Abstract
A number of transcriptional pathways regulating fetal lung development are active during repair of the injured lung. We hypothesized that C/EBPα, a transcription factor critical for lung maturation, plays a role in protection of the alveolar epithelium following hyperoxic injury of the mature lung. Transgenic CebpαΔ/Δ mice, in which Cebpα was conditionally deleted from Clara cells and type II cells after birth, were developed. While no pulmonary abnormalities were observed in the CebpαΔ/Δ mice (7–8 wk old) under normal conditions, the mice were highly susceptible to hyperoxia. CebpαΔ/Δ mice died within 4 days of exposure to 95% oxygen in association with severe lung inflammation, altered maturation of surfactant protein B and C, decreased surfactant lipid secretion, and abnormal lung mechanics at a time when all control mice survived. mRNA microarray analysis of isolated type II cells at 0, 2, and 24 h of hyperoxia demonstrated the reduced expression of number of genes regulating surfactant lipid and protein homeostasis, including Srebf, Scap, Lpcat1, Abca3, Sftpb, and Napsa. Genes influencing cell signaling or immune responses were induced in the lungs of CebpαΔ/Δ mice. C/EBPα was required for the regulation of genes associated with surfactant lipid homeostasis, surfactant protein biosynthesis, processing and transport, defense response to stress, and cell redox homeostasis during exposure to hyperoxia. While C/EBPα did not play a critical role in postnatal pulmonary function under normal conditions, C/EBPα mediated protection of the lung during acute lung injury induced by hyperoxia.
Keywords: Vnn1, GSH, microarray, Napsa, surfactant
many of the transcriptional programs involved in establishing the structure and differentiation of the lung during in utero development are also expressed in the postnatal lung and are activated during lung injury and repair. CCAAT enhancer binding protein α (C/EBPα) is a member of a family of basic leucine zippers that serves an important role in normal organ development in the fetus. In the mouse lung, Cebpα is expressed in alveolar type II cells, alveolar macrophages, and airway epithelial cells from embryonic day (E) 15.5 (3). We have recently demonstrated that lung-specific, conditionally targeted deletion of Cebpα using SP-C-rtTA system in the developing fetal mouse lung caused death soon after birth from respiratory distress related to failed lung maturation. C/EBPα, FOXA2, and TTF1 share transcriptional targets crucial for lung development and surfactant synthesis, and the normal expression of Cebpα was dependent on both FOXA2 and TTF1 (35).
C/EBPα plays an important role in regulation of cell proliferation, cell differentiation, and lipid biosynthesis in most tissues (10, 31, 57). While diverse roles of C/EBPα have been identified in other mature organs and in tumorigenesis in vivo (27, 52), its role in the mature lung is unknown. We hypothesize that C/EBPα plays an important role in lung protection from respiratory epithelial cell injury in the mature lung. In the present study, we induced lung injury by exposing mice to hyperoxia. The use of high concentrations of oxygen, necessary for survival of some patients with pulmonary disease, contributes to acute and chronic lung injury. Because the entire surface area of lung epithelium is directly exposed to oxygen, hyperoxia causes epithelial and endothelial cell damage. Hyperoxia-induced lung injury is a complex process associated with changes in expression of a number of genes mediating cytoprotection. Lung epithelial cell damage occurs within 24 h of oxygen exposure, before the onset of morphological injury. During this phase, intracellular metabolism of oxygen is increased, and reactive oxygen and nitrogen species are generated. Increases in reactive oxygen species induce rapid changes in endothelial and epithelial cell metabolism. Crosslinking of membrane proteins, peroxidation of lipids, inhibition of cellular phosphatase, DNA fragmentation, and influx of inflammatory cells into the air space then occurs (45). The transcriptional targets of C/EBPα that mediate protection during hyperoxia are currently unknown.
Of the surfactant proteins, SP-B is singularly required for maintenance of lung function and survival. In the normal lung, SP-B protein and SP-B mRNA increased or did not change during short-term exposure to hyperoxia. Susceptibility to hyperoxic lung injury is associated with the levels of SP-B in an experimental model. Adult heterozygous SP-B+/− mice, in which SP-B mRNA and protein were reduced by 50%, were susceptible to hyperoxia lung injury (14). Lung injury associated with surfactant deficiency remains a major cause of pulmonary morbidity and mortality in patients, particularly with decreased SP-B. SP-B concentrations in bronchoalveolar lavage fluid (BALF) from patients with acute lung injury ranged from 25 to 50% of normal (19, 47). From a clinical perspective, activation of cellular pathways maintaining physiological surfactant levels in the alveolus during acute lung injury is critical for protection of lung function.
In the present study, transgenic mice in which the Cebpα was conditionally deleted in the lung were developed using CCSP-rtTA system (herein called CebpαΔ/Δ mice), thereby blocking Cebpα expression in respiratory epithelial cell lining in conducting airways and in a subset of type II alveolar cells. CebpαΔ/Δ mice grow normally without any pulmonary dysfunction, suggesting that C/EBPα does not play a critical role in pulmonary homeostasis in the postnatal lung under normal conditions. In contrast, CebpαΔ/Δ mice were highly susceptible to hyperoxia, with resultant severe pulmonary inflammation and high mortality. In the present study, the role and mechanisms by which C/EBPα influences pulmonary cytoprotection and surfactant homeostasis during hyperoxia injury are identified.
MATERIALS AND METHODS
Transgenic mice.
Cebpαflox/flox mice were kindly provided by Dr. P. Johnson (National Cancer Institute, Frederick, MD) (30) and were then maintained in a mixed C57/BL6 and FVB/N background. Recombination of loxP sites was accomplished by expressing Cre recombinase using (tetO)7 CMV-Cretg/tg activator mice, and recombination of the floxed allele in the presence of doxycycline was achieved using CCSP-rtTA−/tg (line 1) activator mice (46). Triple transgenic mice were generated by mating CCSP-rtTA−/tg, Cebpαflox/flox to (tetO)7 CMV-Cretg/tg, Cebpαflox/flox. PCR was performed on genomic DNA from the tail using primers previously reported (35, 46). All mice were studied at ages 7–8 wk under protocols approved by the Institutional Animal Care and Use Committee at Cincinnati Children's Hospital Research Foundation. Mice were kept in a pathogen-free environment until oxygen exposure.
Histology and immunohistochemistry.
Mice were killed by an intraperitoneal injection of pentobarbital (100 mg/kg) followed by exsanguination. Lungs were inflated with 4% paraformaldehyde in PBS through the trachea at 25 cmH2O for fixation and immersed in the same fixative. Tissue was fixed overnight, washed with PBS, dehydrated in a series of alcohols, and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin for histology. Immunohistochemistry for C/EBPα was performed using C/EBPα rabbit polyclonal IgG 1:5,000 (Santa Cruz Biotechnology, Santa Cruz, CA) and for SP-B proprotein was performed using rabbit polyclonal antibody (Seven Hills Bioreagents, Cincinnati, OH) (1:1,500). Electron microscopy was performed on lung tissue from CebpαΔ/Δ and littermate control mice after fixation in glutaraldehyde as previously described (35).
Validation of mRNAs and proteins.
Quantitative RT-PCR was analyzed using TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA). All probes, including the probe for β-actin as the endogenous control, were selected from the list of Applied Biosystems. Twenty-five nanograms of input cDNA were used for each lung tissue or type II cell sample. Changes in C/EBPα protein by Western blot were determined in type II cells isolated from CebpαΔ/Δ and control mice using collagenase (53). Oxidative stress-related genes were analyzed on type II cells isolated from CebpαΔ/Δ and control mice after exposure to hyperoxia for 69 h using commercially available targeted cDNA array of 84 oxidative stress genes (oxidative stress PCR array; SuperArray Bioscience, Frederick, MD) according to the manufacturer's instructions.
Hyperoxia, lung mechanics, BALF, and tissue analysis.
CebpαΔ/Δ and littermate control mice were exposed either to 95% O2 or to room air up to 69 h, the timing based on previous observations regarding the duration of oxygen exposure inducing respiratory distress in 95% O2 (24, 58). Lung mechanics were studied in tracheostomized mice under anesthesia by intraperitoneal injection of 0.1 ml/10 g body wt of a mixture containing 40 mg/ml ketamine and 2 mg/ml xylazine with computerized FlexiVent system (SCIREQ Scientific Respiratory Equipment, Montreal, Quebec, Canada) as described previously (22, 24).
Mice were given pentobarbital sodium intraperitoneally and killed by exsanguination. Five 1-ml aliquots of 0.9% NaCl were flushed into the lungs, withdrawn by syringe three times for each aliquot, and pooled. Total volume of BALF was recorded, which was similar between groups. The lavaged lung tissue was removed and homogenized in 2 ml of 0.9% NaCl. Total protein in aliquots of BALF from each mouse was measured by the methods of Lowry et al. (33). Lung homogenates were centrifuged at 1,500 g, and IL-1β, IL-6, and macrophage inflammatory protein-2 (MIP-2) were quantitated in supernatant and BALF using quantitative murine sandwich ELISA kits (R&D Systems, Minneapolis, MN). The content of SP-A, SP-B, pro-SP-B, SP-C, and SP-D, in the same volume of BALF for each surfactant protein, was determined by Western blot analysis. Separation was carried out under nonreducing conditions for SP-B and under reducing conditions for SP-A, SP-C, and SP-D as described previously (24, 35). Protein bands were quantitated by densitometric analyses with Alpha Imager 2000 documentation and ImageQuant analyses software. Data were expressed relative to control mice in air, which was given a value of 1.
Aliquots of BALF and lung tissue homogenate were extracted with chloroform/methanol (2:1), and saturated phosphatidylcholine (Sat PC) was isolated with osmium tetroxide (37), followed by measurement of phosphorus (2, 23). By intraperitoneal injection, CebpαΔ/Δ and control mice in air and after 24-h exposure to hyperoxia (n = 5/group) were given 10 μl/g body wt containing 0.7 μCi [3H]palmitic acid that was stabilized in 0.9% NaCl with 5% human serum albumin (25). Mice were continuously exposed to hyperoxia for hyperoxia group, or air for comparison group, and killed at 8 h after radiolabeled surfactant phospholipid precursor injection. BALF was recovered from each animal, and then lung tissue was homogenized in saline. Sat PC was isolated from the BALF and lung homogenate as described above. Radioactivity in isolated Sat PC from BALF and lung homogenate after BAL was measured to study the incorporation of radiolabeled surfactant precursor into Sat PC. The percent secretion of radiolabeled Sat PC was calculated as the percentage of radioactivity in BALF Sat PC relative to the radioactivity in total lung Sat PC.
Statistics.
Results for the above studies were expressed as means ± SE. Statistical differences were determined using unpaired Student's t-test. Comparisons among groups were assessed by ANOVA with Tukey's test used for post hoc analyses. Differences were considered significant at the 5% level.
RNA microarray analysis.
Methods for RNA isolation and data analysis were as described previously (62). CebpαΔ/Δ and control mice were exposed to hyperoxia for 0 h (baseline), 2 h, and 24 h. Short hyperoxia exposure was chosen for RNA microarray analysis to detect direct effect and early response genes. Type II cells were then isolated from one male and one female mouse and combined as one pooled sample. mRNA was extracted from three independent pools of isolated type II cells at each time point using RNeasy Mini Kit (Qiagen, Valencia, CA). The cRNA was then hybridized to the Mouse Gene 1.0 ST Array (Affymetrix, Santa Clara, CA) according to the manufacturer's protocol. The RNA quality and quantity assessment, probe preparation, labeling, hybridization, and image scan were carried out in the Cincinnati Children's Hospital Medical Center Affymetrix Core using standard procedure. Data were further analyzed using GeneSpring GX 9.0 (Agilent Technologies, Santa Clara, CA). Differentially expressed genes in CebpαΔ/Δ and control mice at duration of hyperoxia were identified using Student's t-test with the threshold of P ≤0.01 and fold change ≥1.5. The minimal expression level for the probe sets was 35–100 percentiles for at least 66% of samples to remove probes biased heavily by background.
Cluster discovery and gene ontology analysis.
Differentially expressed genes in air and after 2- and 24-h exposure to 95% O2 were combined and subjected to a principal components analysis, which captured the structure variance using fewer variables and revealed the most dominant patterns in the data set (i.e., Eigen vectors of the similarity matrix of the entities) and grouped each entity to the most correlated patterns. All mRNAs associated with the same principal component comprised a cluster. The changes in expression profiling patterns with exposure time and genotypes were dissected through the clustering analysis. Genes from distinct clusters were then subject to gene ontology analysis. Ontology categories were considered significant when a Fisher's exact test was P ≤ 0.01 and gene hits ≥10.
Pathway analysis and network construction.
Overrepresented functions, pathways, as well as potential protein/protein or protein/DNA interactions, were identified using Ingenuity Pathway Analysis (IPA; Ingenuity Systems, Redwood City, CA). IPA software mapped the differentially expressed genes identified from the microarray experiment onto the interactome according to Ingenuity Pathway Knowledge Base, which is the largest curated database from published literature on mammalian biology. Relationships between genes, proteins, small molecules, complexes, cells, processes, and diseases were manually extracted and curated from >200,000 peer-reviewed papers. Genetic networks preferentially enriched of input genes were generated based on their connectivity. Statistical scores were calculated to rank the resulting networks and pathways using Fisher's right-tailed exact test. Customized gene networks were constructed by merging and expending the top ranked networks focusing on elucidating the cytoprotection role and transcriptional mechanism of C/EBPα in mature lung under hyperoxia stress.
RESULTS
Deletion of Cebpα in CebpαΔ/Δ mice lung.
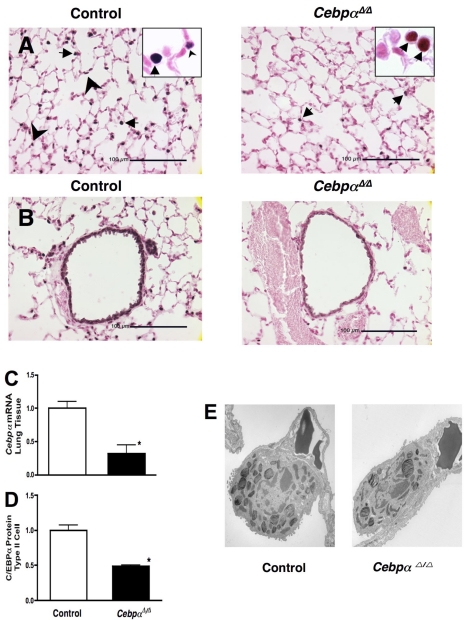
The rtTA system was used for control of Cebpα expression in vivo (18, 28, 32). CCSP was used as a promoter and CCSPrtTAtg/−, (tetO)7 CMV-Cretg/−, Cebpαflox/flox mice (CebpαΔ/Δ mice) were generated in which the Cebpα was permanently deleted from intrapulmonary respiratory epithelial cells upon administration of doxycycline in the chow to the dam from E0 and continued doxycycline treatment to postnatal day 14. Continuous treatment of the dams with doxycycline in chow provided enough doxycycline to the newborns through the milk (46). CCSP is expressed in Clara cells from approximately E16-E17 gestation. The CCSP-rtTA transgene is also active in a subset of type II cells after birth (41, 63). Thus, in this CCSP-rtTA system for CebpαΔ/Δ mice, deletion of Cebpα occurred in Clara cells from E16-E17 gestation and in subsets of type II cells after birth. In contrast to SP-C-rtTA CebpαΔ/Δ mice that died at birth from pulmonary immaturity and respiratory failure (35), in the present study Cebpα was not deleted from type II epithelial cells in the fetus, and lung development was normal in CebpαΔ/Δ mice. CebpαΔ/Δ mice survived, grew normally, and had a normal life span under normal conditions. Cebpαflox/flox littermates lacking either rtTA or Cre genes served as controls, and there were no genotype-related differences between these two controls. In control mice, immunohistochemical staining for Cebpα was detected primarily in type II epithelial cells (Fig. 1A, arrowheads), airway epithelial cells (Fig. 1B), and alveolar macrophages (Fig. 1A, arrows). Substantial loss of C/EBPα staining was observed in type II cells and airway epithelial cells in adult 8-wk-old CebpαΔ/Δ mice. As expected from the specificity of the CCSP promoter, C/EBPα was similarly immunostained in alveolar macrophages (Fig. 1A, arrows) in CebpαΔ/Δ and control mice. Therefore, microarray and RT-PCR array were analyzed on isolated type II cells rather than lung tissue. In CebpαΔ/Δ mice, Cebpα mRNA expression in lung tissue was significantly decreased (Fig. 1C). Likewise, C/EBPα protein in isolated type II cells was significantly decreased to one-half in CebpαΔ/Δ mice (Fig. 1D). The purity of the type II cell preparation from mice was typically >90%, as assessed by modified papanicolaou stain and immunostaining for pro-SP-C. The majority of microscopically identified contaminating cells in the type II cell preparation was alveolar macrophages (53) in which C/EBPα was highly expressed. CebpαΔ/Δ mice lungs were normal under normal conditions, and no apparent abnormalities were expressed in light and electron photomicrographs of the lung structure (Fig. 2B) including type II cells (Fig. 1E).
Fig. 1.
Decreased C/EBPα expression in adult CebpαΔ/Δ mice in type II cells (A) and conducting airway epithelial cells (B). Dams of CebpαΔ/Δ mice were maintained on doxycycline (in chow) from embryonic day 0. CebpαΔ/Δ mice survived postnatally and were treated with doxycycline until 14 days of age. In littermate control mice, C/EBPα was detected in alveolar type II cells (arrowheads) and conducting airways, whereas nuclear staining of respiratory epithelial cells was absent or markedly decreased in CebpαΔ/Δ mice assessed at 7 wk of age. Immunohistochemical staining for C/EBPα was detected in alveolar macrophages (arrows) in both control and CebpαΔ/Δ mice, indicating the specificity of gene deletion in the respiratory epithelium. There were no changes in lung morphology in CebpαΔ/Δ mice under normal conditions. C: decreased expression of Cebpα mRNA in 7-wk-old CebpαΔ/Δ mice lungs. Cebpα mRNA in lung was decreased in CebpαΔ/Δ mice compared with that in control mice analyzed by RT-PCR (n = 3/group). D: decreased C/EBPα protein (by Western blot) in isolated type II cells. The majority of microscopically identified contaminating cells in isolated type II cells was alveolar macrophages in which C/EBPα was detected in CebpαΔ/Δ mice by immunohistochemistry (A). In this system, nearly 70% of Cebpα gene deletion occurs in respiratory epithelial cells from CebpαΔ/Δ mice. Type II cells isolated from 1 male and 1 female mouse were pooled. N = 3 pool/group, *P < 0.01 vs. control. E: electron microscopy was performed on lungs from control and CebpαΔ/Δ mice in room air at 7 wk of age. The ultrastructure of the lung of CebpαΔ/Δ mice, including type II cells, is similar to that of control mice. Shown are representative electron microphotographs of n = 3 mice/group.
Fig. 2.
A: Kaplan-Meier plot of survival of CebpαΔ/Δ and control mice in hyperoxia. Seven-week-old CebpαΔ/Δ mice and littermate control mice were exposed to 95% O2. Survival of CebpαΔ/Δ mice in hyperoxia (n = 12) was significantly decreased compared with controls (n = 11). P < 0.01 by log-rank test. B: lung morphology (hematoxylin and eosin staining) in room air and after 69-h exposure to 95% O2. Deletion of Cebpα did not influence lung morphology and was similar between control and CebpαΔ/Δ mice in room air (AIR). After exposure to hyperoxia (O2), control mice showed only minor histological changes (perivascular edema). More severe lung inflammation was observed in CebpαΔ/Δ mice in hyperoxia, including thickened alveoli, epithelial necrosis, and air space enlargement. Photomicrographs are representative of n = 4/group. C: increased protein from bronchoalveolar lavage fluid (BALF) in CebpαΔ/Δ mice exposed to hyperoxia. Total protein contents were similarly low in control and CebpαΔ/Δ mice in room air. After oxygen exposure, protein was significantly increased in the BALF from CebpαΔ/Δ mice, suggesting increased alveolar capillary leak. N = 4/group.
CebpαΔ/Δ mice were susceptible to oxygen-induced injury.
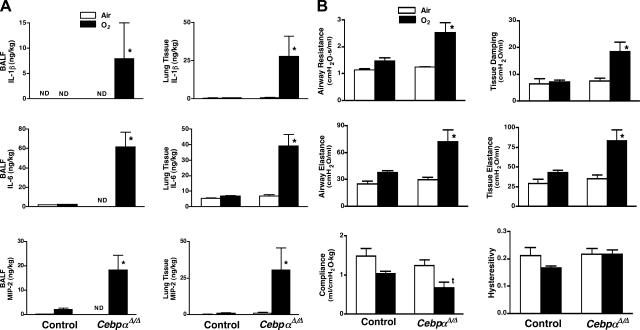
Seven-week-old CebpαΔ/Δ mice and littermate control mice were placed in 95% O2. Since the entire surface area of lung epithelium is directly exposed to O2, hyperoxia induces both acute and chronic lung injuries. Survival studies indicated that CebpαΔ/Δ mice were highly susceptible to hyperoxia, and all the CebpαΔ/Δ mice died within 4 days, a time at which 100% of the control mice survived (Fig. 2A). Lung inflammation, mechanics, and morphology were studied after a 69-h exposure to 95% O2. Inflammation, severe alveolar space enlargement, perivascular edema, and thickened alveoli were observed in CebpαΔ/Δ mice (Fig. 2B). Minor histological changes (slight perivascular edema) were observed in control mice after oxygen exposure. After hyperoxia exposure, protein content in BALF from CebpαΔ/Δ mice was significantly increased (Fig. 2C), suggesting increased protein permeability in CebpαΔ/Δ mice, and IL-1β, IL-6, and MIP-2 in BALF and lung homogenates were increased in CebpαΔ/Δ mice (Fig. 3A). While pulmonary mechanics were unaltered in CebpαΔ/Δ mice under normoxic conditions, exposure to 95% O2 for 69 h resulted in a marked deterioration in pulmonary mechanics (Fig. 3B). Lung compliance was decreased in association with a marked increase in airway resistance, airway elastance, tissue damping, and tissue elastance, consistent with severe respiratory dysfunction in CebpαΔ/Δ mice during hyperoxia.
Fig. 3.
A: increased IL-1β, IL-6, and MIP-2 in BALF and lung homogenate of CebpαΔ/Δ mice after exposure to 95% O2 for 69 h. Levels of each cytokine were significantly increased in the CebpαΔ/Δ mice after hyperoxia. *P < 0.01 vs. others by ANOVA. ND, not detectable; n = 4/group. B: abnormal pulmonary mechanics in CebpαΔ/Δ mice following hyperoxia. Lung mechanics were assessed using FlexiVent System in control and CebpαΔ/Δ mice exposed to room air or 95% O2 for 69 h. Lung mechanics were similar in control and CebpαΔ/Δ mice in room air. After exposure to 95% O2, airway resistance, airway elastance, tissue damping, and tissue elastance were increased and compliance decreased significantly in CebpαΔ/Δ mice. *P < 0.01 vs. others, tP < 0.05 vs. air groups by ANOVA. N = 4/group.
Surfactant homeostasis was altered in CebpαΔ/Δ mice after exposure to hyperoxia.
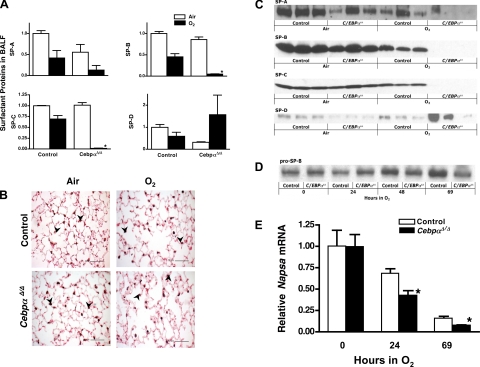
C/EBPα plays an important role in lipid metabolism and lipid biosynthesis in adipocyte and liver (55). Since alveolar type II epithelial cells synthesize surfactant and C/EBPα is highly expressed in type II cells, C/EBPα is thought to play a role in surfactant lipid synthesis (38). Deletion of C/EBPα did not influence Sat PC levels in the postnatal mouse lung under normal conditions (Fig. 4A). The influence of Cebpα deletion in surfactant homeostasis during the acute phase (24 h) of hyperoxia lung injury was studied. While Sat PC was unchanged in control mice after a 24-h exposure to hyperoxia, Sat PC in BALF was decreased in CebpαΔ/Δ mice by 50% (Fig. 4A, O2 24 h), indicating altered surfactant lipid metabolism by deletion of C/EBPα. Body weights were similar in control (25.7 ± 1.0 g) and CebpαΔ/Δ mice (23.0 ± 0.8 g). Mice were given body weight-adjusted doses of [3H]palmitic acid by intraperitoneal injection 24 h after exposure to hyperoxia or in air and 8 h after injection; the amount of labeled Sat PC was measured in BALF and lung tissue after BAL. This time point was considered optimal to measure the net incorporation of precursors into Sat PC and secretion of labeled Sat PC into the alveoli (26). Under normal conditions in air, Sat PC synthesis and secretion were normal in C/EBPα mice, and radiolabeled Sat PC in BALF and lung tissue was similar to that of control mice (data not shown). While incorporation of radiolabeled precursor into Sat PC in lung tissue was similar in control and CebpαΔ/Δ mice in hyperoxia, [3H]Sat PC in BALF and calculated % secretion were significantly decreased in CebpαΔ/Δ mice (Fig. 4B). This decrease in surfactant secretion was associated with abnormal ultrastructure of lamellar bodies in CebpαΔ/Δ mice type II cells (Fig. 4C) after exposure to hyperoxia for 24 h. While 75% of type II cells were normal, 4 out of 15 type II cells in a blindly selected area had fewer and/or smaller lamellar bodies (Fig. 4C), similar to that seen in patients (7) and mice (9, 40) with decreased expression of lipid transport protein ABCA3, critical for surfactant homeostasis. There was no influence of 24-h hyperoxia to the ultrastructure of type II cells in control mice (Fig. 4C). After a 69-h exposure to hyperoxia, Sat PC concentrations were: control mice (n = 4) BALF (6.8 ± 0.6 μmol/kg), lung tissue (20.5 ± 1.0 μmol/kg), and CebpαΔ/Δ mice (n = 4) BALF (7.5 ± 0.7 μmol/kg), lung tissue (20.9 ± 0.3 μmol/kg). After longer (69 h) exposure to hyperoxia, Sat PC in BALF from control mice decreased by 45%, whereas in CebpαΔ/Δ mice, Sat PC in BALF remained as low as that observed following 24 h of hyperoxia.
Fig. 4.
A: while surfactant metabolism in CebpαΔ/Δ mice was normal in air, saturated phosphatidylcholine (Sat PC) was decreased in CebpαΔ/Δ mice BALF after 24-h exposure to hyperoxia. Sat PC in lung tissue after BAL was similar between control and CebpαΔ/Δ mice. *P < 0.05 vs. others, n = 5/group. B: intraperitoneally injected [3H]palmitic acid after 24-h exposure to hyperoxia, incorporated into Sat PC 8 h after injection was significantly decreased in BALF. Percentage of [3H]Sat PC in BALF relative to total lung was decreased in CebpαΔ/Δ mice suggesting decreased surfactant Sat PC secretion in hyperoxia. *P < 0.05 vs. control, n = 5/group. C: under 24-h hyperoxia stress, deletion of Cebpα influenced lamellar body formation in type II epithelial cells. C, a: size and number of lamellar bodies were unchanged in type II cells from control mice after 24-h exposure to hyperoxia. C, b and c: representative electron micrographs of heterogeneity of lamellar body formation in type II cells from CebpαΔ/Δ mice after 24-h hyperoxia are shown. CebpαΔ/Δ mice exhibited decreased number of lamellar bodies (b) or smaller-size lamellar bodies (c). Shown are representative electron micrographs of n = 3/groupscale; bars = 2 μm.
Total volume of BALF recovered by lavage was similar between groups. Surfactant proteins in the BALF were analyzed by Western blot and expressed relative to control mice in air. Under normoxic conditions, the content of mature SP-B and SP-C in BALF from CebpαΔ/Δ mice was similar to that in control mice BALF (Fig. 5, A and C). Mature SP-C was significantly decreased in CebpαΔ/Δ mice after exposure to hyperoxia for 69 h. Mature SP-B in BALF was decreased in CebpαΔ/Δ mice (0.4 ± 0.1, n = 4) relative to control mice (1.0 ± 0.2, P < 0.05, n = 4) after 24 h and was further decreased after 69 h of hyperoxia (Fig. 5. A and C). In contrast, there were no obvious differences in pro-SP-B immunostaining between control and CebpαΔ/Δ mice in air and 69-h hyperoxia (Fig. 5B). Content of pro-SP-B in the same quantity of supernatant of the lung homogenate after centrifugation was determined by Western blot (Fig. 5D), and protein bands were quantitated. Pro-SP-B levels were similar in control and CebpαΔ/Δ mice lungs after 24-h exposure to hyperoxia (control 1.0 ± 0.2, CebpαΔ/Δ 1.4 ± 0.3, P = 0.3, n = 4), suggesting that cleavage of pro-SP-B to mature SP-B was likely influenced by deletion of Cebpα. SP-B is synthesized as a proprotein and processed to the functional, mature SP-B in type II cells by multiple proteolytic enzymes including napsin A (gene: Napsa) (20, 59, 61), cathepsin H (Ctsh) (8), and pepsinogen C (Pgc) (15, 17). Similar to SP-B, these proteolytic enzymes likely play a role in the maturation process of SP-C (8). Napsa and Ctsh mRNA were significantly decreased, whereas Pgc was increased in CebpαΔ/Δ mice as demonstrated by microarray analyses of type II cells isolated after 24-h exposure to 95% O2. A decrease in relative expression of Napsa mRNA in hyperoxia was confirmed by RT-PCR (Fig. 5E) supporting the concept that C/EBPα may influence SP-B maturation (processing) from pro-SP-B during hyperoxia via regulation of Napsa.
Fig. 5.
A: decreased mature SP-B and SP-C in CebpαΔ/Δ mice following hyperoxia. Mature surfactant proteins in the same volume BALF were quantitated by Western blot analyses. After exposure to 95% O2 for 69 h, SP-B and SP-C were markedly decreased in CebpαΔ/Δ mice. Values are presented as relative to control mice in air (value given as 1). *P < 0.05 vs. others; n = 3/group. B: pro-SP-B immunostaining on control and CebpαΔ/Δ mice lungs in room air and after 69-h exposure to 95% O2. Pro-SP-B immunostaining in alveolar type II epithelial cells (arrowheads) was similar for all the groups, suggesting altered maturation of SP-B from pro-SP-B in CebpαΔ/Δ mice in hyperoxia. Shown are representative microphotographs of n = 4/group. C: representative Western blot for SP-A, SP-B, SP-C, and SP-D in air and after 69-h exposure to hyperoxia; n = 3/group. D: representative Western blot for pro-SP-B after 0- (baseline), 24-, 48-, and 69-h exposure to 95% O2. E: relative expression of Napsa by RT-PCR in CebpαΔ/Δ and control mice lungs during exposure to hyperoxia. Decrease in Napsa mRNA was significantly faster in CebpαΔ/Δ mice than control mice in hyperoxia.*P < 0.05 vs. control; n = 5 or 6/group.
Identification of differentially expressed genes in alveolar type II epithelial cells from CebpαΔ/Δ mice by microarray analyses.
The complete microarray dataset was submitted to Gene Expression Omnibus (acc. no. GSE14917). Comparison analyses of major enriched functional categories were performed using IPA software to study the role of C/EBPα in pulmonary homeostasis under air (0 h) and at 2 and 24 h in hyperoxia (Fig. 6). Under air and 2 h in hyperoxia, deletion of Cebpα from lung epithelial cells had a modest effect on mRNA expression in type II cells. mRNAs influenced by C/EBPα were mainly those associated with cellular movement, cell-cell interaction, and development categories. In contrast, after exposure to 95% O2 for 24 h, we identified 2,851 differentially expressed mRNAs altered by deletion of Cebpα. The most significantly influenced categories were shifted to cell signaling (primarily calcium-mediated) and immune response.
Fig. 6.
Comparison analysis of genomic responses induced by exposure to 95% O2 for 0 h (baseline), 2 h, and 24 h. Differences in mRNAs in the lung of CebpαΔ/Δ and control mice were identified through microarray analysis and subject to functional enrichment analysis using Ingenuity Pathway Analysis (IPA). Highly significant genes were selected by Fisher's Exact Test; P < 1.0E-4. The statistical significances in each biological function were presented using negative log transform of P value; n indicates the total gene numbers in each biological function category.
Lung morphology, function, and survival studies indicated that CebpαΔ/Δ mice were highly susceptible to oxygen-induced injury. Consistent with these observations, a group of oxidative stress-related mRNAs were significantly altered in CebpαΔ/Δ mice compared with control mice after 24 h of hyperoxia (Table 1). All of those genes detected by microarray were similarly affected after 69-h exposure to hyperoxia when analyzed by commercial RT-PCR array (Table 1). Cellular antioxidants including glutathione peroxidase 1 and 8 (Gpx1, Gpx8), peroxiredoxin 3 (Prdx3), prostaglandin-endoperoxide synthase 2 (Ptgs2, also known as Cox-2), glutathione S-transferase pi 1 and zeta 1 (Gstp1 and Gstz1), prion protein (Prnp), and thioredoxin reductase 3 (Txnrd3) were decreased, whereas extracellular glutathione peroxidases (Gpx3 and 6) and superoxide dismutase 3 (Sod3) were increased in CebpαΔ/Δ mice compared with control mice after 24- and 69-h exposure to hyperoxia. Expression of genes involved in the metabolism of reactive oxygen species (Sod3, Nox3, Nox4, Fmo1, Tpo) and oxygen transporters Hbq1 and Vim1 were induced in CebpαΔ/Δ mice during hyperoxia. The Vanin family of proteins are involved in GSH-dependent oxidative stress response and acute inflammatory response (34). Both Vnn1 and Vnn3 mRNA expression analyzed by microarray was significantly decreased (−3.5-fold and −2.1-fold, respectively) in CebpαΔ/Δ mice after 24-h exposure to hyperoxia, which was confirmed by RT-PCR (Table 1): Vnn1 (control 1.0 ± 0.18, CebpαΔ/Δ 0.28 ± 0.02, n = 3 pooled sample, P < 0.05) and Vnn3 (control 1.0 ± 0.09, CebpαΔ/Δ 0.37 ± 0.02, n = 3 pooled sample, P < 0.05).
Table 1.
Oxidative stress-related genes in type II cells and average ratios between CebpαΔ/Δ and control mice
| Symbol | Description |
Microarray Analyses |
RT-PCR
|
||
|---|---|---|---|---|---|
| 0 h | 2 h | 24 h | 69 h | ||
| Aass | Aminoadipate-semialdehyde synthase | 1.12 | 0.83 | 0.49 | 0.44 |
| Ehd2 | EH-domain containing 2 | 1.03 | 1.06 | 0.51 | 1.17 |
| Ehd3 | EH-domain containing 3 | 0.93 | 1.04 | 1.60 | N/A |
| Fmo1 | Flavin containing monooxygenase 1 | 0.99 | 2.05 | 1.93 | 1.54 |
| Gpx1 | Glutathione peroxidase 1 | 1.10 | 0.88 | 0.46 | 0.76 |
| Gpx3 | Glutathione peroxidase 3 | 1.27 | 1.76 | 2.28 | 2.99 |
| Gpx6 | Glutathione peroxidase 6 | 1.00 | 0.86 | 1.98 | 2.74 |
| Gpx8 | RIKEN cDNA 2310016C16 gene | 0.91 | 0.70 | 0.52 | 0.81 |
| Hbq1 | RIKEN cDNA F830116E18 gene | 0.86 | 0.89 | 1.97 | 4.07 |
| II19 | Interleukin-19 | 0.94 | 0.90 | 0.67 | 0.57 |
| Nostrin | Nitric oxide synthase trafficker | 1.01 | 1.98 | 1.72 | N/A |
| Nox3 | NADPH oxidase 3 | 1.01 | 0.81 | 1.78 | N/A |
| Nox4 | NADPH oxidase 4 | 0.97 | 0.99 | 1.64 | 2.34 |
| Prdx3 | Peroxiredoxin 3 | 1.23 | 1.01 | 0.51 | 0.72 |
| Ptgs2 | Prostaglandin-endoperoxide synthase 2 | 0.95 | 0.94 | 0.61 | 1.32 |
| Sod3 | Superoxide dismutase 3, extracellular | 1.33 | 1.25 | 2.04 | 2.67 |
| Tmod1 | Tropomodulin 1 | 1.01 | 0.84 | 0.43 | 1.02 |
| Tpo | Throid peroxidase | 0.93 | 0.92 | 1.52 | 2.84 |
| Txnrd3 | Thioredoxin reductase 3 | 1.05 | 0.84 | 0.52 | 0.92 |
| Vim | Vimentin | 1.02 | 1.65 | 1.88 | 1.48 |
| Vnn1 | Vanin 1 | 0.84 | 0.45 | 0.27 | (0.28)* N/A |
| Vnn3 | Vanin 3 | 0.77 | 0.67 | 0.40 | (0.37)* N/A |
Time course microarray analysis was performed using isolated type II cells after 0-, 2-, and 24-h hyperoxia exposure. RT-PCR was analyzed using commercial RT-PCR array for oxidative stress genes on isolated type II cells after 69-h hyperoxia exposure.
Analyzed by RT-PCR after 24-h hyperoxia. N = 3 pooled samples. N/A, not available by RT-PCR array.
Comparison of the dynamic mRNA profiling between CebpαΔ/Δ and control mice after 0, 2, and 24 h of hyperoxia.
To further understand the molecular mechanisms mediating the susceptibility of the CebpαΔ/Δ mice to hyperoxia, we compared the dynamic mRNA profiling between CebpαΔ/Δ and control mice after 0-, 2-, and 24-h exposure to hyperoxia. We combined 5,416 differentially expressed probe sets from the mRNA microarray analysis (map to 2,984 unique genes) from each condition and divided them into four subgroups using hierarchical clustering. Figure 7A shows the heat map of hierarchical clustering results. Data were dissected into four clusters (C1-C4) based on their expression similarity analyzed by Euclidean Distance with complete linkage method. Figure 7B shows the corresponding clustering profiles, representing the changes in group mean and standard deviation after 0-, 2-, and 24-h exposure to hyperoxia.
Fig. 7.
Dynamic mRNA profiling between CebpαΔ/Δ and control mice after 0-, 2-, and 24-h hyperoxia exposure. A: the heat map of hierarchical clustering results. B: corresponding line graph of each cluster calculated by group mean and standard deviation after 0-, 2-, and 24-h hyperoxia.
Clusters were further analyzed by functional classification and network construction using IPA. The analysis was concentrated on two major clusters, C2 and C3, for the present study, consisting of 82% of all the differentially expressed genes. C2 mapped 1,406 genes that were moderately induced in CebpαΔ/Δ mice after 24-h hyperoxia. Inflammatory/immune response and cell signaling (primarily calcium and or/ G protein-mediated) were the most significant functional categories with P values of 1.5E-12 and 1.3E-9, respectively. Eicosanoid signaling pathway was the top-ranked (P = 4.6E-5) canonical pathway, which showed significant overlapping with C2 genes (Fig. 8). A number of genes in eicosanoid signaling pathway, including multiple members of the phospholipase A2 family (Pla2g3, Pla2g10, Pla2g2e, Pla2g2f) and phospholipase A2 receptor 1 (Pla2r1), arachidonate lipoxygenases 12b and 15b (Alox12b, Alox15b), prostaglandin D and F receptors, prostaglandin D2 synthase (Ptgdr, Ptgfr, and Ptgds), leukotriene B4 receptor 2 (Ltb4r2), and thromboxane A2 receptor (Tbxa2r), were induced by 24-h exposure to hyperoxia.
Fig. 8.
Cluster 2 genes significantly overlapped with those in the eicosanoid signaling pathway (P = 4.6E-05). The significance is calculated using IPA software based on the likelihood measurement for a given pathway associated with the dataset by random chance. After 24-h hyperoxia challenge, genes increased in CebpαΔ/Δ mice are indicated by up arrows; genes decreased in CebpαΔ/Δ mice are indicated by short down arrows.
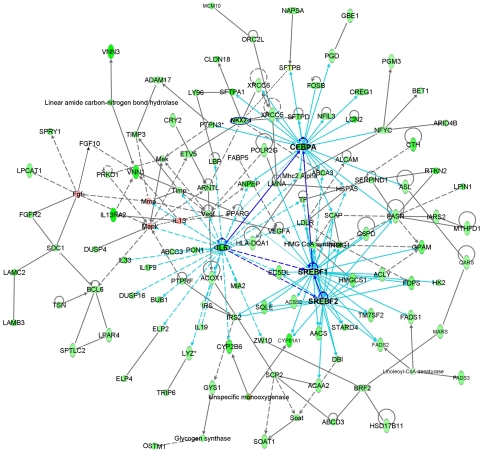
C3 consists of 1,125 genes whose mRNA levels were decreased in CebpαΔ/Δ mice after hyperoxia exposure. Lipid metabolism was the most significant functional category (P = 2.19E-11, 121 genes). Figure 9 represents a gene network compiled from merging lipid metabolism-related networks from IPA analysis of C3 genes. Many genes that are known to be involved in surfactant protein synthesis, processing, and regulation (Sftpa1, Sftpb, Sftpd, Lpcat1, Napsa, Nkx2-1), lamellar body assembling and transport (Abca3, Fabp5, Slc38a4, Stard4), and lipid biosynthesis and metabolism (Scap, Srebf1, Srebf2, Insig1, Acss2, Acly, Fasn, Fdps, Fads1, Fads2, Gpam, Soat1, Ldlr) were negatively correlated with the length of exposure to hyperoxia. Cebpα, Nkx2-1, Srebf1/Srebf2 and IL6 are likely the key transcription factors regulating cluster genes in response to the exposure to hyperoxia in CebpαΔ/Δ mice compared with that in control mice. Decreased expression of IL6 in CebpαΔ/Δ mice type II cells after 24-h exposure to hyperoxia was confirmed by RT-PCR on isolated type II cells (control 1.0 ± 0.10, CebpαΔ/Δ 0.27 ± 0.04, n = 3 pooled sample, P < 0.05).
Fig. 9.
Biological association networks of cluster 3 genes revealed by IPA. Genes/proteins are represented by nodes, and the biological relationship between 2 nodes is represented by an edge (line). A solid line indicates direct interaction; a dashed line is indirect interaction; A B indicates binding; A → B indicates A is the cause of B; A
B indicates binding; A → B indicates A is the cause of B; A B indicates A inhibits B, and
B indicates A inhibits B, and  indicates self-regulation. The mRNA expression of cluster 3 genes was negatively correlated with the length of hyperoxia exposure time and color coded in green. Positively correlated genes are color coded in pink. Many genes in the network were known to be involved in synthesis, processing, and regulation of surfactant proteins and lipids.
indicates self-regulation. The mRNA expression of cluster 3 genes was negatively correlated with the length of hyperoxia exposure time and color coded in green. Positively correlated genes are color coded in pink. Many genes in the network were known to be involved in synthesis, processing, and regulation of surfactant proteins and lipids.
These microarray analyses demonstrated that C/EBPα regulates the expression of genes influencing protection and/or recovery from hyperoxia-induced lung injury in that C/EBPα is required for surfactant homeostasis and postnatal lung function during hyperoxic stress.
DISCUSSION
C/EBPα is required for lung function and surfactant homeostasis during oxygen-induced lung injury.
Our previous studies demonstrated that C/EBPα is required for the lung maturation in late gestation, regulating the synthesis of both surfactant lipids and proteins (35). Interestingly, postnatal deletion of Cebpα did not alter lung function, Sat PC, or surfactant protein levels under normal conditions. Under hyperoxia stress, however, surfactant homeostasis and survival were significantly altered in adult CebpαΔ/Δ mice consistent with the important role of prenatal C/EBPα in surfactant homeostasis seen at birth (31). Differentially expressed cluster of genes in CebpαΔ/Δ vs. control mice was negatively correlated with the duration of exposure to hyperoxia. As summarized in microarray network in Fig. 10, Cebpα, Srebf1/Srebf2, Nkx2.1, and IL6 are likely the important regulators of this gene network. Genes in this cluster include surfactant protein genes (Sftpa1, Sftpb, and Sftpd), genes involved in surfactant protein synthesis, processing, and regulation (Lpcat1, Napsa, and Nkx2-1), lamellar body assembly, surfactant, or lipid transport (Abca3, Fabp5, Slc38a4, Stard4), and lipid biosynthesis and metabolism (Scap, Srebf1, Srebf2, Insig1, Acss2, Acly, Fasn, Fdps, Fads1, Fads2, Gpam, Soat1, Ldlr). Many of these lipid-related target genes were overlapped with the influenced genes identified in our previous study of transgenic mice in which the Scap gene (a master regulator of lipid synthesis) was deleted in the respiratory epithelium (6). C/EBPα interacts with Nkx2.1 and regulates lipid biosynthesis and surfactant homeostasis during hyperoxia, at least partially through SCAP/SREBP signaling pathway. In isolated type II cells, IL6 mRNA expression in CebpαΔ/Δ mice was decreased to 30% of that in control mice after 24-h exposure to hyperoxia. In contrast, IL-6 protein in BALF and lung tissue from hyperoxia-exposed CebpαΔ/Δ mice was increased in association with increased number and activation of inflammatory cells in the lung. Significantly decreased Sat PC in BALF, abnormalities in lamellar body ultrastructure in type II cells, and altered SP-B maturation from pro-SP-B were also demonstrated in CebpαΔ/Δ mice after 24-h exposure to hyperoxia. In full-term infants with hereditary ABCA3 deficiency (7), lamellar body structure was abnormal, SP-B proprotein was strongly expressed in type II cells, and immunostaining for mature SP-B was less intense, suggesting that the altered maturation of SP-B seen in CebpαΔ/Δ mice is perhaps related to decreased napsin A and ABCA3 in type II cells. Together, C/EBPα is required for surfactant homeostasis and postnatal lung function during hyperoxia.
Fig. 10.
Summary of microarray network. Nkx2-1, Srefb1/2, and IL6 are likely the key coregulators of the C/EBPα network. C/EBPα and Nkx2-1 regulate genes involved in surfactant biosynthesis, processing, transport, and assembly. In the network, C/EBPα interacts with Srefb1/2 to control phospholipid and cholesterol biosynthesis and with IL6 to regulate genes in acute/defense response and influences cell redox states.
C/EBPα is required for maintenance of cell redox homeostasis under oxidative stress.
Glutathione (GSH) is the key protective antioxidant in the lung. Alterations of lung GSH metabolism or balance of cell redox state (GSH/GSSG) are considered as a central feature of many inflammatory lung diseases including lung injury induced by hyperoxia (21, 39, 49, 50). Regulation of intracellular GSH level in response to oxidative stress is achieved via the activation of various transcription factors (such as NF-κB, AP-1, and Nrf2), redox sensor, and signal transduction pathways (such as JNK/p38/MAPK) (51, 60). Genes involved in the glutathione metabolism, response to oxidative stress, and cell redox homeostasis were significantly altered in CebpαΔ/Δ mice after exposure to hyperoxia (Table 1). Interestingly, genes that regulate intracellular GSH level including Gpx1, Gpx8, Gstp1, Gstz1, and Txnrd3 were decreased, whereas extracellular antioxidants including Gpx3, Gpx6, and Sod3 were increased in CebpαΔ/Δ mice under hyperoxia. Among these, Gstp1, the primary isoform of glutathione S-transferases (GSTs) in lung epithelium, plays a key role in cellular protection against oxidative stress and is a known biomarker for susceptibility of airway inflammation, including childhood asthma (16, 36, 56). Gpx1 is the major cytosolic and mitochondrial glutathione peroxidase, abundantly and ubiquitously expressed in many tissues where it plays a role in the primary protection of cells during acute oxidative stress (13). Gpx3, on the other hand, is the major extracellular glutathione peroxidase detected in kidney, lung, heart, breast, and placenta (11).
The Vanin family of proteins is epithelial ectoenzymes with pantetheinase activities and produces cysteamine, a powerful antioxidant compound (34, 48). Multiple studies demonstrated that Vanins are important proteins regulating GSH-dependent responses to oxidative injury and likely play important cytoprotective roles in islet and thymus cells under oxidative or inflammatory stress (4, 48, 54). Vanin-1 (Vnn1) is an epithelial sensor of oxidative stress that regulates endogenous GSH levels, influences the redox status, triggers acute inflammation response, and influences the cell fate in response to hyperoxia injury in both thymic and gut epithelial cells (4, 5). In the present study, both Vnn1 and Vnn3 mRNA expression were significantly decreased in CebpαΔ/Δ mice type II cells during hyperoxia. Both human and mouse Vnn1 gene promoters contain C/EBPα binding sites, suggesting that Vnn1 may be the direct transcriptional target of C/EBPα and may be the oxidative stress sensor in pulmonary type II epithelium cells. The cytoprotective effect of C/EBPα against hyperoxia is likely to be mediated through the transcriptional regulation of Vanin to trigger a variety of cellular redox-sensitive signaling processes.
Deletion of Cebpα altered eicosanoid signaling pathway.
The eicosanoid signaling pathway was significantly induced in CebpαΔ/Δ mice after exposure to hyperoxia. Genes encoding multiple members of phospholipase A2 family (sPLA2s: Pla2g3, Pla2g5, Pla2g10, Pla2g2c, Pla2g2e, Pla2g2f) and phospholipase A2 receptor 1 (Pla2r1), arachidonate lipoxygenases 12b and 15b (Alox12b, Alox15b), prostaglandin D and F receptors, prostaglandin D2 synthase (Ptgdr, Ptgfr, and Ptgds), leukotriene B4 receptor 2 (Ltb4r2), and thromboxane A2 receptor (Tbxa2r) were all induced in CebpαΔ/Δ mice after 24-h hyperoxia exposure. sPLA2s hydrolyze cellular phospholipids to release arachidonic acid, which is a critical and initial step in the eicosanoid signaling pathway. The sPLA2 family represents a group of structurally related, disulfide-rich, Ca2+-dependent, low-molecular-weight enzymes with a catalytic histidine (29, 44). The phospholipase A2 lipolytic enzymes Pla2g5 and Pla2g10 catalyze the calcium-dependent hydrolysis of the 2-acyl groups in 3-sn-phosphoglycerides, releasing arachidonic acid from cell membrane phospholipids (42, 43) and play a role in lipid-mediated inflammation in the lung (1, 12). Pla2g2a is the only member in the sPLA2 family whose transcriptional regulation has been extensively studied, and the activity of both the human and mouse Pla2g2a promoters is known to be regulated by C/EBP family members (1).
In conclusion, while the expression of C/EBPα in respiratory epithelial cells is required for normal fetal lung maturation, respiratory epithelial cell-specific deletion of C/EBPα in the postnatal mouse lung did not alter lung morphology, gene expression, or function under normal conditions. In contrast, CebpαΔ/Δ mice were susceptible to hyperoxia, causing acute lung injury and respiratory failure, supporting the concept that C/EBPα plays an important role in enhancing epithelial cell survival, surfactant lipid homeostasis, and SP-B and -C maturation under oxidative stress. These are the novel findings that define the important role of C/EBPα in postnatal pulmonary homeostasis.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-095464 (M. Ikegami, J. A. Whitsett, Y. Xu) and HL-090156 (J. A. Whitsett).
Acknowledgments
We thank Benjamin Feldman and Yanhua Wang for excellent technical assistance.
REFERENCES
- 1.Andreani M, Olivier JL, Berenbaum F, Raymondjean M, Bereziat G. Transcriptional regulation of inflammatory secreted phospholipases A(2). Biochim Biophys Acta 1488: 149–158, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett GR Phosphorus assay in column chromatography. J Biol Chem 234: 466–468, 1959. [PubMed] [Google Scholar]
- 3.Berg T, Didon L, Nord M. Ectopic expression of C/EBPalpha in the lung epithelium disrupts late lung development. Am J Physiol Lung Cell Mol Physiol 291: L683–L693, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Berruyer C, Martin FM, Castellano R, Macone A, Malergue F, Garrido-Urbani S, Millet V, Imbert J, Dupre S, Pitari G, Naquet P, Galland F. Vanin-1−/− mice exhibit a glutathione-mediated tissue resistance to oxidative stress. Mol Cell Biol 24: 7214–7224, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berruyer C, Pouyet L, Millet V, Martin FM, LeGoffic A, Canonici A, Garcia S, Bagnis C, Naquet P, Galland F. Vanin-1 licenses inflammatory mediator production by gut epithelial cells and controls colitis by antagonizing peroxisome proliferator-activated receptor gamma activity. J Exp Med 203: 2817–2827, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besnard V, Wert SE, Stahlman MT, Postle AD, Xu Y, Ikegami M, Whitsett JA. Deletion of scap in alveolar type II cells influences lung lipid homeostasis and identifies a compensatory role for pulmonary lipofibroblasts. J Biol Chem 284: 4018–4030, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brasch F, Schimanski S, Muhlfeld C, Barlage S, Langmann T, Aslanidis C, Boettcher A, Dada A, Schroten H, Mildenberger E, Prueter E, Ballmann M, Ochs M, Johnen G, Griese M, Schmitz G. Alteration of the pulmonary surfactant system in full-term infants with hereditary ABCA3 deficiency. Am J Respir Crit Care Med 174: 571–580, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Brasch F, Ten Brinke A, Johnen G, Ochs M, Kapp N, Muller KM, Beers MF, Fehrenbach H, Richter J, Batenburg JJ, Buhling F. Involvement of cathepsin H in the processing of the hydrophobic surfactant-associated protein C in type II pneumocytes. Am J Respir Cell Mol Biol 26: 659–670, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Bruder E, Hofmeister J, Aslanidis C, Hammer J, Bubendorf L, Schmitz G, Rufle A, Buhrer C. Ultrastructural and molecular analysis in fatal neonatal interstitial pneumonia caused by a novel ABCA3 mutation. Mod Pathol 20: 1009–1018, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev 5: 1538–1552, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Chu FF, Esworthy RS, Doroshow JH, Doan K, Liu XF. Expression of plasma glutathione peroxidase in human liver in addition to kidney, heart, lung, and breast in humans and rodents. Blood 79: 3233–3238, 1992. [PubMed] [Google Scholar]
- 12.Curfs DM, Ghesquiere SA, Vergouwe MN, van der Made I, Gijbels MJ, Greaves DR, Verbeek JS, Hofker MH, de Winther MP. Macrophage secretory phospholipase A2 group X enhances anti-inflammatory responses, promotes lipid accumulation, and contributes to aberrant lung pathology. J Biol Chem 283: 21640–21648, 2008. [DOI] [PubMed] [Google Scholar]
- 13.De Haan JB, Crack PJ, Flentjar N, Iannello RC, Hertzog PJ, Kola I. An imbalance in antioxidant defense affects cellular function: the pathophysiological consequences of a reduction in antioxidant defense in the glutathione peroxidase-1 (Gpx1) knockout mouse. Redox Rep 8: 69–79, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Epaud R, Ikegami M, Whitsett JA, Jobe AH, Weaver TE, Akinbi HT. Surfactant protein B inhibits endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol 28: 373–378, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Foster C, Aktar A, Kopf D, Zhang P, Guttentag S. Pepsinogen C: a type 2 cell-specific protease. Am J Physiol Lung Cell Mol Physiol 286: L382–L387, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Fryer AA, Hume R, Strange RC. The development of glutathione S-transferase and glutathione peroxidase activities in human lung. Biochim Biophys Acta 883: 448–453, 1986. [DOI] [PubMed] [Google Scholar]
- 17.Gerson KD, Foster CD, Zhang P, Zhang Z, Rosenblatt MM, Guttentag SH. Pepsinogen C proteolytic processing of surfactant protein B. J Biol Chem 283: 10330–10338, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gossen M, Bujard H. Efficacy of tetracycline-controlled gene expression is influenced by cell type: commentary. Biotechniques 19: 213–217, 1995. [PubMed] [Google Scholar]
- 19.Gregory TJ, Longmore WJ, Moxley MA, Whitsett JA, Reed CR, Fowler AA, Hudson LD, Maunder RJ, Crim C, Hyers TM. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest 88: 1976–1981, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guttentag S, Robinson L, Zhang P, Brasch F, Buhling F, Beers M. Cysteine protease activity is required for surfactant protein B processing and lamellar body genesis. Am J Respir Cell Mol Biol 28: 69–79, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Haddad JJ, Harb HL. l-gamma-Glutamyl-l-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: a signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol Immunol 42: 987–1014, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Hardie WD, Davidson C, Ikegami M, Leikauf GD, Le Cras TD, Prestridge A, Whitsett JA, Korfhagen TR. EGF receptor tyrosine kinase inhibitors diminish transforming growth factor-α-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L1217–L1225, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Hess HH, Derr JE. Assay of inorganic and organic phosphorus in the 0.1–5 nanomole range. Anal Biochem 63: 607–613, 1975. [DOI] [PubMed] [Google Scholar]
- 24.Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, Perl AK, Hull WM, Wert SE, Whitsett JA. Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Invest 113: 28–37, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikegami M, Falcone A, Whitsett JA. STAT-3 regulates surfactant phospholipid homeostasis in normal lung and during endotoxin-mediated lung injury. J Appl Physiol 104: 1753–1760, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Ikegami M, Ueda T, Hull W, Whitsett JA, Mulligan RC, Dranoff G, Jobe AH. Surfactant metabolism in transgenic mice after granulocyte macrophage-colony stimulating factor ablation. Am J Physiol Lung Cell Mol Physiol 270: L650–L658, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Johnson PF Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci 118: 2545–2555, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lubbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA 93: 10933–10938, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat 68–69: 3–58, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Lee YH, Sauer B, Johnson PF, Gonzalez FJ. Disruption of the c/ebp alpha gene in adult mouse liver. Mol Cell Biol 17: 6014–6022, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lekstrom-Himes J, Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem 273: 28545–28548, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Lewandoski M Conditional control of gene expression in the mouse. Nat Rev Genet 2: 743–755, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951. [PubMed] [Google Scholar]
- 34.Maras B, Barra D, Dupre S, Pitari G. Is pantetheinase the actual identity of mouse and human vanin-1 proteins? FEBS Lett 461: 149–152, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Martis PC, Whitsett JA, Xu Y, Perl AK, Wan H, Ikegami M. C/EBP (alpha) is required for lung maturation at birth. Development 133: 1155–1164, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Maruyama T, Iizuka H, Tobisawa Y, Shiba T, Matsuda T, Kurohane K, Imai Y. Influence of local treatments with capsaicin or allyl isothiocyanate in the sensitization phase of a fluorescein-isothiocyanate-induced contact sensitivity model. Int Arch Allergy Immunol 143: 144–154, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Mason RJ, Nellenbogen J, Clements JA. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res 17: 281–284, 1976. [PubMed] [Google Scholar]
- 38.Mason RJ, Pan T, Edeen KE, Nielsen LD, Zhang F, Longphre M, Eckart MR, Neben S. Keratinocyte growth factor and the transcription factors C/EBP alpha, C/EBP delta, and SREBP-1c regulate fatty acid synthesis in alveolar type II cells. J Clin Invest 112: 244–255, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastruzzo C, Crimi N, Vancheri C. Role of oxidative stress in pulmonary fibrosis. Monaldi Arch Chest Dis 57: 173–176, 2002. [PubMed] [Google Scholar]
- 40.Matsuzaki Y, Besnard V, Clark JC, Xu Y, Wert S, Ikegami M, Whitsett J. STAT3 regulates ABCA3 expression and influences lamellar body formation in alveolar type II cells. Am J Respir Cell Mol Biol 38: 551–558, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meneghetti A, Cardoso WV, Brody JS, Williams MC. Epithelial marker genes are expressed in cultured embryonic rat lung and in vivo with similar spatial and temporal patterns. J Histochem Cytochem 44: 1173–1182, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Munoz NM, Kim YJ, Meliton AY, Kim KP, Han SK, Boetticher E, O'Leary E, Myou S, Zhu X, Bonventre JV, Leff AR, Cho W. Human group V phospholipase A2 induces group IVA phospholipase A2-independent cysteinyl leukotriene synthesis in human eosinophils. J Biol Chem 278: 38813–38820, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Murakami M, Koduri RS, Enomoto A, Shimbara S, Seki M, Yoshihara K, Singer A, Valentin E, Ghomashchi F, Lambeau G, Gelb MH, Kudo I. Distinct arachidonate-releasing functions of mammalian secreted phospholipase A2s in human embryonic kidney 293 and rat mastocytoma RBL-2H3 cells through heparan sulfate shuttling and external plasma membrane mechanisms. J Biol Chem 276: 10083–10096, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Murakami M, Kudo I. Diversity and regulatory functions of mammalian secretory phospholipase A2s. Adv Immunol 77: 163–194, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Perkowski S, Sun J, Singhal S, Santiago J, Leikauf GD, Albelda SM. Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am J Respir Cell Mol Biol 28: 682–696, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res 11: 21–29, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Pison U, Seeger W, Buchhorn R, Joka T, Brand M, Obertacke U, Neuhof H, Schmit-Neuerburg KP. Surfactant abnormalities in patients with respiratory failure after multiple trauma. Am Rev Respir Dis 140: 1033–1039, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Pitari G, Malergue F, Martin F, Philippe JM, Massucci MT, Chabret C, Maras B, Dupre S, Naquet P, Galland F. Pantetheinase activity of membrane-bound Vanin-1: lack of free cysteamine in tissues of Vanin-1 deficient mice. FEBS Lett 483: 149–154, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Rahman I Inflammation and the regulation of glutathione level in lung epithelial cells. Antioxid Redox Signal 1: 425–447, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal 7: 42–59, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem 234–235: 239–248, 2002. [PubMed] [Google Scholar]
- 52.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365: 561–575, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rice WR, Conkright JJ, Na CL, Ikegami M, Shannon JM, Weaver TE. Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol 283: L256–L264, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Roisin-Bouffay C, Castellano R, Valero R, Chasson L, Galland F, Naquet P. Mouse vanin-1 is cytoprotective for islet beta cells and regulates the development of type 1 diabetes. Diabetologia 51: 1192–1201, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Rosen ED The molecular control of adipogenesis, with special reference to lymphatic pathology. Ann NY Acad Sci 979: 143–158; discussion 188–196, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Spiteri MA, Bianco A, Strange RC, Fryer AA. Polymorphisms at the glutathione S-transferase, GSTP1 locus: a novel mechanism for susceptibility and development of atopic airway inflammation. Allergy 55, Suppl 61: 15–20, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Takiguchi M The C/EBP family of transcription factors in the liver and other organs. Int J Exp Pathol 79: 369–391, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tokieda K, Whitsett JA, Bachurski C, Wert SE, Hull WM, Iwamoto HS. SP-B deficient mice are susceptible to hyperoxic lung injury. Am J Respir Cell Mol Biol 21: 463–472, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Ueno T, Linder S, Na CL, Rice WR, Johansson J, Weaver TE. Processing of pulmonary surfactant protein B by napsin and cathepsin H. J Biol Chem 279: 16178–16184, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Walters DM, Cho HY, Kleeberger SR. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: a potential role for Nrf2. Antioxid Redox Signal 10: 321–332, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Weaver TE, Lin S, Bogucki B, Dey C. Processing of surfactant protein B proprotein by a cathepsin D-like protease. Am J Physiol Lung Cell Mol Physiol 263: L95–L103, 1992. [DOI] [PubMed] [Google Scholar]
- 62.Xu Y, Ikegami M, Yanhna W, Matsuzaki Y, Whitsett JA. Gene expression and biological processes influenced by deletion of STAT3 in pulmonary type II cells. BMC Genomics 8: 455, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou L, Lim L, Costa RH, Whitsett JA. Thyroid transcription factor-1, hepatocyte nuclear factor-3 beta, surfactant protein B, C, and Clara cell secretory protein in developing mouse lung. J Histochem Cytochem 44: 1183–1193, 1996. [DOI] [PubMed] [Google Scholar]