Abstract
The relative contribution of inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs) and ryanodine receptors (RyRs) to agonist-induced Ca2+ signaling in mouse airway smooth muscle cells (SMCs) was investigated in lung slices with phase-contrast or laser scanning microscopy. At room temperature (RT), methacholine (MCh) or 5-hydroxytryptamine (5-HT) induced Ca2+ oscillations and an associated contraction in small airway SMCs. The subsequent exposure to an IP3R antagonist, 2-aminoethoxydiphenyl borate (2-APB), inhibited the Ca2+ oscillations and induced airway relaxation in a concentration-dependent manner. 2-APB also inhibited Ca2+ waves generated by the photolytic release of IP3. However, the RyR antagonist ryanodine had no significant effect, at any concentration, on airway contraction or agonist- or IP3-induced Ca2+ oscillations or Ca2+ wave propagation. By contrast, a second RyR antagonist, tetracaine, relaxed agonist-contracted airways and inhibited agonist-induced Ca2+ oscillations in a concentration-dependent manner. However, tetracaine did not affect IP3-induced Ca2+ release or wave propagation nor the Ca2+ content of SMC Ca2+ stores as evaluated by Ca2+-release induced by caffeine. Conversely, both ryanodine and tetracaine completely blocked agonist-independent slow Ca2+ oscillations induced by KCl. The inhibitory effects of 2-APB and absence of an effect of ryanodine on MCh-induced airway contraction or Ca2+ oscillations of SMCs were also observed at 37°C. In Ca2+-permeable SMCs, tetracaine inhibited agonist-induced contraction without affecting intracellular Ca2+ levels indicating that relaxation also resulted from a reduction in Ca2+ sensitivity. These results indicate that agonist-induced Ca2+ oscillations in mouse small airway SMCs are primary mediated via IP3Rs and that tetracaine induces relaxation by both decreasing Ca2+ sensitivity and inhibiting agonist-induced Ca2+ oscillations via an IP3-dependent mechanism.
Keywords: lung slices, Ca2+-induced Ca2+ release, membrane depolarization, tetracaine
in airway smooth muscle cells (SMCs), agonist-induced contraction is associated with an elevation in intracellular Ca2+ concentration ([Ca2+]i). However, this increase of [Ca2+]i is not steady but occurs as repetitive Ca2+ waves propagating along the length of the SMCs (6, 33, 43, 44). The basic mechanism underlying these Ca2+ oscillations consists of a cyclic Ca2+ release from, and reuptake to, the sarcoplasmic reticulum (SR) via specialized receptor/Ca2+ channels and Ca2+ pumps together with a Ca2+ influx to replace any Ca2+ lost across the cell membrane to the extracellular space (8).
There are two major types of Ca2+ release channels on the SR of airway SMCs, inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs) and ryanodine receptors (RyRs) (1, 2, 31). Each type of receptors exists as three different isomers, all of which have been identified in the airway SMCs (19, 24, 31, 55). Although these receptors differ in structure and molecular weight, their functional activity, to mediate Ca2+ release from the SR, is similar. However, each receptor type is regulated in a different manner. IP3Rs are activated by IP3 (22), produced from phosphatidylinositol 4,5-bisphosphate (PIP2) by Gαq-activated PLCβ in response to G protein-coupled receptor (GPCR) agonists. In addition, the open probability of the IP3R is also modulated by [Ca2+]i; increasing [Ca2+]i serves to enhance IP3R opening, but high [Ca2+]i can reduce the open probability of some IP3Rs (10, 22, 27). In contrast to the IP3R, the opening of the RyRs is primarily modulated by [Ca2+]i. However, SR luminal Ca2+ concentration may also serve to regulate the RyR and IP3R (21, 22). In addition, the second messenger, cyclic ADP-ribose (cADPR), also has been implicated in the activation of RyRs in association with agonist-induced Ca2+ signaling (29).
To explore the regulation of agonist-induced Ca2+ oscillations of SMCs, it is crucial to first understand the mechanisms mediating the cyclic Ca2+ release from SR. Generally, it is acknowledged that the initiation of SMC Ca2+ oscillations is mediated by Ca2+ release from SR via the IP3R (7, 9, 37). However, it has been a long-standing debate in airway or other types of SMCs as to whether RyRs are involved and are required to maintain cyclic Ca2+ release and the propagation of Ca2+ waves by Ca2+-induced Ca2+ release (CICR) (14, 31, 44, 46, 57) or, alternatively, if the entire process was exclusively accomplished by activated IP3R without significant RyRs involvement (34, 36, 50).
The aim of the present research is to determine the relative contribution of the IP3Rs and RyRs to agonist-induced intracellular Ca2+ signaling of airway SMCs. Most previous investigations have relied on pharmacological approaches, using RyR inhibitors, such as ryanodine, dantrolene, and tetracaine, and IP3R inhibitors, such as xestospongin C (XeC) and 2-aminoethoxydiphenyl borate (2-APB). Unfortunately, all of these drugs have some nonspecific effects that will confound results if the assays are not sufficiently specific (49). Consequently, to diminish the misinterpretation of experimental data and obtain a consistent mechanism, we have examined the action of these pharmacological agents on the IP3Rs or RyRs in a number of different conditions.
A second type of Ca2+ signaling occurs in airway SMCs, namely agonist-independent, KCl-induced Ca2+ oscillations. From our previous studies (43), we found that KCl induced slow frequency, long-lasting Ca2+ oscillatory waves that result from the sequential events of depolarization-induced Ca2+ influx, overload of the Ca2+ store, and subsequent Ca2+ release via sensitized RyRs. Because these Ca2+ signals do not appear to involve the IP3R, they serve as a useful comparison for agonist-induced Ca2+ oscillations.
For these studies, we have used mouse lung slices to simultaneously measure Ca2+ signaling and airway contraction. We found that the IP3R antagonist, 2-APB, could relax airways contracted with methacholine (MCh) or 5-hydroxytryptamine (5-HT) and inhibit agonist-induced Ca2+ oscillations. The inhibitory effect of 2-APB was also confirmed by its ability to inhibit IP3-induced Ca2+ release. On the other hand, the RyR antagonist, ryanodine had little effect on agonist-induced airway contraction or Ca2+ oscillations or IP3-induced Ca2+ release. However, ryanodine did inhibit KCl-induced slow Ca2+ oscillations. By contrast, tetracaine, another reported RyR antagonist, clearly inhibited agonist-induced airway contraction and Ca2+ signaling but had little effect on IP3-induced Ca2+ release or the Ca2+ content of the SR. Moreover, tetracaine significantly reduced the agonist-induced increase in Ca2+ sensitivity of the SMCs. Therefore, it appears that tetracaine, although often used as a RyR inhibitor in channel studies, inhibits agonist-induced airway contraction by modulating receptor-G protein interaction to decrease both IP3 production and Ca2+ sensitivity. From these data, we suggest that in mouse airway SMCs the IP3R serves as the primary receptor in the initiation and maintenance of intracellular Ca2+ signaling during agonist-induced airway contraction. An involvement of RyRs is not required in agonist-induced Ca2+ responses in normal physiological conditions. However, it is not known whether the RyR may become involved and change IP3R-mediated Ca2+ signaling of airway SMCs in disease conditions such as airway hyperresponsiveness.
MATERIALS AND METHODS
Materials.
Cell culture reagents were obtained from GIBCO-Invitrogen; Oregon Green 488 BAPTA-1 AM was obtained form Molecular Probes-Invitrogen; and caged-iso-Ins(1,4,5)P3/propionoxymethyl ester (PM) was obtained from Alexis Biochemicals (San Diego, CA) and dissolved in DMSO, aliquoted, and frozen at −80°C. Other reagents were obtained from Sigma-Aldrich or Calbiochem. Hanks-Balanced Salt Solution (HBSS) was supplemented with 20 mM HEPES buffer (sHBSS) and adjusted to pH 7.4.
Lung slices.
The preparation of lung slices has been previously described in detail (43). Briefly, male BALB/c mice (7–10 wk; Charles River Breeding Laboratories, Needham, MA) were killed by intraperitoneal injection of 0.3 ml of pentobarbital sodium (Nembutal) as approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School. After the thoracic cavity was opened, the lungs were inflated with ∼1.3-ml warm (37°C) ∼2% agarose in sHBSS via a catheter cannulated into the trachea. The agarose was followed by a final injection of a bolus of air (∼0.2 ml) to flush the agarose out of the airways and into the distal alveoli. After the agarose was gelled with cold sHBSS (∼4°C), the lung lobes were removed and sectioned into ∼140-μm thick slices with a vibratome. Slices were maintained in DMEM and antibiotics at 37°C and 10% CO2 for up to 3 days.
To conduct experiments at 37°C, the perfusion system and microscope were enclosed in a Plexiglas chamber and heated with a custom-built heater consisting of two heater elements (AF20-300-120-1D-K-10-3.1; Farnam Custom Products, Philadelphia, PA) coupled with circulating fans (1976K37; McMaster-Carr Supply, Chicago, IL). The temperature was monitored and regulated by a T thermocouple (HTTC36-T-14G-6; Omega Engineering, Stamford, CT) and a thermal controller (CC-A10; Farnam Custom Products).
Measurement of airway contraction and relaxation.
Lung slices were transferred to a large cover glass on a Plexiglas support, and a nylon mesh with a small hole cut in it, to expose the selected airway, was placed over the slice to hold it in position. A second, smaller cover glass, edged with silicone grease, was placed on the top of nylon mesh to form a sealed chamber. Experimental solutions, selected by electronic valves under computer control, were perfused through the chamber under gravity flow. Experiments were performed at either RT or 37°C. Phase-contrast images of airway contraction were collected with an inverted microscope (IX71; Olympus America, Melville, NY) with a ×10 objective, a zoom adapter, and a CCD camera (RS-170 video in conjunction with a frame grabber; Picolo; Euresys, Itasca, IL) at 1 frame every 2 s. Images were captured by Video Savant (IO Industries, London, Ontario, Canada), an image-acquisition software package, and stored on a hard drive. Airway contraction was measured by determining the change in lumen area, with respect to the initial lumen area, by pixel summing with custom-written macros (43).
Measurement of intracellular Ca2+ signaling.
Approximately 15 lung slices were placed in 2 ml of sHBSS containing 20 μM Oregon Green 488 BAPTA-1 AM, 100 μM sulfobromophthalein (an inhibitor of anion exchanges to reduce dye extrusion), and 0.1% Pluronic F-127 and incubated in the dark for 1 h at 30°C. The slices were washed in sHBSS with 100 μM sulfobromophthalein for an additional 30 min at 30°C to allow for dye deesterification. The lung slices were placed in the perfusion chamber as described above. The intracellular Ca2+ signaling was examined with either a custom-built video-rate confocal microscope or 2-photon scanning laser microscope as previously described (4, 43). Fluorescent images were recorded at a rate of 15 or 30 images per second. Fluorescence intensity of the SMCs was determined with respect to time (image number) from the average gray value of user-selected regions of interest (ROI; 6 × 6 pixels) within the cells with custom-written software. Fluorescence intensity was expressed in absolute units or in relative terms as a fluorescence ratio (F/F0) normalized to the initial fluorescent intensity (F0).
Flash photolysis of caged-IP3.
Flash photolysis of caged-IP3 was used to experimentally increase the [IP3]i. Lung slices were initially loaded with Oregon Green 488 BAPTA-1 AM as described above and subsequently incubated with 2 μM caged-IP3 for 1 h in sHBSS containing 0.1% Pluronic F-127 and 100 μM sulfobromophthalein followed by deesterification for 30 min in sHBSS containing 100 μM sulfobromophthalein. The details of the flash photolysis setup have also been previously described (2). Briefly, a UV flash was generated by an electronic shutter from a filtered (330 caged-IP3 nm) mercury arc lamp and focused as point source into the microscope. The intensity of flash was regulated by neutral density filters. The spot size could be adjusted with an iris diaphragm.
Preparation of Ca2+-permeabilized lung slices.
To clamp the [Ca2+]i of airway SMCs at a constant level, it is necessary to increase the permeability of the plasma membrane to Ca2+. Typically, this has been achieved by the application of detergents or toxins. Unfortunately, these agents frequently increase membrane permeability to other cellular constituents. To overcome this problem, we have developed an alternative approach using caffeine and ryanodine to only increase the permeability of cell membrane to Ca2+; the use and verification of this method has been previously described in detail (4). Briefly, lung slices were simultaneously exposed to 20 mM caffeine and 50 μM ryanodine for 5 min. This treatment is believed to lock the RyRs in the open state and thereby brings about the depletion of the SR Ca2+ store. This, in turn, leads to a persistent Ca2+ influx via store-operated channels to elevate the [Ca2+]i. As a result, the extracellular Ca2+ concentration is used to determine the [Ca2+]i. The constant elevated [Ca2+]i of permeabilized cells was monitored and confirmed by Ca2+ imaging. Lung slices treated with caffeine and ryanodine remain viable for experimentation for >5 h, and during this time the effect of the treatment was irreversible.
Data analysis.
Results are expressed as means ± SE. Statistical significance was determined by using Student's or paired Student's t-test.
RESULTS
Experiments were only performed on intact airways (100- to 200-μm diameter, midposition of the respiratory tract; Ref. 5) with an epithelium displaying active ciliary beating and with a lumen free of agarose (Fig. 1A). We examined the airway responses to two different agonists (MCh and 5-HT) and KCl to avoid the possibility of characterizing mechanisms that were only specific to single agonist. In addition, because experiments have the best chance of detecting changes in behavior if they are performed in the responsive region of the concentration-response curves, we used agonist concentrations (200 nM) that we (2, 6, 43) have previously found to induce nonsaturated responses of airway contraction and SMC Ca2+ signaling.
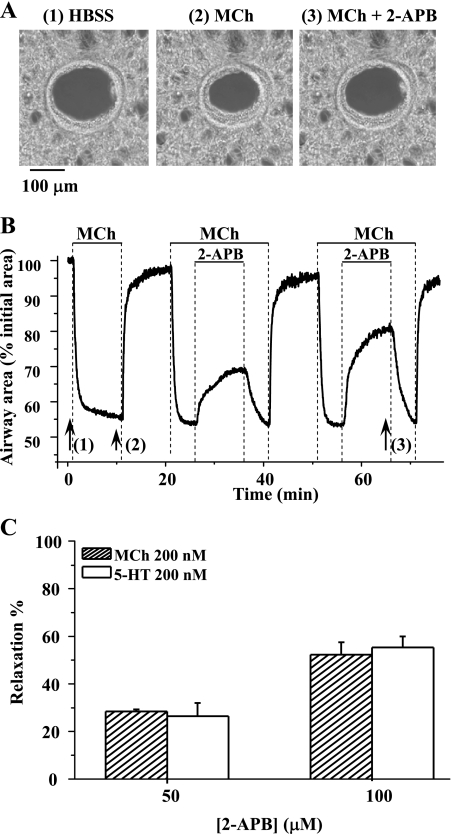
Fig. 1.
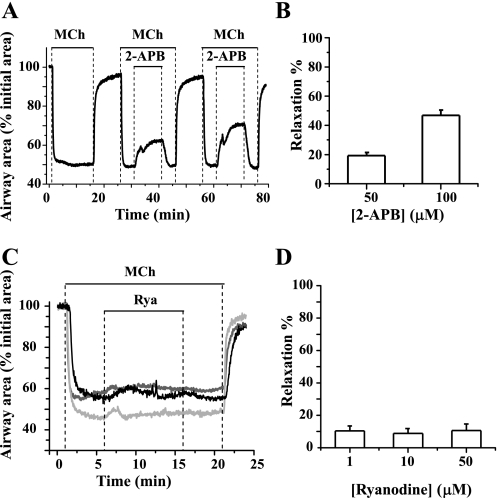
The effect of 2-aminoethoxydiphenyl borate (2-APB) on agonist-induced airway contraction. A: phase-contrast images of the same airway (at times indicated by numbered arrows in B) showing 1) the relaxed initial state of the airway, 2) the contractile state of the airway induced by 200 nM methacholine (MCh), and 3) the relaxation induced by 100 μM 2-APB. B: the change in airway area with respect to time showing the airway contraction in response to 200 nM MCh and the subsequent relaxation induced by either 50 or 100 μM 2-APB. The magnitude of airway relaxation increased as the concentration of 2-APB increased. C: summary of the relaxation induced in airways contracted with 200 nM MCh or 200 nM 5-hydroxytryptamine (5-HT) by 50 and 100 μM 2-APB. The magnitude of the relaxation was measured after 10 min of 2-APB exposure and is expressed as a percentage of the contracted luminal area induced by agonists. Each column represents the mean ± SE from 6 different experiments on different slices from 3 mice.
Effect of 2-APB on agonist-induced airway contraction.
To explore the role of the IP3R in agonist-induced airway contraction, the airway responses to the IP3R antagonist 2-APB were examined. When exposed to 200 nM MCh, the airways responded by contracting and reducing their luminal area within 2 min, after which the contractile state stabilized (at 40–50% of initial luminal area) while MCh was perfused. Removal of MCh resulted in airway relaxation (Fig. 1, A and B). When the same airways, again contracted with MCh, were exposed to 2-APB, they quickly relaxed; relaxation was fast in the first 2 min but subsequently slow (Fig. 1B). The airways recontracted when 2-APB was removed. The amount of relaxation induced by 2-APB is expressed as the percentage increase in luminal area normalized to the contracted area induced by MCh. After 10 min, the airways relaxed 28.44 ± 0.83 and 52.33 ± 5.13% in response to 50 and 100 μM 2-APB, respectively (Fig. 1C). Similar responses of airway contraction (52.0 ± 8.0% related to initial area) and 2-APB-induced relaxation (26.43 ± 5.51 and 55.25 ± 4.72% of contracted area at 50 and 100 μM) were obtained when the contractile agonist was changed to 5-HT (200 nM) (Fig. 1C). These results suggest that the activity of the IP3R contributes to airway contraction.
Effect of ryanodine and tetracaine on agonist-induced airway contraction.
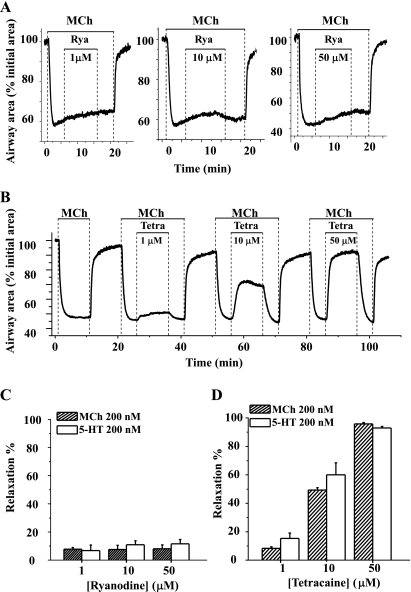
To similarly explore the role of the RyR in agonist-induced airway contraction, the airway responses to antagonists of the RyR (ryanodine and tetracaine) were examined. After the airways were contracted with 200 nM MCh, the addition of ryanodine, at concentrations ranging from 1 to 50 μM, had little effect on the airway contractile response (Fig. 2). During the course of these experiments, the airway showed a small, steady relaxation rate (∼8% over 10 min), but this process was not altered by the addition of increasing concentrations of ryanodine (Fig. 2, A and C). Similarly, ryanodine (1, 10, or 50 μM) had little effect on 5-HT-induced (200 nM) contractile responses (Fig. 2C). These results suggest that the RyR is not involved in agonist-induced airway contraction.
Fig. 2.
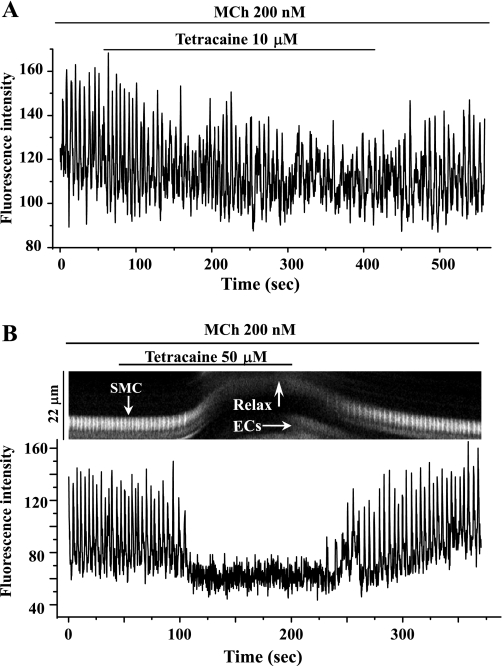
Effects of ryanodine and tetracaine on agonist-induced airway contraction. Representative experiments showing airway contraction in response to 200 nM MCh and the subsequent responses to different concentrations of ryanodine (Rya; A; 1, 10, or 50 μM) or tetracaine (Tetra; B; 1, 10, or 50 μM) are shown. At all concentrations tested, ryanodine had no obvious effect on MCh-induced contraction. Because the effects of ryanodine are irreversible, 3 individual lung slices were used to test the effects of each concentration of ryanodine. By contrast, tetracaine relaxed MCh-contracted airways in a concentration-dependent manner. Because the effects of tetracaine are reversible, the same lung slice was used to examine the effects of multiple concentrations of tetracaine. A summary of the airway responses to ryanodine (C) and tetracaine (D) in the presence of MCh or 5-HT is shown. Relaxation was measured after 10 min of exposure to ryanodine or tetracaine and is expressed as a percentage of the contracted luminal area induced by the specific agonist. Each column represents the mean ± SE from ≥6 different experiments on different slices from >3 mice.
Surprisingly, in contrast to the effects of ryanodine, tetracaine, an antagonist used in other studies to implicate the RyR in airway contraction, induced a significant relaxation of both MCh- and 5-HT-induced airway contraction. In response to tetracaine, contracted airways quickly relaxed to a plateau level within 3 min in a concentration-dependent manner; a near full relaxation was induced by 50 μM tetracaine (Fig. 2, B and D). This tetracaine effect was readily reversible; the airways quickly recontracted when tetracaine was removed (Fig. 2B).
Effect of 2-APB on agonist-induced Ca2+ oscillations.
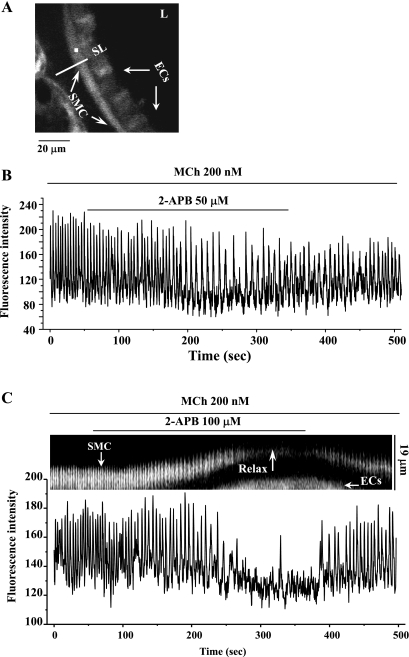
In response to 200 nM MCh, airway SMCs displayed an initial elevation of [Ca2+]i followed by cyclic increases of [Ca2+]i in the form of oscillatory Ca2+ waves. These repetitive Ca2+ oscillations usually originated from one end of the SMC and propagated the full length of the cell. The frequency of Ca2+ oscillations stabilized after ∼2 min of MCh exposure. These changes in the intracellular Ca2+ signaling have been previously shown to closely correlate with the contractile response of the airway SMCs (2, 43).
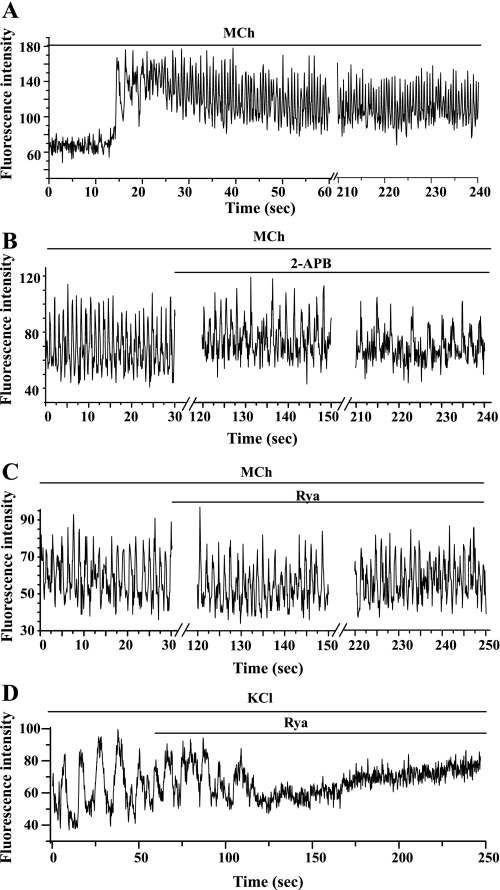
The subsequent exposure to 2-APB gradually slowed the Ca2+ oscillations (from 20.5 ± 1.8 to 15.8 ± 2.6 min−1 after 5 min of exposure at 50 μM, n = 4 experiments from different airways from 3 mice; Fig. 3B) or nearly stopped the Ca2+ oscillations (at 100 μM; Fig. 3C). When 2-APB was removed, the Ca2+ oscillations resumed and gradually increased their frequency to approach the original rate after 3 min. The time course of this inhibition (∼2 min) and restoration of the Ca2+ signaling correlates with the time taken for the airways to relax and recontract in response to 2-APB (Fig. 3C). A similar cessation of 5-HT-induced (200 nM) Ca2+ oscillations by 100 μM 2-APB was observed in three different airways from two mice.
Fig. 3.
The effect of 2-APB on the Ca2+ signaling of airway smooth muscle cells (SMCs) induced by 200 nM MCh. A: a fluorescence image of part of an airway obtained by confocal microscopy. L, lumen; ECs, epithelial cells; SL, scan line. B: the intracellular Ca2+ signaling occurring within airway SMC [recorded from a region of interest (ROI) of about 6 × 6 pixels as represented by the white square indicated in A] in response to 200 nM MCh and 50 μM 2-APB; a representative trace from 4 slices from 3 mice. C: line-scan (top, constructed by sequentially aligning the pixels from the SL shown in A) and the Ca2+ signaling (bottom) showing the effect of 100 μM 2-APB on 200 nM MCh-induced contraction and Ca2+ signaling of airway SMCs. Representative data are from 8 different slices from 4 mice. MCh induced high-frequency Ca2+ oscillations within the SMC and airway contraction. 2-APB at 50 μM (B) or 100 μM (C) either slowed or nearly stopped the Ca2+ oscillations and induced relaxation (Relax). On 2-APB removal, the Ca2+ oscillations resumed.
Effect of ryanodine and tetracaine on agonist-induced Ca2+ oscillations.
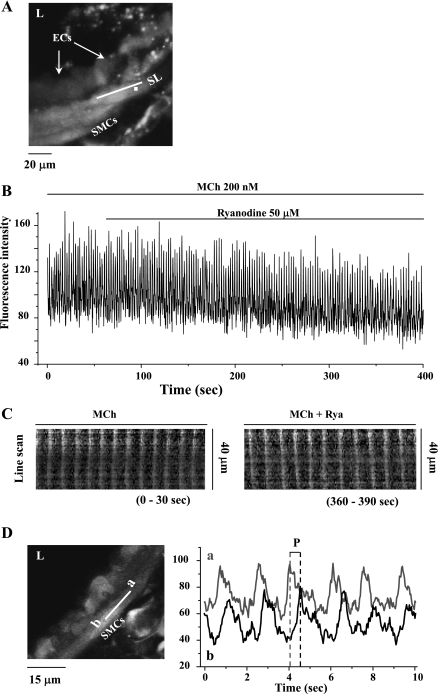
In keeping with the observation that ryanodine had no effect on airway contraction, ryanodine (examined with 1, 10, or 50 μM) had no significant effect on the frequency of MCh-induced Ca2+ oscillations (Fig. 4B) during the 5 min of observation. The Ca2+ oscillation frequency was 20.9 ± 2.8 min−1 in MCh alone compared with 19.5 ± 2.3 min−1 after 5-min exposure of 50 μM ryanodine in presence of MCh (n = 10 different airways from 5 mice). Similarly, the propagation of the oscillatory Ca2+ wave, as examined by line-scan analysis along the longitudinal axis of SMC (Fig. 4C), was not altered by 5 min of ryanodine exposure. Likewise, the propagation velocity of Ca2+ wave, determined by the time taken for the wave to propagate between two ROIs (e.g., Fig. 4D, left, “a” and “b”), was not influenced by ryanodine. In response to 200 nM MCh, Ca2+ wave velocity was 27.63 ± 2.76 and 27.60 ± 3.37 μm/s in the absence or presence of 50 μM ryanodine, respectively (n = 5 SMCs from different slices from 2 mice). Ryanodine also had no significant effect on the frequency of the Ca2+ oscillation of SMCs induced by 200 nM 5-HT. The Ca2+ oscillation frequency was 20.2 ± 2.7 min−1 before and 19.2 ± 3.0 min−1 after 5-min exposure to 50 μM ryanodine (n = 4 airways from different slices from 2 mice).
Fig. 4.
The effect of 50 μM ryanodine on Ca2+ signaling induced by 200 nM MCh in airway SMCs. A: a fluorescence image of part of an airway obtained by confocal microscopy under resting conditions. B: changes in intracellular Ca2+ concentration ([Ca2+]i) with respect to time (from a ROI; white square in A) in a SMC in response to 200 nM MCh and 50 μM ryanodine. MCh induced high-frequency Ca2+ oscillations that were unaffected by the addition of ryanodine. Representative data are from ≥10 different slices from 5 mice. C: line-scan plots of the propagated changes in [Ca2+]i (Ca2+ waves) following exposure to MCh (0–30 s) and ryanodine (360–390 s). Line-scans were constructed by sequentially aligning the pixels from the SL shown in A, parallel to the longitudinal axis of the SMC. D: the propagation velocity of the Ca2+ waves was measured by determining the period (P) between sequential changes in Ca2+ in regions “a” and “b” (right) of the same SMCs (left). Ryanodine (50 μM) had no significant effect on the frequency of MCh-induced Ca2+ oscillations, the appearance of the Ca2+ waves, or the propagation velocity of the Ca2+ waves.
By contrast, but consistent with its relaxant action, tetracaine at 10 μM slowed the frequency of MCh-induced (200 nM) Ca2+ oscillations within 2 min (Fig. 5A; from 23.2 ± 3.5 to 14.6 ± 1.0 min−1 after 5 min of exposure). In addition, the period and waveform of these slowed Ca2+ oscillations also became irregular. Higher concentrations (50 μM) of tetracaine stopped the Ca2+ oscillations after 1 min. Airway relaxation was tightly coupled to this cessation of the Ca2+ oscillations (Fig. 5B). The Ca2+ oscillations were restored to normal on tetracaine removal (n = 4, from different slices from 3 mice). A similar complete inhibitory action of 50 μM tetracaine on 5-HT-induced Ca2+ oscillations was observed (data not shown).
Fig. 5.
The effect of tetracaine on Ca2+ signaling in airway SMCs induced by MCh. High-frequency Ca2+ oscillations induced in airway SMCs by 200 nM MCh were either slowed by 10 μM tetracaine (A) or stopped by 50 μM tetracaine (B, bottom). The line-scan plot (B, top) shows that the cessation of the Ca2+ oscillations (white vertical lines) is associated with the relaxation of the SMC (upward deflection of oscillation trace). The resumption of the Ca2+ oscillation is correlated with the recontraction of the SMC. Representative data are from 4 different slices from 3 mice.
Second messenger mediating Ca2+ oscillations.
In many cases, Ca2+ oscillations, involving repetitive Ca2+ release and reuptake from the SR, are mediated by agonist-induced production of IP3 acting on the IP3R. A similar process of sensitizing the RyR with the second messenger cADPR can also occur and has been proposed as a regulatory mechanism of agonist-induced Ca2+ oscillations in airway SMCs. Because both the IP3R and RyR are implicated in airway SMC Ca2+ signaling, we performed studies to determine the predominant messenger mediating agonist-induced Ca2+ oscillations in mouse airway SMCs.
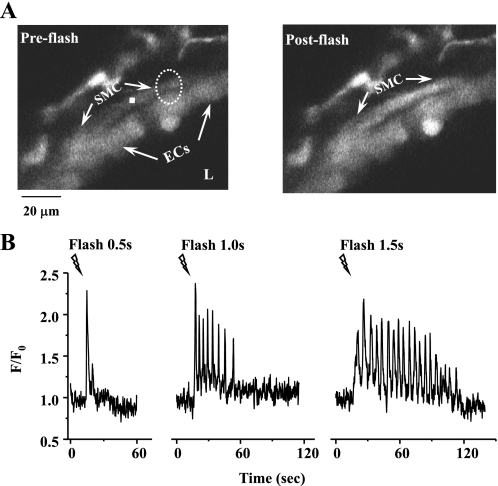
We examined the effect of the photolytic release of IP3 (uncaging) on airway SMCs and found that the increase in [IP3]i, following exposure to UV light, induced repetitive Ca2+ waves that propagated from the illuminated area to the rest of the SMC (Fig. 6A). The frequency and number of these Ca2+ oscillations was increased by an increase in the UV exposure time (Fig. 6B). These IP3-induced Ca2+ oscillations mimicked concentration-dependent agonist-induced Ca2+ oscillations and were accompanied by a contractile response.
Fig. 6.
Effect of photolytic release of inositol 1,4,5-trisphosphate (IP3) from caged-IP3 on Ca2+ signaling of airway SMCs. A: fluorescent images of part of an airway obtained by confocal microscopy showing the intracellular Ca2+ signaling of airway SMC before (left) and after (right) the UV flash. Dashed white oval represents the position and size of the zone of UV illumination. B: intracellular Ca2+ oscillatory waves induced by photolysis of caged-IP3. When the flash time was extended from 0.5 to 1.5 s, the Ca2+ signaling increased from a single Ca2+ transient to multiple Ca2+ oscillations. F/F0, fluorescence ratio.
Because cADPR is not cell-permeable, similar sensitization experiments of the RyR with cADPR were not possible. Consequently, we took the opposite approach and applied the cell-permeable competitive inhibitor of cADPR, 8-bromo-cADPR (8-Br-cADPR), and examined its effect on MCh-induced SMC Ca2+ signaling and airway contraction. No significant changes in the Ca2+ oscillations or contractile responses of the airways were observed during 20-min incubation with 100 μM 8-Br-cADPR (data not shown). Collectively, these results with caged-IP3 and 8-Br-cADPR suggest that IP3 is the primary intracellular mediator responsible for the generation of Ca2+ oscillations in mouse airway SMCs.
Effect of 2-APB on IP3-induced Ca2+ release.
In view of our earlier result that 2-APB inhibited agonist-induced Ca2+ oscillations (Fig. 3), we investigated the effect of 2-APB on IP3-induced Ca2+ release. It is important to note that in these experiments, the duration of the UV flash used for photolysis was reduced to 0.2 s so that it only induced a single Ca2+ wave within the SMC (Fig. 6B). This adjustment allowed us to release a small but similar amount of IP3 multiple times in the same SMC and thereby compare changes in the Ca2+ signaling of the same SMC before and after application of 2-APB.
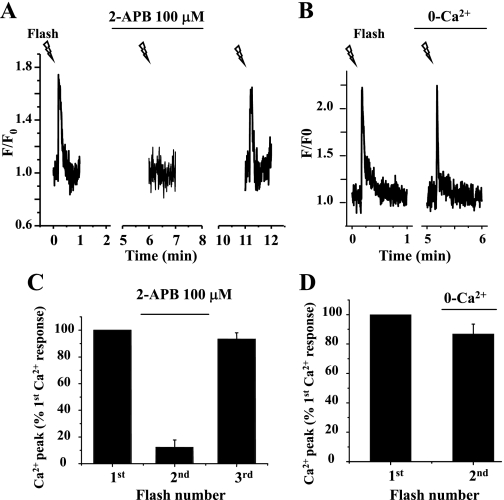
Under these conditions, the first UV flash induced a single Ca2+ transient in the SMC (Fig. 7). However, after 3 min of exposure to 100 μM 2-APB, the elevation of [Ca2+]i in response to a second photolysis of caged-IP3 was virtually eliminated, and no Ca2+ wave was observed in SMCs. When the 2-APB was removed, the third UV flash of similar intensity once again induced a transient increase in [Ca2+]i and a propagating Ca2+ wave that was indistinguishable from that induced by the first UV flash (Fig. 7, A and C).
Fig. 7.
Effect of 2-APB on Ca2+ waves induced by the flash photolysis of caged-IP3. The Ca2+ response of the SMC induced by the repetitive flash photolysis (0.2-s exposure; lightning bolts) of caged-IP3 in lung slices incubated with 100 μM 2-APB (A) or zero extracellular Ca2+ (0-Ca2+; B) for 3 min is shown. Summary of the changes in SMC Ca2+ signaling (peak florescence intensity) induced by IP3 in the presence of 2-APB (C) or absence of extracellular Ca2+ (D) is shown. Exposure to 2-APB virtually abolished the Ca2+ response to IP3, whereas the removal of extracellular Ca2+ had little effect on IP3-induced Ca2+ signaling. Data represent the means ± SE from 5 different slices from 2 mice.
In addition to its action on the IP3R, 2-APB also appears to inhibit store-operated Ca2+ entry (SOC) (11), and this could reduce the amount of Ca2+ stored within the SR. Consequently, to rule out the possibility that emptying of internal Ca2+ stores during the course of experiments was responsible for the abolishment of IP3-induced Ca2+ release by 2-APB, we repeated the above experiments in the absence of Ca2+ entry by the removal of extracellular Ca2+. As shown in Fig. 7, B and D, the absence of Ca2+ entry had little effect on the magnitude of the Ca2+ transient induced by a second release of IP3. However, the duration of the IP3-induced Ca2+ transient was shortened in absence of Ca2+ entry (Fig. 7B). This result indicated that some emptying of Ca2+ store had occurred due to Ca2+ leakage, and this resulted in the earlier termination of the Ca2+ release from SR. An alternative explanation is that Ca2+ removal from the cytoplasm was enhanced by the increased gradient between the intracellular and extracellular Ca2+ concentration. A second possible action of 2-APB is the inhibition of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), which again could empty Ca2+ stores. However, we found by using caffeine to induce the release of internal Ca2+ that the Ca2+ store retained 85.1 ± 3.7% of its Ca2+ charge after 5 min in 2-APB (by comparing the peak value of the caffeine-induced Ca2+ wave before and after exposure to 2-APB, n = 4 different airways from 2 mice). These results are consistent with the hypothesis that 2-APB acts primarily by inhibiting IP3-induced Ca2+ release via the IP3R.
Effect of ryanodine and tetracaine on IP3-induced Ca2+ release.
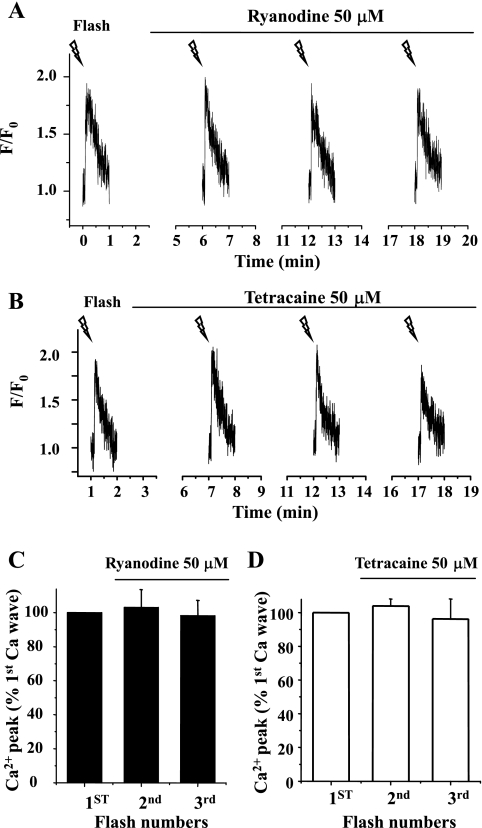
The idea that IP3 is releasing Ca2+ via the IP3R was further corroborated by the observations that the extended incubation of airway SMCs with 50 μM ryanodine (>5 min; Fig. 8A) and 50 μM tetracaine (Fig. 8B) had no effect on repetitive flash-induced Ca2+ transients or the ability to propagate Ca2+ waves in SMC. These results also indicated that the RyRs do not contribute to the Ca2+ transients initiated by IP3 and were consistent with the action of ryanodine on Ca2+ oscillations. In addition, it also implies that the inhibitory effect of tetracaine on agonist-induced Ca2+ oscillations was not mediated by inhibiting Ca2+ release via the IP3R. Alternative explanations are that tetracaine leads to the depletion of the internal Ca2+ store (25) or that it decreases the production of IP3.
Fig. 8.
Effect of ryanodine and tetracaine on Ca2+ waves induced by flash photolysis of caged-IP3. Representative experiments showing the Ca2+ changes induced by repetitive flash-photolysis of caged-IP3 of airway SMCs in lung slices incubated with 50 μM ryanodine (A) and 50 μM tetracaine (B) are shown. Summary of changes in the Ca2+ signals (peak fluorescence intensity, expressed as the ratio of the Ca2+ response before and after drug exposure) induced by IP3 in the presence of 50 μM ryanodine (C) or 50 μM tetracaine (D) is shown. Neither ryanodine nor tetracaine blocked the Ca2+ increases induced by IP3. Each column represents the mean ± SE from 5 different slices from 2 mice.
Effect of tetracaine on refilling of the internal Ca2+ store.
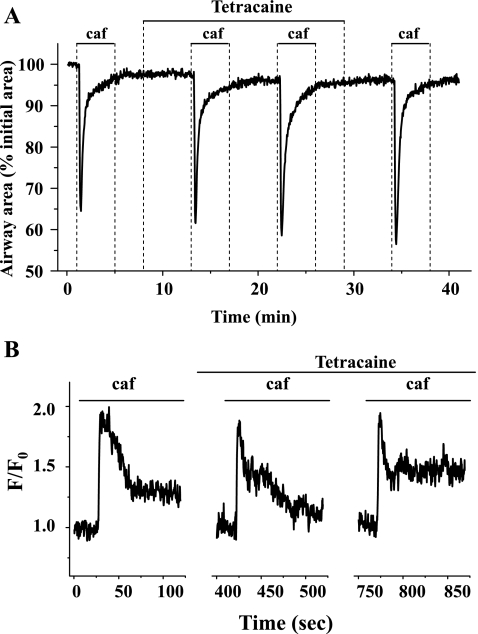
To investigate the effect of tetracaine on the Ca2+ capacity of SR, we examined the contractile responses and Ca2+ signaling of airway SMCs to repetitive stimuli of caffeine (Fig. 9). In these experiments, we found that the airway contractile response to 20 mM caffeine was similar before, during, or after exposure of the airways to 50 μM tetracaine (Fig. 9A). A comparable set of results was obtained with respect to the Ca2+ release induced by caffeine (Fig. 9B). Tetracaine (50 μM) was found to have little effect on the caffeine-induced Ca2+ transients. These results indicated that tetracaine did not reduce the Ca2+ capacity of the SR, and this argues against the possibility that tetracaine inhibited agonist-induced Ca2+ oscillations by the depletion of internal Ca2+ stores.
Fig. 9.
The effect of tetracaine on caffeine (caf)-induced Ca2+ release. Representative experiments demonstrating contraction (A) and Ca2+ signaling (B) of airway SMCs induced by repetitive exposure to 20 mM caffeine in the presence of 50 μM tetracaine are shown. Tetracaine had no significant effect on caffeine-induced contraction or Ca2+ release. Representative data are from 4 different slices from 2 mice.
So far, we have demonstrated that tetracaine does not inhibit Ca2+ oscillations by inactivating IP3Rs or by depleting Ca2+ stores. The alternative possibility that tetracaine is acting as a RyR antagonist to reduce agonist-induced Ca2+ oscillations also appears unlikely in view of our findings that ryanodine, a well-documented specific antagonist of the RyR, had no effect on agonist-induced Ca2+ oscillations. As a result, we are forced to hypothesize that, in intact SMCs, tetracaine acts via a mechanism other than RyR inhibition even though tetracaine may serve as a RyR antagonist in studies with isolated channels or cell extracts.
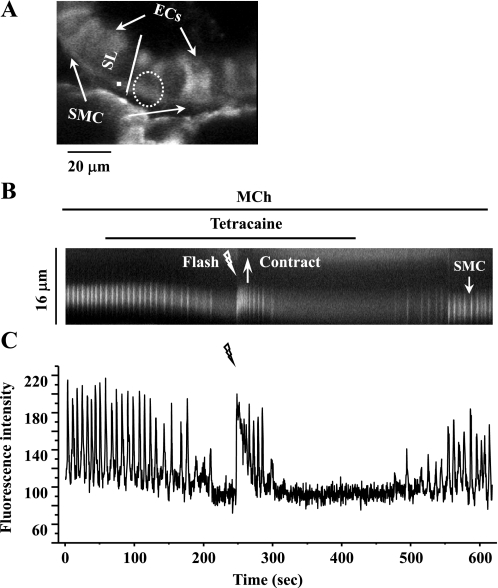
Consequently, we hypothesized that tetracaine decreases the amount of IP3 available to drive the Ca2+ oscillations. To test this idea, we used photolysis to increase the [IP3]i in cells in which the agonist-induced oscillations had been inhibited with tetracaine (Fig. 10). In response to flash photolysis, the Ca2+ oscillations were reinitiated and lasted for ∼1 min. This Ca2+ response was associated with a transient recontraction of the SMCs.
Fig. 10.
Effect of flash photolysis of caged-IP3 on SMCs treated with MCh and tetracaine. A: a fluorescence image of part of an airway obtained by confocal microscopy under resting conditions. Dashed white oval represents the position and size of the zone of UV illumination. The intracellular Ca2+ signaling of a SMC in response to a UV flash (1.0 s) in the presence of 200 nM MCh and 50 μM tetracaine is represented by a line-scan plot (B), constructed by sequentially aligning the pixels along a SL (white line indicated in A) across the SMC and ECs and changes in [Ca2+]i in respect to time (C; from a ROI, white square in A). Photolysis of caged-IP3 reinitiated Ca2+ oscillations and a transient contraction in the presence of MCh and tetracaine. Representative data are from 4 different airways from 2 mice.
Effect of tetracaine on agonist-induced contraction of permeabilized SMCs.
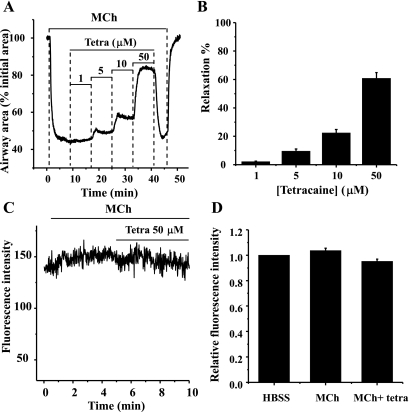
The idea that tetracaine acts by another mechanism is further supported by the observation that a greater airway relaxation is induced by tetracaine compared with 2-APB (at low concentrations; Figs. 1B and 2B). The fact that the Ca2+ oscillations are slowed to a similar extent by tetracaine and 2-APB (Figs. 3B and 5A) implies that the Ca2+ sensitivity of the airway has been decreased by tetracaine. To investigate this possibility, we examined the responses of lung slices permeabilized to Ca2+ with caffeine and ryanodine.
After permeabilizing the SMCs to Ca2+, their [Ca2+]i was set at an elevated level by perfusion with sHBSS (extracellular Ca2+ = 1.3 mM). Under conditions of elevated [Ca2+]i, these permeabilized mouse airways are fully relaxed. This response is fully described in previous work (4) and has been ascribed to a Ca2+-dependent decrease in Ca2+ sensitivity. Importantly, during the subsequent contractile and relaxant responses induced by agonists or tetracaine, there was no significant change in the intracellular Ca2+ signal in these permeabilized SMCs (Fig. 11, C and D). When the permeabilized airways were exposed to MCh, they responded with a significant contraction. Because the Ca2+ levels did not change, this contraction is attributed to a MCh-induced increase in Ca2+ sensitization. In the presence of MCh, exposure to tetracaine relaxed the airway in a concentration-dependent manner, from 2.10 ± 0.42% at 1 μM tetracaine to 60.78 ± 4.09% at 50 μM tetracaine (Fig. 11, A and B). This relaxation indicated that tetracaine also acts by reducing airway Ca2+ sensitivity.
Fig. 11.
The effect of tetracaine on MCh-induced contraction and the [Ca2+]i of permeabilized airway SMCs. The airway SMCs were permeabilized to Ca2+ by treatment with 20 mM caffeine and 50 μM ryanodine and washed with HBSS supplemented with 20 mM HEPES buffer (sHBSS) for 10 min. A representative trace (A) and summary data (B) showing the effect of tetracaine on the airway contracted with 200 nM MCh are shown. Increasing concentrations of tetracaine relaxed the airway. The extent of relaxation is expressed as a percentage of contracted lumen area induced by MCh. Data represent the means ± SE from 4 different slices from 2 mice. A representative trace (C) and summary data (D) demonstrate that in permeabilized SMCs, the [Ca2+]i remained at a high constant level throughout the experiments (the [Ca2+]i was expressed as ratio of the levels before and after drug exposure in D). Data represent the means ± SE from 4 different slices from 2 mice.
Effect of ryanodine and tetracaine on KCl-induced airway contraction and Ca2+ signaling of SMCs.
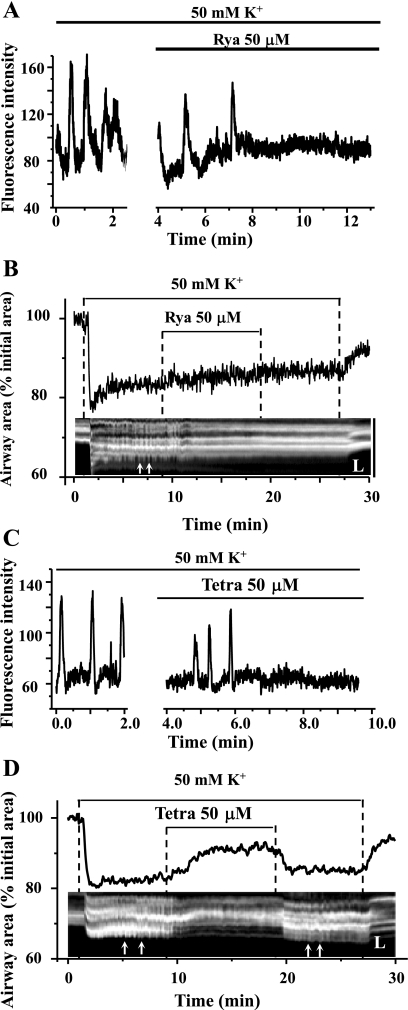
Although ryanodine and tetracaine had no effect on IP3-induced Ca2+ release in airway SMCs, it is necessary to demonstrate, if we are to imply that RyRs are not involved, that these antagonists can block the Ca2+ signals and contractile responses of SMCs when RyRs do play a major role in the underlying mechanism. Therefore, we examined the effect of ryanodine and tetracaine on the Ca2+ and contractile responses of SMCs induced by membrane depolarization. We (43) have previously demonstrated, and as shown in Fig. 12A, that membrane depolarization in response to KCl (50 or 100 mM) induced very slow frequency and prolonged Ca2+ oscillations (2–3 min−1). We concluded these Ca2+ oscillations resulted from the overloading of the SR with Ca2+ due to a continuous Ca2+ influx stimulated by depolarization. When the SR Ca2+ content was high, spontaneous RyR openings were amplified into whole cell Ca2+ waves by CICR. In presence of 50 μM ryanodine, these KCl-induced Ca2+ oscillations slowed down and stopped after 4 min, whereas the basal level of [Ca2+]i gradually increased (Fig. 12A). Tetracaine also blocked the KCl-induced Ca2+ oscillations, but this stoppage was not accompanied by an elevation of baseline level of Ca2+ (Fig. 12C).
Fig. 12.
Effect of ryanodine and tetracaine on KCl-induced Ca2+ oscillations and contraction of airway SMCs. A and C: representative data showing that slow frequency Ca2+ oscillations are induced in airway SMCs in response to 50 mM KCl and that these Ca2+ oscillations are inhibited by 50 μM ryanodine (A) or 50 μM tetracaine (C). The baseline [Ca2+]i remained elevated in response to ryanodine but not tetracaine. B and D: representative data showing effect of ryanodine and tetracaine on KCl-induced changes in airway lumen area (top traces) and unsynchronized transient SMC contractions or twitching (small white arrows in the bottom line-scan image). Tetracaine had a significant relaxant effect on KCl-induced airway contraction, whereas ryanodine did not. Both tetracaine (reversibly) and ryanodine (irreversibly) inhibited SMC twitching. The line-scan images were obtained from phase-contrast images along a SL across the airway wall and lumen. Representative data are from 4 different slices from 2 mice.
These slow frequency, unsynchronized KCl-induced Ca2+ oscillations resulted in the twitching of individual SMCs and, because of a lack of a coordinated contractile effort, this resulted in a minimal airway contraction (∼20% reduction of luminal area). In the presence of either ryanodine or tetracaine, the twitching of individual SMC stopped. However, the airway only relaxed in the presence of tetracaine (Fig. 12, B and D). These responses are consistent with the observation that ryanodine, but not tetracaine, induced an elevation of basal [Ca2+]i after the cessation of the KCl-induced Ca2+ oscillations. In keeping with the irreversible binding of ryanodine to the RyR, twitching of the SMCs did not reoccur on the removal of ryanodine (Fig. 12B). By contrast, SMC twitching resumed after tetracaine removal, a result indicating a reversible weak binding to the RyR (Fig. 12D). These results demonstrate the effectiveness of ryanodine and tetracaine in blocking KCl-induced Ca2+ signaling.
Influence of temperature on the activity of IP3Rs and RyRs.
To rule out the possibility that the insensitivity of the RyR to antagonists is a temperature-dependent effect, we examined the contribution of the IP3Rs and RyRs to MCh-induced Ca2+ oscillations and contraction in airway SMCs at 37°C.
In response to 200 nM MCh at 37°C, the extent of airway contraction was similar to that observed at RT (Fig. 13A; ∼45%; Ref. 3). This agonist-induced contraction was associated with a similar elevation of [Ca2+]i in the form of Ca2+ oscillations (Fig. 14A). However, the frequency of the Ca2+ oscillations induced by 200 nM MCh at 37°C was much higher (∼67 min−1) than that at RT (∼20 min−1) (3). These repetitive Ca2+ waves started frequently from one end of the SMC and propagated along the entire cell. The propagating velocity of the waves was 94.6 ± 6.9 μm/s (n = 5 different slices from 3 mice), much greater than that at RT (P < 0.05). The fast Ca2+ oscillations were clearly observed in the presence of MCh and persisted while MCh was present for >5 min.
Fig. 13.
Influence of temperature on the action of 2-APB and ryanodine. Representative responses showing the change in airway area with respect to time on exposure to 200 nM MCh and subsequently 50 and 100 μM 2-APB (A) or 1 μM (light gray line), 10 μM (gray line), or 50 μM (black line) ryanodine (C) at 37°C are shown. Summaries of the relaxation induced in airways contracted with 200 nM MCh by 50 and 100 μM 2-APB (B) or 1, 10, or 50 μM ryanodine (D) at 37°C. The extent of relaxation (percent) was measured after 10 min of 2-APB or ryanodine exposure. Each column represents the mean ± SE from ≥5 different experiments from 3 mice.
Fig. 14.
Influence of temperature on MCh-induced Ca2+ signaling. Representative responses showing the intracellular Ca2+ signaling of airway SMCs in response to 200 nM MCh (A) and subsequently 100 μM 2-APB (B) or 50 μM ryanodine (C) at 37°C are shown. MCh induced rapid (∼60 min−1) and sustained Ca2+ oscillations. After the MCh-induced Ca2+ oscillations had stabilized (∼3 min), 2-APB or ryanodine was added to the lung slice. The Ca2+ oscillations were inhibited by 2-APB (B) but unaffected by ryanodine (C) after 3 min. Representative data are from ≥6 slices from 3 mice for each antagonist. In contrast, Ca2+ oscillations induced by KCl (50 mM) were rapidly stopped by 50 μM ryanodine (D), and this was associated with an increase in baseline Ca2+. Representative data are from ≥5 different airways from 3 mice.
When the IP3R antagonist 2-APB was added to the MCh (200 nM)-contracted airway SMCs at 37°C, a concentration-dependent relaxation was observed (Fig. 13, A and B). A relaxation of 19.3 ± 2.1 and 46.8 ± 3.8% was induced by 50 and 100 μM 2-APB, respectively, after 10 min. In keeping with this relaxation response, 100 μM 2-APB, at 37°C, slowed the fast MCh-induced Ca2+ oscillations of airway SMCs from 69.3 ± 5.2 to 33.5 ± 4.3 min−1 after 3 min of exposure (Fig. 14B; n = 6 different airways from 3 mice). When 2-APB was removed, the frequency of the Ca2+ oscillations gradually increased to the original rate. Although 2-APB reduced both MCh-induced airway contraction and Ca2+ oscillations at 37°C, this inhibitory action was reduced compared with its action at RT (compare Figs. 1 and 3 with Figs. 13 and 14; P < 0.05 for 50 and 100 μM 2-APB). This apparent attenuation of the inhibitory effect of 2-APB most likely results from the relative increase in the frequency of the Ca2+ oscillations at an equal concentration of MCh at 37°C.
When airways contracted with MCh (200 nM) at 37°C were exposed to ryanodine (1, 10, or 50 μM), airway contraction was not substantially affected by any concentration of ryanodine (Fig. 13, C and D). Similarly, 50 μM ryanodine did not significantly alter the frequency of MCh-induced Ca2+ oscillations (71.7 ± 6.2 min−1) after 3 min (68.5 ± 5.0 min−1, n = 9 different airways from 4 mice; Fig. 14C) following the propagation of >200 Ca2+ waves along the SMCs. This is equivalent to a period of ∼10 min at RT where the Ca2+ oscillations have a frequency of ∼20 min−1. These results suggest the RyR does not take part in nor is influenced by agonist-induced Ca2+ oscillations.
However, over a longer time (>5-min exposure to 50 μM ryanodine at 37°C), the Ca2+ waves associated with the Ca2+ oscillations became less regular and did not propagate the full length of the cell. In addition, the baseline [Ca2+]i was slowly elevated. These changes in the Ca2+ signaling were not associated with relaxation of SMCs. By contrast, 50 μM ryanodine quickly stopped KCl-induced Ca2+ oscillations (original frequency = 5.0 ± 0.6 min−1, n = 5 slices from 3 mice at 37°C) after the propagation of 3–5 Ca2+ waves with the last Ca2+ wave occurring at 82.5 ± 8.0 s after ryanodine exposure (Fig. 14D), a response similar to that found at RT. This fast action of ryanodine on KCl-induced oscillations indicates that ryanodine interacts quickly with Ca2+-activated RyRs and, importantly, implies that the much delayed changes in the form of the agonist-induced Ca2+ waves result from an indirect action of ryanodine. The increased baseline of [Ca2+]i indicates a Ca2+ leakage and subsequent depletion of Ca2+ from the SR. We suggest the most likely explanation for this is a temperature-dependent increase in the random opening of the RyR that allows ryanodine binding. Therefore, at 37°C, a faster accumulation of RyRs, in an open state can occur to drain the SR of Ca2+.
DISCUSSION
In this study, we examined the contribution of IP3R and RyR to agonist-induced Ca2+ signaling and contraction of airway SMCs. The IP3Rs and RyRs appear to be colocalized in SMCs (7, 39), and, as a result, these receptors may interact during SMC Ca2+ signaling. Consequently, pharmacological agents that selectively block either the IP3Rs or RyRs have been frequently used to determine the role of each receptor. However, the interpretation of such experiments requires careful consideration in view of the nonspecific effects of these agents. For example, although ryanodine can increase or decrease the conductance of the RyR in a concentration-dependent manner, this action can also alter the Ca2+ content of the SR (4, 34, 53). As a result, the impact of ryanodine on Ca2+ signaling may be due to a direct action on the RyR or indirect action on IP3R-mediated Ca2+ signals that use the SR Ca2+ store. Similarly, tetracaine, in addition to inhibiting RyR, acts as a local anesthetic and has been reported to block N-methyl-d-aspartate receptors (41) and K+ channels (32). Likewise, in addition to acting as a cell-permeant IP3R antagonist, 2-APB may also inhibit SOC and SERCA activity, which has the result of increasing Ca2+ leakage from the SR (40, 42). In addition to nonspecific effects associated with the IP3R antagonist XeC (16, 48), another difficulty associated with XeC is its low solubility. In previous studies, we (6) reported that the prior prolonged incubation of lung slices with XeC prevented agonist-induced Ca2+ signaling. However, in our short-term perfusion experiments reported here with the maximal possible concentration of XeC (20 μM), we did not observe an inhibition of MCh-induced airway contraction (data not shown). This suggests that low concentrations of antagonist are insufficient to immediately compete with high local concentration of agonist-generated IP3. Because experiments with a long-term exposure to XeC are more susceptible to nonspecific effects, we believe short-term responses are likely to be more reliable. Heparin, another commonly used IP3R antagonist, has the disadvantages of membrane impermeability, an action on RyRs and the ability to uncouple G protein signaling pathways (15, 20, 49). Although there is clearly no exclusive experimental drug or method, if we are going to understand airway hyperresponsiveness and how it develops in asthma, it is imperative that we understand the contribution of IP3Rs and RyRs to airway contraction. Consequently, we have carefully examined the effects of inhibiting IP3Rs and RyRs in response to a variety of different stimuli, including agonists, caged-IP3, and membrane depolarization, to control for the nonspecific effects of these receptor antagonists.
We and others have shown that airway SMC contraction, induced by agonists, is mediated via Ca2+ oscillations (29, 47). Consequently, we initially examined the effect of IP3R or RyR antagonists on these two basic responses. When 2-APB was applied to airways contracted with agonist, the airways displayed a concentration-dependent relaxation. This relaxation was associated with a slowing or near cessation of the Ca2+ oscillations. It was also noticed that the airway was not completely relaxed when a high concentration of 2-APB was applied and the intracellular Ca2+ signaling was significantly diminished. This phenomenon can be interpreted by the high Ca2+ sensitivity in the presence of agonist. Because 2-APB had no effect on Ca2+ sensitization, the airway could maintain a significant contraction with a small elevation of [Ca2+]i. This relaxant effect is different to that of β2-agonists that inhibit both Ca2+ signaling and Ca2+ sensitivity. By contrast, the addition of ryanodine to agonist-contracted airways had no effect on either their contractile state or the frequency of the Ca2+ oscillations in their SMCs. We anticipated that the velocity of the Ca2+ waves would either slow or break up into discrete Ca2+ domains (i.e., Ca2+ puffs) if a contribution by the RyRs was inhibited. However, the velocity or form of the propagating Ca2+ waves associated with the Ca2+ oscillations was unaffected by ryanodine. These results strongly indicate that the IP3Rs primarily contribute to the generation and propagation of agonist-induced Ca2+ oscillations. This conclusion is consistent with the finding that the number of IP3Rs exceeds that of RyRs by 10-fold in SMCs (54). In addition, under physiological conditions, [Ca2+]i only has to increase by submicromolar concentrations (<1 μM) to activate the IP3R in the presence of IP3 (30). By contrast, RyRs often require [Ca2+]i increases up to 15 μM for activation (12, 21). Therefore, the propagation of Ca2+ waves by CICR via sensitized IP3Rs is likely to occur at lower thresholds of Ca2+.
We recognize that the noninvolvement of the RyR in agonist-induced Ca2+ signaling is in contrast to current ideas forwarded by other investigators from studies with airway SMCs from mouse bronchi (19), rabbit trachea (28), porcine trachea (14, 31, 44, 45), and canine (23) or human bronchi (13). Although it is appealing to attribute this variation to fundamental differences in SMCs between species or even between different airway generations, the more likely scenario is, unfortunately, the misleading, nonspecific effects of the pharmacological agents used to obtain the experimental results. To resolve this conundrum, our aim here has been to address, in detail, the known nonspecific effects of the antagonists and the implications that they raise for Ca2+ signaling in SMCs.
The first important response is the inhibition of the IP3R with 2-APB. The inhibition of Ca2+ oscillations by 2-APB, supported by the observations with flash photolysis that 2-APB inhibited IP3-induced Ca2+ release, is strong evidence for the expected action of 2-APB on the IP3R. However, 2-APB has been reported to significantly inhibit SOC (11, 42) and thereby the replenishment of Ca2+ stores. We investigated this idea and found some effect of 2-APB on Ca2+ stores, as judged by caffeine-induced Ca2+ release. Therefore, to ensure that the slowing of the Ca2+ oscillations or the failure of IP3 to evoke a Ca2+ transient was not the result of store depletion, we examined the ability of IP3 to release Ca2+ from the SR under conditions that would mimic the full abolishment of SOC, i.e., zero extracellular Ca2+. In the absence of extracellular Ca2+, for an equal amount of time as 2-APB exposure, the photolytic release of IP3 elicited a similar Ca2+ response, confirming that SOC inhibition was not a concern. Similarly, we considered the action of 2-APB on the depletion of the Ca2+ store via an inhibition of SERCA activity and ruled out this possibility by demonstrating that the Ca2+ store was sufficiently replenished to maintain a caffeine-induced Ca2+ response in presence of 2-APB. In addition, compared with our previous studies, 2-APB inhibited the Ca2+ oscillations more quickly than by Ca2+-store depletion induced by zero extracellular Ca2+. Consequently, these experiments collectively confirm the primary function of 2-APB as an IP3R antagonist and emphasize the major role of IP3R in airway SMC Ca2+ oscillations.
On the other side of the hypothesis, the failure of ryanodine to influence the Ca2+ oscillations in any way indicates that there is little involvement of RyRs in agonist-induced Ca2+ oscillations of mouse intrapulmonary airway SMCs. An important feature of the action of ryanodine is that it can only combine with an open RyR, a process called use dependency (21). The fact that no effects were observed with ryanodine implies that the RyRs were not activated (or open) at any time during the Ca2+ oscillation process. If RyRs made a major contribution, we would have expected to see similar results to those we have obtained with caffeine and ryanodine treatment of airway SMCs, i.e., the RyRs will be activated by caffeine and locked in the open state by ryanodine, which would lead to the depletion of the internal store and continuous Ca2+ influx. Under these conditions, the Ca2+ oscillations would stop, and [Ca2+]i would remain at a sustained high level. If RyR made a minor contribution, this might be observed in the characteristics of the propagated Ca2+ waves, but again no changes were observed. We also confirmed that ryanodine had no effect on IP3-induced Ca2+ release and Ca2+ wave propagation with the photolytic release of IP3. Collectively, all of these results exclude the direct involvement of RyRs in IP3-induced CICR and Ca2+ waves in airway SMCs. A similar conclusion was also reached in colon SMCs where localized IP3-mediated Ca2+ release could not propagate as Ca2+ waves by RyRs in the vicinity (38).
Although we have ruled out a direct action of ryanodine on IP3-induced Ca2+ signaling, it is worth emphasizing that ryanodine may indirectly inhibit IP3R-mediated Ca2+ release. A prolonged exposure to ryanodine, especially at 37°C, which would enhance random channel gating, would provide sufficient time for the stochastic ”trapping“ of the RyRs in the open state (random openings of the RyR followed by ryanodine binding). This would lead to the gradual depletion of the Ca2+ content of SR to which IP3Rs and RyRs have common access and the interference of IP3-induced Ca2+ oscillations. This indirect inhibitory effect of ryanodine may also account for our original observations (45-min exposure time; Ref. 6) and those by others for the inhibitory effect of ryanodine on agonist-induced Ca2+ oscillations.
An alternative mechanism for the activation of RyRs in airway SMCs is an agonist-stimulated increase in cADPR. Acting as a functional equivalent of IP3 for IP3R, cADPR generated from β-NAD by ADP-ribosyl cyclase located as a cell surface receptor (CD38) is believed to facilitate the activation of RyRs via FK506-binding protein 12.6 (FKBP12.6) (17, 18, 45, 52). The cADPR/CD38 pathway of Ca2+ release is thought to be recruited by receptor-coupled G proteins (29), but the details are largely unknown. Although the poor membrane permeability of cADPR precluded its direct use in this study, a membrane-permeable competitive inhibitor of cADPR (8-Br-cADPR) had no effect on the MCh- or 5-HT-induced contractile responses or Ca2+ signaling of mouse airway SMCs in lung slices. Therefore, the contribution of cADPR-RyR pathway to agonist-induced Ca2+ oscillations under normal physiological conditions appears to be minimal in mouse airway SMCs.
Another means to activate RyRs is overloading the internal Ca2+ stores (21, 35). This condition can be experimentally induced by KCl-mediated membrane depolarization to stimulate Ca2+ influx via voltage-dependent Ca2+ channels. The consequence of a cyclic Ca2+ store overload coupled to RyR activation and store emptying was slow frequency Ca2+ oscillations (43). As might be expected, ryanodine abolished these KCl-induced slow Ca2+ oscillations of airway SMCs by blocking the activated RyR. It also increased the baseline [Ca2+]i by eliciting additional Ca2+ leakage from SR via the open RyRs. The importance of this result is that it indicates that the inhibitory effects of ryanodine can be observed in lung slices and that the lack of a response to ryanodine in experiments with agonists is not the result of drug impotency. The positive inhibitory effect of ryanodine is also confirmed by its joint action with caffeine in inhibiting the RyR in the open state.
In addition to demonstrating that IP3R is the major contributor to Ca2+ oscillations in mouse airway SMCs, our data also indicate that agonist-induced Ca2+ oscillations are qualitatively similar at RT and 37°C. Only the speed of the Ca2+ oscillations increased, suggesting the fundamental mechanisms underlying the Ca2+ oscillations are not altered by temperature, although it is likely that the kinetics of the process are changed. As a result, the immediate effects of ryanodine or 2-APB on agonist-induced Ca2+ oscillations were similar at RT and 37°C. This behavior is in contrast to vascular SMCs in which agonist-induced Ca2+ oscillations occurred at RT but changed to a uniform increase in [Ca2+]i at higher temperature (32°C) in association with the development of myogenic tone in SMCs (56). The higher [Ca2+]i was believed to inactivate the IP3R.
A surprising finding in our study was the inhibitory effects of tetracaine on agonist-induced Ca2+ signaling. The conventional inhibitor effect of tetracaine on RyRs was confirmed in airway SMCs by the cessation of KCl-induced Ca2+ oscillations, although this action was relatively weak because it could be reversed with high concentrations of caffeine. More importantly, tetracaine appeared to mediate its action by altering Ca2+ signaling and Ca2+ sensitization. In other studies, tetracaine has been suggested to block IP3-mediated Ca2+ release at high concentrations (1 mM) (36, 51). However, we found that 50 μM tetracaine does not affect Ca2+ release in response to IP3. Therefore, we suggest the cessation of agonist-induced Ca2+ oscillations in presence of tetracaine results from an absence of IP3. This idea is supported by the reinitiation of Ca2+ oscillations when IP3 was made available by flash photolysis. Another nonspecific effect of tetracaine is inhibition of membrane ion channels and Ca2+ influx, but this appears to be minimal in airway SMCs because repetitive exposures to caffeine in the presence of tetracaine induced similar contractile and Ca2+ responses and had no effect on the [Ca2+]i of permeabilized SMCs. Consequently, we propose that tetracaine acts at the membrane level to inhibit the receptor-coupled dual pathways that regulate the activation of PLCβ for IP3 production and G protein-coupled activation of Rho kinase to modulate Ca2+ sensitization. Similar actions of tetracaine have been reported previously (26) to explain how tetracaine may act as a local anesthetic. Collectively, these results indicated that tetracaine, and other similar local anesthetic agents, such as procaine, inhibit the Ca2+ responses of mouse airway SMCs in a manner unrelated to the RyRs. More importantly, these conclusions emphasize that caution is required when explaining the effects of tetracaine on airway SMC physiology of other species. In conclusion, our experiments demonstrate that the IP3R plays the major role in GPCR agonist-induced Ca2+ oscillations and that RyR-mediated CICR does not appear to be involved in normal physiological conditions.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants HL-71930 and HL-087401 to M. J. Sanderson.
REFERENCES
- 1.Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L909–L917, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bai Y, Sanderson MJ. Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a cAMP-mediated inhibition of the IP3 receptor. Respir Res 7: 34, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai Y, Sanderson MJ. The contribution of Ca2+ signaling and Ca2+ sensitivity to the regulation of airway smooth muscle contraction is different in rats and mice. Am J Physiol Lung Cell Mol Physiol 296: L947–L958, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol 291: L208–L221, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bai Y, Zhang M, Sanderson MJ. Contractility and Ca2+ signaling of smooth muscle cells in different generations of mouse airways. Am J Respir Cell Mol Biol 36: 122–130, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergner A, Sanderson MJ. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol 119: 187–198, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berridge MJ Inositol trisphosphate and calcium signalling. Nature 361: 315–325, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351: 751–754, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 16: 1145–1150, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Cannell MB, Cheng H, Lederer WJ. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J 67: 1942–1956, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai JM, Kuo KH, Leo JM, Pare PD, van Breemen C, Lee CH. Acetylcholine-induced asynchronous calcium waves in intact human bronchial muscle bundle. Am J Respir Cell Mol Biol 36: 600–608, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Dai JM, Kuo KH, Leo JM, van Breemen C, Lee CH. Mechanism of ACh-induced asynchronous calcium waves and tonic contraction in porcine tracheal muscle bundle. Am J Physiol Lung Cell Mol Physiol 290: L459–L469, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Dasso LL, Taylor CW. Heparin and other polyanions uncouple alpha 1-adrenoceptors from G-proteins. Biochem J 280: 791–795, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Smet P, Parys JB, Callewaert G, Weidema AF, Hill E, De Smedt H, Erneux C, Sorrentino V, Missiaen L. Xestospongin C is an equally potent inhibitor of the inositol 1,4,5-trisphosphate receptor and the endoplasmic-reticulum Ca2+ pumps. Cell Calcium 26: 9–13, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Deshpande DA, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose-mediated Ca2+ signaling contributes to airway smooth muscle hyper-responsiveness. FASEB J 17: 452–454, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande DA, White TA, Dogan S, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose signaling: role in the regulation of calcium homeostasis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 288: L773–L788, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Du W, Stiber JA, Rosenberg PB, Meissner G, Eu JP. Ryanodine receptors in muscarinic receptor-mediated bronchoconstriction. J Biol Chem 280: 26287–26294, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+-release channels. Trends Pharmacol Sci 15: 145–149, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev 82: 893–922, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerthoffer WT, Murphey KA, Khoyi MA. Inhibition of tracheal smooth muscle contraction and myosin phosphorylation by ryanodine. J Pharmacol Exp Ther 246: 585–590, 1988. [PubMed] [Google Scholar]
- 24.Haberichter T, Roux E, Marhl M, Mazat JP. The influence of different InsP(3) receptor isoforms on Ca2+ signaling in tracheal smooth muscle cells. Bioelectrochemistry 57: 129–138, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Hille B Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol 69: 497–515, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollmann MW, Difazio CA, Durieux ME. Ca-signaling G-protein-coupled receptors: a new site of local anesthetic action? Reg Anesth Pain Med 26: 565–571, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Iino M Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol 95: 1103–1122, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iizuka K, Yoshii A, Dobashi K, Horie T, Mori M, Nakazawa T. InsP3, but not novel Ca2+ releasers, contributes to agonist-initiated contraction in rabbit airway smooth muscle. J Physiol 511: 915–933, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jude JA, Wylam ME, Walseth TF, Kannan MS. Calcium signaling in airway smooth muscle. Proc Am Thorac Soc 5: 15–22, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaftan EJ, Ehrlich BE, Watras J. Inositol 1,4,5-trisphosphate (InsP3) and calcium interact to increase the dynamic range of InsP3 receptor-dependent calcium signaling. J Gen Physiol 110: 529–538, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kannan MS, Prakash YS, Brenner T, Mickelson JR, Sieck GC. Role of ryanodine receptor channels in Ca2+ oscillations of porcine tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol 272: L659–L664, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Komai H, McDowell TS. Local anesthetic inhibition of voltage-activated potassium currents in rat dorsal root ganglion neurons. Anesthesiology 94: 1089–1095, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Kuo KH, Dai J, Seow CY, Lee CH, van Breemen C. Relationship between asynchronous Ca2+ waves and force development in intact smooth muscle bundles of the porcine trachea. Am J Physiol Lung Cell Mol Physiol 285: L1345–L1353, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Lamont C, Wier WG. Different roles of ryanodine receptors and inositol (1,4,5)-trisphosphate receptors in adrenergically stimulated contractions of small arteries. Am J Physiol Heart Circ Physiol 287: H617–H625, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Lukyanenko V, Subramanian S, Gyorke I, Wiesner TF, Gyorke S. The role of luminal Ca2+ in the generation of Ca2+ waves in rat ventricular myocytes. J Physiol 518: 173–186, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacMillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle cells. J Physiol 569: 533–544, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarron JG, Bradley KN, MacMillan D, Muir TC. Sarcolemma agonist-induced interactions between InsP3 and ryanodine receptors in Ca2+ oscillations and waves in smooth muscle. Biochem Soc Trans 31: 920–924, 2003. [DOI] [PubMed] [Google Scholar]
- 38.McCarron JG, MacMillan D, Bradley KN, Chalmers S, Muir TC. Origin and mechanisms of Ca2+ waves in smooth muscle as revealed by localized photolysis of caged inositol 1,4,5-trisphosphate. J Biol Chem 279: 8417–8427, 2004. [DOI] [PubMed] [Google Scholar]
- 39.McGeown JG Interactions between inositol 1,4,5-trisphosphate receptors and ryanodine receptors in smooth muscle: one store or two? Cell Calcium 35: 613–619, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Missiaen L, Callewaert G, De Smedt H, Parys JB. 2-Aminoethoxydiphenyl borate affects the inositol 1,4,5-trisphosphate receptor, the intracellular Ca2+ pump and the non-specific Ca2+ leak from the non-mitochondrial Ca2+ stores in permeabilized A7r5 cells. Cell Calcium 29: 111–116, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Nishizawa N, Shirasaki T, Nakao S, Matsuda H, Shingu K. The inhibition of the N-methyl-d-aspartate receptor channel by local anesthetics in mouse CA1 pyramidal neurons. Anesth Analg 94: 325–330, table of contents, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Peppiatt CM, Collins TJ, Mackenzie L, Conway SJ, Holmes AB, Bootman MD, Berridge MJ, Seo JT, Roderick HL. 2-Aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell Calcium 34: 97–108, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol 125: 535–553, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prakash YS, Kannan MS, Sieck GC. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol Cell Physiol 272: C966–C975, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Prakash YS, Kannan MS, Walseth TF, Sieck GC. Role of cyclic ADP-ribose in the regulation of [Ca2+]i in porcine tracheal smooth muscle. Am J Physiol Cell Physiol 274: C1653–C1660, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Ruehlmann DO, Lee CH, Poburko D, van Breemen C. Asynchronous Ca2+ waves in intact venous smooth muscle. Circ Res 86: E72–E79, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thorac Soc 5: 23–31, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Solovyova N, Fernyhough P, Glazner G, Verkhratsky A. Xestospongin C empties the ER calcium store but does not inhibit InsP3-induced Ca2+ release in cultured dorsal root ganglia neurones. Cell Calcium 32: 49–52, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Taylor CW, Broad LM. Pharmacological analysis of intracellular Ca2+ signalling: problems and pitfalls. Trends Pharmacol Sci 19: 370–375, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Tazzeo T, Zhang Y, Keshavjee S, Janssen LJ. Ryanodine receptors decant internal Ca2+ store in human and bovine airway smooth muscle. Eur Respir J 32: 275–284, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Tovey SC, Dyer JL, Godfrey RE, Khan SZ, Bilmen JG, Mezna M, Michelangeli F. Subtype identification and functional properties of inositol 1,4, 5-trisphosphate receptors in heart and aorta. Pharmacol Res 42: 581–590, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Wang YX, Zheng YM, Mei QB, Wang QS, Collier ML, Fleischer S, Xin HB, Kotlikoff MI. FKBP12.6 and cADPR regulation of Ca2+ release in smooth muscle cells. Am J Physiol Cell Physiol 286: C538–C546, 2004. [DOI] [PubMed] [Google Scholar]
- 53.White C, McGeown JG. Carbachol triggers RyR-dependent Ca2+ release via activation of IP(3) receptors in isolated rat gastric myocytes. J Physiol 542: 725–733, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wibo M, Godfraind T. Comparative localization of inositol 1,4,5-trisphosphate and ryanodine receptors in intestinal smooth muscle: an analytical subfractionation study. Biochem J 297: 415–423, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wojcikiewicz RJ Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem 270: 11678–11683, 1995. [DOI] [PubMed] [Google Scholar]
- 56.Zacharia J, Zhang J, Wier WG. Ca2+ signaling in mouse mesenteric small arteries: myogenic tone and adrenergic vasoconstriction. Am J Physiol Heart Circ Physiol 292: H1523–H1532, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang YX. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol 125: 427–440, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]