The latest drug for weight reduction, orlistat (Xenical, Roche, Sweden), was approved for use in Sweden in July 1998, and by September 1999 13 million defined daily doses (360 mg per defined daily dose) had been sold. Steatorrhoea and other gastrointestinal disorders were the most frequently reported adverse reactions in clinical trials.1 Adverse reactions indicating systemic effects have also been reported for orlistat. We report on a case of hypertension associated with the drug.
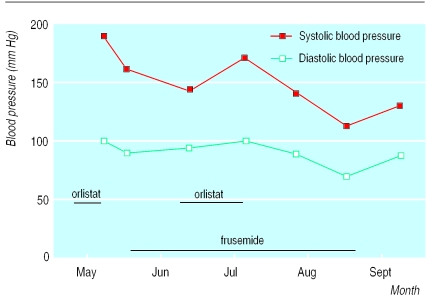
At the beginning of 1999, a 40 year old previously healthy woman commenced orlistat treatment because of obesity. She took sporadic doses for some months and then increased the dosage to 120 mg three times daily during one week in May 1999. She experienced dizziness, peripheral oedema, and pulsating headache and stopped the treatment. On medical examination her blood pressure was 190/100 mm Hg on three different occasions. Her heart rate was regular, at 60 beats/min. She was advised to stop taking orlistat, and a few days later her blood pressure had decreased to 160/90 mm Hg and the oedema had regressed. Laboratory tests, including measurement of thyroid hormone concentrations, were all normal. Treatment with frusemide (furosemide) 30 mg orally daily was started, and the blood pressure decreased to 145/95 mm Hg (figure). The patient restarted orlistat treatment in July 1999. Headache and peripheral oedema recurred, and her blood pressure increased to 170/100 mm Hg. Again, orlistat was discontinued. Her symptoms disappeared, and her blood pressure decreased to 140/90 mm Hg. After another month she experienced dizziness, and her blood pressure was 110/70 mm Hg. After cessation of diuretic treatment her blood pressure stabilised at 130/90 mm Hg, and this remained stable after three months.
Orlistat was considered causal to the hypertension in this patient owing to a positive dechallenge and rechallenge. The mechanism for this reaction is not clear. Fluid retention may be a possibility.
Overall, 13 cases of hypertension associated with orlistat have been reported to the manufacturer, but information on blood pressure measurements and follow up was limited in these cases. Although some of the patients had a history of hypertension, others, as in our case, had not. We have informed the Medical Products Agency, which is the Swedish regulatory body overseeing the safety of medicines.
Figure.
Blood pressure in relation to orlistat and frusemide treatment
Footnotes
Competing interests: None declared.
References
- 1.Tonstad S, Pometta D, Erkelens DW, Ose L, Moccetti T, Schouten JA, et al. The effect of the gastrointestinal lipase inhibitor, orlistat, on serum lipids and lipoproteins in patients with primary hyperlipidaemia. Eur J Clin Pharmacol. 1994;46:405–410. doi: 10.1007/BF00191901. [DOI] [PubMed] [Google Scholar]