Abstract
Intact alveolar barrier function is associated with better outcomes in acute lung injury patients; however, the regulation of alveolar epithelial paracellular transport during lung injury has not been extensively investigated. This study was undertaken to determine whether changes in tight junction claudin expression affect alveolar epithelial barrier properties and to determine the mechanisms of altered expression. In anesthetized mice exposed to ventilator-induced lung injury, claudin-4 was specifically induced among tight junction structural proteins. Real-time PCR showed an eightfold increase in claudin-4 expression in the lung injury model. To examine the role of this protein in barrier regulation, claudin-4 function was inhibited with small interfering RNA (siRNA) and a blocking peptide derived from the binding domain of Clostridium perfringens enterotoxin (CPEBD). Inhibition of claudin-4 decreased transepithelial electrical resistance but did not alter macromolecule permeability in primary rat and human epithelial cells. In mice, CPEBD decreased air space fluid clearance >33% and resulted in pulmonary edema during moderate tidal volume ventilation that did not induce edema in control peptide-treated mice. In vitro phorbol ester induced a ninefold increase in claudin-4 expression that was dependent on PKC activation and the JNK MAPK pathway. These data establish that changes in alveolar epithelial claudin expression influence paracellular transport, alveolar fluid clearance rates, and susceptibility to pulmonary edema. We hypothesize that increased claudin-4 expression early in acute lung injury represents a mechanism to limit pulmonary edema and that the regulation of alveolar epithelial claudin expression may be a novel target for acute lung injury therapy.
Keywords: alveolar epithelium, ion transport, paracellular transport, tight junction, pulmonary edema, acute respiratory distress syndrome
acute lung injury (ALI) is characterized by the loss of alveolar barrier function and pulmonary edema (35). ALI, including the acute respiratory distress syndrome (ARDS), is a common clinical problem with a mortality rate near 40% (22). There are no effective pharmacological therapies for patients with ALI.
Pulmonary edema formation in ALI results from increased epithelial and endothelial permeability and decreased clearance of edema fluid by the alveolar epithelium. These barrier functions require paracellular tight junctions; however, our current understanding of the structural components and regulation of tight junctions in the alveolar epithelium is insufficient. The claudin family of proteins forms the intercellular barrier at tight junctions, and differences in claudin expression account for differences in permeability and ion selectivity of paracellular pores among epithelia in the nephron, large airways, and other organs (4, 31). For example, previous studies in renal epithelial cells have shown that claudin-4 forms a high-resistance, low-permeability tight junction that favors anion-selective paracellular ion movement (13, 30). Others have reported that changes in claudin expression in response to environmental stimuli may account for changes in epithelial barrier properties in the gastrointestinal tract (23).
The primary objective of this study was to determine whether claudin proteins were differentially expressed in the lung during experimental ALI and whether changes in claudin expression account for differences in alveolar barrier properties relevant to pulmonary edema formation and clearance. Initial studies demonstrated that claudin-4 expression was specifically increased in the lung in ALI. We then tested the functional contribution of claudin-4 to alveolar barrier properties and the mechanisms for the induction of claudin-4 expression in a mouse model of ALI and in primary human and rat alveolar epithelial cells. The cellular mechanisms for the induction of claudin-4 were studied using phorbol ester, an activator of multiple canonical signaling pathways previously shown to be important in ALI and barrier regulation (25, 29) and specific small molecule and peptide inhibitors.
MATERIALS AND METHODS
Mouse model of ventilator-induced ALI.
Animal protocols were approved by the local Institutional Animal Care and Use Committee. C57BL/6 mice (20–25 g) were anesthetized with 4% isoflurane and ketamine/xylazine (90/10 mg/kg), a tracheotomy tube made of polyethylene tubing (PE90; Becton Dickinson) was placed, and the mice were connected to a mechanical ventilator (Harvard Apparatus). Lung injury was induced using our previously reported model of ventilator-induced lung injury (11) with slight modification. Briefly, mice were ventilated with a high tidal volume (20 ml/kg, peak airway pressure 20 cmH2O, without end-expiratory pressure), a moderate tidal volume (8 ml/kg, peak airway pressure 12 cmH2O including an end-expiratory pressure of 3 cmH2O and equal minute volume), or were allowed to breathe spontaneously for 3 h. The moderate volume, noninjurious mechanical ventilation group was included as an additional control.
Gene expression microarray, real-time PCR, and Western blotting.
To determine whether genes for proteins important in tight junction structure, function, or regulation were differentially expressed in ALI, we compared whole genome mRNA expression (Affymetrix 430 2.0 arrays) in lungs from mice ventilated with high tidal volume to that of spontaneously breathing mice (n = 5 in each group). Differential expression of claudin-4 was validated by a separate comparison of new subjects using real-time PCR (ABI 7500). These studies compared spontaneously breathing mice with mice ventilated with moderate and high tidal volume ventilation. Briefly, lung tissue was removed and homogenized, RNA was extracted (RNeasy; Qiagen), and cDNA was prepared (SuperScript III; Invitrogen). Real-time PCR data were obtained using SYBR Green (Invitrogen) and compared with standard curves prepared from serial dilutions of samples and normalized to β-actin expression as previously described. Claudin-4, -3, and -18 protein expression was compared by Western blotting using rabbit anti-claudin primary antibodies (Invitrogen). Cell lysates were separated by SDS-PAGE under reducing conditions and transferred to nitrocellulose membranes. Membranes were blocked with milk and immunoblotted with primary antibody overnight. Blot images were analyzed with ImageJ (W. S. Rasband, National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij). Densitometry data normalized to β-actin data obtained from identically loaded and concurrently run immunoblots are reported.
Measurement of lung injury.
Pulmonary edema was measured using a gravimetric technique and reported as excess lung water (16). Alveolar barrier permeability to albumin was measured as the flux of radiolabeled albumin into the extravascular spaces of the lung, as previously described (9). Alveolar epithelial fluid clearance was measured as the change in total protein concentration of an intratracheally instilled tracer over time, as previously reported (8).
Primary cell culture.
Primary rat alveolar type II cells were isolated by elastase digestion and mechanical dissociation as previously described (9) and cultured in Transwells (cat. no. 3413; Corning) with DME-H21 medium supplemented with 10% fetal bovine serum. Primary human distal lung epithelial cells (DLECs; Clonetics) were seeded in a 75-cm2 flask and then grown to confluence in Transwells. These are cytokeratin-positive squamous epithelial cells with many features of alveolar type II cells, including surfactant production, lamellar bodies, and high-resistance tight junctions (20). These cells were used to determine whether there was consistency between rat and human cells in the paracellular transport measures. The human cells were cultured in supplemented growth medium (SAGM; Clonetics) containing bovine pituitary extract (30 μg/ml), hydrocortisone (0.5 μg/ml), EGF (0.5 ng/ml), epinephrine (0.5 μg/ml), transferrin (10 μg/ml), insulin (5 μg/ml), retinoic acid (0.1 ng/ml), triiodothyronine (6.5 ng/ml), gentamicin (50 μg/ml), amphotericin B (50 ng/ml), and 0.05% bovine serum albumin. Transepithelial electrical resistance was measured using an epithelial voltohmmeter (EVOM; World Precision Instruments), and in vitro permeability was determined using fluorescently labeled dextran as previously described (11).
Generation of Clostridium perfringens enterotoxin binding domain.
Clostridium perfringens enterotoxin (CPE) protein contains a cytotoxic domain and a binding domain that binds to the second extracellular loop of claudins 3 and 4 but not to other claudins. In intestinal epithelial cells, CPE binding results in endocytosis and degradation of claudin-4 (27, 32). Previous studies have shown that a peptide consisting of the binding domain of CPE binds claudin-4 and removes it from the cell surface but is not cytotoxic (27, 28). Therefore, we cloned and expressed a CPE binding domain peptide (CPEBD) as an inhibitor of claudin-4 and -3 function using standard procedures (12). C. perfringens [American Type Culture Collection (ATCC)] was grown at 37°C in anaerobic conditions on blood agar plates, and genomic DNA was isolated (Genomic-tip; Qiagen). A portion of the gene corresponding to amino acids 192–319 was cloned by PCR using the following primers: forward 5′-TCTACAGATATAGAAAAAGAAATCCTT-3′, reverse 5′-CATACTGTCTTTTGTAAATTAATTTGA-3′. The PCR product was ligated into a vector containing an NH2-terminal hexahistidine tag (pCRT7/NT; Invitrogen). Following sequence confirmation, the peptide was expressed in Escherichia coli (TOP10; Invitrogen), purified by immobilized metal affinity chromatography on a nickel column (His-Bind; Pierce), transferred into PBS by ion exchange chromatography in a PE-10 column (Amersham), and purified of endotoxin (Detoxi-Gel affinity column; Pierce). Purified peptide (14 kDa) was confirmed by gel electrophoresis. A control peptide containing neither the cytotoxic nor the binding domain, corresponding to amino acids 192–290 of the CPE protein, was also produced using the same forward primer and the reverse primer 5′-TTAGCTTATATCAACATAATGATCTTTTAC-3′. Cytotoxicity was determined by measuring lactate dehydrogenase (LDH) activity in media from cells treated with the CPE peptides. After initial dose-finding studies, CPEBD and control peptide (100 μg) were given to mice intraperitoneally 18 and 2 h before in vivo experimental procedures. In the in vitro studies, CPEBD (10 μg/ml) in complete growth medium was placed in both the apical and basolateral chambers of the Transwells, and measures of barrier function were made 2 h later.
Immunostaining.
Primary rat alveolar type II epithelial cells and human primary type II-like cells (DLECs) were cultured on collagen-coated glass, fixed in ice-cold 1:1 methanol and acetone, blocked for 2 h with milk, incubated overnight with primary antibodies for claudins 3, 4, 18, and occludin (Zymed), and then incubated for 1 h with FITC- or Texas red-labeled secondary antibodies (Invitrogen). Images were visualized on a confocal fluorescence microscope (Leica LCS).
Inhibition of claudin-4 expression with siRNA.
RNAs corresponding to the DNA sequence 5′-CGCACAGACAAGCCTTAC-3′ of human claudin-4 were synthesized (Sigma-Proligo) based on a previously published sequence (19). A sequence without known homology to any human gene (5′-AATTCTCCGAACGTGTCAC-3′) was used as a nonsilencing control (Qiagen). Primary human cells were transfected with small interfering RNA (siRNA; 20 nM) using cationic lipid (HiPerFect; Qiagen) in a ratio of 3 μl/μg suspended in Optimem. Cells were incubated in transfection medium for 4 h and then returned to complete growth medium. Cells were transfected 1 day after plating when at >80% confluence. Claudin expression and barrier properties were evaluated 72 h later. During protocol development, transfection efficiency was determined using a fluorescent-labeled control siRNA construct. Knockdown of claudin-4 mRNA and protein expression were confirmed with real-time PCR and Western blotting. Expression of claudin-3 and claudin-18 mRNA were measured to confirm specificity of claudin-4 knockdown.
MAPK activation and inhibition.
Total and phosphorylated JNK were measured by Western blotting in whole lung and primary cell lysates using specific antibodies (total JNK, Calbiochem; phospho-JNK, Invitrogen). PMA (Sigma) was used to induce JNK activation in primary human DLECs in vitro. The PKC inhibitor Gö-6850 (Calbiochem) was used to confirm that the PMA-mediated effects required PKC. Two small molecule inhibitors with different specificities for non-JNK kinases (SP-600125 and AS601245; Calbiochem) were used to inhibit JNK activity and were compared with vehicle-only controls. In addition, a peptide inhibitor of JNK-mediated phosphorylation of the protein jun that was conjugated to HIV TAT peptide to allow cell entry (JIP-TAT; Calbiochem) was used to inhibit JNK activity and was compared with a control peptide-TAT (AnaSpec). The effect of p38 MAPK inhibition with SB-253080 or SB-202190 and ERK1/2 MAPK inhibition with PD-98059 on PMA-mediated claudin-4 induction was also examined.
Statistics.
Differences between groups were compared using unpaired t-tests or ANOVA with post hoc Student-Newman-Keuls correction for multiple comparisons. Differences within a single group were compared with paired t-tests. Data are reported as means ± SD or means ± SE where indicated.
RESULTS
Differential expression of genes for tight junction proteins in ALI.
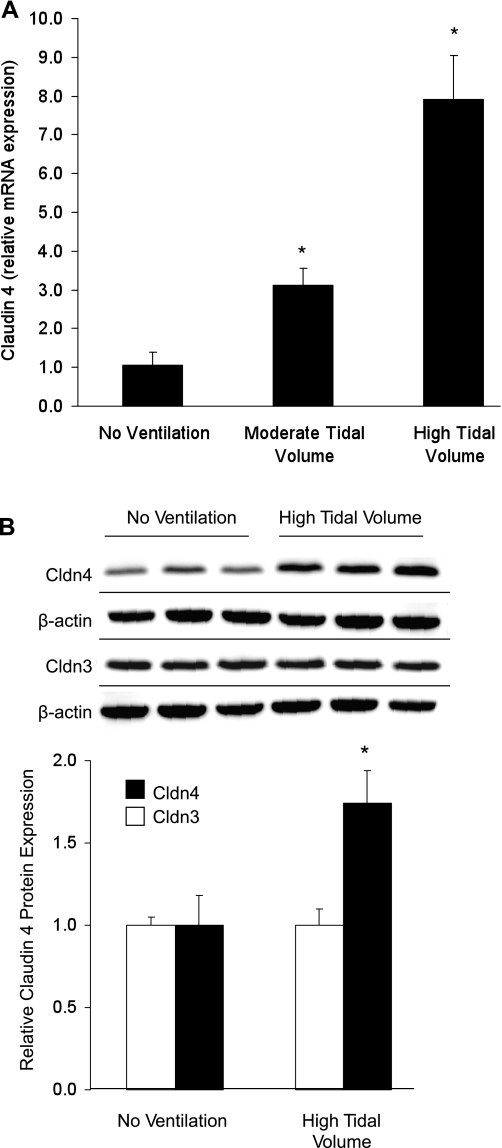
Whole lung mRNA expression microarray analysis showed that mechanical ventilation with a high tidal volume for 3 h resulted in differential expression of claudin-4. Using a twofold induction and statistical significance defined by P < 0.05 by the false discovery method, the effect on claudin-4 expression was specific among the 36 genes within the annotated gene otology term “tight junction” (GO:0005923), which includes all claudins, occludin, zona occludins 1, 2, and 3, and other genes. Gene microarray data are available in the Gene Expression Omnibus database (acc. no. GSE11434; http://www.ncbi.nlm.nih.gov/geo/). Quantitative real-time PCR confirmed that claudin-4 mRNA expression increased during lung injury and during noninjurious, moderate tidal volume ventilation compared with spontaneous ventilation (Fig. 1A). Western blotting demonstrated a significant and specific increase in claudin-4 protein expression after 3 h of high tidal volume mechanical ventilation (Fig. 1B). Claudin-3 protein expression was unchanged. Western blotting also showed no change in claudin-18 expression with high tidal volume ventilation (1.0 ± 0.11 without ventilation compared with 1.0 ± 0.31 with ventilation, relative expression normalized to β-actin, n = 3 in each group; P > 0.05).
Fig. 1.
Claudin-4 (Cldn4) mRNA and protein expression in acute lung injury. A: real-time PCR demonstrated a dose-response increase in claudin-4 mRNA abundance in whole mouse lungs following 3 h of moderate or high tidal volume ventilation (normalized to β-actin) (*P < 0.05 compared with all other groups; n = 6 in each group). B: mechanical ventilation with high tidal volume for 3 h also significantly increased claudin-4 protein expression as shown by this Western blot. Densitometry revealed a significant 75% increase in claudin-4 protein and no change in claudin-3 protein expression with high tidal volume ventilation. Claudin-18 protein expression was also unchanged by mechanical ventilation (see text).
Effect of claudin-4 inhibition with CPEBD on alveolar epithelial barrier properties and ALI.
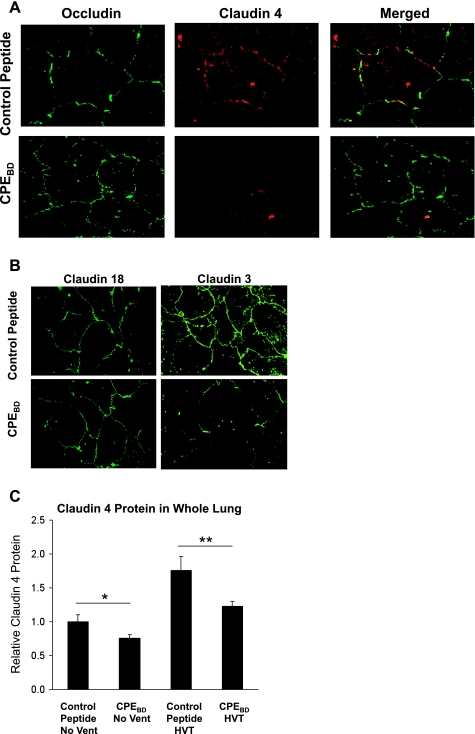
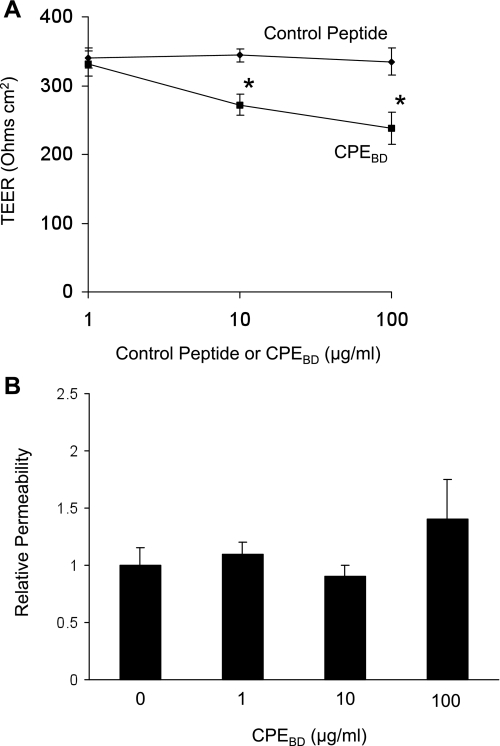
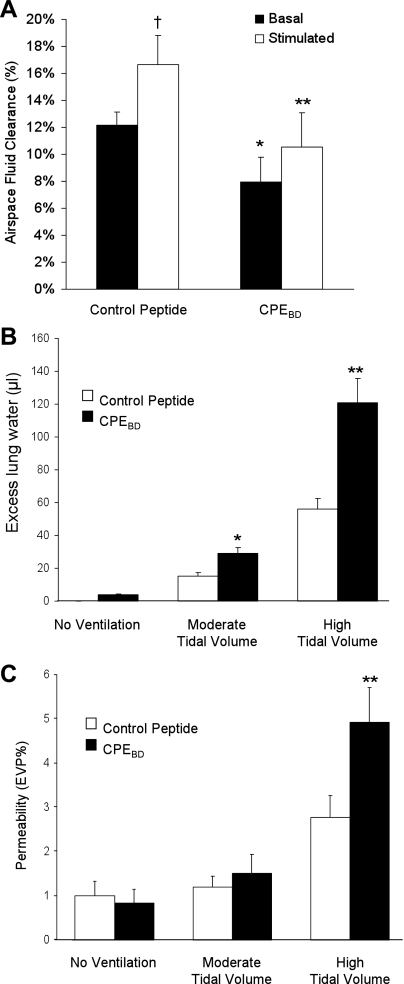
Immunostaining showed that CPEBD (10 μg/ml) decreased claudin-4 expression at tight junctions without affecting occludin (Fig. 2A). CPEBD decreased claudin-3 expression but did not affect claudin-18 distribution (Fig. 2B). Consistent with these data, Western blotting showed that CPEBD significantly decreased claudin-4 protein levels in whole mouse lungs (Fig. 2C). A similar effect was seen with claudin-3. Although ventilation did not increase claudin-3 protein expression, claudin-3 protein expression by Western blot was decreased in mice treated with CPEBD and ventilated with high tidal volume ventilation compared with controls (1.0 ± 0.4 compared with 0.42 ± 0.07, n = 3 in each group; P = 0.06). CPEBD did not affect claudin-18 expression in whole lung by Western blot analysis (1.0 ± 0.32 with control peptide normalized to β-actin compared with 1.16 ± 0.26 with CPEBD, n = 3 in each group; P > 0.05). CPEBD also induced a dose-dependent decrease in transepithelial electrical resistance in primary rat type II cells (Fig. 3A). In human type II-like DLECs, CPEBD (10 μg/ml) decreased transepithelial resistance (249 ± 12 Ω·cm2 with control peptide compared with 196 ± 20 Ω·cm2 with CPEBD, means ± SE, n = 3 replicates of 6 wells each; P < 0.05). CPEBD did not affect permeability to large (40-kDa) molecules (Fig. 3B). CPEBD was not cytotoxic as measured by LDH levels in media; LDH activity did not differ among untreated cells, control peptide-treated cells, and CPEBD-treated cells (LDH activity was 30 ± 4 IU/l with CPEBD and 31 ± 6 IU/l in control peptide-treated primary rat type II cells and 27 ± 4 IU/l in media-only controls, means ± SE, n = 3 replicates of 6 wells each; P > 0.05). In mouse studies in vivo, CPEBD resulted in significantly lower basal and β-adrenergic agonist-stimulated alveolar fluid clearance (Fig. 4A). CPEBD did not increase baseline lung water (pulmonary edema; Fig. 4B) or albumin flux (protein permeability; Fig. 4C); however, CPEBD resulted in increased pulmonary edema during moderate or high tidal volume ventilation (Fig. 4B). Protein permeability was also greater during high tidal volume ventilation in CPEBD-treated animals (Fig. 4C). Neither CPEBD-treated mice nor control peptide-treated mice demonstrated weight loss (data not shown), physical distress, or air space neutrophilia before mechanical ventilation. Air space neutrophil counts were 1.2 ± 0.9 in control peptide-treated mice and 1.8 ± 0.8 in CPEBD-treated mice (P > 0.05).
Fig. 2.
Blocking claudin-4 with a Clostridium perfringens binding domain peptide (CPEBD) alters paracellular permeability without dissociating tight junctions. A: CPEBD did not affect occludin (green) distribution but decreased claudin-4 (red) abundance at tight junctions in primary rat alveolar type II cells. The merged images on the right show the colocalization of claudin-4 and occludin at cell-cell junctions (yellow) in the control peptide-treated cells. B: claudin-18 expression was also not changed by CPEBD, however, CPEBD decreased claudin-3 expression. These data indicate that CPEBD results in a decrease in claudin-4 at tight junctions but does not entirely disrupt the tight junction. C: CPEBD also decreased claudin-4 protein levels in mouse whole lung without mechanical ventilation (*P < 0.05) and after 3 h of high tidal volume (HVT) ventilation (n = 3 per group; **P < 0.05). CPEBD decreased claudin-3 expression but did not affect claudin-18 protein expression in whole lungs (see text).
Fig. 3.
Effect of CPEBD on transepithelial electrical resistance (TEER) and permeability in vitro. A: CPEBD decreased transepithelial electrical resistance in a dose-dependent fashion in primary rat type II epithelial cells (means ± SE; *P < 0.05 compared with control peptide; n = minimum of 3 replicates of 6 wells each). B: CPEBD did not increase paracellular permeability to large (40-kDa) molecules indicating that paracellular transport properties were altered, but tight junction function was not lost (*P < 0.05 compared with control peptide; n = minimum of 3 replicates of 6 wells each, means ± SE).
Fig. 4.
Effect of the loss of claudin-4 on air space fluid clearance, pulmonary edema, and albumin flux in vivo. A: blocking claudin-4 with CPEBD in vivo decreased basal and β-adrenergic-stimulated air space fluid clearance. CPEBD decreased basal air space fluid transport (% instilled volume cleared in 30 min) in unventilated mice (black bars). CPEBD also decreased β-adrenergic agonist-stimulated (terbutaline, 10−5 M) alveolar fluid clearance (white bars) (*P < 0.05 compared with control peptide basal clearance and **P < 0.05 compared with control peptide-stimulated clearance; n = 6–9 in each group). β-Adrenergic agonist increased fluid clearance in control peptide-treated mice (†P < 0.05) but not in CPEBD-treated mice. B: blocking claudin-4 with CPEBD in vivo increases pulmonary edema during injury. CPEBD (black bars) resulted in increased pulmonary edema (excess lung water) during either moderate or high tidal volume mechanical ventilation. CPEBD did not significantly affect baseline lung water in the absence of mechanical ventilation. C: CPEBD did not affect permeability to albumin at baseline or during moderate tidal volume ventilation. However, CPEBD increased lung injury severity with high tidal volume mechanical ventilation as measured by albumin flux expressed as the percent of the plasma volume in the extravascular spaces of the lung [extravascular plasma equivalents (EVP%)]. *P < 0.05 compared with control peptide (white bars), **P < 0.05 compared with all other groups; n = 6–9 in each group. Therefore, inhibition of claudin-4 function with CPEBD decreased air space fluid clearance, increased susceptibility to pulmonary edema with mechanical ventilation, and resulted in more severe ventilator-induced lung injury.
Inhibition of claudin-4 expression with siRNA.
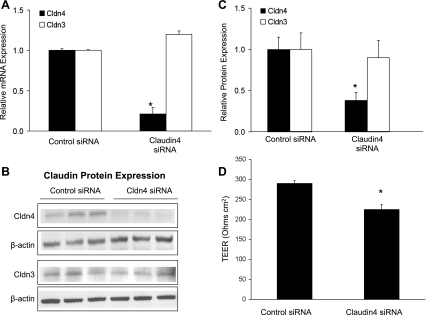
Claudin-4 siRNA induced an 80% decrease in claudin-4 mRNA expression compared with nonsilencing siRNA control in human primary cells (Fig. 5A). Claudin-4 siRNA significantly decreased claudin-4 protein expression compared with control siRNA (Fig. 5, B and C). Claudin-4 siRNA did not significantly affect claudin-3 mRNA abundance (Fig. 5A) or protein levels (Fig. 5, B and C). Claudin-4 siRNA had no effect on claudin-18 protein levels [1.0 ± 0.4 in control siRNA-treated cells and 1.1 ± 0.2 in claudin-4 siRNA-treated cells (means ± SD of densitometry normalized to β-actin), n = 3 in each group]. As with CPEBD, claudin-4 knockdown with siRNA resulted in significantly decreased transepithelial electrical resistance (Fig. 5D).
Fig. 5.
Claudin-4 knockdown with small interfering RNA (siRNA). To extend and confirm the CPEBD studies by specifically blocking only claudin-4 expression, primary human type II-like distal lung epithelial cells (DLECs) were transfected with siRNA targeted to claudin-4. A: at 72 h posttransfection, claudin-4 siRNA (20 nM) decreased claudin-4 mRNA abundance without affecting claudin-3 expression (*P < 0.05 compared with nonsilencing control siRNA without homology to any known human gene; n = minimum of 3 replicates of 6 wells each, means ± SE). B: claudin-4 siRNA significantly decreased claudin-4 but not claudin-3 protein expression. C: densitometry of Western blot data normalized to β-actin showed a significant decrease in claudin-4 protein with targeted siRNA (n = 3 per group; P < 0.05; means ± SD) but no change in claudin-3 protein levels. D: claudin-4 knockdown decreased transepithelial electrical resistance in human cells with an effect size comparable with CPEBD (*P < 0.05 compared with nonsilencing control siRNA; n = minimum of 3 replicates of 6 wells each, means ± SE). These data support the hypothesis that claudin-4 expression increases transepithelial resistance and decreases paracellular ion transport.
Induction of claudin-4 expression by JNK.
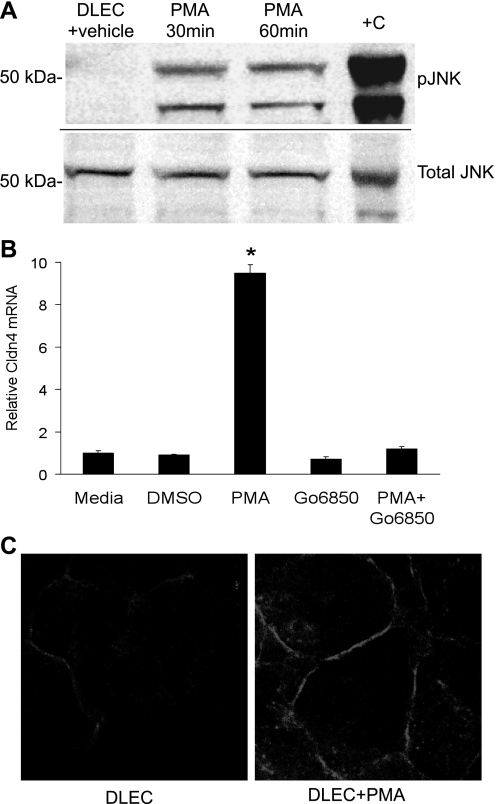
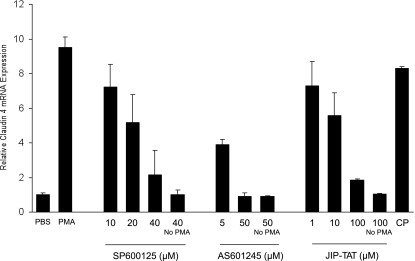
The potential role of MAPK pathways, including JNK, in the induction of claudin-4 expression was examined as claudin-4 was induced early in ALI along with genes comprising stress response pathways known to be regulated by MAPK pathways. JNK was of particular interest because a recent report indicated that JNK may positively influence epithelial repair in a wound healing model in primary airway epithelial cells (37). As others have reported (15), we confirmed that mechanical ventilation resulted in phosphorylation of JNK in whole lung. Therefore, to investigate the mechanisms for claudin-4 induction in vitro, the phorbol ester PMA was used to activate MAPKs, including JNKs, in primary human DLECs (Fig. 6A). PMA induced a ninefold increase in claudin-4 mRNA by 4 h, and this was associated with increased claudin-4 protein expression that localized to tight junctions (Fig. 6, B and C). PMA-mediated PKC activation was required for claudin-4 induction as the classic and novel PKC isoform inhibitor Gö-6850 blocked the effect (Fig. 6B). PKC-induced expression of claudin-4 was blocked in a dose-dependent fashion by JNK inhibition with SP-600125, AS601245, or JIP-TAT peptide (Fig. 7). Inhibition of ERK1/2 with PD-98059 (20 μM) or p38 with either SB-253080 (40 μM) or SB-202190 (10 μM) had no effect on PMA-induced claudin-4 mRNA expression.
Fig. 6.
PMA induces the JNK MAPK pathway and claudin-4 expression. A: in human type II-like DLECs, PMA-induced JNK1 (46 kDa) and JNK2 (54 kDa) phosphorylation (pJNK) at 30 and 60 min compared with vehicle (DMSO). UV-treated 293 cell extracts (Cell Signaling) were used as a positive control (+C). The same samples were tested for phosphorylated JNK and total JNK on separate, identically loaded polyacrylamide gels run concurrently. B: PMA induced a 9-fold increase in claudin-4 mRNA expression at 4 h that was completely inhibited by the classic and novel PKC inhibitor Gö-6850 (1 μM; *P < 0.05; n = 4 replicates of 6 wells each, means ± SE). C: PMA increased claudin-4 protein abundance primarily at the plasma membrane at 4 h.
Fig. 7.
PMA-mediated claudin-4 induction requires the JNK pathway. A: inhibition of the JNK MAPK pathway with each of 3 inhibitors (SP-600125, AS601245, and JIP-TAT peptide) blocked the PMA-induced increase in claudin-4 expression in human DLECs in a dose-dependent fashion (*P < 0.05; n = minimum of 3 replicates of 6 wells each, means ± SE). CP, control peptide TAT. Inhibitors of ERK1/2 or p38 had no effect on PMA-mediated claudin-4 mRNA expression.
DISCUSSION
Previous clinical studies have shown that preservation of alveolar epithelial barrier properties, including permeability and alveolar epithelial sodium and fluid transport, correlates with better outcomes in ALI patients (18, 36). Although maintenance of barrier properties requires intact epithelial tight junctions, relatively little is known about the claudins expressed in the alveolar epithelium, including whether claudin expression is regulated in response to changes in the local environment such as acute inflammation, mechanical stress, oxidative stress, or injury. Recent studies have begun to define the claudins expressed in the lung (3, 6, 34) and how environmental stimuli may influence claudin expression (7), but the role of claudins in barrier regulation in ALI is uncertain. The present study was undertaken to determine whether differential expression of tight junction claudins is a mechanism for regulation of alveolar barrier properties in ALI.
We found that claudin-4 was among the genes differentially expressed early in ventilator-induced lung injury, suggesting that changes in claudin-4 expression at the transcriptional level may be a mechanism for the regulation of tight junction function during ALI. In our array study, no other claudin showed a >2-fold change in mRNA expression. We then tested the potential contribution of claudin-4 to tight junction properties by using CPEBD and siRNA to inhibit claudin-4 functions. Decreased claudin-4 expression resulted in leakier tight junctions, decreased alveolar fluid clearance, and increased pulmonary edema with mechanical ventilation. Therefore, we propose that the change in claudin-4 expression during lung injury could reflect an adaptive response of the alveolar epithelium, acting to protect barrier function and limit edema during injury.
This hypothesis is supported by our loss of function studies. We cloned and expressed the binding domain of CPE (corresponding to amino acids 290–319 of the protein) as a claudin-4-blocking peptide and confirmed that CPEBD removed claudin-4 from tight junctions of alveolar epithelial cells without altering the distribution of other tight junction proteins (occludin and claudin-18). CPEBD decreased transepithelial electrical resistance consistent with leakier tight junctions and greater paracellular ion movement. CPEBD did not alter permeability to large molecules, further supporting the finding that CPEBD did not completely dissociate tight junctions. Because CPEBD affects both claudins 3 and 4, we used siRNA to more specifically test the functional contribution of claudin-4 to barrier properties. Claudin-4 protein expression was decreased by nearly 70% with siRNA in primary human type II-like cells, but expression of claudin-3 was not significantly changed. The decrease in claudin-4 expression was associated with significantly lower transepithelial electrical resistance, and the change in transepithelial electrical resistance was comparable with the effect of CPEBD. Although the full effect of CPEBD cannot be solely attributed to a decrease in claudin-4 expression with certainty, these data show that blocking claudin-4 with either CPEBD or siRNA results in increased paracellular leak and suggest a role for claudin-4 in maintaining a tight alveolar barrier. In addition, claudin-3 expression did not change with ventilator-induced lung injury. Consistent with our findings, previous studies in primary alveolar epithelial type II cells and Madin-Darby canine kidney (MDCK) cells have shown that EGF increases transepithelial electrical resistance and also increases claudin-4 expression (3, 26), although it is not clear whether claudin-4 accounted for the full effect of EGF on transepithelial resistance.
In mice, CPEBD decreased basal and β-adrenergic agonist-stimulated alveolar fluid clearance without increasing baseline excess lung water or albumin permeability. Alveolar fluid transport requires a sodium-potassium ATPase-generated sodium gradient across the epithelium (17). Because claudin-4 has been shown to limit paracellular sodium transport (30), increased claudin-4 expression may maximize alveolar fluid clearance. In addition, under both basal conditions and with β-adrenergic stimulation, alveolar fluid transport also requires the transepithelial movement of chloride. Under basal conditions, much of this chloride movement is thought to occur through the paracellular pathway (17). Regulation of the paracellular pathway is poorly understood; however, claudin-4 forms anion (chloride)-selective paracellular pores in MDCK and LLC-PK1 cells (13, 30, 33). Our data are consistent with a similar function for claudin-4 in the alveolar epithelium: the formation of a chloride-selective/sodium-restrictive paracellular pathway would promote transepithelial fluid transport by limiting paracellular sodium flux and favoring the transepithelial chloride transport required for electrical neutrality during sodium active transport. Furthermore, one recent study reported that in H441 cells, increased claudin-4 expression coincided with increased transepithelial fluid transport following stimulation with dexamethasone (24). Therefore, it appears that one function of increased claudin-4 expression in ALI could be to facilitate transepithelial sodium transport and limit pulmonary edema, although other roles for the change in claudin-4 expression cannot be excluded.
Consistent with the alveolar fluid clearance data, mice given CPEBD were more susceptible to pulmonary edema during moderate tidal volume ventilation, which was not injurious to mice given the control peptide. It is not certain to what extent the increase in pulmonary edema was the result of impaired alveolar fluid clearance or increased edema formation, but both mechanisms may play a role. With high tidal volume ventilation, CPEBD-treated mice had increased pulmonary edema and increased permeability to albumin. Why did CPEBD increase albumin permeability in vivo with high tidal volume ventilation but not in vitro? A likely possibility is that in ventilator-induced lung injury, increased pulmonary edema resulting from impaired air space fluid clearance or increased edema formation would increase lung injury by amplifying the detrimental mechanical forces in the ventilated lung (10). Although CPEBD is expected to affect claudin-4 and -3 expression in all epithelia, treated mice did not develop a phenotype of intestinal barrier impairment. Mice did not develop diarrhea or lose weight. Furthermore, CPEBD-treated mice did not develop evidence of lung inflammation at baseline in that baseline lung albumin flux and air space neutrophil counts were normal. It is likely that the loss of claudins 4 and 3 did not alter intestinal barrier permeability to large molecules such as LPS; CPEBD lacks a cytotoxic domain and did not induce cell necrosis. These data show that although claudin-4 may not be required to prevent pulmonary edema at baseline, inhibition of claudins 4 and 3 limited alveolar fluid transport and increased susceptibility to edema and injury during mechanical ventilation.
The induction of claudin-4 gene expression during lung injury was similar to other genes regulated by stress response pathways activated early in ALI, including MAPK pathways (9, 15, 21, 29). Therefore, to examine the mechanisms for the observed induction of claudin-4 expression, we tested the contribution of the canonical MAPK pathways to claudin-4 expression. Using a phorbol ester to activate PKC and MAPKs (including JNK) in vitro, we found a ninefold induction of claudin-4. Using two specific small molecule inhibitors of JNK and a peptide inhibitor of jun phosphorylation, we further found that JNK activity was required for the PKC-mediated induction of claudin-4 expression. Inhibition of ERK1/2 or p38 did not affect claudin-4 expression. This finding may seem unexpected, as JNK activation can induce proinflammatory pathways associated with edema formation; however, recent data have demonstrated a broader role for JNK in epithelial function, including promoting repair (37). We propose that one function of JNK activation is to activate a protective pathway involving claudin-4 expression, although a role for claudin-4 in epithelial repair cannot be excluded. The specific transcription factor pathways involved in claudin-4 regulation may differ from those important to the expression of proinflammatory pathways and remain to be investigated. It is possible that the in vitro PMA experiments did not fully model the in vivo signaling pathways important to claudin-4 expression, and both positive and negative results from the inhibitor studies should be interpreted with appropriate caution. Although there was an induction of claudin-4 expression with PMA, the effect of PMA on transepithelial electrical resistance was not assessed. Therefore, it is not certain whether the increase in claudin-4 expression resulted in a functional change in barrier properties in these conditions. It is possible that the increase in claudin-4 did not affect paracellular transport and that other effects of PMA stimulation could influence alveolar barrier properties in these experiments. The objective of these experiments was to target potential mechanisms for the induction of claudin-4 in lung cells. Future studies should assess how the specific overexpression of claudin-4 influences barrier properties in the alveolar epithelium.
Although changes in ion channel and transporter expression in the injured lung have been reported by our group and others (1, 2, 5, 9, 14, 21), whether regulation of the paracellular pathway is important to lung fluid balance in ALI is uncertain. Tight junction constituents are regulated at multiple levels (31); however, to our knowledge, this is the first report to demonstrate increased claudin-4 mRNA and protein expression during ALI. These data support a protective role for claudin-4 in the alveolar epithelium, one that promotes high-resistance tight junctions and higher rates of alveolar fluid clearance and limits edema. Because previous clinical studies have highlighted the importance of intact barrier function to clinical outcomes, advancing our understanding of alveolar barrier regulation in ALI may provide insight into new therapeutic strategies for ALI and pulmonary edema.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant HL-88440 (J. A. Frank).
REFERENCES
- 1.Adir Y, Factor P, Dumasius V, Ridge KM, Sznajder JI. Na,K-ATPase gene transfer increases liquid clearance during ventilation-induced lung injury. Am J Respir Crit Care Med 168: 1445–1448, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Borok Z, Hami A, Danto SI, Lubman RL, Kim KJ, Crandall ED. Effects of EGF on alveolar epithelial junctional permeability and active sodium transport. Am J Physiol Lung Cell Mol Physiol 270: L559–L565, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Chen SP, Zhou B, Willis BC, Sandoval AJ, Liebler JM, Kim KJ, Ann DK, Crandall ED, Borok Z. Effects of transdifferentiation and EGF on claudin isoform expression in alveolar epithelial cells. J Appl Physiol 98: 322–328, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol 285: L1166–L1178, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell 13: 3218–3234, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daugherty BL, Mateescu M, Patel AS, Wade K, Kimura S, Gonzales LW, Guttentag S, Ballard PL, Koval M. Developmental regulation of claudin localization by fetal alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L1266–L1273, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez AL, Koval M, Fan X, Guidot DM. Chronic alcohol ingestion alters claudin expression in the alveolar epithelium of rats. Alcohol 41: 371–379, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finley N, Norlin A, Baines DL, Folkesson HG. Alveolar epithelial fluid clearance is mediated by endogenous catecholamines at birth in guinea pigs. J Clin Invest 101: 972–981, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank J, Roux J, Kawakatsu H, Su G, Dagenais A, Berthiaume Y, Howard M, Canessa CM, Fang X, Sheppard D, Matthay MA, Pittet JF. Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem 278: 43939–43950, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Frank JA, Matthay MA. Science review: mechanisms of ventilator-induced injury. Crit Care 7: 233–241, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank JA, Pittet JF, Wray C, Matthay MA. Protection from experimental ventilator-induced acute lung injury by IL-1 receptor blockade. Thorax 63: 147–153, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Graslund S, Nordlund P, Weigelt J, Hallberg BM, Bray J, Gileadi O, Knapp S, Oppermann U, Arrowsmith C, Hui R, Ming J, Dhe-Paganon S, Park HW, Savchenko A, Yee A, Edwards A, Vincentelli R, Cambillau C, Kim R, Kim SH, Rao Z, Shi Y, Terwilliger TC, Kim CY, Hung LW, Waldo GS, Peleg Y, Albeck S, Unger T, Dym O, Prilusky J, Sussman JL, Stevens RC, Lesley SA, Wilson IA, Joachimiak A, Collart F, Dementieva I, Donnelly MI, Eschenfeldt WH, Kim Y, Stols L, Wu R, Zhou M, Burley SK, Emtage JS, Sauder JM, Thompson D, Bain K, Luz J, Gheyi T, Zhang F, Atwell S, Almo SC, Bonanno JB, Fiser A, Swaminathan S, Studier FW, Chance MR, Sali A, Acton TB, Xiao R, Zhao L, Ma LC, Hunt JF, Tong L, Cunningham K, Inouye M, Anderson S, Janjua H, Shastry R, Ho CK, Wang D, Wang H, Jiang M, Montelione GT, Stuart DI, Owens RJ, Daenke S, Schutz A, Heinemann U, Yokoyama S, Bussow K, Gunsalus KC. Protein production and purification. Nat Methods 5: 135–146, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem 281: 36117–36123, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Lecuona E, Saldias F, Comellas A, Ridge K, Guerrero C, Sznajder JI. Ventilator-associated lung injury decreases lung ability to clear edema in rats. Am J Respir Crit Care Med 159: 603–609, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Li LF, Yu L, Quinn DA. Ventilation-induced neutrophil infiltration depends on c-Jun N-terminal kinase. Am J Respir Crit Care Med 169: 518–524, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. J Clin Invest 116: 1615–1623, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 82: 569–600, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 142: 1250–1257, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Mima S, Tsutsumi S, Ushijima H, Takeda M, Fukuda I, Yokomizo K, Suzuki K, Sano K, Nakanishi T, Tomisato W, Tsuchiya T, Mizushima T. Induction of claudin-4 by nonsteroidal anti-inflammatory drugs and its contribution to their chemopreventive effect. Cancer Res 65: 1868–1876, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura M, Matute-Bello G, Liles WC, Hayashi S, Kajikawa O, Lin SM, Frevert CW, Martin TR. Differential response of human lung epithelial cells to fas-induced apoptosis. Am J Pathol 164: 1949–1958, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roux J, Kawakatsu H, Gartland B, Pespeni M, Sheppard D, Matthay MA, Canessa CM, Pittet JF. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem 280: 18579–18589, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Russo MA, Hogenauer C, Coates SW Jr, Santa Ana CA, Porter JL, Rosenblatt RL, Emmett M, Fordtran JS. Abnormal passive chloride absorption in cystic fibrosis jejunum functionally opposes the classic chloride secretory defect. J Clin Invest 112: 118–125, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shlyonsky V, Goolaerts A, Van Beneden R, Sariban-Sohraby S. Differentiation of epithelial Na+ channel function. An in vitro model. J Biol Chem 280: 24181–24187, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Siflinger-Birnboim A, Johnson A. Protein kinase C modulates pulmonary endothelial permeability: a paradigm for acute lung injury. Am J Physiol Lung Cell Mol Physiol 284: L435–L451, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Singh AB, Harris RC. Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J Biol Chem 279: 3543–3552, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J Cell Biol 147: 195–204, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi A, Kondoh M, Masuyama A, Fujii M, Mizuguchi H, Horiguchi Y, Watanabe Y. Role of C-terminal regions of the C-terminal fragment of Clostridium perfringens enterotoxin in its interaction with claudin-4. J Control Release 108: 56–62, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Uhlig U, Haitsma JJ, Goldmann T, Poelma DL, Lachmann B, Uhlig S. Ventilation-induced activation of the mitogen-activated protein kinase pathway. Eur Respir J 20: 946–956, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107: 1319–1327, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 68: 403–429, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Van Itallie CM, Betts L, Smedley JG 3rd, McClane BA, Anderson JM. Structure of the claudin-binding domain of Clostridium perfringens enterotoxin. J Biol Chem 283: 268–274, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol 285: F1078–F1084, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol 29: 62–70, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001. [DOI] [PubMed] [Google Scholar]
- 37.White SR, Tse R, Marroquin BA. Stress-activated protein kinases mediate cell migration in human airway epithelial cells. Am J Respir Cell Mol Biol 32: 301–310, 2005. [DOI] [PubMed] [Google Scholar]