Abstract
Inhibitory M2 muscarinic receptors on airway parasympathetic nerves normally limit acetylcholine release. Viral infections decrease M2 receptor function, increasing vagally mediated bronchoconstriction. Since retinoic acid deficiency causes M2 receptor dysfunction, we tested whether retinoic acid would prevent virus-induced airway hyperreactivity and prevent M2 receptor dysfunction. Guinea pigs infected with parainfluenza virus were hyperreactive to electrical stimulation of the vagus nerves, but not to intravenous acetylcholine, indicating that hyperreactivity was due to increased release of acetylcholine from parasympathetic nerves. The muscarinic agonist pilocarpine, which inhibits vagally mediated bronchoconstriction in control animals, no longer inhibited vagally induced bronchoconstriction, demonstrating M2 receptor dysfunction. Treatment with all-trans retinoic acid (1 mg/kg) prevented virus-induced hyperreactivity and M2 receptor dysfunction. However, retinoic acid also significantly reduced viral titers in the lungs and attenuated virus-induced lung inflammation. In vitro, retinoic acid decreased M2 receptor mRNA expression in both human neuroblastoma cells and primary cultures of airway parasympathetic neurons. Thus, the protective effects of retinoic acid on airway function during viral infection appear to be due to anti-inflammatory and antiviral mechanisms, rather than to direct effects on M2 receptor gene expression.
Keywords: asthma, parainfluenza, vagus, parasympathetic, cholinergic
viral infections are a major cause of asthma attacks, both in children (14) and adults (3). Even in the absence of underlying asthma, naturally occurring viral infections increase airway reactivity. This virus-induced hyperreactivity is blocked by atropine, indicating that it is mediated by increased reflex bronchoconstriction (8).
We (10) and others (5) have previously shown that viral infection increases vagally mediated bronchoconstriction in virus-infected animals. This is caused by loss of function of inhibitory M2 muscarinic receptors on parasympathetic neurons (10). These inhibitory M2 receptors normally decrease acetylcholine release onto M3 receptors on airway smooth muscle, subsequently decreasing contraction and bronchoconstriction (11). Inhibitory M2 receptors are found on airway parasympathetic neurons in guinea pigs (11), humans (21), and all other species tested thus far (1, 4, 15, 25). Blocking neuronal M2 receptors with selective antagonists increases vagally mediated bronchoconstriction by as much as eightfold, whereas stimulating them substantially suppresses vagally mediated bronchoconstriction (11).
Vitamin A deficiency has been shown to cause airway hyperreactivity and M2 muscarinic receptor dysfunction in guinea pigs (19) that can be reversed by repleting vitamin A (18). Furthermore, the demonstration by Massaro and Massaro (17) that treatment with retinoic acid caused lung regeneration in rats with experimental emphysema has led to interest and clinical trials of all-trans retinoic acid in humans with chronic obstructive lung disease. Exacerbations of COPD are frequently associated with viral infections (24), thus we examined whether retinoic acid would also protect against virus-induced hyperreactivity by protecting neuronal M2 muscarinic receptor function.
MATERIALS AND METHODS
Animals.
Pathogen-free female Dunkin-Hartley guinea pigs (300–350 g; Hilltop Animal Farms, Scottsdale, PA) were shipped in filtered crates and housed in high-efficiency particulate-filtered air. All animals were handled in accordance with the standards established by the U.S. Animal Welfare Act set forth in the National Institutes of Health guidelines and were approved by the Johns Hopkins University and Oregon Health and Science University Animal Care and Use Committees.
Virus stock solutions.
Stock solutions of parainfluenza type 1 (Sendai virus, VR-105, American Type Culture Collection) and of human influenza A/Port Chalmers/72 (H3N2) were grown in rhesus monkey kidney cell monolayers and prepared and titered in fresh rhesus monkey kidney cell monolayers as described previously (10).
Viral infection and retinoic acid treatment of guinea pigs.
Animals were infected with parainfluenza virus by instilling intranasally 105 times the amount of virus required to infect 50% of rhesus monkey kidney cell monolayers (TCID50). Controls were inoculated with uninfected culture media. To ensure that retinoic acid did not affect the ability to establish an infection in vivo, 1 mg/kg all-trans retinoic acid was administered intraperitoneally 2 and 3 days after viral infection to some animals. Control groups were injected with vehicle only. Retinoic acid was checked for endotoxin using the limulus amebocyte lysate assay (HyCult Biotechnology) and was found to be undetectable.
Viral infection was monitored by infecting rhesus monkey kidney cells with dilutions of lung homogenates from all animals as previously described (10). Viral infection of isolated cells was detected by hemadsorption as previously described (10). Viral content was determined as the amount of lung homogenate required to produce infection in 50% of rhesus monkey kidney monolayers (the TCID50). In some experiments, we titered virus by real-time RT-PCR as described below, which, in our hands, yielded similar results to the rhesus monkey kidney cell method.
Vagal reactivity and M2 receptor function.
Four days after infection, guinea pigs were anesthetized with urethane (1.8 g/kg ip). Heart rate and blood pressure were measured via a carotid artery cannula. Both jugular veins were also cannulated for administration of drugs. Both vagus nerves were cut and the distal ends placed on shielded electrodes immersed in a pool of mineral oil. Body temperature was maintained at 37°C using a heating blanket. Animals were paralyzed with succinylcholine (10 ml·kg−1·min−1 iv) and ventilated (tidal vol 1 ml/100 g body wt at 100 breaths/min) via a tracheal cannula (using a constant volume pump, Harvard Apparatus, South Natick, MA). All animals were treated with guanethidine (20 mg/kg iv) and propranolol (1 mg/kg iv) 20 min before physiological measurements to block sympathetic nerves.
Bronchoconstriction [measured as an increase in pulmonary inflation pressure (Ppi) via a pressure transducer (Becton Dickinson) on a sidearm of the tracheal cannula] was induced by stimulating the vagi electrically at 2–25 Hz, 0.2-ms pulse duration, 10 V, 5-s pulse train at 2-min intervals. Bronchoconstriction was also induced by acetylcholine (1–10 μg/kg iv) administered to vagotomized animals to test the function of postjunctional M3 receptors on airway smooth muscle. Blockade of vagally induced and acetylcholine-induced bronchoconstriction by atropine indicated that they were mediated by acetylcholine and muscarinic receptors.
Neuronal M2 receptor function was measured by the ability of a muscarinic agonist, pilocarpine (0.01–100 μg/kg iv), to inhibit vagally induced bronchoconstriction in a dose-dependent manner. For these experiments, the vagi were electrically stimulated at 2 Hz, 0.2-ms pulse duration, 44 pulses/train. The voltage (between 5 and 15 V) was chosen in the absence of pilocarpine to induce an increase in Ppi of 15–25 mmH2O above baseline. The effect of pilocarpine on vagally induced bronchoconstriction was measured as a ratio of bronchoconstriction in the presence of pilocarpine to bronchoconstriction in the absence of pilocarpine. Voltages were not different between groups.
Lung lavage.
At the end of the experiment, guinea pigs were killed, and the lungs were lavaged five times with 10-ml aliquots of PBS via the tracheal cannula. Differential cell counts were obtained using cytospin slides stained with Diff-Quik (Scientific Products, McGaw Park, IL).
Cell lines and primary cell cultures.
SK-N-SH human neuroblastoma cells (from American Type Culture Collection) were maintained in culture at 37°C/5% CO2 for not more than 10 serial passages using EMEM culture media supplemented with 10% FBS. Primary cultures of airway parasympathetic neurons were initiated from guinea pig tracheas and grown on matrigel in serum-free medium for 1 wk as previously described (9).
Primary cultures of airway epithelial cells were prepared from human tracheas (obtained from organ donors via the Pacific Northwest Transplant Bank) and from guinea pig tracheas. Tracheas were immersed overnight at 4°C in DMEM + 1% PSA that contained 0.5% pronase and then warmed to 37°C for 30 min. Disaggregated epithelial cells were separated from underlying tissues by gentle washing with DMEM + 5% FBS + 1% PSA and plated on collagen-coated plates. Four hours later, media was replaced with LHC8 + 1% PSA and subsequently changed at 48- to 72-h intervals until infection.
Retinoic acid treatment and viral infection of epithelial cell cultures.
Retinoic acid (1 μM) was applied to cell cultures daily for a total of 4 days. At 2 days of treatment, cell monolayers were inoculated with various amounts of parainfluenza virus (for guinea pig tracheal epithelial cells) or influenza virus (for cultures of human airway epithelial cells or A549 lung epithelial cells) for 1 h and washed twice with HBSS. Cells were harvested for assay 2 days later.
Real-time RT-PCR.
Total RNA was isolated from cell cultures (RNeasy, Qiagen), and 0.5–2 μg total RNA was reverse transcribed using random primers (Superscript III, Invitrogen). Real-time PCR comparison of mRNA transcript levels was made using SYBR green with ROX correction (Quantitect, Qiagen, and iTaq, Bio-rad) in a 96-well thermal cycler (Mx3000P, Stratagene) with fluorescence detected at 72°C. Standard cycling parameters used were 94°C for 20 s, 58°C for 30 s, 72°C for 30 s, and the reactions were continued for 45 cycles. The following primers were used: human IL-8 (5′TCTGCAGCTCTGTGTGAAGG3′) and (5′TGTGGTCCACTCTCAATCACTC3′), human retinoid inducible gene-I (RIG-I) (5′TGGCATATTGACTGGACGTG3′) and (5′TGCCTTCATCAGCAACTGAG3′), guinea pig M2 muscarinic receptor (5′TTTTCCAATGCTGCTGTCAC3′) and (5′GGCATGTTGTTGTTGTTTGG3′), human M2 muscarinic receptor (5′TTAAAGTCAACCGCCACCTC3′) and (5′CAAAGGTCACACACCACAGG3′), human RING-4 (a gene we have found to be induced by retinoic acid) (5′AGCTTTGCCAACGAGGAG3′) and (5′GTGACAAGGTTCCCACT3′), and 18S ribosomal RNA (5′GTAACCCGTTGAACCCCATT3′) and (5′CCATCCAATCGGTAGTAGCG3′). When possible, primer pairs were designed to cross the splice junction for an intron of a minimum of 1-kb size to reduce the possibility of amplifying genomic DNA. Serial tenfold dilutions of randomly selected cDNAs were analyzed for each PCR plate to validate the replicable range and amplification efficiency across experiments.
Virus RNA was quantified using primers directed at the matrix protein for parainfluenza: (5′ATGCGGCTGATCTTCTCACT3′) and (5′CTTTGCCACGACATTAGGGT3′) and influenza A: (5′CATCCTGTTGTATATGAGGCCCAT3′) and (5′GGACTGCAGCGTAGACGCTT3′). Total cellular RNAs were isolated and cDNAs synthesized as above using random hexamer primers. cDNAs were then frozen at −20°C for 30 min, and RNase H treated at 37°C for 30 min and then assayed by real-time PCR. cDNA standards were prepared from the viral stock solutions used for experimental infections, and serial tenfold dilution of this cDNA was analyzed for each PCR plate to calculate the viral content in TCID50 relative to hemadsorption in rhesus monkey kidney cells.
Melting curve analysis was used routinely to confirm single products for each reaction. Representative PCR products were verified using agarose gel extraction (Qiagen, Valencia, CA) followed by direct automated sequencing (Applied Biosystems) and BLAST analysis (NCBI).
Drugs and reagents.
Acetylcholine chloride, atropine, guanethidine, pilocarpine, propranolol, all-trans retinoic acid, succinylcholine chloride, and urethane were purchased from Sigma (St. Louis, MO). Rhesus monkey kidney cells were purchased from Viromed (Minneapolis, MN). Culture media, FBS, trypsin-EDTA, penicillin-streptomycin-amphotericin B, and l-glutamine were obtained from Invitrogen. Pronase and human placental collagen, type IV, were obtained from Sigma. BSA was obtained from Fisher Scientific. Insulin-transferrin-selenenite was obtained from Mediatech.
Statistical analysis.
All data were expressed as means ± SE. Pilocarpine, frequency and acetylcholine responses as well as dose response curves for gene expression and viral replication were analyzed using two-way ANOVAs for repeated measures. Lung lavage cell counts were analyzed using ANOVA. A P value of <0.05 was considered significant. All statistical analyses were made with the software package Statview 4.5 (Abacus Concepts, Berkeley, CA).
RESULTS
Effect of retinoic acid on virus-induced hyperreactivity.
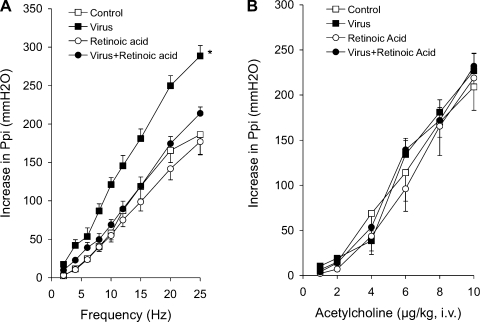
Electrical stimulation of both vagus nerves caused frequency-dependent bronchoconstriction that was significantly potentiated 4 days after infection with parainfluenza virus type 1 (Sendai virus, Fig. 1A). Virus-induced hyperreactivity was mediated by nerves since in vagotomized animals, bronchoconstriction induced by intravenous acetylcholine was not potentiated by viral infection (Fig. 1B). Treatment with all-trans retinoic acid (1 mg/kg ip) on days 2 and 3 after infection prevented virus-induced hyperreactivity to nerve stimulation (Fig. 1A) but had no effect on acetylcholine-induced bronchoconstriction (Fig. 1B) or on responses to vagal stimulation or acetylcholine in uninfected animals. Neither viral infection nor retinoic acid altered vagally induced or acetylcholine-induced bradycardia (data not shown).
Fig. 1.
Virus-induced hyperreactivity is prevented by retinoic acid. A: electrical stimulation (10 V, 0.2 ms, 5 s) of both vagi caused frequency-dependent bronchoconstriction, measured as an increase in pulmonary inflation pressure in controls (open squares). This is not affected by treatment with retinoic acid (1 mg/kg ip, open circles). Vagally induced bronchoconstriction was significantly potentiated by parainfluenza virus infection (closed squares). Retinoic acid completely prevented virus-induced hyperreactivity. B: intravenous acetylcholine-induced bronchoconstriction (open squares) was not altered by parainfluenza virus infection (closed squares), retinoic acid (open circles), or retinoic acid + virus infection (closed circles). *Significantly different from control; n = 4–5.
Effect of retinoic acid on virus-induced neuronal M2 muscarinic receptor dysfunction.
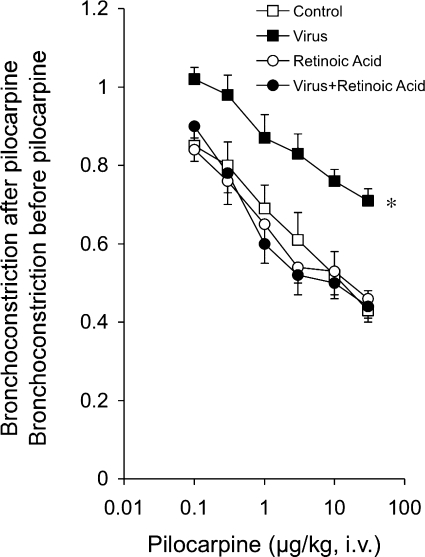
By stimulating M2 receptors, the muscarinic agonist pilocarpine inhibited vagally induced bronchoconstriction in control animals, demonstrating functional neuronal M2 receptors (Fig. 2). In virus-infected animals, the ability of neuronal M2 receptors to limit acetylcholine release is impaired as pilocarpine did not decrease vagally mediated bronchoconstriction in these animals. Retinoic acid restored the response to pilocarpine in virus-infected animals to control levels, indicating that M2 receptor function had been restored. Mock infection of animals with UV-inactivated virus stocks had no effect on airway reactivity or airway inflammation measured 4 days later (data not shown).
Fig. 2.
Retinoic acid prevented virus-induced M2 receptor dysfunction. Reproducible bronchoconstriction was produced by stimulation of both vagi (2 Hz, 0.2-ms pulse, for 22 s at 1-min intervals). Pilocarpine inhibited vagally induced bronchoconstriction in control (open squares) but not parainfluenza virus-infected guinea pigs (closed squares). Retinoic acid (1 mg/kg ip) did not affect M2 receptor function in uninfected animals (open circles) but prevented M2 receptor dysfunction in virus-infected animals (closed circles). *Significantly different from control; n = 4–6.
Effect of retinoic acid on virus-induced inflammation in the lungs.
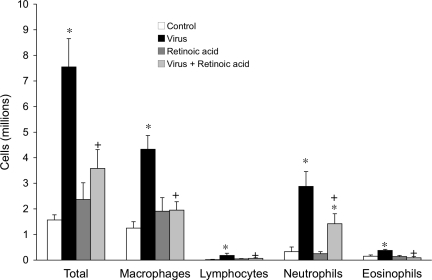
Viral infection significantly increased all inflammatory cells recovered in bronchoalveolar lavage (Fig. 3). Retinoic acid prevented virus-induced increases in macrophages, lymphocytes, and eosinophils, and attenuated the virus-induced increase in neutrophils. Retinoic acid did not affect inflammatory cell populations in uninfected control animals.
Fig. 3.
Retinoic acid inhibited the virus-induced influx of inflammatory cells in bronchoalveolar lavage. Parainfluenza virus infection (black bars) significantly increased all inflammatory cells in bronchoalveolar lavage compared with uninfected controls (open bars). Retinoic acid (1 mg/kg) significantly inhibited increases in all inflammatory cell types in virus-infected (light gray) but not uninfected controls (dark gray). *Significantly different from control, +significantly different from infected; n = 3–7.
Effect of retinoic acid on viral infection and replication.
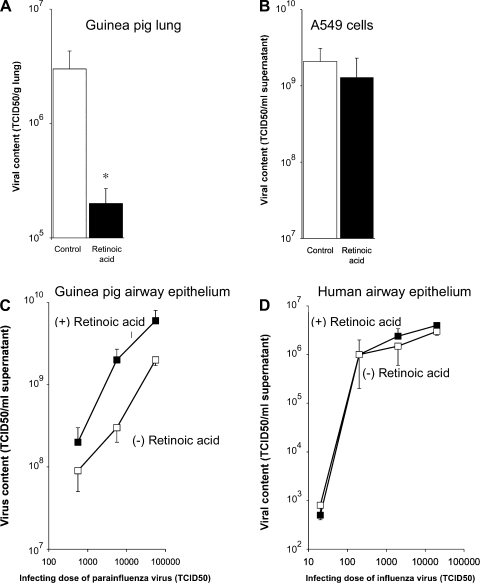
The concentration of virus recovered from infected guinea pig lungs was significantly decreased by retinoic acid treatment (Fig. 4A). In contrast, retinoic acid did not inhibit viral infection or replication in vitro in A549 cells infected with influenza virus (Fig. 4B), primary cultures of guinea pig airway epithelial cells infected with parainfluenza (Fig. 4C), or in primary cultures of human airway epithelial cells infected with influenza virus (Fig. 4D). Thus the in vivo antiviral effect of retinoic acid does not appear to be the result of a direct effect on the infected epithelial cells.
Fig. 4.
Effects of retinoic acid on viral replication. A: retinoic acid (1 mg/kg ip) significantly reduced parainfluenza virus titers in lungs from infected guinea pigs. Results represent the multiple of the amount of virus required to produce infection in 50% of rhesus monkey kidney cells (TCID50) normalized to lung wet weight (*significantly different from no retinoic acid, n = 6). In contrast, retinoic acid did not affect replication of influenza virus in A549 cells (B), parainfluenza virus in primary cultures of guinea pig airway epithelial cells (C), or the replication of influenza virus in primary cultures of human airway epithelial cells (D).
Effect of retinoic acid on M2 receptor gene expression in cultured neurons.
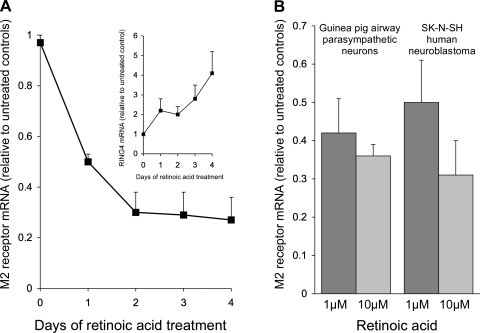
Retinoic acid (1 μM) decreased M2 receptor gene expression in BE(2)-M17 human neuroblastoma cells (Fig. 5A). Expression was decreased by one-half after 24 h of retinoic acid and by 80% after 3 days. This did not appear to be a toxic effect of the retinoic acid or a global effect on mRNA synthesis, as the expression of RING-4 (an MHC-related gene we have found to be induced by retinoic acid) was substantially increased over the same time period (Fig. 5A, inset). In a second neuroblastoma cell line, SK-N-SH, and in primary cultures of guinea pig airway parasympathetic neurons, retinoic acid also decreased M2 receptor gene expression in a dose-dependent fashion (Fig. 5B). Thus the ability of retinoic acid to prevent virus-induced M2 receptor dysfunction does not appear to be due to a direct effect of retinoic acid on M2 receptor gene expression.
Fig. 5.
Retinoic acid decreased M2 receptor mRNA expression in cultured neurons. Treatment with retinoic acid (1 μM) decreased M2 receptor mRNA expression in BE(2)-M17 human neuroblastoma cells over 4 days (A). Expression of RING-4 was increased over the same time period (A, inset). Retinoic acid also decreased M2 receptor mRNA expression in SK-N-SH human neuroblastoma cells (B; n = 5) and in primary cultures of human parasympathetic neurons (B; n = 3).
Effect of retinoic acid on IL-8 and RIG-I gene expression in virus-infected airway epithelial cell cultures.
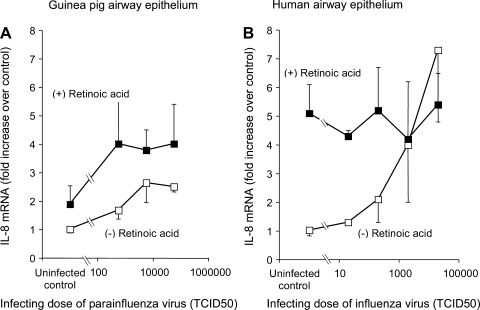
As we have previously shown (7), viral infection substantially increased the expression of IL-8 in airway epithelial cell cultures from both humans and guinea pigs (Fig. 6). Retinoic acid also induced IL-8 mRNA expression and did not suppress virus-induced induction of IL-8 mRNA expression (Fig. 6). Thus the in vivo anti-inflammatory effect of retinoic acid does not appear to be due to suppression of IL-8 expression by virus-infected epithelial cells.
Fig. 6.
A: virus infection increased IL-8 expression in primary cultures of airway epithelial cells from guinea pigs (A) and humans (B). Retinoic acid (1 μM) increased IL-8 expression in uninfected cells, and in parainfluenza virus-infected cells, retinoic acid potentiated the increase in IL-8 expression.
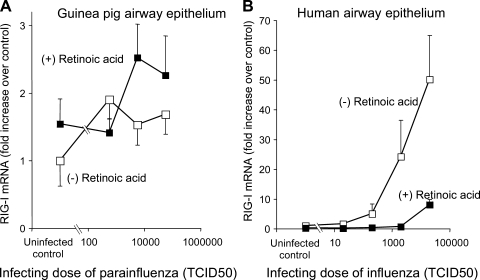
The antiviral helicase RIG-I was induced by viral infection in cultured airway epithelium in correlation with the inoculating dose of virus (Fig. 7). However, despite its name, RIG-I was not induced by retinoic acid. Furthermore, retinoic acid decreased RIG-I expression in virus-infected human airway epithelial cells (Fig. 7). Other RNA helicase family members MDA-5 and LGP-2 were also inducible by virus infection but not by retinoic acid (data not shown).
Fig. 7.
Retinoic acid did not induce retinoic acid inducible gene-I (RIG-I) expression in either uninfected or parainfluenza-infected primary cultures of guinea pig airway epithelium (open squares: without retinoic acid; closed squares: with retinoic acid, 1 μM). B: in primary cultures of human airway epithelial cells, infection with influenza virus induced RIG-I expression. This induction was suppressed by retinoic acid (open squares: without retinoic acid; closed squares: with retinoic acid, 1 μM).
DISCUSSION
Viral infection causes hyperreactivity to vagal stimulation. As we have previously shown, this is due to increased acetylcholine release from parasympathetic nerves, resulting from dysfunction of inhibitory M2 muscarinic receptors on the nerves (10). In contrast, the M3 muscarinic receptors on airway smooth muscle are unaffected by viral infection, as bronchoconstriction in response to exogenous acetylcholine is not increased. Treating virus-infected animals with retinoic acid (1 mg/kg) 2 days after infection prevented virus-induced hyperreactivity (Fig. 1) and protected M2 receptor function measured 2 days later (Fig. 2). In terms of the potential therapeutic use of retinoic acid, it is significant that the retinoic acid was given after viral infection was initiated.
Retinoic acid blocked virus-induced influx of macrophages, lymphocytes, and eosinophils, and attenuated virus-induced neutrophilia (Fig. 3). We have previously shown that eosinophils do not contribute to virus-induced hyperreactivity unless animals are also sensitized to antigen (2). However, macrophages do contribute to virus-induced hyperreactivity and M2 receptor dysfunction since depleting them, by pretreating with liposome-encapsulated clodronate, prevented both hyperreactivity and M2 receptor dysfunction in virus-infected animals (16). While the mechanism of macrophage-induced M2 receptor dysfunction is a topic of continuing investigation in our lab, we have recently found that TNFα decreases M2 receptor expression in primary cultures of both human and guinea pig airway parasympathetic neurons, as well as in human neuroblastoma cells. This effect appears to be primarily the result of decreased message stability (23). Neutrophils are also important in virus-induced hyperreactivity since cyclophosphamide, which depletes granulocytes, blocks virus-induced hyperreactivity in about one-half of the animals so treated (12), this effect being dependent on the viral titers in the lungs. Thus, a mechanism for retinoic acid-induced protection in viral infection is by anti-inflammatory effects.
The mechanisms of the anti-inflammatory effect of retinoic acid in virus-infected lungs are, however, unknown. We have previously shown that IL-8, a proinflammatory cytokine, is strongly induced by viral infection of airway epithelial cells (7). However, retinoic acid failed to inhibit virus-induced IL-8 gene expression in cultured airway epithelial cells. Our results are in agreement with Chang et al. (6), who demonstrated that retinoic acid increased IL-8 expression in airway epithelial cells. Thus, a direct effect of retinoic acid on epithelial IL-8 production cannot be invoked to explain the anti-inflammatory effects of retinoic acid in vivo.
The mechanisms of the anti-inflammatory effect of retinoic acid in virus-infected lungs are unknown. Retinoic acid has been described to inhibit expression of VCAM (20) and to inhibit the activity of phospholipase A2 and the release of arachidonic acid (13). Recent reports also suggest a role for retinoic acid in promoting the differentiation of T regulatory cells (22). Further investigation will be required to determine whether these mechanisms are responsible for inhibiting the inflammatory response to viral infections.
In addition to its anti-inflammatory effects, retinoic acid also significantly suppressed viral replication in the lungs in vivo, as measured by recovery of virus from lung homogenates (Fig. 4A). However, retinoic acid did not exert a similar effect in vitro. Viral replication was not suppressed by retinoic acid in either pulmonary epithelial A549 cells (Fig. 4B) or in primary cultures of guinea pig (Fig. 4C) or human airway epithelial cells (Fig. 4D). Sendai infection induced RIG-I in cultured airway epithelium (Fig. 7). RIG-I is an important molecule in the initiation of antiviral defenses that responds to double-stranded RNA and viral infection. However, despite its name, RIG-I was not induced by retinoic acid. Surprisingly, a review of the published literature also failed to reveal evidence that RIG-I is actually induced by retinoic acid.
Airway reactivity is often linked to function of neuronal M2 muscarinic receptors. Loss of M2 function results in hyperreactivity (10). Because vitamin A deficiency causes M2 muscarinic receptor dysfunction (19), we tested the effect of retinoic acid on M2 receptor gene expression. In primary cultures of guinea pig airway parasympathetic neurons and two different human neuroblastoma lines [SK-N-SH and BE(2)-M17], we found that retinoic acid substantially reduced M2 receptor gene expression (Fig. 5). This did not appear to be a global effect of retinoic acid on mRNA synthesis, as retinoic acid substantially increased expression of RING-4 in the same cultures. Thus, direct induction of M2 receptor gene expression by retinoic acid in airway parasympathetic neurons is not likely to be involved in amelioration of virus-induced airway hyperreactivity by retinoic acid. We do not know the mechanism of decreased M2 receptor expression in response to retinoic acid, but the slow time course of the decrease (Fig. 6) suggests that it may not be a direct effect of retinoic acid receptors on the M2 receptor promoter.
Thus, we have shown that retinoic acid prevents virus-induced airway hyperreactivity and M2 receptor dysfunction. Protection of M2 function was unlikely due to increased expression of M2 receptors, since in cultured neurons M2 gene expression was decreased by retinoic acid. Although retinoic acid decreased viral titers in vivo, the antiviral effects of retinoic acid are not due to direct effects on airway epithelial cells. Retinoic acid is anti-inflammatory and decreases macrophages in the lungs, which may account for decreased viral replication. The finding that retinoic acid decreases viral replication, inflammation, and airway hyperreactivity when given after initiation of infection suggests the potential for postinfection treatment in humans.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants HL-55543, HL-54659, HL-61013, HL-71795, and PPG-HL-10342, and by a grant from the American Heart Association.
Acknowledgments
We thank Bethany Yost, Fiona Coulson, Elizabeth Bivins-Smith, and Isabella Jacoby for technical and scientific assistance. We thank the members of the Pacific Northwest Transplant Bank, Portland, Oregon, for procuring and supplying tracheas from organ donors.
REFERENCES
- 1.Aas P, Maclagan J. Evidence for prejunctional M2 muscarinic receptors in pulmonary cholinergic nerves in the rat. Br J Pharmacol 101: 73–76, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp Med 190: 1465–1478, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atmar RL, Guy E, Guntupalli KK, Zimmerman JL, Bandi VD, Baxter BD, Greenberg SB. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med 158: 2453–2459, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Blaber LC, Fryer AD, Maclagan J. Neuronal muscarinic receptors attenuate vagally-induced contraction of feline bronchial smooth muscle. Br J Pharmacol 86: 723–728, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner CK, Songsiridej V, Dick EC, Busse WW. In vivo and in vitro studies on the use of the guinea pig as a model for virus-provoked airway hyperreactivity. Am Rev Respir Dis 132: 305–310, 1985. [DOI] [PubMed] [Google Scholar]
- 6.Chang MM, Harper R, Hyde DM, Wu R. A novel mechanism of retinoic acid-enhanced interleukin-8 gene expression in airway epithelium. Am J Respir Cell Mol Biol 22: 502–510, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Choi AM, Jacoby DB. Influenza virus A infection induces interleukin-8 gene expression in human airway epithelial cells. FEBS Lett 309: 327–329, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Empey DW, Laitinen LA, Jacobs L, Gold WM, Nadel JA. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis 113: 131–139, 1976. [DOI] [PubMed] [Google Scholar]
- 9.Fryer AD, Elbon CL, Kim AL, Xiao HQ, Levey AI, Jacoby DB. Cultures of airway parasympathetic nerves express functional M2 muscarinic receptors. Am J Respir Cell Mol Biol 15: 716–725, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Fryer AD, Jacoby DB. Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol 102: 267–271, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fryer AD, Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol 83: 973–978, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fryer AD, Yarkony KA, Jacoby DB. The effect of leukocyte depletion on pulmonary M2 muscarinic receptor function in parainfluenza virus-infected guinea-pigs. Br J Pharmacol 112: 588–594, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hope WC, Patel BJ, Fiedler-Nagy C, Wittreich BH. Retinoids inhibit phospholipase A2 in human synovial fluid and arachidonic acid release from rat peritoneal macrophages. Inflammation 14: 543–559, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O'Toole S, Myint SH, Tyrrell DA, Holgate ST. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 310: 1225–1229, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen GL, White CW, Takeda K, Loader JE, Nguyen DD, Joetham A, Groner Y, Gelfand EW. Mice that overexpress Cu/Zn superoxide dismutase are resistant to allergen-induced changes in airway control. Am J Physiol Lung Cell Mol Physiol 279: L350–L359, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Lee AM, Fryer AD, van Rooijen N, Jacoby DB. Role of macrophages in virus-induced airway hyperresponsiveness and neuronal M2 muscarinic receptor dysfunction. Am J Physiol Lung Cell Mol Physiol 286: L1255–L1259, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Massaro GD, Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med 3: 675–677, 1997. [DOI] [PubMed] [Google Scholar]
- 18.McGowan SE, Holmes AJ, Smith J. Retinoic acid reverses the airway hyperresponsiveness but not the parenchymal defect that is associated with vitamin A deficiency. Am J Physiol Lung Cell Mol Physiol 286: L437–L444, 2004. [DOI] [PubMed] [Google Scholar]
- 19.McGowan SE, Smith J, Holmes AJ, Smith LA, Businga TR, Madsen MT, Kopp UC, Kline JN. Vitamin A deficiency promotes bronchial hyperreactivity in rats by altering muscarinic M(2) receptor function. Am J Physiol Lung Cell Mol Physiol 282: L1031–L1039, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Migita H, Satozawa N, Lin JH, Morser J, Kawai K. RORalpha1 and RORalpha4 suppress TNF-alpha-induced VCAM-1 and ICAM-1 expression in human endothelial cells. FEBS Lett 557: 269–274, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Minette PA, Barnes PJ. Prejunctional inhibitory muscarinic receptors on cholinergic nerves in human and guinea pig airways. J Appl Physiol 64: 2532–2537, 1988. [DOI] [PubMed] [Google Scholar]
- 22.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317: 256–260, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Nie Z, Jacoby DB, Fryer AD. Etanercept prevents airway hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. Br J Pharmacol 156: 201–210, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL, Wedzicha JA. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164: 1618–1623, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Zhang XY, Robinson NE, Zhu FX. Modulation of ACh release from airway cholinergic nerves in horses with recurrent airway obstruction. Am J Physiol Lung Cell Mol Physiol 276: L769–L775, 1999. [DOI] [PubMed] [Google Scholar]