Abstract
Normal alveolarization has been studied in rodents using detailed morphometric techniques and loss of function approaches for growth factors and their receptors. However, it remains unclear how these growth factors direct the formation of secondary septae. We have previously developed a transgenic mouse model in which expression of a soluble dominant-negative FGF receptor (dnFGFR) in the prenatal period results in reduced alveolar septae formation and subsequent alveolar simplification. Retinoic acid (RA), a biologically active derivative of vitamin A, can induce regeneration of alveoli in adult rodents. In this study, we demonstrate that RA induces alveolar reseptation in this transgenic mouse model and that realveolarization in adult mice is FGF dependent. Proliferation in the lung parenchyma, an essential prerequisite for lung regrowth was enhanced after 14 days of RA treatment and was not influenced by dnFGFR expression. During normal lung development, formation of secondary septae is associated with the transient presence of α-smooth muscle actin (αSMA)-positive interstitial myofibroblasts. One week after completion of RA treatment, αSMA expression was detected in interstitial fibroblasts, supporting the concept that RA-initiated realveolarization recapitulates aspects of septation that occur during normal lung development. Expression of dnFGFR blocked realveolarization with increased PDGF receptor-α (PDGFRα)-positive cells and decreased αSMA-positive cells. Taken together, our data demonstrate that FGF signaling is required for the induction of αSMA in the PDGFRα-positive myofibroblast progenitor and the progression of alveolar regeneration.
Keywords: transgenic mouse model, interstitial myofibroblast, emphysema, platelet derived growth factor receptor
secondary septation in normal lung development: The parenchyma of the newborn mouse and rat lung is still in the saccular stage of development. There are no alveoli, and the primary septae are thick and contain a double capillary network (7). At the beginning of alveolarization, small ridges appear along the primary septae. These ridges increase in height to become the secondary septae that subdivide the air spaces into smaller units, the alveoli. Immature primary and secondary septae contain two capillary layers, one on each side of a central layer of interstitium, which consists of elastin deposits and the interstitial myofibroblast. Although the supporting septum is immature, successive rounds of alveolar expansion and subdivision occur, resulting in the large increase in alveolar number that occurs between birth and 21 days of age. In the adult lung, septae are thin, containing only a single capillary layer, and the interstitial myofibroblast has disappeared (7).
Retinoic acid (RA)-synthesizing enzymes, receptors, and cytoplasmic proteins are abundant during alveolar septation (30). Mice with deletions in RA receptors fail to form alveoli normally (34, 49). Retinol-rich lipofibroblasts are present in the alveolar wall during the period of normal septation and diminish after septation has been completed (55). Interstitial lipofibroblasts, which “traffic” the lipids and store retinoids (44), are positioned in close apposition to alveolar type II cells (17). Lipofibroblasts express PDGF receptor-α (PDGFRα), and lack of these lipofibroblasts results in failed septation in PDGFRα knockout mice (5, 24). Boström, Lindahl, and others (5, 24, 54) hypothesized that lipofibroblasts are the progenitors of the interstitial myofibroblasts, the cellular core of the newly forming alveolar septum. Recent confocal microscopy by McGowan et al. (35) revealed that lipofibroblasts with high lipid content are located at the base of the alveolar septum and express low levels of PDGFRα. The same studies show that cells expressing high levels of PDGFRα have characteristics of myofibroblasts and are located at the alveolar entry ring (35). Interstitial myofibroblasts have the morphology of fibroblasts (21) but contain contractile elements as shown by ultrastructural analysis (1). They also express α-smooth muscle actin (αSMA) (23). It remains unclear how the formation of new septae is regulated and whether the same processes are used during reseptation in repair of the postnatal lung.
RA and lung regeneration.
RA, the biologically active derivative of vitamin A, is known to be involved in lung development and maintenance of alveolar structures in the adult (3, 11, 13, 30, 33, 36). Many clinical studies and animal models support the importance of dietary retinoids in lung morphogenesis and repair (43, 46–48). It has been known for many decades that vitamin A deprivation in rats causes squamous metaplasia of the conducting airway epithelium that can be reversed by vitamin A restoration (3, 61). As seen in vitamin A-depleted animals, infants who develop bronchopulmonary dysplasia (BPD) show squamous metaplasia in conducting airways and arrested alveologenesis. In a multicenter clinical trial, vitamin A supplementation caused a small but significant reduction in the incidence of BPD (40). RA treatment of adult rats, previously made emphysematous by the intratracheal instillation of elastase or perinatal dexamethasone treatment, reinitiated septation and realveolarization (19, 31). The effects of RA treatment on alveolar regeneration have been subject to numerous studies with mixed success (4, 16, 19, 25, 26, 28, 29, 32, 38, 53). Differences in the pharmacokinetics of retinoid metabolism between strains may be a partial explanation for the negative studies (51). The molecular mechanisms involved in normal alveolar septation and new septum formation during alveolar repair remain unclear. In this study, we used RA to induce realveolarization and to determine the role of FGF signaling in the formation of secondary septa.
FGF signaling during alveolarization, lung injury, and lung repair.
Growth factors are key regulators of alveolar formation and have also been implicated in repair mechanisms to restore lung integrity after acute lung injury. In mice, diffuse alveolar damage induced by hyperoxia is associated with increased lung levels of FGF7 and FGF2 that promote type II cell proliferation and facilitate repair of the damaged epithelium (6, 9). Moreover, intratracheal instillation or intravenous injection of FGF7 induced alveolar type II cell proliferation and reduced mortality after exposure to hyperoxia in mice and rats (2, 39). FGF receptor (FGFR) signaling is important for normal alveolar septation, since mice deficient in both FGFR3 and FGFR4 do not form secondary septae (59). Moreover, using a conditional transgenic mouse model to express a soluble dominant-negative FGFR (dnFGFR), we demonstrated that FGFR signaling is critical before the initiation of alveolar septation but not important during normal alveolarization, thus implicating a role of FGF signaling in lung progenitor cell expansion and/or commitment (20).
In the present study, we used a conditional transgenic mouse model to study the role of FGF signaling in realveolarization induced by RA treatment. We demonstrate that αSMA expression is induced in PDGFRα-positive interstitial fibroblasts during realveolarization, thus recapitulating the developmental program. Concurrent expression of a secreted dnFGFR had no effect on proliferation but blocked induction of αSMA expression in the secondary crests resulting in an accumulation of PDGFRα and inhibition of realveolarization.
MATERIALS AND METHODS
Transgenic animals used.
The conditional system consists of two transgenes: the activator transgene and the operator transgene. In the activator line, the 3.7-kb human surfactant protein C (SFTPC) promoter [FVB.Cg-Tg(SFTPC-rtTA)5Jaw/J; The Jackson Laboratory] drives expression of the reverse tet activator to the epithelial cells in the lung (41). The operator line has the transgene of interest, a soluble dnFGFR under the control of the tet operator (tetOdnFGFR-Hfc) (20). In the presence of doxycycline (dox), the reverse tet activator can bind the operator sequence and activate expression of dnFGFR (20). Gene activation is detected as early as 16 h after dox treatment in this activator line (41). Wild-type and single transgenic littermates showed no alveolar simplification after dox treatment and served as controls along with untreated double transgenic (DbTg) mice. All animal studies were conducted on FVB/N mice unless otherwise mentioned. For each treatment group, experiments were performed on at least three to five animals of each genotype to meet statistical requirements (Fig. 1).
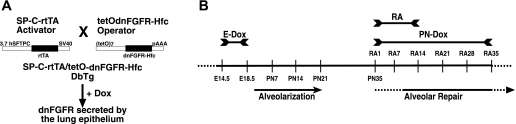
Fig. 1.
A: schematic drawing of the mouse breeding to generate double transgenic (DbTg) mice. Heterocygote mice of the activator line, SP-C-rtTA line 1, are crossed to heterocygote mice of the operator line, tetOdnFGFR-Hfc. According to Mendelian inheritance, only 25% of the offspring carry both transgenes, DbTg mice. Only in DbTg progenies doxycycline (dox) treatment activates dominant-negative FGF receptor (dnFGFR) expression specifically in the lung epithelium (20). B: timeline of dox and retinoic acid (RA) treatment to induce alveolar simplification and alveolar repair, respectively, in DbTg mice. Dox treatment from embryonic day 14.5 (E14.5) to E18.5 (E-Dox) induces expression of dnFGFR and inhibits postnatal alveolarization. RA treatment from postnatal day 35 (PN35) to PN48 induces alveolar repair. Postnatal dox treatment from PN35 throughout RA treatment (PN-Dox) induces dnFGFR expression and inhibits alveolar repair. Alveolar repair in the presence and absence of FGF signaling was assessed in DbTg mice after embryonic inhibition of FGF signaling 7, 14, 21, 28, and 35 days after initiation of RA treatment (RA7, RA14, RA21, RA28, and RA35). SV40, simian virus 40.
PDGFRαGFP/WT mice.
B6.129S4-Pdgfratm11(EGFP)Sor/J mice were provided by Dr. Philippe Soriano (Fred Hutchinson Cancer Research Center, Seattle, WA). This mouse line carries a green fluorescent protein (GFP) allele fused to a histone 2B (H2B) moiety introduced into the PDGFRα endogenous locus. The H2B domain targets nuclear localization of GFP in cells that express endogenous PDGFRα. Genotyping of all alleles was done by polymerase chain reaction (18, 20).
Animal care.
All transgenic mice were housed in a barrier facility with purified air and water and were provided with either autoclaved or irradiated food containing dox in accordance with federal and institutional guidelines. Mice were supplied with food and water ad libitum and were exposed to a 12:12-h light-dark cycle. Mice were routinely screened for viral and bacterial infections by placing sentinels in their cages. The animal experiments were approved by the Cincinnati Children's Hospital institutional animal care and use committee (IACUC), 7B11078, accreditation number A3108-01 (11/13/07). Euthanizing of mice with ketamine, xylazine, and acepromazine has been approved by and is in accordance with guidelines specified by the American Veterinary Medical Association.
RA treatment.
All trans-RA (Sigma) was dissolved in DMSO and peanut oil (Sigma) at a dose of 2 μg/g body wt, administered daily in a 50-μl intraperitoneal injection for a period of 10 days from postnatal day 35 (PN35) to PN46 with a 2-day break on PN40 and PN41 (27, 60). As control, DbTg mice received DMSO/peanut oil only [DbTg, dox treatment from embryonic day 14.5 (E14.5) to E18.5 (E-Dox), and DMSO]. Lungs were harvested 7, 14, 21, 28, and 35 days after initiation of RA treatment (RA7, RA14, RA21, RA28, and RA35). Please note that RA was given the first 12 days of the treatment regimen (RA7 and RA14), and progression of lung regeneration was assessed 1, 2, and 3 wk (RA21, RA28, and RA35) thereafter.
Time line of dox and RA treatment.
Pregnant dams from the breeding scheme in Fig. 1 were treated with dox in the food (625 mg/kg; Harlan Teklad, Madison, WI) from gestational age E14.5 to E18.5 (41). Activation of the dnFGFR from E14.5 to E18.5 results in reproducible emphysema in the adult lung. These mice are referred to as DbTg, E-Dox (20). A subset of these mice was subsequently treated with RA starting on PN35. These mice are referred to as DbTg, E-Dox, RA. Postnatal dox treatment from PN35 throughout RA treatment (PN-Dox) induces dnFGFR expression in most alveolar type II cells in the adult lung. These mice are referred to as DbTg, E-Dox, RA, PN-Dox. After embryonic dnFGFR expression, RA-mediated alveolar repair was assessed on RA7, RA14, RA21, RA28, and RA35 (Fig. 1).
Tissue harvest.
Before euthanasia, mice were killed by lethal injection of ketamine, xylazine, and acepromazine. For optimal lung histology, lungs were inflation-fixed with 4% paraformaldehyde in PBS at 25 cmH2O pressure via a tracheal cannula. All lungs were inflated with closed chests to ensure the same inflation volume and subsequent comparable histology of fixed tissue. Lungs were fixed overnight at 4°C. All lungs were washed with PBS, dehydrated through a graded series of ethanol solutions, and processed for paraffin embedding. Sections (5 μm) were loaded onto polysine slides for analysis.
Morphometric point and intersection counting analysis.
To quantify alveolar simplification, fractional air space area by morphometric point intersection analysis was determined on histological sections of inflation-fixed adult lungs 3 wk after completion of RA treatment (RA35). For each animal, three to five sections from different parts of the lung, showing all five lobes, were analyzed. A 120-point grid was overlaid over five to seven random pictures of each lobe. The alveolar space was calculated as percentage of grid intersections over alveolar space vs. alveolar tissue. Each symbol represents the average percentage of air space of one animal. Values for each individual animal were averaged per experimental group (58).
Immunohistochemistry.
Fluorescent immunohistochemistry was performed on paraffin sections as previously described (64). The following antibodies have been used. Phospho-histone H3: 1:500, polyclonal rabbit anti-phospho-histone H3, H5110-14B, from United States Biological, Swampscott, MA, with secondary antibody Alexa Fluor 568-conjugated goat anti-rabbit IgG, Molecular Probes-Invitrogen, Carlsbad, CA. αSMA immunohistochemistry: 1:1,000 monoclonal mouse from Sigma-Aldrich, A5228, with secondary antibody Alexa Fluor 594-conjugated donkey anti-mouse IgG1, Molecular Probes-Invitrogen, and DAPI containing mounting media. PDGFRα: 1:250 polyclonal rabbit anti-PDGFRα from Santa Cruz Biotechnology, sc-338, with secondary antibody Alexa Fluor 488-conjugated goat anti-rabbit, Molecular Probes-Invitrogen.
Expression of phospho-histone H3, αSMA, and PDGFRα was determined by microscopy using dual-fluorescent labeling and a Zeiss Axioplan 2 Imaging Universal Microscope with an AxioCam MRm black and white digital camera (AxioVision 4.3) and with an ApoTome slider for pseudoconfocal imaging. Images were captured by Zeiss AxioVision processed in Adobe Photoshop, and composites were made using Macromedia FreeHand. Colocalization of nuclear GFP and immunohistochemistry for αSMA were assessed using a Nikon C1si three-channel and spectral confocal microscope with a Nikon DXM 1200 color camera; images were processed with EZ-C1 (Nikon) and AutoQuant (Media Cybernetics) for deconvolution.
Elastin.
Elastin content was estimated by using the Fastin Elastin Assay according to the manufacturer's recommendations (Biocolor, Newtownabbey, Northern Ireland).
Mitotic index by phospho-histone H3 staining.
The mitotic index was assessed by fluorescent immunohistochemistry for phospho-histone H3-positive cells before and during RA treatment. Three to five animals per group were analyzed. From each animal, we double-labeled (phospho-histone H3 and DAPI) 6 sections, which were spaced by 40–60 μm and contained all 5 lobes. Five random, nonoverlapping ×10 magnification fields from each slide were analyzed for phospho-histone H3-positive nuclei, and an average of 7,500 nuclei were counted per mouse. Nuclei of large airways, bronchioles, and larger vessels were excluded. The mitotic index was expressed as the ratio of phospho-histone H3-positive nuclei per 1,000 counted nuclei. ANOVA was used for comparison of mitotic indices, and the Student-Newman-Keuls test of the means was used for pairwise comparison of significance. Nuclei of large airways, bronchioles, and larger vessels were excluded. Differences (means ± SE) were assessed by Student's t-test.
Morphometric analysis of αSMA and PDGFRα expression 1 wk after completion of RA treatment.
To quantify induction of interstitial myofibroblasts by morphometric analysis, immunohistochemistry for αSMA (red) and PDGFRα (green) was performed on histological sections of inflation-fixed adult lungs 1 wk after completion of RA treatment (RA21). Three to seven animals were evaluated from each treatment group from control mice (single and DbTg mice with no embryonic dox treatment), DbTg mice after embryonic dox treatment (DbTg, E-Dox), and DbTg mice with embryonic and adult dox treatment (DbTg, E-Dox, PN-Dox). For each animal, we triple-labeled (αSMA, PDGFRα, and DAPI) histological sections and analyzed 10–12 random, nonoverlapping ×10 magnification picture frames from each slide. αSMA, PDGFRα staining and DAPI-positive nuclei of large airways, bronchioles, and larger vessels were excluded. The three-dimensional nature of the lung sections cause regions of overlapping nuclei, which were excluded from the counts. αSMA- and PDGFRα-positive staining was expressed as percentage of all DAPI-positive nuclei. ANOVA was used for comparison of percentages, and differences (means ± SE) were assessed by Student's t-test and the Student-Newman-Keuls test of the means for pairwise comparison of significance.
RESULTS
Realveolarization is dependent on FGF signaling.
We (8, 20) previously developed a mouse model in which prenatal inhibition of FGF signaling caused by conditional expression of a soluble dnFGFR results in alveolar simplification, and emphysema in the adult mouse. To test whether RA induces realveolarization in the emphysema model, histological and morphometric analysis were performed on lungs after prenatal dnFGFR expression and postnatal RA treatment. The RA treatment protocol and morphometric analyzes were previously described by Hind and Maden (19). Mice were first made emphysematous by embryonic dox treatment. At 35 days of age, mice were subjected to 10 days of subcutaneous RA injections (DbTg, E-Dox, RA; see timeline in Fig. 1). Three weeks after RA treatment (RA35), effects on realveolarization were assessed by hematoxylin-eosin (H&E) staining (Fig. 2) and by morphometric point intersection analysis of fractional air space area (Fig. 3). In DbTg control mice (no dox, no RA), the normal fractional air space was 65%. Air space was increased to 75% in mice with emphysema. RA treatment induced alveolar regeneration, and air space returned to 65% (Figs. 2C, 2F, and 3). Expression of dnFGFR during and after RA treatment (RA1 to RA35) inhibited alveolar regeneration, and alveolar air space remained at 75%. Morphometric analysis revealed that RA treatment induced realveolarization and that concurrent dnFGFR expression inhibited realveolarization. Expression of dnFGFR in the postnatal lung did not affect alveolar air space in adult mice (20).
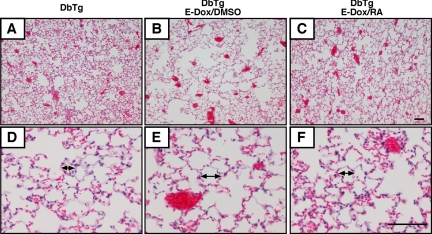
Fig. 2.
RA improves alveolar simplification in DbTg mice. Lung histology was analyzed by hematoxylin-eosin (H&E) staining of DbTg mice at RA35. A and D show normal lung, no prenatal dox and RA treatment from PN35 to PN48. B and E show that prenatal dox induced dnFGFR expression and caused emphysema. C and F show that RA treatment resolved emphysema. Arrows indicate average alveolar size. Scale bar = 100 μm.
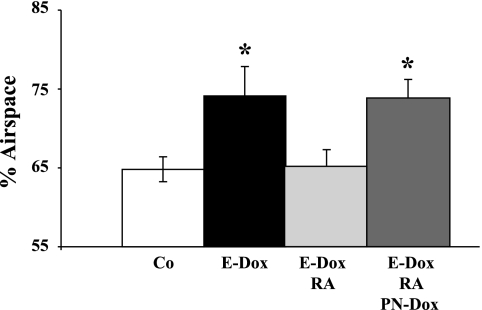
Fig. 3.
dnFGFR expression inhibits realveolarization. Fractional air space area by morphometric point intersection analysis was performed on histological sections of RA35 DbTg and single transgenic control mice. E-Dox, dox from E14.5 to E18.5 to induce prenatal dnFGFR expression; RA, RA treatment from PN35 to PN48; (Co) DMSO, control treatment from PN35 to PN48; PN-Dox, dox treatment starting at PN35 throughout repair induces dnFGFR expression. Expression of the soluble dnFGFR during this inhibits realveolarization (*P ≤ 0.05; n = 3).
Elastin fibers are an important component of the parenchymal architecture of lungs and are increased in FGFR3 and FGFR4 double knockout mice (59). To determine whether FGF signaling affected elastin expression during realveolarization, elastin protein concentrations were assessed at completion of RA treatment (RA15) and 1 wk thereafter (RA21). Expression of dnFGFR did not change elastin concentration in RA15 lungs (no dox: 21.7 ± 0.6 μg/mg protein vs. on dox: 24.5 ± 6.6 μg/mg; P = 0.998) or in RA21 lungs (no dox: 26.5 ± 2.1 μg/mg vs. on dox: 31.0 ± 6.1 μg/mg; P = 0.217). These data demonstrate that expression of dnFGFR does not significantly increase elastin expression at RA15 or RA21.
FGF does not regulate proliferation after RA-mediated repair.
During normal septation, the lung grows rapidly from PN3 to PN10 and continues to grow thereafter, maintaining a constant lungs-to-body weight ratio (Ref. 56; Perl, unpublished data). The mitotic index in adult lungs is extremely low (56). To test whether dnFGFR expression interfered with proliferation, the mitotic index of the lung parenchyma was determined before and after RA treatment (Fig. 4). We first assessed baseline proliferation in emphysematous lungs in the presence and absence of dnFGFR (Fig. 4A). The mitotic index in the lung parenchyma of normal age-matched control mice was 0.98 ± 0.002. Prenatal inhibition of FGF signaling (E-Dox) caused emphysematous lungs in adult mice, and the mitotic index was increased to 1.87 ± 0.043. Inhibition of FGF signaling by expression of dnFGFR in adult emphysematous lungs significantly reduced the mitotic index to 0.81 ± 0.060 (P = 0.00019). These data demonstrate that adult emphysematous lungs have a higher mitotic index at baseline, which is not sufficient to induce alveolar regeneration and moreover is dependent on FGF signaling.
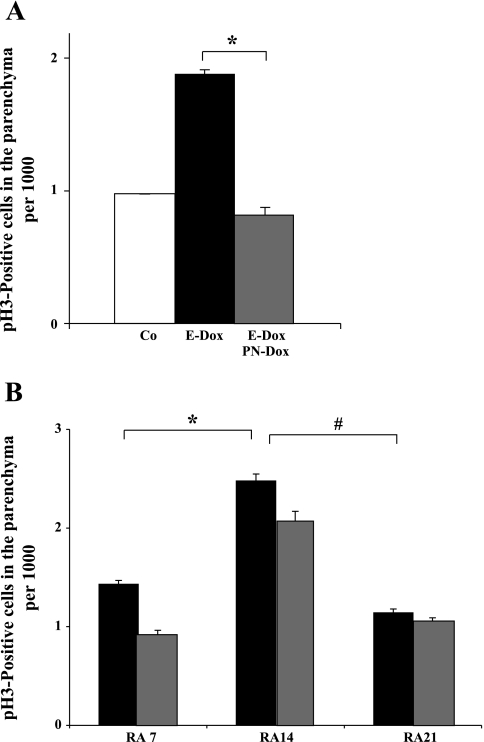
Fig. 4.
The mitotic index in the parenchyma of adult lungs was determined by immunohistochemistry for phospho-histone H3 (pH3)-positive cells. White column: DbTg. Black columns: DbTg, E-Dox. Gray columns: DbTg, E-Dox, PN-Dox. Each column represents data from 3 to 4 animals. A: no RA: prenatal dnFGFR expression resulted in significantly elevated proliferation in adult lungs compared with normal transgenic mice. The increase in proliferation was dependent on FGF signaling (*P = 0.00019; n = 3–4). B: RA7 and RA14: compared with RA7, proliferation in the lung was significantly increased after 14 days of RA treatment (*P < 0.0001; n = 3). RA21: 1 wk after completion of RA treatment, proliferation was significantly (#P < 0.0001; n = 3) decreased compared with proliferation after RA14. Dox-mediated expression of dnFGFR (gray columns) did not influence the proliferation index at RA7, RA14, or RA21 (P ≥ 0.1; n = 3). Differences (means ± SE) were assessed by Student's t-test.
To further understand the role of cellular proliferation in RA-mediated realveolarization, we determined the mitotic index at RA7, RA14, and 1 wk after completion of RA treatment (RA21) (Fig. 4B). After 7 days of RA treatment, the proliferation index was 1.4 ± 0.037. After 14 days of RA, the mitotic index peaked at 2.48 ± 0.070 and was significantly reduced 1 wk after completion of RA treatment (1.14 ± 0.040). When dnFGFR was expressed during RA treatment, the mitotic index was not significantly different compared with regenerating lung parenchyma (P > 0.1; RA7: 0.94 ± 0.028; RA14: 2.08 ± 0.086; RA21: 1.07 ± 0.021). These data show that the RA-mediated increase in mitotic index was independent of dnFGFR expression and implicate other roles of FGF in alveolar regeneration.
Induced αSMA expression in interstitial fibroblasts during realveolarization is dependent on FGF signaling.
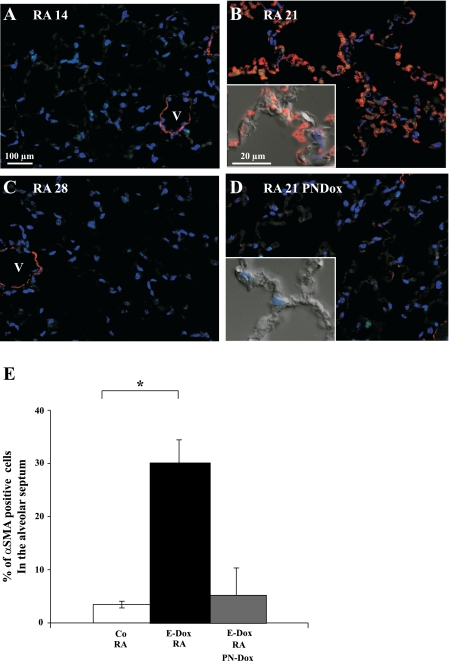
αSMA is a cytological marker for interstitial myofibroblasts in the newly forming, immature alveolar septum (7). αSMA and elastic fibers accumulate in the alveolar entry ring, a preferred location of PDGFRα expressing cells (35). To determine whether RA treatment induces myofibroblast differentiation during reseptation, immunohistochemistry for αSMA was performed on lung sections of adult RA-treated mice (Fig. 5). αSMA expression in fibroblasts associated with airways and arteries was unchanged in any of the treatment groups and thus served as an internal control for antibody staining. αSMA expression in the alveolar septae was absent at the end of RA treatment (RA14; Fig. 5A), was increased 1 wk after RA treatment was completed (RA21; Fig. 5B), and was absent again 2 wk after completion of RA treatment (RA28; Fig. 5C). Expression of the dnFGFR receptor during and after RA treatment inhibited αSMA expression in the regenerating alveolar septae (RA21 PN-Dox; Fig. 5D). The number of αSMA-expressing interstitial myofibroblasts was determined by morphometric analysis 1 wk after completion of RA treatment (RA21; Fig. 5E). During alveolar regeneration, the percentage of αSMA-positive cells increased significantly from 3.5 to 30.4%. When dnFGFR was expressed, the percentage of αSMA-positive cells did not increase and was comparable with control animals.
Fig. 5.
Alveolar α-smooth muscle actin (αSMA) expression is induced 1 wk after completion of RA treatment. Immunohistochemistry for αSMA (red) was performed on lung sections of adult DbTg mice after prenatal inhibition of FGF signaling and postnatal RA treatment. No αSMA expression in the alveolar septum was found at the end of RA treatment (A; RA14). One week after completion of RA treatment, αSMA expression was detected in the alveolar interstitium (B; RA21). Expression thereafter decreased and was not detected 2 wk after completion of RA treatment (C; RA28). dnFGFR expression (PN-Dox) suppressed induction of αSMA (D). Peribronchiolar and perivascular αSMA expression was not affected at any time point and served as an internal control of the immunohistochemistry staining. Scale bar = 100 μm and 20 μm in the inset. V, vessel. Red: αSMA. Blue: DAPI. E: morphometric analysis of septal αSMA expression in RA21 lungs. White column: control: DbTg mice, no dox, RA: 3.46% (± 0.64%) of the cells expressed αSMA (n = 4). Black column: DbTg, E-Dox: 30.40% (± 4.04%) of the cells expressed αSMA (*P < 0.001; n = 7). Gray column: DbTg, E-Dox, PN-Dox: expression of dnFGFR suppressed expression of αSMA. Compared with DbTg, E-Dox animals, only 5.13% (±5.2%) of the cells expressed αSMA (P = 0.38; n = 3).
Inhibition of FGF signaling increases the number of PDGFRα-positive fibroblasts in the alveolar interstitium.
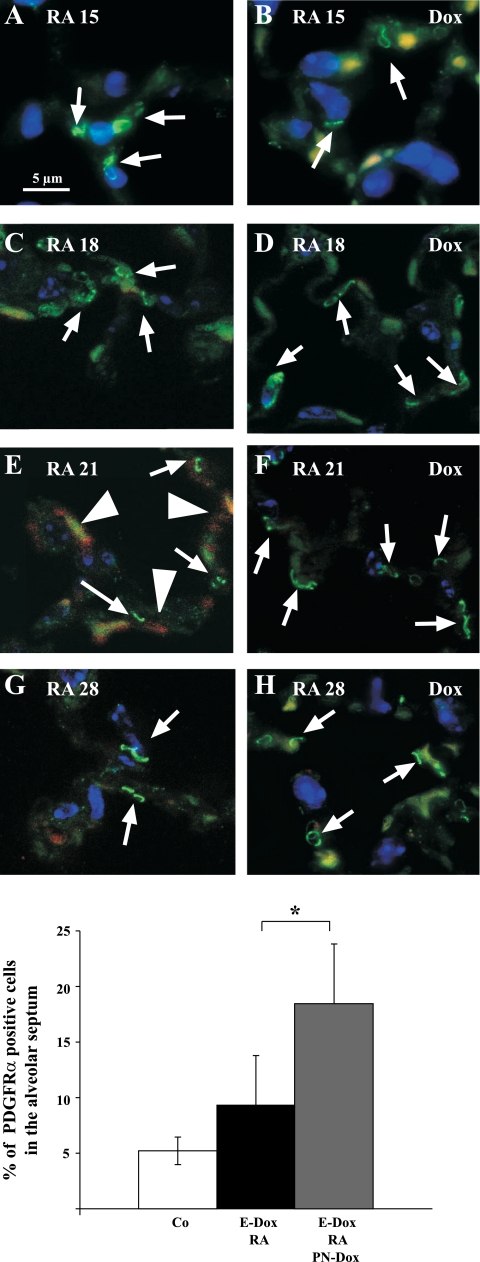
Boström et al. (5), Lindahl et al. (24), and McGowan and Torday (37) hypothesized that the PDGFRα-positive lipofibroblast differentiates into the αSMA-positive interstitial myofibroblast during alveolarization. Recent studies by McGowan et al. (35) show that during normal alveolarization the abundance of PDGFRα-expressing cells increases as the alveolar septae form. Lipofibroblasts expressed low levels of PDGFRα, whereas myofibroblasts expressed high levels of PDGFRα during normal alveolarization (35). To test whether expression of dnFGFR influences PDGFRα expression in interstitial fibroblasts during alveolar regeneration, we performed dual immunohistochemistry for PDGFRα (green) and αSMA (red) expression at completion of RA treatment (RA15) and 3, 7, and 14 days thereafter (RA18, RA21, and RA28) (Fig. 6). During normal regeneration, PDGFRα was detected at all time points (Fig. 6, A, C, E, and G), whereas the number of αSMA-expressing cells was maximal at RA21 (Fig. 6E). In the presence of dnFGFR, expression of PDGFRα was detected at all time points (Fig. 6, B, D, F, and H; dox), but αSMA staining was not detected (Fig. 6F).
Fig. 6.
αSMA expression but not PDGF receptor-α (PDGFRα) expression is suppressed by dnFGFR. Immunohistochemistry is shown for αSMA (red, cytoplasmic), PDGFRα (green, punctuated signal on the cell membrane), and DAPI (blue, nuclear) on DbTg mice after prenatal inhibition of FGF signaling and 3, 7, and 14 days after RA treatment. Expression of PDGFRα (arrows) on the cell membrane of some alveolar fibroblasts can be found at all time points after RA treatment is finished (A, C, E, and G). In the presence of FGF signaling, αSMA (arrowheads) expression was induced 7 days after RA treatment (E). dnFGFR was expressed during RA treatment and alveolar repair (B, D, F, and H). Expression of dnFGFR suppressed αSMA after RA treatment (F and H). More cells expressed PDGFRα (arrows) 1 wk after completion of RA treatment (compare D, F, and H with C, E, and G). Scale bar = 5 μm. Autofluorescence of red blood cells is found in the red and green channels and results in a yellow signal. I: morphometric analysis of PDGFRα expression in RA21 lungs revealed a significant increase in PDGFRα-expressing cells when dnFGFR was expressed. White column: control: 5.21% (±1.23%) of the cells express PDGFRα (n = 4). Black column: DbTg, E-Dox: 9.43% (±4.35%) of the cells express PDGFRα (P < 0.068; n = 7). Gray column: DbTg, E-Dox, PN-Dox: dnFGFR expression increases percentage of PDGFRα cells 18.32% (±5.52%). *P < 0.0044; n = 3.
The number of PDGFRα-positive cells in the lung interstitium was quantified by morphometrics. One week after RA treatment was completed, the percentage of PDGFRα-positive cells in the lung interstitium was similar to untreated normal lungs (Fig. 6, E and I). Expression of dnFGFR during lung regeneration significantly increased the percentage of PDGFRα-positive cells (Fig. 6, F and I). To determine whether myofibroblasts in regenerating septae express PDGFRα, immunohistochemistry for αSMA (red) and PDGFRα (green) was performed on RA21 lungs. Colocalization of PDGFRα and αSMA expression in the same cells was found 1 wk after RA treatment was completed (RA21; Fig. 7A). No αSMA staining was detected in PDGFRα-positive cells when dnFGFR was expressed. To identify PDGFRα-positive cells by nuclear GFP signal, PDGFRαGFP/WT mice were crossed into our emphysema model and subjected to RA-mediated regeneration (18). The nuclear GFP signal corresponds with PDGFRα gene transcription and not PDGFRα protein expression and was expected to appear earlier in alveolar regeneration, and therefore confocal microscopy was performed on triple transgenic lungs at RA18. Nuclear GFP and cytosolic αSMA (red) were colocalized in interstitial fibroblasts as early as 4 days after completion of RA treatment (Fig. 7B), which confirmed the presence of dual expressing cells in the regenerating alveolar septum. No colocalization could be found in the presence of dnFGFR expression. These data demonstrate that dnFGFR inhibits the induction of αSMA in PDGFRα-positive cells during alveolar repair.
Fig. 7.
Dual PDGFRα- and αSMA-positive cells during alveolar regeneration. A: immunohistochemistry for αSMA (red), PDGFRα (green), and DAPI (blue) DbTg lungs 7 days after completion of RA treatment. B: confocal microscopy after immunohistochemistry for αSMA (red) on triple transgenic RA18 lungs [nuclear green fluorescent protein (GFP) in PDGFRαGFP/WT] demonstrate existence of double-positive PDGFRα and αSMA cells. Expression of dnFGFR during alveolar repair results in no dual positive. Scale bar = 5 μm.
DISCUSSION
RA enhances alveolar regeneration in some but not all animal models of lung injury (4, 16, 19, 25, 26, 28, 29, 32, 38, 50, 53). In this study, we show that RA reinitiates formation of new alveolar septae in emphysema caused by prenatal inhibition of FGF signaling. We demonstrated that lungs with increased alveolar air space, due to prenatal block of FGF signaling, have increased proliferation but do not repair unless treated with RA. Analysis of the temporal course of alveolar regeneration revealed induction of αSMA, a marker for interstitial myofibroblasts, 1 wk after completion of RA treatment. These data support the concept that RA-induced alveolar regeneration recapitulates some aspects of alveolar development. Using immunohistochemistry and confocal microscopy, we confirmed that αSMA was induced in PDGFRα-expressing fibroblasts during alveolar regeneration. Expression of dnFGFR had no effect on proliferation during RA repair, blocked αSMA induction, and increased PDGFRα-positive fibroblasts, and alveoli did not regenerate. These data suggest that FGF signaling is required for myofibroblast differentiation as a requisite step in alveolar formation. The lack of fibrotic lesions and the obvious plasticity of existing septae and progenitor cell pools in this emphysema model makes it a valuable tool to study molecular processes and signaling pathways in alveolar reseptation.
The dnFGFR used in this study is a truncated version of FGFR2b and has been demonstrated to inhibit signaling of FGF1, FGF3, FGF7, and FGF10 but not FGF2, FGF4, epidermal growth factor, hepatocyte growth factor, or insulin-like growth factor (8). Dox-mediated expression of dnFGFR by epithelial cells creates an extracellular trap that binds and inactivates a specific subset of FGFs (FGF1, FGF3, FGF7, and FGF10) that are bound and inactivated before they can interact with the native FGFRs on both the epithelium and the mesenchyme. FGF1, FGF2, FGF7, FGF9, FGF10, and FGF18 are all expressed in the developing lung (45). FGF1, FGF2, and FGF7 are expressed in the adult lung, but little is known about the expression of other FGFs or whether FGFs play a role in alveolar regeneration (12, 62).
Since FGFs are known for their mitogenic role in development and repair, the impact of FGF signaling on cellular proliferation in the absence and presence of RA mediated realveolarization was analyzed. The mitotic index in emphysematous DbTg lungs was increased twofold in the absence of RA. Despite the increase in proliferation, these animals do not form new septae without RA treatment. Expression of dnFGFR, which is known to block signaling of FGF1, FGF3, FGF7, and FGF10 (8), significantly reduced proliferation in the absence of RA but not in the presence of RA treatment.
Since the mitotic index was already increased in adult emphysematous lungs and dnFGFR expression did not affect proliferation after RA treatment, we further analyzed molecular changes during alveolar reseptation, which were regulated by FGF. Based on the known role of the interstitial myofibroblast in normal alveolarization, we investigated myofibroblast differentiation during alveolar regeneration. Our data demonstrate that αSMA was induced in the interstitial fibroblast during alveolar reseptation. Since αSMA expression was observed 1 wk after completion of RA, it is unlikely that RA directly induces αSMA expression.
There are at least three different types of myofibroblasts in the adult lung, vascular, bronchiolar, and interstitial. Very little is known about the lineage relationship of the bronchiolar, vascular, or interstitial myofibroblasts and whether different FGFs and FGFRs serve different functions. It was previously shown that FGFR2 influences commitment of smooth muscle cell progenitors (14). We show that expression of a truncated FGFR2b isoform, which is known to block FGF1, FGF3, FGF7, and FGF10 but not FGF9 signaling, inhibited expression of αSMA in interstitial fibroblasts during reseptation. Yi et al. (63) demonstrated an inhibitory role of FGF9 on the differentiation of the peribronchiolar myofibroblast during early lung development. FGF9 is expressed in the epithelium of the developing bronchi and the mesothelial cells and signals to the mesenchyme through the FGFR2IIIc isoform (10, 15, 57). Expression of the dnFGFR in our system had no effect on peribronchiolar or perivascular αSMA expression.
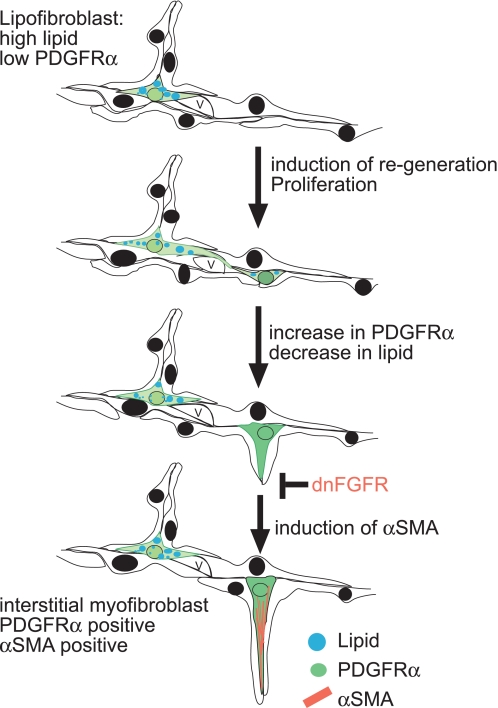
Alveolar staining for αSMA- and PDGFRα-positive cells is missing specifically from lungs lacking PDGFRα (5, 22, 24, 52). Therefore, Lindahl et al. (24) hypothesized that the PDGFRα-positive mesenchymal cells in the perinatal lung may be precursors of alveolar myofibroblasts or provide signals that induce septum formation, matrix deposition, and the differentiation of myofibroblasts. McGowan et al. (35) demonstrated that during alveolar development fibroblasts with high expression levels of PDGFRα contain low levels of lipids, express αSMA, and are preferentially located at the alveolar entry ring. Our data identified cells in the regenerating alveoli that express both αSMA and PDGFRα, suggesting that reseptation recapitulates aspects of normal alveolarization. In this paper, we provide evidence that inhibition of FGF signaling inhibits the induction of αSMA in the PDGFRα-positive cell and results in an increase in PDGFRα-positive cells. Our data show that the peak of proliferation after RA-induced realveolarization is at RA14, whereas the increase in PDGFRα-positive cells in the absence of FGF signaling is observed at RA18, thus suggesting that the increase of PDGFRα-positive cells is due to a lack in differentiation rather than an increase in proliferation. This finding is supported by data from McGowan et al. (35) that demonstrate that PDGFRα-expressing cells are not proliferating more rapidly than cells that do not express the receptor and that there is an inverse correlation between an age-related increase in PDGFRα-positive cells and decrease in proliferative PDGFRα-expressing cells during normal alveolarization. Based on our data and data published by Lindahl and McGowan (24, 35), we propose the following conceptual model of realveolarization. After reseptation is initiated by RA, downstream mediators induce differentiation of the PDGFRα-positive myofibroblast progenitor to the αSMA-positive myofibroblast, which is FGF dependent (Fig. 8). Because of the lack of molecular or genetic tools to specifically label subsets of lung fibroblasts and their progenitors, it is not possible to perform lineage studies at present. Until new molecular or genetic tools are available to specifically label subsets of fibroblasts that might serve as progenitor cells and to follow their daughter cells in lung development and lung regeneration, the lineage relationship of the interstitial fibroblasts will remain unclear.
Fig. 8.
Conceptual model of RA-induced reseptation and the role of FGF in the induction of αSMA in a PDGFRα-positive progenitor cell. RA induces proliferation after 14 days, and after 18 days the number of low lipid and PDGFRα-positive cells (green) increases. Based on Lindahl's and McGowan's data (24, 35), we propose that the PDGFRα-positive cell, which is low in lipids, is a precursor cell for the interstitial myofibroblast and requires FGF signaling to induce expression of αSMA. Our data show that in the presence of FGF signaling, PDGFRα-positive cells induce αSMA (red) expression and that expression of dnFGFR inhibits induction of αSMA in PDGFRα-positive cells, resulting in a block of myofibroblast differentiation and increase of PDGFRα-positive cells.
In the future, this transgenic mouse model will allow valuable studies on lung regeneration without the initiation of lung fibrosis and facilitate dissection of the molecular regulators of alveolar repair.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant 5P50 HL-56387-07 and American Heart Association Grant 0755563B.
Acknowledgments
We thank Dave Loudy, Carrie Ripberger, and Lily Cong for their technical assistance, Jeffrey Whitsett and Malcolm Maden for support, and Sheila Bell, Cindy Bachurski, and James Bridges for their review of the manuscript.
Present address of E. Gale: MRC Clinical Science Centre, Hammersmith Hospital, Imperial College, London, UK.
REFERENCES
- 1.Adler KB, Low RB, Leslie KO, Mitchell J, Evans JN. Contractile cells in normal and fibrotic lung. Lab Invest 60: 473–485, 1989. [PubMed] [Google Scholar]
- 2.Barazzone C, Donati YR, Rochat AF, Vesin C, Kan CD, Pache JC, Piguet PF. Keratinocyte growth factor protects alveolar epithelium and endothelium from oxygen-induced injury in mice. Am J Pathol 154: 1479–1487, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baybutt RC, Hu L, Molteni A. Vitamin A deficiency injures lung and liver parenchyma and impairs function of rat type II pneumocytes. J Nutr 130: 1159–1165, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Belloni PN, Garvin L, Mao CP, Bailey-Healy I, Leaffer D. Effects of all-trans-retinoic acid in promoting alveolar repair. Chest 117: 235S–241S, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Törnell J, Heath JK, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85: 863–873, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Buch S, Han RN, Liu J, Moore A, Edelson JD, Freeman BA, Post M, Tanswell AK. Basic fibroblast growth factor and growth factor receptor gene expression in 85% O2-exposed rat lung. Am J Physiol Lung Cell Mol Physiol 268: L455–L464, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Burri PH Postnatal development and growth. In: The Lung: Scientific Foundations, edited by Crystal RG and West JB. Philadelphia, PA: Lippincott-Raven, 1997, p. 1013–1026.
- 8.Celli G, LaRochelle WJ, Mackem S, Sharp R, Merlino G. Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J 17: 1642–1655, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charafeddine L, D'Angio CT, Richards JL, Stripp BR, Finkelstein JN, Orlowski CC, LoMonaco MB, Paxhia A, Ryan RM. Hyperoxia increases keratinocyte growth factor mRNA expression in neonatal rabbit lung. Am J Physiol Lung Cell Mol Physiol 276: L105–L113, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Colvin JS, Feldman B, Nadeau JH, Goldfarb M, Ornitz DM. Genomic organization and embryonic expression of the mouse fibroblast growth factor 9 gene. Dev Dyn 216: 72–88, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Cook VJ, Coxson HO, Mason AG, Bai TR. Bullae, bronchiectasis and nutritional emphysema in severe anorexia nervosa. Can Respir J 8: 361–365, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Cordon-Cardo C, Vlodavsky I, Haimovitz-Friedman A, Hicklin D, Fuks Z. Expression of basic fibroblast growth factor in normal human tissues. Lab Invest 63: 832–840, 1990. [PubMed] [Google Scholar]
- 13.Coxson HO, Chan IH, Mayo JR, Hlynsky J, Nakano Y, Birmingham CL. Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med 170: 748–752, 2004. [DOI] [PubMed] [Google Scholar]
- 14.De Langhe SP, Carraro G, Warburton D, Hajihosseini MK, Bellusci S. Levels of mesenchymal FGFR2 signaling modulate smooth muscle progenitor cell commitment in the lung. Dev Biol 299: 52–62, 2006. [DOI] [PubMed] [Google Scholar]
- 15.del Moral PM, De Langhe SP, Sala FG, Veltmaat JM, Tefft D, Wang K, Warburton D, Bellusci S. Differential role of FGF9 on epithelium and mesenchyme in mouse embryonic lung. Dev Biol 293: 77–89, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Fujita M, Ye Q, Ouchi H, Nakashima N, Hamada N, Hagimoto N, Kuwano K, Mason RJ, Nakanishi Y. Retinoic acid fails to reverse emphysema in adult mouse models. Thorax 59: 224–230, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gewolb IH, Torday JS. High glucose inhibits maturation of the fetal lung in vitro. Morphometric analysis of lamellar bodies and fibroblast lipid inclusions. Lab Invest 73: 59–63, 1995. [PubMed] [Google Scholar]
- 18.Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol 23: 4013–4025, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hind M, Maden M. Retinoic acid induces alveolar regeneration in the adult mouse lung. Eur Respir J 23: 20–27, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Hokuto I, Perl AK, Whitsett JA. Prenatal, but not postnatal, inhibition of fibroblast growth factor receptor signaling causes emphysema. J Biol Chem 278: 415–421, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Kapanci Y, Assimacopoulos A, Irle C, Zwahlen A, Gabbiani G. “Contractile interstitial cells” in pulmonary alveolar septa: a possible regulator of ventilation-perfusion ratio? Ultrastructural, immunofluorescence, and in vitro studies. J Cell Biol 60: 375–392, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klinghoffer RA, Hamilton TG, Hoch R, Soriano P. An allelic series at the PDGFalphaR locus indicates unequal contributions of distinct signaling pathways during development. Dev Cell 2: 103–113, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Leslie KO, Mitchell JJ, Woodcock-Mitchell JL, Low RB. Alpha smooth muscle actin expression in developing and adult human lung. Differentiation 44: 143–149, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Lindahl P, Karlsson L, Hellstrom M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development 124: 3943–3953, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Lucey EC, Goldstein RH, Breuer R, Rexer BN, Ong DE, Snider GL. Retinoic acid does not affect alveolar septation in adult FVB mice with elastase-induced emphysema. Respiration 70: 200–205, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Maden M Retinoids have differing efficacies on alveolar regeneration in a dexamethasone-treated mouse. Am J Respir Cell Mol Biol 35: 260–267, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Maden M, Hind M. Retinoic acid in alveolar development, maintenance and regeneration. Philos Trans R Soc Lond B Biol Sci 359: 799–808, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.March TH, Bowen LE, Finch GL, Nikula KJ, Wayne BJ, Hobbs CH. Effects of strain and treatment with inhaled all-trans-retinoic acid on cigarette smoke-induced pulmonary emphysema in mice. COPD 2: 289–302, 2005. [PubMed] [Google Scholar]
- 29.March TH, Cossey PY, Esparza DC, Dix KJ, McDonald JD, Bowen LE. Inhalation administration of all-trans-retinoic acid for treatment of elastase-induced pulmonary emphysema in Fischer 344 rats. Exp Lung Res 30: 383–404, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Massaro GD, Massaro D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol Lung Cell Mol Physiol 270: L305–L310, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Massaro GD, Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med 3: 675–677, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Massaro GD, Massaro D. Retinoic acid treatment partially rescues failed septation in rats and in mice. Am J Physiol Lung Cell Mol Physiol 278: L955–L960, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Massaro GD, Radaeva S, Clerch LB, Massaro D. Lung alveoli: endogenous programmed destruction and regeneration. Am J Physiol Lung Cell Mol Physiol 283: L305–L309, 2002. [DOI] [PubMed] [Google Scholar]
- 34.McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol 23: 162–167, 2000. [DOI] [PubMed] [Google Scholar]
- 35.McGowan SE, Grossmann RE, Kimani PW, Holmes AJ. Platelet-derived growth factor receptor-alpha-expressing cells localize to the alveolar entry ring and have characteristics of myofibroblasts during pulmonary alveolar septal formation. Anat Rec (Hoboken) 291: 1649–1661, 2008. [DOI] [PubMed] [Google Scholar]
- 36.McGowan SE, Harvey CS, Jackson SK. Retinoids, retinoic acid receptors, and cytoplasmic retinoid binding proteins in perinatal rat lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 269: L463–L472, 1995. [DOI] [PubMed] [Google Scholar]
- 37.McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol 59: 43–62, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Meshi B, Vitalis TZ, Ionescu D, Elliott WM, Liu C, Wang XD, Hayashi S, Hogg JC. Emphysematous lung destruction by cigarette smoke. The effects of latent adenoviral infection on the lung inflammatory response. Am J Respir Cell Mol Biol 26: 52–57, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Panos RJ, Bak PM, Simonet WS, Rubin JS, Smith LJ. Intratracheal instillation of keratinocyte growth factor decreases hyperoxia-induced mortality in rats. J Clin Invest 96: 2026–2033, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearson E, Bose C, Snidow T, Ransom L, Young T, Bose G, Stiles A. Trial of vitamin A supplementation in very low birth weight infants at risk for bronchopulmonary dysplasia. J Pediatr 121: 420–427, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res 11: 21–29, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Robbins ST, Fletcher AB. Early vs delayed vitamin A supplementation in very-low-birth-weight infants. JPEN J Parenter Enteral Nutr 17: 220–225, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Schultz CJ, Torres E, Londos C, Torday JS. Role of adipocyte differentiation-related protein in surfactant phospholipid synthesis by type II cells. Am J Physiol Lung Cell Mol Physiol 283: L288–L296, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol 66: 625–645, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Shenai JP, Chytil F, Jhaveri A, Stahlman MT. Plasma vitamin A and retinol-binding protein in premature and term neonates. J Pediatr 99: 302–305, 1981. [DOI] [PubMed] [Google Scholar]
- 47.Shenai JP, Chytil F, Stahlman MT. Liver vitamin A reserves of very low birth weight neonates. Pediatr Res 19: 892–893, 1985. [DOI] [PubMed] [Google Scholar]
- 48.Shenai JP, Kennedy KA, Chytil F, Stahlman MT. Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. J Pediatr 111: 269–277, 1987. [DOI] [PubMed] [Google Scholar]
- 49.Snyder JM, Jenkins-Moore M, Jackson SK, Goss KL, Dai HH, Bangsund PJ, Giguere V, McGowan SE. Alveolarization in retinoic acid receptor-beta-deficient mice. Pediatr Res 57: 384–391, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Srinivasan G, Bruce EN, Houtz PK, Bruce MC. Dexamethasone-induced changes in lung function are not prevented by concomitant treatment with retinoic acid. Am J Physiol Lung Cell Mol Physiol 283: L275–L287, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Stinchcombe SV, Maden M. Retinoic acid induced alveolar regeneration: critical differences in strain sensitivity. Am J Respir Cell Mol Biol 38: 185–191, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Sun T, Jayatilake D, Afink GB, Ataliotis P, Nister M, Richardson WD, Smith HK. A human YAC transgene rescues craniofacial and neural tube development in PDGFRalpha knockout mice and uncovers a role for PDGFRalpha in prenatal lung growth. Development 127: 4519–4529, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Tepper J, Pfeiffer J, Aldrich M, Tumas D, Kern J, Hoffman E, McLennan G, Hyde D. Can retinoic acid ameliorate the physiologic and morphologic effects of elastase instillation in the rat? Chest 117: 242S–244S, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Torday JS, Torres E, Rehan VK. The role of fibroblast transdifferentiation in lung epithelial cell proliferation, differentiation, and repair in vitro. Pediatr Pathol Mol Med 22: 189–207, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Vaccaro C, Brody JS. Ultrastructure of developing alveoli. I. The role of the interstitial fibroblast. Anat Rec 192: 467–479, 1978. [DOI] [PubMed] [Google Scholar]
- 56.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol Lung Cell Mol Physiol 275: L110–L117, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol 258: 169–184, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Weibel ER Sterological Methods: Practical Methods of Biological Morphometry. New York: Academic, 1979, vol. 1, p. 9–196. [Google Scholar]
- 59.Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development 125: 3615–3623, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Wert SE, Yoshida M, LeVine AM, Ikegami M, Jones T, Ross GF, Fisher JH, Korfhagen TR, Whitsett JA. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci USA 97: 5972–5977, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolbach SB, Howe PR. Epithelial repair in recovery from vitamin A deficiancy: an experimental study. J Exp Med 57: 511–526, 1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yano T, Mason RJ, Pan T, Deterding RR, Nielsen LD, Shannon JM. KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am J Physiol Lung Cell Mol Physiol 279: L1146–L1158, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Yi L, Domyan ET, Lewandoski M, Sun X. Fibroblast growth factor 9 signaling inhibits airway smooth muscle differentiation in mouse lung. Dev Dyn 238: 123–137, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou L, Lim L, Costa RH, Whitsett JA. Thyroid transcription factor-1, hepatocyte nuclear factor-3beta, surfactant protein B, C, and Clara cell secretory protein in developing mouse lung. J Histochem Cytochem 44: 1183–1193, 1996. [DOI] [PubMed] [Google Scholar]