Abstract
Acid-sensing ion channels (ASIC) are voltage-insensitive, cationic channels that have recently been identified in vascular smooth muscle (VSM). It is possible that ASIC contribute to vascular reactivity via Na+ and Ca2+ conductance; however, their function in VSM is largely unknown. In pulmonary VSM, store-operated Ca2+ entry (SOCE) plays a significant role in vasoregulatory mechanisms such as hypoxic pulmonary vasoconstriction and receptor-mediated arterial constriction. Therefore, we hypothesized that ASIC contribute to SOCE in pulmonary VSM. We examined SOCE resulting from depletion of intracellular Ca2+ stores with cyclopiazonic acid in isolated small pulmonary arteries and primary cultured pulmonary arterial smooth muscle cells by measuring 1) changes in VSM [Ca2+]i using fura-2 indicator dye, 2) Mn2+ quenching of fura-2 fluorescence, and 3) store-operated Ca2+ and Na+ currents using conventional whole cell patch-clamp configuration in voltage-clamp mode. The role of ASIC was assessed by the use of the ASIC inhibitors, amiloride, benzamil, and psalmotoxin 1, or siRNA directed towards ASIC1, ASIC2, or ASIC3 isoforms. We found that store-operated VSM [Ca2+]i responses, Mn2+ influx, and inward cationic currents were attenuated by either pharmacological ASIC inhibition or treatment with ASIC1 siRNA. These data establish a unique role for ASIC1 in mediating SOCE in pulmonary VSM and provide new insight into mechanisms of VSM Ca2+ entry and pulmonary vasoregulation.
Keywords: degenerin/epithelial Na+ channel, pulmonary hypertension, calcium current, capacitative calcium entry, sodium current, psalmotoxin 1, vascular reactivity
a novel family of voltage-insensitive, cationic channels has recently been identified in vascular smooth muscle (VSM). Members of the degenerin/epithelial Na+ channel (DEG/ENaC) family share amino acid homology and a common topology, consisting of short intracellular NH2 and COOH termini and two membrane-spanning domains separated by a large extracellular domain (43). Despite being highly conserved, this family of ion channels appears to be activated by markedly distinct stimuli and participates in diverse biological processes. Two members of this family, ENaC (α, β, γ, and δ) and acid-sensing ion channels (ASIC 1-4), have been identified in mammals and form homomeric or heteromeric ion channels consisting of three subunits. ENaC proteins are expressed in a diverse range of cell types but are highly expressed in epithelial cells of the kidney, lung, and colon, where they play a rate-limiting role in Na+ and water transport (5, 8, 16, 17, 31, 43). ASICs are widely distributed in the central and peripheral nervous system where they are thought to induce neuronal depolarization and action potential generation in response to decreases in extracellular pH (31, 41, 57). Recently, however, multiple subunits for both ENaC and ASIC have been found in VSM from cerebral, renal, and mesenteric arteries where they play an important role in mechanotransduction of the myogenic response and VSM migration (13, 18, 19, 25, 26). Although these ion channels also appear to be expressed in pulmonary VSM, their function in this cell type is unknown.
Contraction of VSM is dependent on a combination of Ca2+ influx across the plasma membrane, release of Ca2+ from intracellular stores, and sensitization of the contractile apparatus to Ca2+. The release and depletion of Ca2+ from intracellular stores stimulates Ca2+ entry across the plasma membrane through activation of Ca2+-permeable ion channels. This phenomenon is termed store-operated Ca2+ entry (SOCE) or capacitative calcium entry. Unlike many systemic vascular beds, activation of SOCE in the pulmonary circulation elicits smooth muscle contraction, suggesting SOCE plays a significant physiological role in regulation of pulmonary vascular resistance. Indeed, SOCE has been linked to important vasoregulatory mechanisms such as hypoxic pulmonary vasoconstriction and receptor-mediated arterial constriction (50, 60). Furthermore, it has become increasingly evident that intracellular Ca2+ handling pathways in pulmonary VSM and endothelial cells are markedly altered in pulmonary hypertension (14, 27, 40, 55, 65). Although SOCE appears to play a prominent role in the pulmonary circulation, little is known about the channels and proteins involved in mediating SOCE. The goal of this study is to characterize ASIC in pulmonary VSM and determine their contribution to SOCE.
Although DEG/ENaC family members are commonly known for their high permeability to Na+, recent evidence suggests that some channels conduct Ca2+ as well (62, 64). ASIC1a has gained considerable attention as the Ca2+-permeable subunit involved in acidosis-mediated glutamate receptor-independent neuronal injury (62). In addition, another DEG/ENaC family member found in Caenorhabditis elegans, MEC-4, has been shown to conduct Ca2+, which requires Ca2+ release from the endoplasmic reticulum (ER) to promote neuronal death (6, 63). The observations that ASIC1 conduct Ca2+ and MEC-4 is involved in Ca2+ release from the ER support our present findings that ASIC1 contributes to SOCE in pulmonary VSM.
METHODS
All protocols employed in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico School of Medicine (Albuquerque, NM).
Cannulation and Endothelial Disruption of Small Pulmonary Arteries
Male Sprague-Dawley rats (250–300 g body wt, Harlan Industries) were anesthetized with pentobarbital sodium (200 mg/kg ip), and the heart and lungs were exposed by midline thoracotomy. The left lung was removed and immediately placed in ice-cold physiological saline solution (PSS) [pH adjusted to 7.4 with NaOH containing (in mM) 130 NaCl, 4 KC1, 1.2 MgSO4, 4 NaHCO3, 1.8 CaC12, 10 HEPES, 1.18 KH2PO4, 6 glucose, 0.03 EDTA]. Fourth-order intrapulmonary arteries (100- to 150-μm inner diameter, i.d.) of ∼1-mm length and without visible side branches were dissected free and transferred to a vessel chamber (Living Systems, CH-1). The proximal end of the artery was cannulated with a tapered glass pipette, secured in place with a single strand of silk ligature, and gently flushed to remove any blood from the lumen. The vessel lumen was then rubbed with a strand of moose mane to disrupt the endothelium. After cannulation of the distal end, the vessel was stretched longitudinally to approximate its in situ length and pressurized with a servo-controlled peristaltic pump (Living Systems) to 12 mmHg. The effectiveness of endothelial disruption was verified by the lack of a vasodilatory response to ACh (1 μM). The vessel chamber was transferred to the stage of a Nikon Eclipse TS100 microscope where the vessel was superfused with PSS. Bright-field images were obtained with an IonOptix CCD100M camera, and dimensional analysis was performed by IonOptix Sarclen software to measure i.d.
Isolation and Culture of Pulmonary Arterial Smooth Muscle Cells
Fourth-order intrapulmonary arteries were collected and enzymatically digested in reduced Ca2+ HBSS containing papain (9.5 U/ml) and dithiothreitol (1 mM) at 37°C for 30 min. Following digestion, single smooth muscle cells were dispersed by gentle trituration with a fire-polished pipette in Ca2+-free HBSS, and the cell suspension was placed on 25-mm glass coverslips. Pulmonary arterial smooth muscle cells (PASMCs) were either used immediately for experiments or cultured in Ham's F-12 media supplemented with 1% FBS and 1% penicillin/streptomycin for 4–5 days in a humidified atmosphere of 5% CO2-95% air at 37°C. Cellular purity was >95%, as assessed by morphological appearance under phase-contrast microscopy and immunofluorescence staining for smooth muscle α-actin.
Fura-2 Loading of VSM in Isolated Small Pulmonary Arteries and Primary Cultured PASMC
Pressurized arteries were loaded abluminally with the Ca2+-sensitive fluorescent indicator fura-2 AM (2 μM and 0.05% pluronic acid, Molecular Probes) in PSS as previously reported (23, 27, 28). Arteries were incubated in this solution for 45 min and primary cultured PASMC for 15 min at room temperature in the dark and then rinsed for 20 min with PSS (37°C) after the loading period to wash out excess dye and to allow for hydrolysis of AM groups by intracellular esterases. Fura-2-loaded vessels and cells were alternately excited at 340 and 380 nm at a frequency of 1 Hz with an IonOptix Hyperswitch dual excitation light source, and the respective 510-nm emissions were detected with a photomultiplier tube. Background-subtracted 340/380 emission ratios were calculated with IonOptix Ion Wizard software and recorded continuously throughout the experiment, with simultaneous measurement of i.d. from red-wavelength bright-field images for vessels as described above.
Transfection of Primary Cultured PASMC with siRNA
To determine if individual ASIC isoforms contribute to SOCE in pulmonary VSM, we used siRNA (100 nM) to suppress expression as previously described (19). Briefly, PASMC were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Validated siRNA molecules, directed to ASIC1, ASIC2, and ASIC3 (ASIC1: ID 52768; ASIC2: ID 197232; ASIC3: ID 47195), were obtained from Ambion. As a negative control, we 1) studied PASMC following transfection with Lipofectamine alone and 2) used a nontargeting (NT) siRNA control molecule that activates the RNA Induced Silencing Complex (RISC; Dharmacon). Following a 4-h incubation, cultures were supplemented with growth media for 72 h before study. To determine the extent of siRNA suppression of ASIC expression, we used Western blot analysis as described below.
Measurement of VSM SOCE
Ca2+ depletion/repletion.
After fura-2 AM loading, pulmonary arteries or primary cultured PASMC were superfused with Ca2+-free PSS containing 3 mM EGTA to chelate any residual Ca2+ and 50 μM diltiazem to prevent Ca2+ entry through L-type voltage-gated Ca2+ channels (VGCC). We have previously demonstrated that this concentration of diltiazem blocks the increase in [Ca2+]i and vasoconstrictor response to a depolarizing concentration of KCl (50 mM; Ref. 23). In addition, arteries/cells were incubated with the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor cyclopiazonic acid (CPA; 10 μM) to deplete intracellular Ca2+ stores and prevent Ca2+ reuptake. The changes in [Ca2+]i (SOCE) and i.d. (for isolated vessel experiments) were then determined upon repletion of extracellular Ca2+ (1.8 mM) in the continued presence of diltiazem and CPA. Parallel experiments were performed 1) in the presence of the DEG/ENaC family inhibitors amiloride (3, 10, 30 μM) or the amiloride analog benzamil (1, 5, 10 μM); 2) in the presence of the specific ASIC1a inhibitor psalmotoxin 1 (PcTX1; 100 ng/ml); and 3) following transfection with siRNA. Vasoconstrictor responses were calculated as percentages of baseline i.d. VSM [Ca2+]i is represented as a change (Δ) in the fura-2 emission ratios (F340/F380) from baseline.
Mn2+ quenching of fura-2 fluorescence.
SOCE was additionally quantified by quenching of fura-2 fluorescence with Mn2+, which enters the VSM as a Ca2+ surrogate and reduces fura-2 fluorescence on binding to the dye. Pulmonary arteries and primary cultured PASMC were loaded with fura-2, as previously described. Fura-2 was excited at 360 nm, and emission light was recorded at 510 nm. At the excitation wavelength of 360 nm, fura-2 fluorescence intensity is not influenced by [Ca2+]i changes; therefore, changes in fluorescence are assumed to be caused by Mn2+ alone. After stable baseline fluorescence was attained, store-operated channels were activated by superfusing the vessel with Ca2+-free PSS (without EGTA) containing diltiazem (50 μM) and CPA (10 μM) for 15 min. MnCl2 (500 μM) was added to the superfusate for 10 min. Parallel experiments were performed in the presence of amiloride (30 μM), benzamil (10 μM), or PcTX1 (100 ng/ml) and following transfection with siRNA. Mn2+ quenching of fura-2 fluorescence was calculated as percent change in intensity (F; 10 min post-MnCl2) from baseline fluorescence intensity at time 0 (F0).
Measurement of CPA-Induced Ca2+ and Na+ Current
Freshly isolated and primary cultured PASMC were superfused under constant flow (2 ml/min) at room temperature (∼23°C) in an extracellular solution [containing (in mM): 130 sodium methanesulfonate, 1.8 calcium aspartate, 0.5 3,4-diaminopyridine, 10 HEPES, 10 glucose, 0.05 diltiazem, and titrated to pH 7.4 with methane sulfonic acid]. To examine Ca2+ and Na+ currents separately, this extracellular solution was modified either by 1) replacing calcium aspartate with 3.68 mM EGTA to allow Na+ to be the only charge carrier or 2) replacing sodium methanesulfonate with 120 mM tetraethylammonium aspartate and increasing the concentration of calcium aspartate to 5 mM to allow Ca2+ to be the only charge carrier. The intracellular recording solution was composed of the following (in mM): 138 cesium aspartate, 1.15 EGTA, 1 Ca(OH)2, 2 Na2ATP, 10 HEPES, titrated to pH 7.2 with 1 M CsOH. The osmolarity of all solutions was adjusted to 290–300 mosmol/l with sucrose. Currents were recorded in the conventional whole cell patch-clamp configuration in voltage-clamp mode. SERCA was inhibited with the inclusion of CPA (10 μM) to the extracellular solution. Whole cell current was monitored at a holding potential of −50 mV. Voltage steps were applied from −80 to +80 mV in 10-mV increments and 200-ms duration with 2-s intervals before and after stabilization of the CPA-induced inward current. Baseline current was subtracted from each voltage step to obtain accurate store-operated current measurements. A holding potential of 0 mV between each voltage step was chosen to inactivate L-type VGCC that may be present. In addition, these experiments were performed in the presence of diltiazem (50 μM). All current was normalized to cell capacitance to obtain current density (pA/pF), and current voltage (I-V) relationships were generated from the last 50 ms of each stimulus.
Immunofluorescence of ASIC in Lung Sections and PASMC
The right and left lungs were fixed and mounted in paraffin as described previously (29). Lung sections were rehydrated, and antibody-antigen binding was enhanced by an antigen retrieval method in which sections were boiled for 5 min in 50 mM Tris (pH 9.0) buffer. PASMC were enzymatically dissociated as above and fixed in 4% paraformaldehyde for 10 min. All samples were permeabilized with 0.1% Triton X-100 and blocked with 5% normal donkey serum (NDS) in PBS for 1 h before addition of primary antibodies plus 5% NDS in PBS overnight at 4°C. Rabbit anti-ASIC1, rabbit anti-ASIC2, and rabbit anti-ASIC3 antibodies (1:100, Chemicon) were used for immunostaining. All samples were colabeled with mouse anti-smooth muscle α-actin (SMα-actin; 1:200, Sigma). The samples were rinsed with PBS and then exposed to secondary antibody [Cy-5 conjugated donkey anti-mouse IgG (1:100) and Cy-3 conjugated donkey anti-rabbit F(ab′)2 (1:100, Jackson Immunologicals)] in 5% NDS for 1 h. Sample specific binding of the antibody was determined by using antigenic peptide. Samples were treated as above, except the primary antibody was preincubated overnight at 4°C with excess antigenic peptide (+AG; 10 μg/ml for ASIC1 and ASIC3 and 3 μg/ml for ASIC2). Nonspecific binding of the secondary antibody was determined by omitting primary antibody (NP). Samples were examined using the University of New Mexico Cancer Center Fluorescence Microscopy Facility (Zeiss LSM510 confocal microscope; http://hsc.unm.edu/crtc/microscopy/facility.html).
Western Blotting
ASIC expression in pulmonary arteries and primary cultured PASMC was assessed using Western blotting procedures. To obtain sufficient tissue for analysis, intrapulmonary arteries (∼2nd-5th order) from the left and right lungs were dissected from accompanying airways and surrounding lung tissue in PSS. Cultured cells were washed in PBS, and then each sample was homogenized in 10 mM Tris·HCl homogenization buffer containing 255 mM sucrose, 2 mM EDTA, 12 μM leupeptin, 1 μM pepstatin A, 0.3 μM aprotinin, and 1 mM phenylmethylsulfonyl fluoride (all from Sigma). Samples were centrifuged at 10,000 g for 10 min at 4°C to remove insoluble debris. The supernatant was collected, and sample protein concentrations were determined by the Bradford method (Bio-Rad Protein Assay). Control experiments were conducted using different concentrations of protein to ensure linearity of the densitometry curve, which varied from 5 to 50 μg per lane depending on the tissue and antibody. Pulmonary artery lysates and primary cultured PASMC were separated by SDS-PAGE (7.5% Tris·HCl gels, Bio-Rad) and transferred to polyvinylidene difluoride membranes. Blots were blocked for 1 h at room temperature with 5% milk and 0.05% Tween 20 (Bio-Rad) in TBS containing 10 mM Tris·HCl and 50 mM NaCl (pH 7.5). Blots were then incubated overnight at 4°C with rabbit anti-ASIC1, -ASIC2, or -ASIC3 (1:500) and rabbit anti-β-actin (Abcam). For immunochemical labeling, blots were incubated for 1 h at room temperature with goat anti-rabbit IgG-horseradish peroxidase (1:3,000; Bio-Rad). After chemiluminescence labeling (ECL, Pierce), ASIC and β-actin bands were detected by exposing the blots to chemiluminescence-sensitive film (Kodak). Quantification of the bands was accomplished by densitometric analysis of scanned images (SigmaGel software, SPSS). To determine specificity of the ASIC antibodies, samples were treated as above except the primary antibody was preincubated overnight at 4°C with excess antigenic peptide. ASIC bands from siRNA-transfected cells were normalized to those of β-actin.
Calculations and Statistics
All data are expressed as means ± SE. Values of n refer to 1) number of animals in each group for isolated pulmonary arteries, 2) number of 35-mm dishes derived from at least three rats for primary cultured PASMC, and 3) number of cells from at least three animals for electrophysiology experiments. A one-way ANOVA was used to make comparisons when appropriate. If differences were detected by ANOVA, individual groups were compared with the Student-Newman-Keuls test. A probability of P < 0.05 was accepted as significant for all comparisons.
RESULTS
Although amiloride and benzamil are routinely used to inhibit DEG/ENaC family members (31), there are potential complications of these pharmacological inhibitors that we have successfully resolved in validation studies described in the Supplemental data section (Figs. S1-S3; Supplemental data for this article is available online at the AJP-Lung web site).
Inhibition of ENaC/ASIC Attenuates SOCE in Pulmonary VSM
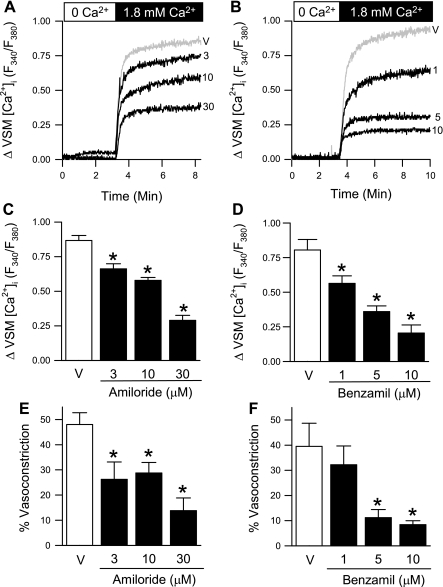
SOCE is thought to be an important mode of Ca2+ entry in pulmonary VSM leading to vasoconstriction (51). We assessed SOCE by two means: Ca2+ depletion/repletion and Mn2+ quenching of fura-2 fluorescence protocols. VSM [Ca2+]i increases when [Ca2+]o is replenished (SOCE) in isolated small pulmonary arteries (Fig. 1, A and B). The ENaC/ASIC inhibitors amiloride and benzamil reduced SOCE in a concentration-dependent manner, summarized in Fig. 1, C and D. Consistent with findings from Robertson et al. (51) and previous work from our laboratory (23), we observed SOCE-mediated vasoconstriction (40–50% of baseline i.d.), which was also attenuated by amiloride and benzamil (Fig. 1, E and F). For the remaining experiments in this study, only the highest concentrations of amiloride (30 μM) and benzamil (10 μM) were used.
Fig. 1.
ENaC/acid-sensing ion channel (ASIC) inhibitors attenuate store-operated Ca2+ entry (SOCE) and associated constriction in isolated small pulmonary arteries in a concentration-dependent manner. Representative traces of SOCE in the presence of amiloride (A; 3–30 μM) or benzamil (B; 1–10 μM). Mean data for SOCE-induced changes in vascular smooth muscle (VSM) [Ca2+]i (C and D; F340/F380) and SOCE-induced vasoconstriction (%baseline inner diameter; E and F). Experiments were performed in the presence of cyclopiazonic acid (CPA) (10 μM) and diltiazem (50 μM). Values are means ± SE; n = 5/group. *P < 0.05 vs. vehicle group (V).
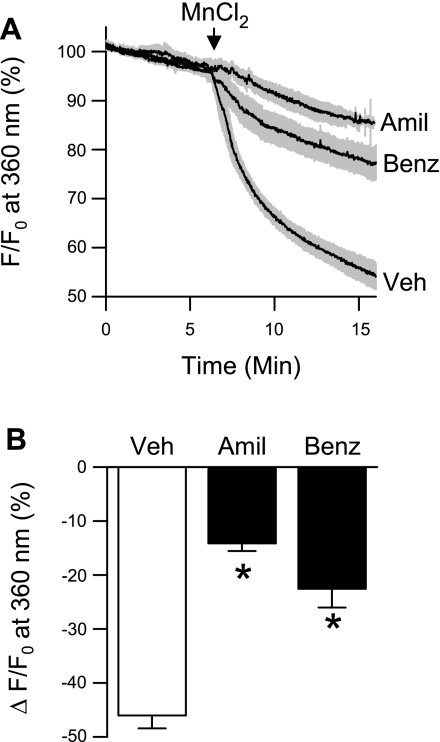
Similar to effects of amiloride and benzamil on responses to Ca2+ repletion, Mn2+ quenching of fluorescence was markedly decreased in small pulmonary arteries following pretreatment with amiloride or benzamil (Fig. 2, A and B). Figure 2A depicts temporal changes in fura-2 fluorescence before and after addition of MnCl2. Mn2+ resulted in a progressive quenching of the fura-2 signal. Approximately 46% of the fura-2 signal was quenched 10 min after addition of MnCl2 (Fig. 2B). In contrast, MnCl2 resulted in only ∼14–22% quenching of fura-2 fluorescence in the presence of amiloride or benzamil. These data suggest that amiloride and benzamil prevent store depletion-induced Mn2+ entry, and by extrapolation Ca2+ entry, into pulmonary VSM.
Fig. 2.
ENaC/ASIC inhibition reduces Mn2+ quenching of fura-2 fluorescence in response to store depletion in isolated small pulmonary arteries. A: percent change in fura-2 fluorescence at 360-nm excitation. Traces are means ± SE (represented by gray regions) in the presence of amiloride (Amil; 30 μM), benzamil (Benz; 10 μM), or vehicle (Veh). B: magnitude of quenching 10 min after MnCl2 (500 μM) administration. Experiments were performed in the presence of CPA (10 μM) and diltiazem (50 μM). Values are means ± SE; n = 5/group. *P <0.05 vs. vehicle. F, fluorescence intensity; F0, baseline fluorescence intensity at time 0.
Since ion channel expression and function can be altered by cell culture, we repeated SOCE measurements in primary cultured PASMC to verify inhibitory effects of amiloride and benzamil in this preparation. Consistent with our findings in isolated pulmonary arteries, both amiloride and benzamil significantly reduced changes in VSM [Ca2+]i (Fig. 3A) and Mn2+ quenching of fura-2 fluorescence (Fig. 3B) in primary cultured PASMC.
Fig. 3.
SOCE in primary cultured pulmonary arterial smooth muscle cells (PASMC) is attenuated by ENaC/ASIC inhibition. SOCE-induced changes in VSM [Ca2+]i (F340/F380) (A) and magnitude of Mn2+ quenching 10 min after MnCl2 (500 μM) administration (B) in the presence of amiloride (Amil; 30 μM), benzamil (Benz; 10 μM), or vehicle (Veh). All experiments were performed in the presence of CPA (10 μM) and diltiazem (50 μM). Values are means ± SE; n = 5/group. *P < 0.05 vs. vehicle group.
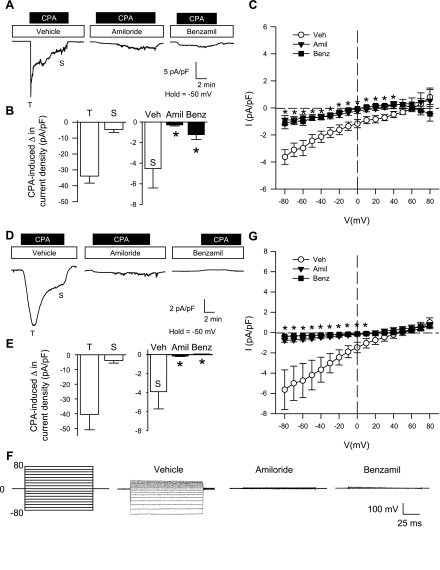
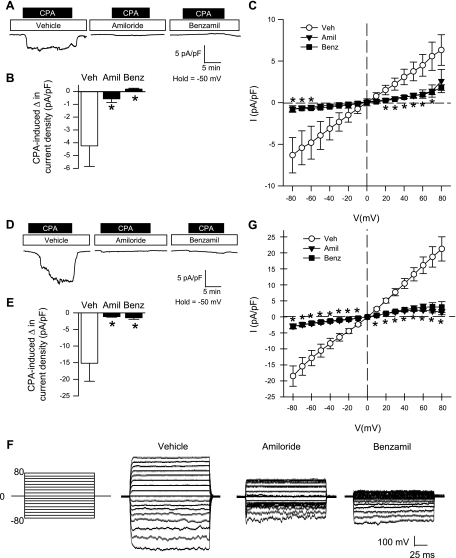
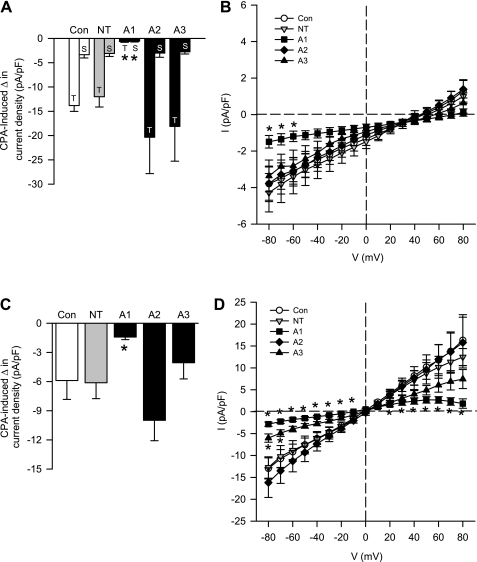
CPA-Induced Ca2+ and Na+ Current Is Diminished By ENaC/ASIC Inhibition in Freshly Isolated and Primary Cultured PASMC
From a holding potential of −50 mV, the addition of CPA to the extracellular solution resulted in a transient (T) and sustained (S) inward Ca2+ current under vehicle conditions that was similar in both freshly isolated (Fig. 4, A and B) and primary cultured (Fig. 4, D and E) PASMC. Preincubation with amiloride or benzamil abolished the transient current and largely diminished the sustained Ca2+ current (Fig. 4, B and E). Steady-state Ca2+ current was also examined in response to a voltage-step protocol (−80 to 80 mV) before and after the addition of CPA. I-V relationships generated from the voltage-step protocol revealed a significant reduction in the inward Ca2+ current by amiloride or benzamil at potentials from −80 to 40 mV for freshly isolated PASMC (Fig. 4C) and −80 to 10 mV for primary cultured PASMC (Fig. 4G). Current density was similar between freshly isolated (Fig. 4C) and primary cultured (Fig. 4G) PASMC, and there was no time-dependent activation or inactivation of this current, suggesting the absence of VGCC activity in this preparation. In addition, the current-voltage relations revealed inward rectification of the Ca2+ current with a positive reversal potential (Erev; 45–60 mV) and a leftward shift in the Erev with amiloride or benzamil (10–30 mV). The inward rectification and positive Erev are consistent with known characteristics of store-operated currents (46).
Fig. 4.
ENaC/ASIC inhibition diminishes CPA-induced Ca2+ current density in both freshly isolated (A–C) and primary cultured (D–G) PASMC. A and D: representative traces showing the effect of amiloride (30 μM), benzamil (10 μM), or vehicle on CPA-induced Ca2+ current density (pA/pF) from a holding potential of −50 mV. B and E: summary data for transient (T) and sustained (S) change in CPA-induced current density for vehicle group (left) and summary data for sustained CPA-induced change in current density in the presence of amiloride, benzamil, or vehicle (right). C and G: current (pA/pF)-voltage (mV) relationship derived from voltage steps (−80 to +80 mV) from a holding potential of 0 mV. F: representative voltage steps showing the effect of vehicle, amiloride, or benzamil on CPA-induced Ca2+ current density (pA/pF) from a holding potential of 0 mV in PASMC from primary culture. Values are means ± SE; n = 6–7/group. *P < 0.05 amiloride and benzamil vs. vehicle group.
Since ENaC/ASIC have high Na+ permeability and store-operated Ca2+ release-activated Ca2+ (CRAC) channels are known to be permeable to monovalent cations in the absence of external divalent cations (3, 7, 32), we examined CPA-induced Na+ current. CPA resulted in a sustained, but not transient, inward Na+ current in both freshly isolated (Fig. 5, A–C) and primary cultured (Fig. 5, D–G) PASMC held at −50 mV. Pretreatment of PASMC with amiloride and benzamil blocked store-mediated Na+ current (Fig. 5, B and E). Furthermore, these inhibitors were found to acutely reverse CPA-induced Na+ current (Fig. S4, Online Supplement). I-V relationships demonstrated an effect of both amiloride and benzamil to significantly reduce inward Na+ current at potentials from −80 to −60 mV in freshly isolated PASMC (Fig. 5C) and −80 to 10 mV in primary cultured PASMC (Fig. 5G). Interestingly, the CPA-induced Na+ current was considerably smaller in freshly isolated (Fig. 5, B and C) compared with primary cultured (Fig. 5, E and G) PASMC. Consistent with the characteristic I-V relationship of nonselective cation channels and previous reports of store-operated Na+ current through store-operated channels (33), CPA-induced Na+ current was ohmic with a Erev near 0 mV.
Fig. 5.
ENaC/ASIC inhibition diminishes CPA-induced Na+ current density in both freshly isolated (A–C) and primary cultured (D–G) PASMC. A and D: representative traces showing the effect of amiloride (30 μM), benzamil (10 μM), or vehicle on CPA-induced Na+ current density (pA/pF) from a holding potential of −50 mV. B and E: summary data for the CPA-induced change in current density in the presence of amiloride, benzamil, or vehicle. C and G: current (pA/pF)-voltage (mV) relationship derived from voltage steps (−80 to +80 mV) from a holding potential of 0 mV. F: representative voltage steps showing the effect of vehicle, amiloride, or benzamil on CPA-induced Na+ current density (pA/pF) from a holding potential of 0 mV in PASMC from primary culture. Values are means ± SE; n = 9–10/group. *P < 0.05 vs. vehicle group.
Expression of ASIC in the Pulmonary Vasculature
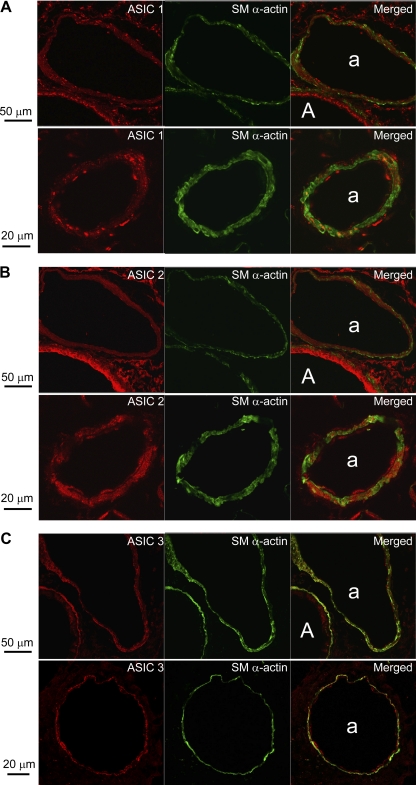
ENaC and ASIC expression has been identified in cultured VSM cells and in the cerebral, renal, and mesenteric vasculature (13, 15, 18, 25, 26). However, their presence in pulmonary VSM has not been previously examined. Figure 6 depicts images obtained by confocal microscopy of ASIC-1, -2, and -3 with simultaneous SMα-actin immunofluorescence in lung sections. ASIC1 (Fig. 6A) and ASIC3 (Fig. 6C) immunofluorescence was largely punctate in appearance, with ASIC1 showing clusters of intense fluorescence. In contrast, ASIC2 (Fig. 6B) displayed a more diffuse expression pattern throughout the lung. Intense ASIC1 and ASIC2 immunofluorescence was present in airway epithelium. However, whereas ASIC1 was more robust at the apical surface of the epithelium, ASIC2 immunoreactivity was more homogeneous throughout the airway epithelial layer. ASIC3 staining appeared greater in both airway and arterial smooth muscle compared with airway epithelium. Colocalization of ASIC1 (Fig. 6A), ASIC2 (Fig. 6B), and ASIC3 (Fig. 6C) with SMα-actin indicates the presence of ASIC within smooth muscle cells. In addition, all ASICs appear to be present in the vascular endothelium, evident by immunofluorescence on the luminal side of the internal elastic lamina (which autofluoresces).
Fig. 6.
Immunofluorescence of ASIC1 (A), ASIC2 (B), and ASIC3 (C) from paraffin-embedded lung sections. ASIC (red) and smooth muscle α-actin (SM α-actin; green) are present in airway (A) and pulmonary arteries (a).
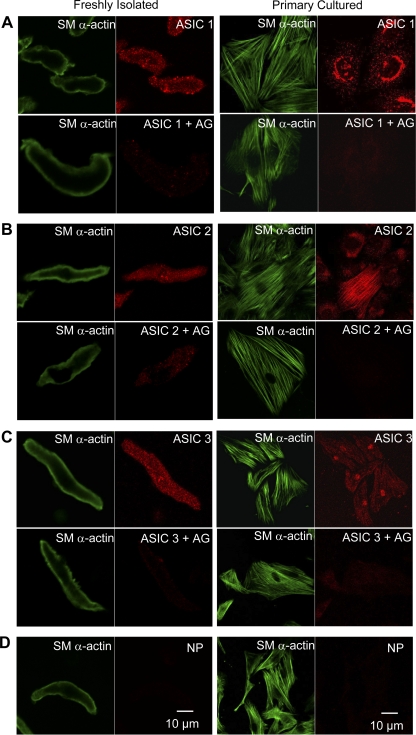
In freshly isolated and primary cultured PASMC, ASIC1 appeared to be expressed throughout the cytosol, with more intense punctate/clustered immunofluorescence in subsarcolemmal (freshly isolated PASMC) and perinuclear regions (Fig. 7A). In contrast, cytosolic expression of ASIC2 was diffuse in acutely isolated cells, but showed distinct perinuclear immunofluorescence in primary cultured PASMC and no observable punctate fluorescence at the cell membrane (Fig. 7B). ASIC3 fluorescence was punctate throughout the cytosol, with nuclear immunofluorescence apparent in cultured cells (Fig. 7C). Immunofluorescence for all three ASIC isoforms was nearly eliminated by preincubating each antibody with the appropriate control peptide (+AG; Fig. 7), thus demonstrating the specificity of the anti-ASIC antibodies. In addition, omitting the anti-ASIC antibody (NP) before secondary antibody incubation revealed a lack of immunofluorescence (Fig. 7D), further illustrating the specificity of the secondary antibody.
Fig. 7.
Immunofluorescence of ASIC1 (A; red), ASIC2 (B; red), ASIC3 (C; red), and SM α-actin (green) in freshly isolated and primary cultured PASMC. Preincubation with the corresponding antigen (+AG) blocks ASIC immunofluorescence. There was no nonspecific binding of the secondary antibody, shown by omitting primary antibody (D; NP).
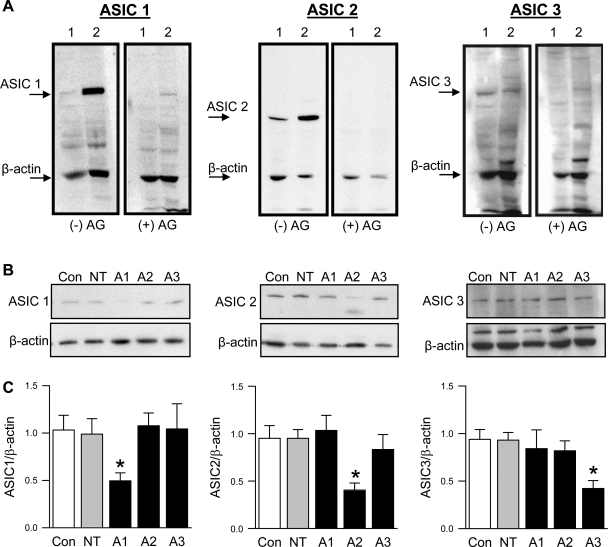
In addition to immunofluorescence, we examined ASIC expression using Western blot analysis. The addition of the control peptide (+AG) identified single bands for ASIC1, ASIC2, and ASIC3 in isolated pulmonary artery homogenates and primary cultured PASMC (Fig. 8A). Western blot analysis was then used to validate knockdown of ASIC expression with siRNA in primary cultured PASMC (Fig. 8, B and C). The siRNA silenced the targeted ASIC isoform without altering expression of the ASIC isoforms.
Fig. 8.
ASIC expression by Western blot analysis. A: representative Western blots of ASIC1 (50 μg protein/lane), ASIC2 (20 μg protein/lane), or ASIC3 (50 μg protein/lane) and β-actin in isolated small pulmonary arteries (lane 1) or primary cultured PASMC (lane 2) in the absence (−AG; left) or presence of corresponding antigen (+AG; right). B: representative Western blots for ASIC1 (A1; 10 μg protein/lane), ASIC2 (A2; 5 μg protein/lane), and ASIC3 (A3; 25 μg protein/lane) and corresponding β-actin. C: summary data for Western analysis of ASIC/β-actin protein expression following transfection with siRNA. Values are means ± SE; n = 6–8/group. *P < 0.05 vs. Con group.
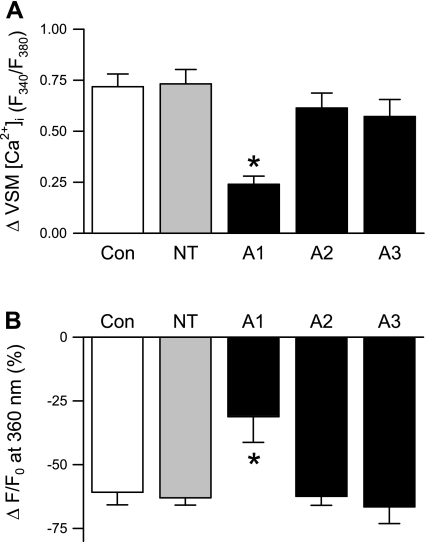
ASIC1 Mediates SOCE in Pulmonary VSM
To determine the involvement of the different ASIC subunits in SOCE, we used siRNA targeted towards ASIC1, ASIC2, and ASIC3 to selectively reduce the expression of each isoform in primary cultures of PASMC. As controls, we examined the effect of nontargeting (NT) siRNA or Lipofectamine alone. Transfection with ASIC1, but not ASIC2 or ASIC3, siRNA diminished SOCE measured by both Ca2+ depletion/repletion (Fig. 9A) and Mn2+ quenching of fura-2 (Fig. 9B) protocols. Consistent with these results, ASIC1 siRNA also attenuated the inward CPA-induced Ca2+ (Fig. 10A) and Na+ current (Fig. 10C). Interestingly, the transient current in the transfected cells (Fig. 10A) appears to be smaller than in nontransfected cells (Fig. 4E), which may be an effect of the transfecting agent. The I-V relationships showed a significant reduction in the inward Ca2+ current at potentials between −80 and −60 mV (Fig. 10B) and inward Na+ current at potentials from −80 to −10 mV (Fig. 10D) in ASIC1 siRNA-transfected cells. In contrast, Ca2+ current was unaltered by either ASIC-2 or -3 siRNA (Fig. 10B). Furthermore, ASIC2 siRNA was without effect on CPA-induced Na+ current, and ASIC3 siRNA significantly reduced Na+ current at potentials from −80 to −70 mV (Fig. 10D).
Fig. 9.
SOCE is attenuated by transfection of ASIC1 siRNA in primary cultured PASMC. SOCE-induced changes in VSM [Ca2+]i (F340/F380) (A) and magnitude of quenching 10 min after MnCl2 (500 μM) administration (B) following transfection of primary cultures with Lipofectamine alone (Con), nontargeting siRNA (NT), or siRNA directed towards ASIC1 (A1), ASIC2 (A2), or ASIC3 (A3). All experiments were performed in the presence of CPA (10 μM) and diltiazem (50 μM). Values are means ± SE; n = 5/group. *P < 0.05 vs. control and NT groups.
Fig. 10.
ASIC1 siRNA diminishes both CPA-induced Ca2+ (A and B) and Na+ (C and D) current density in primary cultured PASMC. Summary data (A) for transient (T) and sustained (S) change in CPA-induced Ca2+ current density and summary data (C) for sustained CPA-induced change in Na+ current density (pA/pF) from a holding potential of −50 mV. Current (pA/pF)-voltage (mV) relationship derived from voltage-step from −80 to +80 from a holding potential of 0 for CPA-induced Ca2+ (B) and Na+ (D) current density. Values are means ± SE; n = 7–8/group. *P < 0.05 vs. control group (B: −80 to −60 mV for A1; D: −80 to −10 and 20 to 80 mV for A1 and −80 to −70 mV for A3).
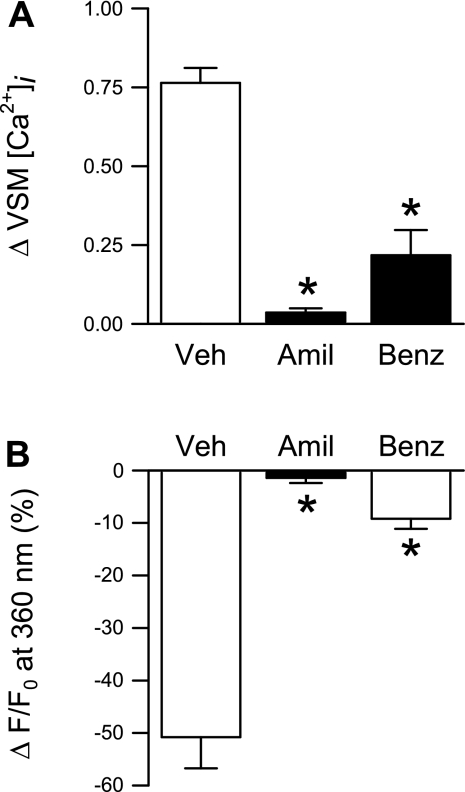
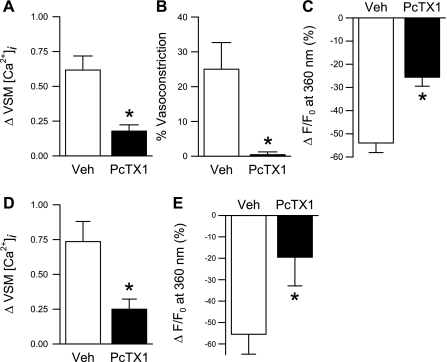
To further validate the role of ASIC1 in SOCE, we performed Ca2+ repletion and Mn2+ quenching of fluorescence experiments in small pulmonary arteries (Fig. 11, A–C) and primary cultured PASMC (Fig. 11, D and E) following pretreatment with the specific ASIC1a inhibitor, PcTX1. PcTX1 diminished changes in [Ca2+]i and Mn2+ quenching in both preparations similar to that observed with ASIC1 siRNA. Together, these data provide evidence that ASIC1 contributes to SOCE in pulmonary VSM.
Fig. 11.
The specific ASIC1 inhibitor, PcTX1, attenuates SOCE. Mean data from isolated small pulmonary arteries (A–C) and primary cultured PASMC (D and E) for SOCE-induced changes in VSM [Ca2+]i (F340/F380); associated vasoconstriction (vessels only) (B); and magnitude of Mn2+ quenching 10 min after MnCl2 (500 μM) administration in the presence of PcTX1 (100 ng/ml) or vehicle (Veh). All experiments were performed in the presence of CPA (10 μM) and diltiazem (50 μM). Values are means ± SE; n = 5–6/group. *P < 0.05 vs. Veh group.
DISCUSSION
The major findings from this study are: 1) ASIC1, ASIC2, and ASIC3 are expressed in pulmonary VSM; 2) the ENaC/ASIC inhibitors amiloride and benzamil attenuate SOCE and associated constriction of small pulmonary arteries; 3) CPA-induced Ca2+ and Na+ currents are inhibited by both amiloride and benzamil; 4) transfection with ASIC1 siRNA, but not ASIC2 or ASIC3 siRNA, decreases SOCE and both Ca2+ and Na+ currents in pulmonary VSM; and 5) the specific ASIC1 inhibitor, PcTX1, similarly reduces SOCE and resultant vasoconstriction. The results from this study demonstrate a novel contribution of ASIC1 to SOCE in pulmonary VSM and advance our understanding of mechanisms regulating pulmonary VSM Ca2+ entry.
Although closely related in structure, members of the ENaC/ASIC family participate in diverse biological processes including Na+/water transport, acid sensation, neurodegeneration, proprioception, taste, learning, memory, mechanosensation, and chemoreception (16, 31, 43, 61). ENaC and ASIC form nonvoltage-gated, cationic channels that are inhibited by tri- and divalent cations, such as Gd3+, La3+, and Ni2+ (2, 30, 49, 54, 56), all of which are known to also inhibit SOCE (46). Recent studies demonstrate that multiple subunits for both ENaC and ASIC are expressed in VSM from cerebral, renal, and mesenteric arteries where they play an important role in mechanotransduction of the myogenic response and VSM migration (13, 15, 18, 19, 24–26). However, there is some functional heterogeneity of these channels among vascular beds. Whereas ENaC contribute substantially to myogenic constriction in cerebral and renal arteries, the role of these channels in myogenic constriction of mesenteric arteries is minimal (13, 24, 25, 26). In addition, ENaC and ASIC are expressed in pulmonary VSM where there is little inherent myogenic tone. These findings suggest ENaC/ASIC may participate in other VSM functions.
Consistent with the notion that ENaC/ASIC have diverse functions in VSM, we found that amiloride and benzamil attenuated SOCE and CPA-induced Ca2+ and Na+ current in pulmonary VSM. Although amiloride and benzamil are routinely used to inhibit DEG/ENaC family members (31), there are a few issues with their use that are briefly discussed here but are further summarized in the online Supplemental data. While the autofluorescence of amiloride and benzamil at 340- to 380-nm wavelengths could complicate interpretation of VSM [Ca2+]i measurements, we found that autofluorescence was similar for each wavelength, and the resultant fura-2 ratio was not significantly affected (Fig. S1, online Supplement). In addition, in situ VSM [Ca2+]i calibrations were not altered by either amiloride, benzamil, or PcTX1 (Fig. S1, online Supplement). We provide evidence in the Supplemental data that inhibition of SOCE by amiloride and benzamil is unlikely due to nonspecific actions of these compounds to inhibit the Na+/Ca2+ exchanger (Fig. S2, online Supplement). Changes in VSM [Ca2+]i and vasoconstriction to the depolarizing stimulus KCl were also unaffected by amiloride or benzamil, suggesting that these inhibitors neither block VGCC nor interfere with detection of changes in VSM [Ca2+]i using fura-2 at the concentrations employed (Fig. S3, online Supplement). Additionally, amiloride has been shown to inhibit members of the transient receptor potential (TRP) channel family (1, 9, 22, 44), which have been implicated in SOCE (46). However, the concentration of amiloride required to block the activity of TRP channels is generally 10- to 100-fold greater (IC50 ranging from 130 to 1,000 μM) than the concentrations used in this study. In view of the potential complications with using amiloride and benzamil, and the lack of information these inhibitors provide about the specific family members and/or subunits contributing to SOCE, we additionally used siRNA approaches to identify the individual channel subtype(s) involved. Considering that the dose-dependent inhibition of SOCE presented in Fig. 1 is consistent with the IC50 for current block of cloned ASIC (10–100 μM for amiloride and 10 μM for benzamil), we examined the specific role of ASIC1, ASIC2, and ASIC3 in mediating SOCE. These studies revealed a role for ASIC1, but not ASIC2 or ASIC3, in mediating SOCE and CPA-induced Ca2+ and Na+ current in pulmonary VSM. Our finding that the specific ASIC1a inhibitor, PcTX1, attenuated SOCE and Mn2+ quenching of fura-2 fluorescence in both isolated pressurized arteries and primary cultured PASMC to a similar degree as ASIC1 siRNA further substantiates the contribution of ASIC1 to SOCE.
ASIC1 has gained considerable attention as the Ca2+-permeable subunit involved in ischemic stroke (62, 64). ASIC1 is the only ASIC member that has been shown to conduct Ca2+; however, the Ca2+ permeability of ASIC remains difficult to determine precisely because Ca2+ can either block or potentiate ASIC currents depending on the extracellular/intracellular Ca2+ concentration and pH (31, 59). Consistent with the notion that ASIC1 conducts Ca2+, the present study demonstrates that inhibition of ASIC1 either pharmacologically or by siRNA attenuates store-operated Ca2+ current. Moreover, we observed amiloride- and benzamil-sensitive acid-induced currents, thus providing further evidence that ASIC form functional channels in pulmonary VSM (Fig. S5, online Supplement).
CPA-induced Na+ current was additionally examined in PASMC because 1) ASIC are highly permeable to Na+, and 2) the use of divalent-free solution has been previously used to obtain much higher store-operated conductance (21, 32, 37). Consistent with these observations, we found that CPA-induced Na+ current was approximately fourfold greater than Ca2+ current. In addition, increasing [Ca2+]o inhibited CPA-induced Na+ current in PASMC (Fig. S6, online Supplement), also a reported characteristic of store-operated current (11). However, we indentified inconsistencies related to our Na+ current measurements, which suggest this monovalent current is not strictly related to SOCE. First, the Na+ current of freshly isolated PASMC was approximately threefold less than Na+ current in primary cultured PASMC. If Na+ was a valid surrogate for Ca2+ current, then we would expect to see this difference between freshly isolated and primary cultured PASMC in all of the other measurements of SOCE (Ca2+ current, store-operated VSM [Ca2+]i responses, Mn2+ influx). Second, ASIC3 siRNA did not diminish store-operated VSM [Ca2+]i responses, Mn2+ influx, or Ca2+ current, but did significantly reduce CPA-induced Na+ current. Together, these data suggest CPA-induced Na+ current may be distinct from the Ca2+ current in this preparation. It has been reported in some instances that the monovalent whole cell current previously attributed to ICRAC is likely mediated by separate, non-CRAC channels (20, 34, 48).
Distinct roles for stromal interaction molecule (STIM) and Orai protein have been defined in mediating SOCE and ICRAC (42, 52, 66). Indeed, recent findings support a role for Orai1/STIM1 mediating SOCE in VSM (10, 38, 47). While there is considerable evidence from numerous laboratories that TRP channels exhibit store-operated activity (46), it has been difficult to determine whether TRP channels are activated by store depletion or by various signals coinciding with depletion. Although several studies support a direct association between TRP channels and STIM1, including a study in pulmonary VSM cells (45), others report little or no functional interaction between TRPC and STIM1 in VSM (10, 12, 39, 47). Furthermore, studies by Li et al. (39) found that STIM1 interacts with a TRPC1-independent channel that contributes substantially to a nonselective cationic current evoked by store depletion. Although this channel is not yet identified, it is likely something other than Orai since it is nonselective, rather than Ca2+ selective, in nature. It is possible this unknown nonselective cationic current is mediated by ASIC1; however, we have not determined whether ASIC1 and STIM1 interact.
We recognize that ASIC1 is probably not a genuine CRAC channel, but rather forms a distinct channel in pulmonary VSM that is sensitive to store depletion, which may also include many of the TRP channel family members. Consistent with the notion that TRP channels are activated indirectly by store depletion, recent studies suggest phosphatidylinositol 4,5-bisphosphate (PIP2) activates SOCE through PKC-dependent phosphorylation of TRPC1 in freshly dispersed rabbit portal vein myocytes (53). In addition, Lefkimmiatis et al (35) demonstrated that store depletion is coupled to enhanced cAMP accumulation and PKA activation through a process that involves STIM1. Interestingly, both PKC and PKA have binding sites on ASIC (4, 36), and, therefore, may similarly facilitate ASIC activity during store depletion.
Regardless of whether ASIC1 is directly or indirectly activated by store depletion, our study has identified a unique role of ASIC1 to mediate Ca2+ entry in pulmonary VSM. Enhanced SOCE has been shown to contribute to hypoxic pulmonary hypertension by facilitating Ca2+ influx and increasing basal [Ca2+]i in PASMC (40, 58). Whether ASIC1 contributes to SOCE associated with hypoxic vasoconstriction and receptor activation in the normal or hypertensive pulmonary circulation remains to be established. Challenges of future studies are to identify the role of ASIC in pulmonary vasoregulation and potential mechanisms by which ASIC contribute to altered pulmonary VSM Ca2+ homeostasis in pulmonary hypertension.
GRANTS
Images in this paper were generated in the University of New Mexico Cancer Center Fluorescence Microscopy Facility, which received support from NCRR 1 S10 RR14668, NSF MCB9982161, NCRR P20 RR11830, NCI P30 CA118100, NCRR S10 RR19287, and NCRR S10 RR016918, the University of New Mexico Health Sciences Center, and the University of New Mexico Cancer Center. This work was also supported by National Heart, Lung, and Blood Institute Grants HL-92598 (to N. L. Jernigan), HL-77876 and HL-88192 (to T. C. Resta), and HL-58124 and HL-07736 (to B. R. Walker), and American Heart Association Grant-In-Aid 0755775Z (to T. C. Resta).
Supplementary Material
Acknowledgments
We thank Tamara Howard for assistance in lung section preparation for immunofluorescence and Minerva Murphy for technical assistance.
REFERENCES
- 1.Alexander SPH, Mathie A, Peters JA. Ion channels. Br J Pharmacol 153: S112–S145, 2008.18311155 [Google Scholar]
- 2.Babinski K, Catarsi S, Biagini G, Seguela P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+. J Biol Chem 275: 28519–28525, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bakowski D, Parekh AB. Monovalent cation permeability and Ca2+ block of the store-operated Ca2+ current I(CRAC)in rat basophilic leukemia cells. Pflügers Arch 443: 892–902, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bashari E, Qadri YJ, Zhou ZH, Kapoor N, Anderson SJ, Meltzer RH, Fuller CM, Benos DJ. Two PKC consensus sites on human acid-sensing ion channel 1b differentially regulate its function. Am J Physiol Cell Physiol 296: C372–C384, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benos DJ, Stanton BA. Functional domains within the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. J Physiol 520: 631–644, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi L, Gerstbrein B, Frokjaer-Jensen C, Royal DC, Mukherjee G, Royal MA, Xue J, Schafer WR, Driscoll M. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat Neurosci 7: 1337–1344, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Braun FJ, Broad LM, Armstrong DL, Putney JW Jr. Stable activation of single Ca2+ release-activated Ca2+ channels in divalent cation-free solutions. J Biol Chem 276: 1063–1070, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Canessa C, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J, Rossier B. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Dai XQ, Ramji A, Liu Y, Li Q, Karpinski E, Chen XZ. Inhibition of TRPP3 channel by amiloride and analogs. Mol Pharmacol 72: 1576–1585, 2007. [DOI] [PubMed] [Google Scholar]
- 10.DeHaven W, Jones B, Petranka J, Smyth J, Tomita T, Bird G, Putney J. TRPC channels function independently of STIM1 and Orai1. J Physiol 587: 2275–2298, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeHaven WI, Smyth JT, Boyles RR, Putney JW Jr. Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem 282: 17548–17556, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich A, Kalwa H, Storch U, Mederos y Schnitzler M, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflügers Arch 455: 465–477, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension 44: 643–648, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, Yuan JXJ. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 285: L1233–L1245, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Gannon KP, Vanlandingham LG, Jernigan NL, Grifoni SC, Hamilton G, Drummond HA. Impaired pressure-induced constriction in mouse middle cerebral arteries of ASIC2 knockout mice. Am J Physiol Heart Circ Physiol 294: H1793–H1803, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Anoveros J, Corey DP. The molecules of mechanosensation. Ann Rev Neurosci 20: 567–594, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev 77: 359–396, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Grifoni SC, Gannon KP, Stec DE, Drummond HA. ENaC proteins contribute to VSMC migration. Am J Physiol Heart Circ Physiol 291: H3076–H3086, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Grifoni SC, Jernigan NL, Hamilton G, Drummond HA. ASIC proteins regulate smooth muscle cell migration. Microvasc Res 75: 202–210, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A. Dissociation of the store-operated calcium current ICRAC and the Mg-nucleotide-regulated metal ion current MagNuM. J Physiol 539: 445–458, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol 465: 359–386, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res 88: 325–332, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Jernigan NL, Broughton BRS, Walker BR, Resta TC. Impaired NO-dependent inhibition of store- and receptor-operated calcium entry in pulmonary vascular smooth muscle after chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 290: L517–L525, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous β- and γENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol 289: F891–F901, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Jernigan NL, LaMarca B, Speed J, Galmiche L, Granger JP, Drummond HA. Dietary salt enhances benzamil-sensitive component of myogenic constriction in mesenteric arteries. Am J Physiol Heart Circ Physiol 294: H409–H420, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Jernigan NL, Walker BR, Resta TC. Chronic hypoxia augments protein kinase G-mediated Ca2+ desensitization in pulmonary vascular smooth muscle through inhibition of RhoA/Rho kinase signaling. Am J Physiol Lung Cell Mol Physiol 287: L1220–L1229, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Jernigan NL, Walker BR, Resta TC. Endothelium-derived reactive oxygen species and endothelin-1 attenuate NO-dependent pulmonary vasodilation following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L801–L808, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Jernigan NL, Walker BR, Resta TC. Pulmonary PKG-1 is upregulated following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 285: L634–L642, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Ji HL, Fuller CM, Benos DJ. Osmotic pressure regulates αβγ-rENaC expressed in Xenopus oocytes. Am J Physiol Cell Physiol 275: C1182–C1190, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Kerschbaum HH, Cahalan MD. Monovalent permeability, rectification, and ionic block of store-operated calcium channels in Jurkat T lymphocytes. J Gen Physiol 111: 521–537, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerschbaum HH, Cahalan MD. Single-channel recording of a store-operated Ca2+ channel in Jurkat T lymphocytes. Science 283: 836–839, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Kozak JA, Kerschbaum HH, Cahalan MD. Distinct properties of CRAC and MIC channels in RBL cells. J Gen Physiol 120: 221–235, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. Store-operated cyclic AMP signalling mediated by STIM1. Nat Cell Biol 11: 433–442, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Leonard AS, Yermolaieva O, Hruska-Hageman A, Askwith CC, Price MP, Wemmie JA, Welsh MJ. cAMP-dependent protein kinase phosphorylation of the acid-sensing ion channel-1 regulates its binding to the protein interacting with C-kinase-1. Proc Natl Acad Sci USA 100: 2029–2034, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lepple-Wienhues A, Cahalan MD. Conductance and permeation of monovalent cations through depletion-activated Ca2+ channels (ICRAC) in Jurkat T cells. Biophys J 71: 787–794, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Jairaman A, Sukumar P, Porter K, Beech D. Orai1 expression and function in vascular smooth muscle cells. FASEB J 22: 965.2, 2008. [Google Scholar]
- 39.Li J, Sukumar P, Milligan CJ, Kumar B, Ma ZY, Munsch CM, Jiang LH, Porter KE, Beech DJ. Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res 103: e97–e104, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin MJ, Leung GPH, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JSK. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Lingueglia E Acid-sensing ion channels in sensory perception. J Biol Chem 282: 17325–17329, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Liou J, Kim ML, Do Heo W, Jones JT, Myers JW, Ferrell JJE, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mano I, Driscoll M. DEG/ENaC channels: a touchy superfamily that watches its salt. Bioessays 21: 568–578, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25: 4052–4061, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng LC, McCormack MD, Airey JA, Singer CA, Keller PS, Shen XM, Hume JR. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol 587: 2429–2442, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parekh AB, Putney JW Jr. Store-operated calcium channels. Physiol Rev 85: 757–810, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. In press. [DOI] [PMC free article] [PubMed]
- 48.Prakriya M, Lewis RS. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J Gen Physiol 119: 487–508, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rafii B, Coutinho C, Otulakowski G, O'Brodovich H. Oxygen induction of epithelial Na+ transport requires heme proteins. Am J Physiol Lung Cell Mol Physiol 278: L399–L406, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Robertson TP, Hague D, Aaronson PI, Ward JPT. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol 525: 669–680, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson TP, Ward JP, Aaronson PI. Hypoxia induces the release of a pulmonary-selective, Ca2+-sensitising, vasoconstrictor from the perfused rat lung. Cardiovasc Res 50: 145–150, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saleh SN, Albert AP, Large WA. Obligatory role for phosphatidylinositol 4,5-bisphosphate in activation of native TRPC1 store-operated channels in vascular myocytes. J Physiol 587: 531–540, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheng S, Perry CJ, Kleyman TR. External nickel inhibits epithelial sodium channel by binding to histidine residues within the extracellular domains of alpha and gamma subunits and reducing channel open probability. J Biol Chem 277: 50098–50111, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Shimoda LA, Wang J, Sylvester JT. Ca2+ channels and chronic hypoxia. Microcirculation 13: 657–670, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Staruschenko A, Dorofeeva N, Bolshakov K, Stockand J. Subunit-dependent cadmium and nickel inhibition of acid-sensing ion channels. J Neurobiol 67: 97–107, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Waldmann R Proton-gated cation channels–neuronal acid sensors in the central and peripheral nervous system. Adv Exp Med Biol 502: 293–304, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Wang WZ, Chu XP, Li MH, Seeds J, Simon RP, Xiong ZG. Modulation of acid-sensing ion channel currents, acid-induced increase of intracellular Ca2+, and acidosis-mediated neuronal injury by intracellular pH. J Biol Chem 281: 29369–29378, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol 289: L5–L13, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 34: 463–477, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Xiong ZG, Chu XP, Simon RP. Ca2+-permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol 209: 59–68, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Xu K, Tavernarakis N, Driscoll M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca2+ release from the endoplasmic reticulum. Neuron 31: 957–971, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA 101: 6752–6757, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang S, Patel HH, Murray F, Remillard CV, Schach C, Thistlethwaite PA, Insel PA, Yuan JXJ. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 292: L1202–L1210, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437: 902–905, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.