101/gad.13.15.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tinker-Kulberg RL, Morgan DO. Pds1 and Esp1 control both anaphase and mitotic exit in normal cells and after DNA damage. Genes Dev. 1999;13(15):1936–1949. doi: 10.1101/gad.13.15.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azzam R, Chen SL, Shou W, Mah AS, Alexandru G, Nasmyth K, Annan RS, Carr SA, Deshaies RJ. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science. 2004;305(5683):516–519. doi: 10.1126/science.1099402. [DOI] [PubMed] [Google Scholar]
- 50.Queralt E, Lehane C, Novak B, Uhlmann F. Downregulation of PP2A(Cdc55) phosphatase by separase initiates mitotic exit in budding yeast. Cell. 2006;125(4):719–732. doi: 10.1016/j.cell.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 51.Visintin R, Stegmeier F, Amon A. The role of the polo kinase Cdc5 in controlling Cdc14 localization. Mol Biol Cell. 2003;14:4486–4498. doi: 10.1091/mbc.E03-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shou W, Azzam R, Chen SL, Huddleston MJ, Baskerville C, Charbonneau H, Annan RS, Carr SA, Deshaies RJ. Cdc5 influences phosphorylation of Net1 and disassembly of the RENT complex. BMC Mol Biol. 2002;3:3. doi: 10.1186/1471-2199-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshida S, Toh-e A. Budding yeast Cdc5 phosphorylates Net1 and assists Cdc14 release from the nucleolus. Biochem Biophys Res Commun. 2002;294:687–691. doi: 10.1016/S0006-291X(02)00544-2. [DOI] [PubMed] [Google Scholar]
- 54.Visintin C, Tomson BN, Rahal R, Paulson J, Cohen M, Taunton J, Amon A, Visintin R. APC/C-Cdh1-mediated degradation of the Polo kinase Cdc5 promotes the return of Cdc14 into the nucleolus. Genes Dev. 2008;22:79–90. doi: 10.1101/gad.1601308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sullivan M, Uhlmann F. A non-proteolytic function of separase links the onset of anaphase to mitotic exit. Nat Cell Biol. 2003;5:249–254. doi: 10.1038/ncb940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reiser V, D'Aquino KE, Ee LS, Amon A. The Stress-activated MAP Kinase Signaling Cascade Promotes Exit from Mitosis. Mol Biol Cell. 2006;17:3136–3146. doi: 10.1091/mbc.E05-12-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeh E, Skibbens RV, Cheng JW, Salmon ED, Bloom K. Spindle dynamics and cell cycle regulation of dynein in the budding yeast, Saccharomyces cerevisiae. J Cell Biol. 1995;130(3):687–700. doi: 10.1083/jcb.130.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu F, Wang Y, Liu D, Li Y, Qin J, Elledge SJ. Regulation of the Bub2/Bfa1 GAP complex by Cdc5 and cell cycle checkpoints. Cell. 2001;107:655–665. doi: 10.1016/s0092-8674(01)00580-3. [DOI] [PubMed] [Google Scholar]
- 59.D’Aquino KE, Monje-Casas F, Paulson J, Reiser V, Charles GM, Lai L, Shokat KM, Amon A. The protein kinase Kin4 inhibits exit from mitosis in response to spindle position defects. Mol Cell. 2005;19:223–234. doi: 10.1016/j.molcel.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Pereira G, Schiebel E. Kin4 kinase delays mitotic exit in response to spindle alignment defects. Mol Cell. 2005;19(2):209–221. doi: 10.1016/j.molcel.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 61.Maekawa H, Priest C, Lechner J, Pereira G, Schiebel E. The yeast centrosome translates the positional information of the anaphase spindle into a cell cycle signal. J Cell Biol. 2007;179(3):423–436. doi: 10.1083/jcb.200705197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaspersen SL, Morgan DO. Cdc14 activates cdc15 to promote mitotic exit in budding yeast. Curr Biol. 2000;10:615–618. doi: 10.1016/s0960-9822(00)00491-7. [DOI] [PubMed] [Google Scholar]
- 63.Seshan A, Bardin AJ, Amon A. Control of Lte1 localization by cell polarity determinants and Cdc14. Curr Biol. 2002;12:2098–2110. doi: 10.1016/s0960-9822(02)01388-x. [DOI] [PubMed] [Google Scholar]
- 64.Pereira G, Schiebel E. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science. 2003;302(5653):2120–2124. doi: 10.1126/science.1091936. [DOI] [PubMed] [Google Scholar]
- 65.D’Amours D, Stegmeier F, Amon A. Cdc14 and condensin control the dissolution of cohesin-independent chromosome linkages at repeated DNA. Cell. 2004;117:455–469. doi: 10.1016/s0092-8674(04)00413-1. [DOI] [PubMed] [Google Scholar]
- 66.Ross KE, Cohen-Fix O. A role for the FEAR pathway in nuclear positioning during anaphase. Dev Cell. 2004;6(5):729–735. doi: 10.1016/s1534-5807(04)00128-5. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan M, Higuchi T, Katis VL, Uhlmann F. Cdc14 phosphatase induces rDNA condensation and resolves cohesin-independent cohesion during budding yeast anaphase. Cell. 2004;117(4):471–482. doi: 10.1016/s0092-8674(04)00415-5. [DOI] [PubMed] [Google Scholar]
- 68.Higuchi T, Uhlmann F. Stabilization of microtubule dynamics at anaphase onset promotes chromosome segregation. Nature. 2005;433(7022):171–176. doi: 10.1038/nature03240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woodbury EL, Morgan DO. Cdk and APC activities limit the spindle-stabilizing function of Fin1 to anaphase. Nat Cell Biol. 2007;9(1):106–112. doi: 10.1038/ncb1523. [DOI] [PubMed] [Google Scholar]
- 70.Widlund PO, Lyssand JS, Anderson S, Niessen S, Yates JR, 3rd, Davis TN. Phosphorylation of the chromosomal passenger protein Bir1 is required for localization of Ndc10 to the spindle during anaphase and full spindle elongation. Mol Biol Cell. 2006;17(3):1065–1074. doi: 10.1091/mbc.E05-07-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D'Amours D, Amon A. At the interface between signaling and executing anaphase--Cdc14 and the FEAR network. Genes Dev. 2004;18(21):2581–2595. doi: 10.1101/gad.1247304. Review. [DOI] [PubMed] [Google Scholar]
- 72.Rahal R, Amon A. Mitotic CDKs control the metaphase-anaphase transition and trigger spindle elongation. Genes Dev. 2008;22:1534–1548. doi: 10.1101/gad.1638308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geil C, Schwab M, Seufert W. A Nucleolus-Localized Activator of Cdc14 Phosphatase Supports rDNA Segregation in Yeast Mitosis. Curr Biol. 2008;18(13):1001–1005. doi: 10.1016/j.cub.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 74.Bembenek J, Yu H. Regulation of the anaphase-promoting complex by the dual specificity phosphatase human Cdc14a. J Biol Chem. 2001;276:48237–48242. doi: 10.1074/jbc.M108126200. [DOI] [PubMed] [Google Scholar]
- 75.Kaiser BK, Zimmerman ZA, Charbonneau H, Jackson PK. Disruption of centrosome structure, chromosome segregation, and cytokinesis by misexpression of human Cdc14A phosphatase. Mol Biol Cell. 2002;13:2289–2300. doi: 10.1091/mbc.01-11-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mailand N, Lukas C, Kaiser BK, Jackson PK, Bartek J, Lukas J. Deregulated human Cdc14A phosphatase disrupts centrosome separation and chromosome segregation. Nat Cell Biol. 2002;4:317–322. doi: 10.1038/ncb777. [DOI] [PubMed] [Google Scholar]
- 77.Berdougo E, Nachury MV, Jackson PK, Jallepalli PV. The nucleolar phosphatase Cdc14B is dispensable for chromosome segregation and mitotic exit in human cells. Cell Cycle. 2008;7(9):1184–1190. doi: 10.4161/cc.7.9.5792. [DOI] [PubMed] [Google Scholar]