Abstract
Recent studies demonstrate that sustained hypoxia induces the robust accumulation of leukocytes and mesenchymal progenitor cells in pulmonary arteries (PAs). Since the factors orchestrating hypoxia-induced vascular inflammation are not well-defined, the goal of this study was to identify mediators potentially responsible for recruitment to and retention and differentiation of circulating cells within the hypoxic PA. We analyzed mRNA expression of 44 different chemokine/chemokine receptor, cytokine, adhesion, and growth and differentiation genes in PAs obtained via laser capture microdissection in adjacent lung parenchyma and in systemic arteries by RT-PCR at several time points of hypoxic exposure (1, 7, and 28 days) in Wistar-Kyoto rats. Analysis of inflammatory cell accumulation and protein expression of selected genes was concomitantly assessed by immunochemistry. We found that hypoxia induced progressive accumulation of monocytes and dendritic cells in the vessel wall with few T cells and no B cells or neutrophils. Upregulation of stromal cell-derived factor-1 (SDF-1), VEGF, growth-related oncogene protein-α (GRO-α), C5, ICAM-1, osteopontin (OPN), and transforming growth factor-β (TGF-β) preceded mononuclear cell influx. With time, a more complex pattern of gene expression developed with persistent upregulation of adhesion molecules (ICAM-1, VCAM-1, and OPN) and monocyte/fibrocyte growth and differentiation factors (TGF-β, endothelin-1, and 5-lipoxygenase). On return to normoxia, expression of many genes (including SDF-1, monocyte chemoattractant protein-1, C5, ICAM-1, and TGF-β) rapidly returned to control levels, changes that preceded the disappearance of monocytes and reversal of vascular remodeling. In conclusion, sustained hypoxia leads to the development of a complex, PA-specific, proinflammatory microenvironment capable of promoting recruitment, retention, and differentiation of circulating monocytic cell populations that contribute to vascular remodeling.
Keywords: vascular remodeling, cytokines, progenitor cells, inflammation, pulmonary hypertension
pulmonary hypertension (PH) is a progressive and often fatal disease characterized by increased pulmonary artery (PA) pressure and vascular resistance (5, 23). All forms of chronic PH demonstrate marked structural remodeling of both large and small PAs, and this remodeling is believed to be a contributing factor to increased vascular resistance and, ultimately, right heart failure (23, 37, 41, 50, 54). Emerging evidence suggests that circulating inflammatory and/or progenitor cells contribute significantly to the remodeling process (1, 6, 9, 13, 28, 32, 33, 35, 44, 45, 49, 54). In fact, recent studies describe inflammation as a characteristic feature of many, if not all, forms of PH in both humans and animal models (9, 13, 32, 44, 45, 49, 54) and report that inflammatory cell accumulation and retention is often greatest in pulmonary perivascular regions, where remodeling is also often prominent (9, 13, 54). The importance of inflammatory cells in the remodeling process is further supported by studies in which generalized anti-inflammatory strategies inhibited the remodeling (6) as well as by experiments where in vivo depletion of a specific pool of circulating leukocytes markedly abrogated perivascular fibrosis and remodeling (13). Yet despite acknowledgement of the important role circulating inflammatory cells play in pulmonary vascular remodeling, little is known regarding factors that facilitate recruitment to and retention of these cells in the pulmonary vasculature.
Similar to chronic inflammatory diseases (e.g., neurodegenerative disease, obesity, asthma, rheumatoid arthritis), accumulating data demonstrate that inflammatory mediators and immune responses participate in the development and progression of systemic vascular diseases such as atherosclerosis (28). Numerous mediators, interacting through complex networks, have been shown to participate in chronic inflammatory diseases, some of which have been likened to persistent “wounds,” including chemokine/chemokine receptors, cytokines, adhesion molecules, matrix proteins, and proteinases (16, 28, 47). Unfortunately, PH has not been extensively studied with regard to these signaling networks. Furthermore, most studies that have investigated mediators involved in PH-associated inflammation have used whole lung tissue extracts to examine the question. However, experimental evidence now strongly suggests that mRNA or protein levels in whole lung homogenates (or any other whole organ) do not accurately reflect specific changes in the vasculature (2, 11, 12, 14, 26, 33, 35, 42, 53, 63). A few recent studies have examined PA-specific expression of proinflammatory factors in animal models of PH, however, these reports focused on only a single or limited number of mediators and did not characterize the complexity of the pulmonary vascular inflammatory microenvironment that appears essential for the accumulation and persistence of inflammatory cell infiltrates and the resultant structural remodeling (26, 33, 35). The goal of the present study, therefore, was to perform a comprehensive analysis of the effects of sustained hypoxia on cytokine, chemokine, and adhesion molecule expression, specifically in the PA over time, and to compare their expression with expression patterns of the same genes in parenchymal lung tissue and systemic vessels. Furthermore, we sought to characterize the nature of inflammatory cell infiltrates observed in the hypoxia-induced inflammatory environment.
To investigate these questions, we chose young (4-wk-old) Wistar-Kyoto rats as an animal model, since this strain develops very severe hypoxic PH, associated with prominent inflammatory cell infiltrates and marked vascular remodeling in both proximal and distal PAs (13). To evaluate hypoxia-induced inflammatory changes in the PA, we chose to analyze mRNA levels of multiple chemokine/chemokine receptors, cytokines, adhesion molecules, and proinflammatory matrix proteins in vessels obtained via laser capture microdissection (LCM) at several time points during the development of PH. The choice of the genes for analysis was based on pilot data derived from Affymetrix array analysis of whole lung gene expression following 3 and 21 days of hypoxia in Wistar-Kyoto rats (see pilot results below) and previous reports demonstrating involvement of specific chemokine, cytokine, adhesion molecule, and matrix protein family members in initiating and sustaining chronic inflammatory diseases (28, 30, 47, 48). We examined the PA specificity of the gene changes by analyzing the expression of many of the genes in microdissected PAs as well as in adjacent lung parenchyma and in systemic vessels (aorta and renal artery) of the same animals. Immunohistochemistry was used to determine the time course and nature of inflammatory cell recruitment and to confirm many of the hypoxia-induced changes in gene expression at the protein level. Finally, to gain further insights into mechanisms potentially involved in the recruitment and/or persistence of inflammatory cells in the hypoxic PA microenvironment, we evaluated the fate of inflammatory cells and the aforementioned mediators following return of the animals to normoxia, since remodeling is known to spontaneously regress (18, 20, 21). This, we presumed, could shed light on the chemokines, cytokines, and adhesion molecules important in retaining inflammatory cells in the vessel wall.
MATERIALS AND METHODS
Animal Model and Tissue Samples
Three-week-old male Wistar-Kyoto rats were purchased from Charles River Laboratories (Wilmington, MA). Animals were housed at ambient altitude [Denver, CO; barometric pressure (PB) = 640 mmHg] for 1 wk before being exposed to hypobaric hypoxia (PB = 380 mmHg; hypoxic group) for the periods of 1 day (24 h), 7 days, and 28 days (n = 7–10 per time point). Age-matched controls were kept at room air (normoxic group; n = 6–7). For the “spontaneous regression of PH” study, animals were exposed to hypobaric hypoxia for 28 days, after which they were returned to normoxic (room air) conditions for the periods of 2 days (48 h) and 56 days (8 wk) (n = 3–7 per time point). At the end of the experiment, animals were anesthetized via intramuscular injection of ketamine (80 mg/kg) and xylazine (16 mg/kg), and right ventricle pressures were taken with the Cardiomax III cardiac output computer (Columbus Instruments, Columbus, OH). Lung tissue was embedded in optimum cutting temperature compound (OCT; Sakura Finetek, Torrance, CA) and frozen for LCM and immunofluorescent staining. Systemic arteries (aortic arch and renal arteries) obtained from four animals per time point were pooled together and placed in RNAlater for RNA isolation (Ambion, Austin, TX). All animal studies were approved by the Institutional Animal Care and Use Committee.
LCM and RNA Isolation
We used the Veritas Microdissection LCM instrument to selectively dissect PAs and perivascular lung parenchyma (Arcturus Engineering, Mountain View, CA). Tissue cryosections (20 μm) were applied to PEN membrane slides (Arcturus Engineering), fixed in 70% ethanol (15 s at −20°C) followed by 100% acetone (5 min at −20°C), dehydrated in graded ethanol (30 s each) and xylene (10 min), and air-dried. The PA was dissected first, and then the perivascular lung parenchyma adjacent to the PA was dissected and placed on a separate cap. Samples from individual animals were collected and analyzed separately to allow for statistical analysis. Captured tissues were incubated in lysis buffer for 30 min at 42°C, and total RNA was extracted with the Qiagen RNeasy Micro Kit with DNase treatment (Qiagen, Valencia, CA). RNA was analyzed with the Agilent 2100 Bioanalyzer Pico Chip (Agilent Technologies, Palo Alto, CA). Only RNA samples with clearly defined 18S and 28S rRNA peaks were used for further analysis.
RNA Amplification and cDNA Synthesis
Amplified cDNA from LCM samples was generated from a minimum of 500 pg of RNA using the WT-Ovation Pico RNA Amplification System (NuGEN Technologies, San Carlos, CA). cDNA from systemic arteries was transcribed with the SuperScript III First-Strand Synthesis System (Invitrogen). cDNA yield was determined by measuring A260 on the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE).
Real-Time PCR
RT-PCR was performed on the 7300 Real-Time PCR System with Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Custom oligonucleotide primers were designed using Primer3 software (sequences listed in Table 1; Ref. 43). Cycling conditions were 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. A disassociation (melt) curve and a no-template control were run for each sample to check for nonspecific product formation and reaction contamination. Genes were normalized to the housekeeping gene hypoxanthine-xanthine phosphoribosyl transferase (HPRT), and fold change relative to normoxic samples was calculated using the 2−ΔΔCt method.
Table 1.
Rat primer sequences for real-time PCR
| Gene | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| 5-Lipoxygenase | TGATGCTAAATGCCACAAGG | AGGTTCTCCATCGCTTTTGA |
| BMP-2 | TGATCACCTGAACTCCACCA | GCTAAGCTCAGTGGGGACAC |
| BMP-6 | AAAGGCTACGCTGCCAACTA | CTCGGGATTCATAAGGTGGA |
| C5 | CCCGATCATCAAGTGGCTAT | TACTCTGTCAGGCCCTCGAT |
| C5R1 | CCAGCATCATACGAAACGTG | CCTGACTCTTCTGGGTGGAG |
| CCL2/MCP-1 | CTGTAGCATCCACGTGCTGT | CCGACTCATTGGGATCATCT |
| CCL4/MIP-1β | CCACTTCCTGCTGCTTCTCT | CAAAGGCTGCTGGTCTCATA |
| CCL5/RANTES | CTGCTGCTTTGCCTACCTCT | CGAGTGACAAAGACGACTGC |
| CCR2 | TTTGATCCTGCCCCTACTTG | TCACAGCCCTATGCCTCTTT |
| CCR5 | GGTCATCTTGAGCCTGATCC | CTTACAGCCCTGTGCCTCTT |
| CX3CL1/fractalkine | CACTTCTGTGCTGACCCAAA | TGTCCACACGCTTCTCAAAC |
| CXCL1/GROα | TGTTGAAGCTTCCCTTGGAC | GAGACGAGAAGGAGCATTGG |
| CXCL12/SDF-1 | TGTGCATTGACCCGAAATTA | TCCTCAGGGGTCTACTGGAA |
| CXCR2 | CGTTCTGGTGACTTTGCTGA | AGCAGGTAGACGTCGGTGAC |
| CXCR4 | TGTTCCAGTTCCAGCACATC | CCTTGGAGTGTGACAGCTTG |
| Endothelin-1 | CGTCCCGTATGGACTAGGAA | GTGAGCACACTGGCATCTGT |
| ET-A receptor | CATGCCTCTGTTGCTGTTGT | TGGTTCTGCTCCTGGTTCTT |
| ET-B receptor | CCCTGAAGCCATAGGTTTTG | TGCATGAAGGCTGTTTTCTG |
| Fibronectin | CAAGGTCCGAGAAGAGGTTG | CCGTGTAAGGGTCAAAGCAT |
| FIZZ-1/RELMα | AGGAAGACCCTCTCATGCAC | CAAAGCCACAAGAACAACCA |
| FLAP | AGAGCACCCCTGGCTACATA | GTCGCTTCCGAAGAAGAAGA |
| FLK-1 | AGCTCAGGTTTTGTGGAGGA | CCAAGAACTCCATGCCCTTA |
| FLT-1 | CAGCACCAAGAGTGACGTGT | GGGTATGGAGAACCCCCTAA |
| HPRT | AAGCTTGCTGGTGAAAAGGA | CAAGGGCATATCCAACAACA |
| HSP-47 | CGGAGAAGCTGAGTTCCAAG | CCAAGGGTGACAGGAGGATA |
| ICAM-1 | CTTCCGACTAGGGTCCTGAA | CTTCAGAGGCAGGAAACAGG |
| IL-18 | ATGCCTGATATCGACCGAAC | GATAGGGTCACAGCCAGTCC |
| IL-1α | AAGAGACCATCCAACCCAGA | TGATGAACTCCTGCTTGACG |
| IL-R1 | TTGTCTCATTGTGCCTCTGC | AAGAGGACAGCTGCGAATGT |
| IL-6 | AGTTGCCTTCTTGGGACTGA | CTGGTCTGTTGTGGGTGGTA |
| IL-6Ra | GCAATTCGAGCTTCGATACC | AAGGCATCATGGATGACACA |
| Integrin-β1 | GGAGTGAATGGGACAGGAGA | TCTGTGAAGCCCAGAGGTTT |
| MMP-2 | AATGGTCGGGAATACAGCAG | TTTGCCGTCCTTCTCAAAGT |
| Osteopontin/OPN | GGTGATAGCTTGGCTTACGG | GGCATCGGGATACTGTTCAT |
| PDGF-A | GCCTTGGAGACAAACCTGAG | AAATGACCGTCCTGGTCTTG |
| PDGF-B | ACACCTCAAACTCGGGTGAC | TCAGTGCCTTCTTGTCATGG |
| PDGF-RA | AGAAGATTGTGCCGCTGAGT | TCCTCGGTTCTGATTTCCAC |
| PDGF-RB | TGGTCTGAGCCACTCACAAG | GACATGAGGGCTTGCTTCTC |
| Periostin | CCCGGCTATATGAGAATGGA | TTCCCTGAGCTTCGAGACAT |
| TGF-β | ATACGCCTGAGTGGCTGTCT | TGAAGCGAAAGCCCTGTATT |
| TIMP-1 | CTGAGAAGGGCTACCAGAGC | TCATCGAGACCCCAAGGTAT |
| TLR-4 | CCCTGGTGTTGGATTTTACG | GGCTACCACAAGCACACTGA |
| TRAIL | GCTTCAGTCAGCACTTCACG | GCAAGCTAGTCCGATTTTGG |
| VCAM-1 | TGCACGGTCCCTAATGTGTA | TGCCAATTTCCTCCCTTAAA |
| VEGF-A | AATCCTGGAGCGTTCACTGT | GCGAGTCTGTGTTTTTGCAG |
Immunostaining
The following cellular markers were analyzed using monoclonal and polyclonal antibodies (MAbs and PAbs, respectively): leukocytic antigens: CD45, CD11b, CD3, OX62, CD45RA, and granulocytes, clone RK-4 (MAbs; Chemicon, Temecula, CA, and Cedarlane Laboratories, Burlington, NC); markers of fibrosis: cellular (ED-A isoform) fibronectin (ED-A-FN MAbs; AbD Serotec, Raleigh, NC), procollagen-associated heat shock protein HSP-47 (MAbs), and tenascin-C (rabbit PAb) (both from EMD Chemicals, San Diego, CA); adhesion molecules: VCAM-1 (goat PAb; Santa Cruz Biotechnology, Santa Cruz, CA); active transforming growth factor-β1 (TGF-β1; chicken PAb; R&D Systems, Minneapolis, MN); CXCL12/stromal cell-derived factor-1 (SDF-1; rabbit PAb; PeproTech EC, London, United Kingdom); VEGF (EMD Chemicals); and osteopontin (OPN; MAb clone MPIIIB101; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA). Appropriate secondary antibodies were used as previously described (13), and staining was examined under a Zeiss fluorescent microscope with an AxioVision digital imaging system (Carl Zeiss MicroImaging, Thornwood, NY).
Quantification of CD11b+ Stained Leukocytes
Lung cryosections were immunostained with the leukocytic cell marker CD11b. The number of CD11b+ cells in the PA wall (CD11b+ cell index) was assessed per unit volume (10-μm radial distance from the external elastic lamina by 500-μm perimeter length).
Statistical Analysis
The relative expression values were calculated using the average of all normoxic values and were log (base 10) transformed. Descriptive statistics were calculated using means and standard errors. One-sample t-tests were performed on the average log-transformed relative expression values at each time point. P values <0.05 were considered to be significant. All analyses were performed using SAS version 9.2 software (SAS Institute, Cary, NC). The mean values for the 28-day hypoxic and the 2-day regression group were compared using a two-sample t-test.
RESULTS
Pilot Data: Microarray Analysis of Inflammatory Gene Expression in the Hypoxic Whole Lung
As a starting point, we wanted to determine inflammatory genes that were upregulated by hypoxia in whole lung tissue at early and late time points. We believed these data, when combined with data from others regarding the molecules involved in generating and maintaining chronic inflammation in a variety of tissues, including systemic blood vessels, would guide the choice of genes to be examined in LCM-generated PA and parenchymal lung tissue. Of the 31,000 probe sets on the Affymetrix Rat 230.2 chip, 21,992 were detected in 3-day and 22,312 in 21-day samples. Genes absent from all samples were removed from analysis. When filtered for fold change with a cutoff of ≥1.5, 3,368 genes from 3-day and 1,584 genes from 21-day were increased in hypoxic compared with normoxic lungs. Genes with Gene Ontology (GO) annotation keyword (inflammation and matrix) were searched in the upregulated gene list and are represented in Supplemental Tables S1 and S2 (available in the data supplement online at the AJP-Lung Cellular and Molecular Physiology web site). Heat maps of genes subcategorized as chemokines and cytokines (Supplemental Fig. S1A), immune regulatory (Supplemental Fig. S1B), and matrix/adhesion (Supplemental Fig. S1C) were created. Some of the genes identified, including CXCL12, CCL2, OPN, IL-6, IL-1, and bone morphogenetic protein (BMP)-2 have been implicated in vascular remodeling. Other genes implicated in chronic vascular inflammation, such as VCAM and ICAM, were notably absent. This information thus provided a guide for our LCM studies and also supported our notion that specific analysis of PA tissue would be necessary to begin to explain the factors involved in initiating and maintaining pulmonary vascular inflammation.
Hypoxia Induces a Progressive Accumulation of Monocytes and Dendritic Cells in the PA Wall
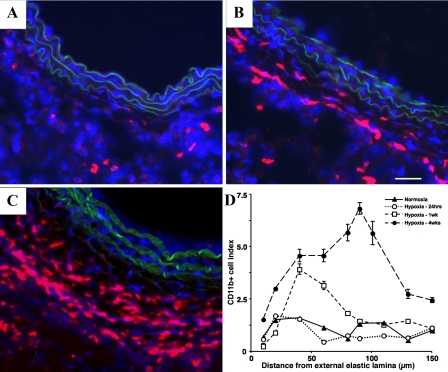
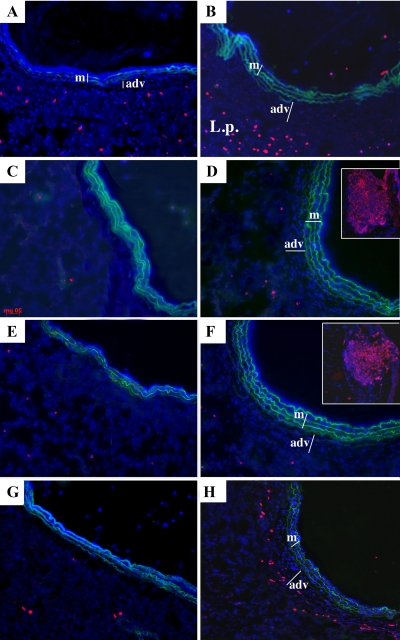
To examine the time course of inflammatory cell appearance in and around the PA, we exposed animals to chronic hypoxia (1–28 days) and performed immunofluorescent staining on lung cryosections for the panleukocytic marker, CD45 (data not shown), CD11b (monocytes and lymphocytes), Alexa 594 (neutrophils), CD3 (T cells), CD45RA (B cells), and OX62 (dendritic cells). In normoxic and 1-day-exposed hypoxic animals, there were few positively stained CD45 and CD11b cells in the adventitia (Fig. 1A; CD45 and CD11b 1-day not shown). However, in 7- and 28-day hypoxic animals, we found a progressive accumulation of CD11b and CD45+ cells in the PA, which correlated with an increase in the thickness of the PA adventitia (Fig. 1, B–D). At none of the time points evaluated were neutrophils (Alexa 594+ cells) observed in the vessel wall, although an increased number of neutrophils were often observed in the lung interstitium (Fig. 2B shows the 28-day time point). We did not observe significant accumulation of T (CD3) or B (CD45RA) cells in the vessel wall at 1, 7, or 28 days (Fig. 2, D and F, 28-day data shown). We did observe an increase in the appearance of OX62+ cells (dendritic cells) in the periadventitial region of vessels from chronically hypoxic animals on days 7 and 28 (Fig. 2H, day 28 shown).
Fig. 1.
Hypoxia induces a progressive accumulation of leukocytic cells expressing CD11b in the rat pulmonary artery (PA) adventitia. Cryosections of PAs from normoxic (A), 7-day hypoxic (B), and 28-day hypoxic (C) rats were stained with antibodies against CD11b (red) and cell nuclei [4′,6′-diamidino-2-phenylindole (DAPI), blue]. Scale bar is 100 μm; all pictures were taken at ×10 magnification. D: quantification of the number of leukocytic cells expressing CD11b (CD11b+ cell index) in the rat PA over time. One-day hypoxic rats show no difference in the number of CD11b+ cells compared with controls, whereas 7- and 28-day hypoxic show a progressive increase in the number of CD11b+ cells. In addition, leukocytic cells are found further away from the external elastic lamina in the 7- and 28-day hypoxic rats due to the increased thickness of the adventitia.
Fig. 2.
Hypoxia induces accumulation of dendritic cells but not of neutrophils and lymphocytes in the PA adventitia. Cryosections of PAs from normoxic rats (left column images: A, C, E, and G) and 28-day hypoxic rats (right column images: B, D, F, and H) were stained with Alexa 594 (red)-conjugated antibodies against neutrophils (A and B), CD3 (T cells; C and D), CD45RA (B cells; E and F), and OX62 (dendritic cells; G and H). Cell nuclei were labeled with DAPI (blue). Green autofluorescence of PA elastic lamellae provide the boundaries of the medial layer (m). The thickened PA adventitia (adv) in samples from hypoxic rats is marked with a bar. Accumulation of neutrophils was clearly observed in the lung parenchyma (L.p.) of hypoxic rats (B) but not within the adventitial layer. T and B lymphocytes did not accumulate in the adventitia (for positive internal control, see insets in D and F) demonstrating bright staining of lymph nodes with the respective antibodies. Chronic (28-day) hypoxia induced significant accumulation of dendritic cells in the periadventitial areas (H).
Hypoxia Induces a Time-Dependent, Complex Proinflammatory Microenvironment in the PA Wall
To begin to determine the factors involved in the recruitment, expansion, and retention of what appear to be principally mononuclear and dendritic cells in the vessel wall, we selectively isolated intralobar PAs by LCM from control and hypoxic animals and performed RT-PCR analysis of PA-derived mRNA. We found that chronic hypoxia induced upregulation of gene expression of a wide spectrum of proinflammatory mediators, including chemokines and their receptors, cytokines, growth and differentiation factors, and adhesion and fibrosis-associated molecules (Table 2).
Table 2.
Genes up- or downregulated in rat PAs isolated by laser capture microdissection
| Gene |
Hypoxic |
Regression
|
||||
|---|---|---|---|---|---|---|
| 1 Day (n = 7) | 7 Days (n = 6) | 28 Days (n = 10) | 2 Days (n = 4) | 56 Days (n = 4) | ||
| Chemokines, receptors, and related genes | BMP-2 | 0.86±0.48 | −0.01±0.13 | 0.75±0.10† | ||
| BMP-6 | 0.77±0.21† | −0.07±0.21 | −0.47±0.26 | |||
| C5 | 0.35±0.62 | −0.68±0.33 | 1.39±0.32† | −0.76±0.69 | −0.04±0.20 | |
| C5R1 | 0.10±0.59 | 0.46±0.14* | 1.13±0.15† | 0.18±0.21 | −0.30±0.76 | |
| CCL2/MCP-1 | −0.07±0.37 | 0.05±0.40 | 1.44±0.23† | 0.30±0.21 | −0.86±0.81 | |
| CCR2 | −0.19±0.58 | 0.48±0.73 | 1.68±0.07† | 1.05±0.43 | −0.82±0.94 | |
| CCL4/MIP-1β | 0.01±0.52 | −0.30±0.40 | 1.21±0.21† | 1.05±0.41 | 0.73±0.34 | |
| CCL5/RANTES | −0.07±0.33 | −1.01±0.36 | −0.09±0.11 | −0.15±0.08 | −0.91±0.52 | |
| CCR5 | −0.29±0.22 | 0.02±0.57 | 2.97±0.16† | 4.32±0.07† | 0.03±0.24 | |
| CXCL12/SDF-1 | 0.52±0.19* | 0.40±0.30 | 1.26±0.12† | −0.27±0.22 | −0.49±0.75 | |
| CXCR4 | 1.82±0.32† | 0.58±0.51 | 1.19±0.26† | 0.07±0.42 | −0.03±0.57 | |
| CXCL1/GROα | 0.61±0.26 | −0.28±0.61 | 0.81±0.19† | |||
| CXCR2 | 1.82±0.62† | −0.55±0.33 | 1.28±0.25† | |||
| CX3CL1/fractalkine | 0.41±0.30 | −0.76±0.41 | −0.08±0.33 | |||
| FIZZ-1 | 0.54±0.43 | 0.75±0.35 | 0.16±0.35 | −0.36±0.57 | 0.67±0.55 | |
| IL-1α | −0.62±0.65 | 0.40±0.96 | 1.04±0.49 | −0.89±0.84 | −1.22±0.24 | |
| IL-R1 | −0.29±0.30 | −0.04±0.53 | 0.34±0.42 | |||
| IL-18 | −0.49±0.42 | 0.24±0.21 | 0.92±0.06† | — | 0.02±0.86 | |
| IL-6 | −0.30±0.41 | 0.51±0.72 | 2.45±0.50† | 1.98±0.68 | 0.18±0.33 | |
| IL-6R | −0.36±0.29 | 0.33±0.60 | 1.34±0.55 | 0.37±0.48 | −0.20±0.16 | |
| TLR-4 | −0.84±0.48 | −1.11±0.34* | −0.18±0.13 | −0.37±0.29 | ||
| TRAIL | 0.42 | −1.62* | 0.23 | −0.69 | — | |
| Adhesion molecules | ICAM-1 | 0.70±0.19* | −0.89±0.24* | 0.41±0.32 | −0.75±0.07† | 0.79±0.55 |
| Integrin-β1 | 0.20±0.16 | 0.04±0.09 | −0.14±0.12 | |||
| Osteopontin | 0.71±0.33 | 1.10±0.09† | 1.39±0.23† | 0.55±0.58 | 0.96±0.61 | |
| VCAM-1 | 0.51±0.13† | 0.85±0.10† | 0.69±0.21† | 0.45±0.24 | 1.33±0.32* | |
| Cell differentiation | 5-LO | 1.12±0.42* | −0.59±0.73 | 1.24±0.18† | 1.06±0.38 | 0.09±1.06 |
| Endothelin-1 | 0.77±0.29* | 0.31±0.14 | 0.53±0.14† | −0.86±0.17* | 0.12±1.01 | |
| ET-A receptor | 0.52±0.25 | 0.23±0.04* | 0.14±0.16 | −0.83±0.43 | −1.89±0.66 | |
| ET-B receptor | −0.14±0.41 | 0.15±0.14 | −0.26±0.14 | −0.35±0.24 | −2.00±1.16 | |
| FLAP | 0.16±0.67 | 0.52±0.13* | 0.98±0.13† | −1.19±0.42 | −0.95±0.78 | |
| TGF-β1 | 1.07±0.16† | 0.29±0.30 | 1.04±0.22† | −1.52±0.75 | 0.27±1.19 | |
| Growth factors and receptors | PDGF-B | 0.92±0.50 | −0.63±0.46 | −0.19±0.36 | ||
| PDGF-RB | 0.15±0.17 | −0.34±0.19 | −0.39±0.14* | |||
| PDGF-A | 1.35±0.15† | 0.07±0.27 | 0.11±0.38 | |||
| PDGF-RA | 0.74±0.25* | 0.22±0.18 | 0.87±0.10† | |||
| VEGF-A | 0.55±0.17* | −0.34±0.35 | 0.10±0.10 | −0.19±0.37 | −3.77±0.29† | |
| FLT-1 | 0.90±0.15† | 0.18±0.20 | 0.36±0.08† | |||
| FLK-1 | 0.78±0.62 | −0.14±0.80 | 0.68±0.20† | |||
| Fibrosis-associated proteins | HSP-47 | 0.88±0.23† | −0.84±0.71 | −0.52±0.33 | ||
| Fibronectin | −0.02±0.09 | −0.05±0.27 | 0.20±0.18 | |||
| MMP-2 | 0.42±0.22 | −0.36±0.20 | 0.08±0.16 | −0.37±0.14 | −2.15±1.25 | |
| Periostin | −0.09±0.18 | −0.01±0.09 | −0.01±0.09 | |||
| TIMP-1 | 0.10±0.30 | 0.13±0.22 | 0.30±0.11* | −2.02±0.43* | 0.54±0.28* | |
Values are means ± SE. Genes were normalized to hypoxanthine-xanthine phosphoribosyl transferase (HPRT), and the log inverse mean fold change ± SE was calculated relative to normoxic animals.
P value between 0.01 and 0.05,
P < 0.01. —, Missing values due to mRNA levels too low to calculate fold change accurately or undetectable via RT-PCR; PAs, pulmonary arteries.
Chemokines/chemokine receptors.
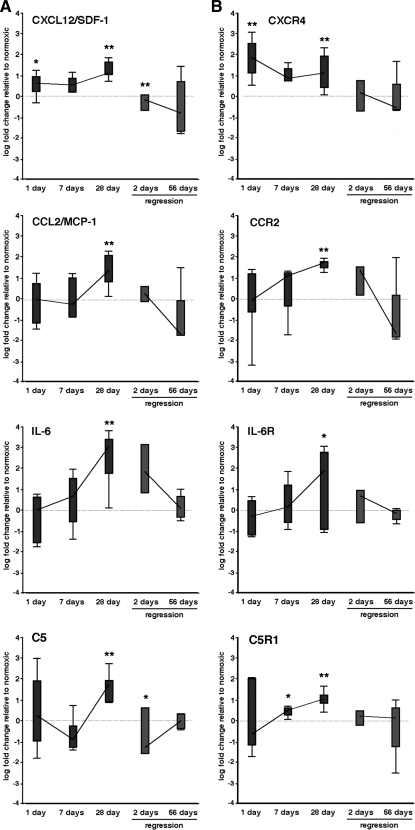
Sustained hypoxia induced the expression of numerous proinflammatory chemokines [including CXCL12/SDF-1, CCL2/monocyte chemoattractant protein-1 (MCP-1), IL-6, and C5] and their respective receptors (CXCR4, CCR2, IL-6R, and C5R1) (Table 2; Fig. 3). Certain variability in gene expression was observed between individual animals, consistent with nearly all other reports using LCM/RT-PCR (14, 19, 29, 35, 60, 61). Importantly, gene expression for some chemokines [CXCL12/SDF-1, CXCL1/growth-related oncogene protein-α (GRO-α), and C5] and their receptors (CXCR4, CXCR2, and C5R1) were upregulated early (1 day) and remained elevated at later time points, whereas other genes [IL-6, CCL2/MCP-1, CCL4/macrophage inflammatory protein-1β (MIP-1β), and IL-1β] and their receptors (IL-6R, CCR2, and CCR5) were only upregulated at later time points (Table 2). Upregulation of chemokines/cytokines at 1 day preceded any noticeable increases in perivascular leukocyte/monocyte accumulation (Fig. 1). We confirmed hypoxia-induced increases of CXCL12/SDF-1 expression by immunostaining (Fig. 5A).
Fig. 3.
Hypoxia induces increases in mRNA levels of cytokines and receptors that may control the recruitment of cells in rat PAs. A and B: distribution of the log-transformed relative expression levels of cytokines (A) and their receptors (B) in PAs isolated by laser capture microdissection (LCM) from hypoxic animals (dark gray) and regression animals (light gray). Bars represent the 25th and 75th percentiles, and whiskers designate 1.5 interquartile ranges. The lines between measurements connect the median values. Stars above the dark gray bars indicate significant deviation of the mean values from the reference line at 0. Stars above the 2- and 28-day regression time points (light gray bars) relate to significant differences between the means for those time points and the 28-day hypoxic values. *P = 0.01–0.05, **P < 0.01. SDF-1, stromal cell-derived factor-1; MCP-1, monocyte chemoattractant protein-1.
Adhesion molecules.
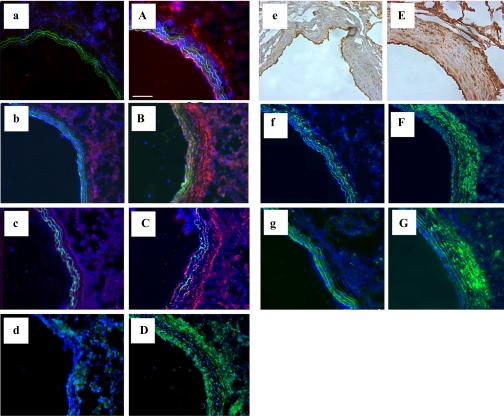
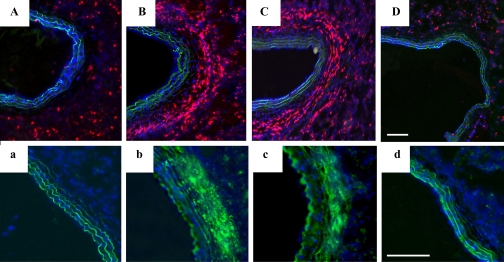
Increases in the gene expression of several adhesion molecules (ICAM-1, OPN, and VCAM-1) within the PA wall were noted at different time points of hypoxic exposure (Table 2; Fig. 4A). ICAM-1 demonstrated an early (1 day) increase that was also observed at 28 days of hypoxia (Table 2). OPN, an inflammation-associated secreted adhesive molecule (46), was upregulated early and remained persistently elevated throughout the hypoxic exposure (Table 2; Fig. 4). Immunostaining demonstrated increased OPN protein accumulation throughout the vessel wall, especially in the perivascular/adventitial regions (Fig. 5). VCAM-1 mRNA expression was upregulated early and remained elevated at 28 days. VCAM-1 upregulation in the hypoxic PA wall was also confirmed by immunostaining (Fig. 5).
Fig. 4.
Hypoxia induces increases in mRNA levels of genes that may control the adhesion, differentiation, and growth of cells in rat PAs. A–C: distribution of the log-transformed relative expression levels of adhesion molecules (A), cell differentiation molecules (B), and growth factors (C) in PAs isolated by LCM from hypoxic animals (dark gray) and regression animals (light gray). Bars represent the 25th and 75th percentiles, and whiskers designate 1.5 interquartile ranges. The lines between measurements connect the median values. Stars above the dark gray bars indicate significant deviation of the mean values from the reference line at 0. Stars above the 28- and 2-day regression time points (light gray bars) relate to the significant difference between the means for those 2 time points and the 28-day hypoxic values. *P = 0.01–0.05, **P < 0.01. TGF-β1, transforming growth factor-β1.
Fig. 5.
Chronic hypoxia induces a proinflammatory and profibrotic microenvironment in rat PAs. Lung sections from normoxic (lowercase letters) and 28-day hypoxic (uppercase letters) rats were stained with antibodies against CXCL12/SDF-1 (a/A; red), osteopontin (b/B; red), VCAM-1 (c/C; red), TGF-β1 (d/D; green), VEGF (e/E; brown), heat shock protein HSP-47 (f/F; green), cellular (ED-A isoform) fibronectin (ED-A-FN; g/G; green) and cell nuclei (DAPI, blue). Scale bar is 100 μm; all pictures were taken at ×10 magnification.
Growth and differentiation factors.
Early (1 day) and persistent increases in mRNA expression of TGF-β1, 5-lipoxygenase (5-LO), 5-LO activating protein (FLAP), endothelin-1 (ET-1), VEGF-A, and PDGF-A and the receptors fms-like tyrosine kinase-1 (Flt-1), fetal liver kinase-1 (Flk-1), and PDGF-RA were observed in hypoxic PA wall (Table 2; Fig. 4). Of these, TGF-β1 and 5-LO demonstrated the most significant increases with time. Upregulation of both TGF-β1 and VEGF-A was confirmed at the protein level by immunostaining (Fig. 5).
Fibrosis-associated molecules.
A number of genes for molecules previously implicated in pulmonary vascular fibrosis [fibronectin, type I procollagen-associated HSP-47, and tissue inhibitor of metalloproteinase (TIMP)-1] demonstrated increased expression at varying time points of hypoxic exposure (Table 2). Immunostaining demonstrated striking hypoxia-induced increases in cellular (ED-A isoform) fibronectin and HSP-47 expression at 28 days (Fig. 4, F and G). However, at the mRNA level, these increases were not statistically significant, underscoring the fact that mRNA expression and protein accumulation are not always directly comparable.
Hypoxia-Induced Inflammation is PA-Specific
To determine if hypoxia-induced upregulation of inflammatory mediators was specific to the PA, we performed RT-PCR analysis on isolated PA tissues, on the adjoining lung parenchyma, and on systemic arteries (aorta and renal artery). Although hypoxia markedly increased expression of chemokines, adhesion molecules, cytokines, and fibrosis-associated molecules in the PA tissue (see Table 2 and Figs. 3 and 4), expression of the same genes in systemic arteries were downregulated, undetectable, or only minimally increased (Table 3). In lung parenchyma, we did observe that some genes were upregulated (CCL2/MCP-1, OPN, CXCR4, IL-6R, TGF-β, HSP-47, and ET-1). Of these, only CCL2 and OPN were found to be upregulated on the Affymetrix arrays (see supplement). Furthermore, only CCL2/MCP-1 and OPN were increased to an extent that was comparable to PA levels (Table 3).
Table 3.
Gene expression levels of cytokines in rat PAs, lung parenchyma, and aortic arch
| Gene |
PA |
Parenchyma
|
Aortic Arch
|
||||
|---|---|---|---|---|---|---|---|
| 1 Day (n = 7) | 28 Days (n = 10) | 1 Day (n = 3) | 28 Days (n = 4) | 1 Day (n = 1) | 28 Days (n = 1) | ||
| Chemokines and receptors | C5 | 0.35±0.62 | 1.39±0.32 | −0.57±0.41 | −0.10±0.08 | −0.26 | — |
| CCL2/MCP-1 | −0.07±0.37 | 1.44±0.23 | 0.11±1.66 | 1.19±0.29 | −0.17 | 0.38 | |
| CXCL12/SDF-1 | 0.52±0.19 | 1.26±0.12 | 0.53±0.47 | −0.29±0.32 | 0.04 | 0.26 | |
| CXCR4 | 1.82±0.32 | 1.19±0.26 | 0.45±0.18 | −0.18±0.12 | 0.02 | −0.09 | |
| FIZZ-1 | 0.54±0.43 | 0.16±0.35 | −0.72±0.24 | −0.06±0.20 | −0.16 | 0.02 | |
| IL-6 | −0.30±0.41 | 2.45±0.50 | −1.35±0.54 | −0.81 | −0.33 | 0.32 | |
| IL-6R | −0.36±0.29 | 1.34±0.55 | −1.18±0.74 | 0.13±0.22 | −0.20 | 0.56 | |
| Adhesion molecule | Osteopontin | 0.71±0.33 | 1.39±0.23 | 0.61±1.23 | 1.71±0.07 | — | — |
| Cell differentiation | Endothelin-1 | 0.77±0.29 | 0.53±0.14 | 0.01±0.27 | 0.10±0.15 | 0.03 | 0.29 |
| TGF-β1 | 1.07±0.16 | 1.04±0.22 | 0.17±0.14 | −0.01±0.16 | −0.10 | −0.03 | |
| Fibrosis-associated protein | HSP-47 | 0.88±0.23 | −0.52±0.33 | 0.75±0.18 | −0.18±0.15 | −0.07 | −0.40 |
Values are log inverse mean fold changes ± SE for PA (n = 10) and parenchyma (n = 4). Results are pooled from n = 4 animals for aortic arch samples. Renal artery samples showed similar gene expression as the aortic arch (data not shown). —, Missing values due to mRNA levels too low to calculate fold change accurately or undetectable via RT-PCR.
Withdrawal of Hypoxic Stimulus Results in Resolution of PA Inflammation and Regression of Vascular Remodeling
On return of chronically (28-day) hypoxic rats to normoxic (room air) conditions for 2 days, immunofluorescent staining showed that the perivascular accumulation of CD11b+ leukocytes was not detectably different from hypoxic rats. However, following 56 days of normoxia, there was no difference in the number of CD11b+ cells compared with nonhypoxia-exposed controls (Fig. 6). Perivascular fibrosis, defined by one of its reliable markers, cellular (ED-A isoform) fibronectin (ED-A-FN), also regressed along the same time course without detectable change at 2 days and near resolution at 56 days (Fig. 6). As expected, PA pressure had returned to normal by 56 days of regression (data not shown).
Fig. 6.
Removal of hypoxic stimulus reverses PA inflammation and remodeling. Cryosections of PAs from normoxic (a/A), 28-day hypoxic (b/B), 48-h regression (c/C), and 8-wk regression (d/D) rats stained with CD11b (uppercase letters, red) and ED-A-FN (lowercase letters, green) and cell nuclei (DAPI, blue) are shown. Scale bar is 100 μm; all pictures were taken at ×10 magnification.
Intralobar PAs were isolated via LCM at 2 and 56 days of the regression process and analyzed by RT-PCR. After 2 days of regression, gene expression of several chemokines/chemokine receptors (CXCL12/SDF-1, C5, and CCR5) and other proinflammatory molecules (ICAM-1, TGF-β1, FLAP, ET-1, ET-RA, VEGF, and TIMP-1) were significantly decreased compared with 28-day hypoxic animals (Table 2; Figs. 3 and 4). Expression of other genes (including IL-6, IL-6R, IL-1α, CCL4/MIP-1β, and C5R) began to decline by 2 days of normoxic reexposure, however, only at 56 days did they approximate age-matched normoxic control levels (Table 2; Figs. 3 and 4). Exceptions from this list included the adhesion and fibrosis molecules (OPN, VCAM-1, ICAM-1, and TIMP-1) for which mRNA levels initially decreased but then reverted to near hypoxic levels at the 56 days time point (Table 2).
DISCUSSION
Previous studies have demonstrated that both chronic hypoxic exposure and monocrotaline treatment lead to the appearance and persistence of inflammatory/progenitor cells in and around both large and small PAs (13, 44). However, little is known of the signals that lead to the recruitment and/or retention of these cells within the diseased PA. The present study demonstrated that sustained hypoxia induces upregulation of a wide range of proinflammatory chemokines, cytokines, growth, differentiation, adhesion, and fibrosis-associated molecules within the PA wall, demonstrating the development and persistence of a hypoxia-induced proinflammatory microenvironment in the PA. The study demonstrates conclusively that the inflammatory response is largely specific for the PA and that it is predominately a response that comprises monocytic derived cells (including macrophages and dendritic cells). We found little evidence for a sustained generalized inflammatory response in lung parenchymal tissue and no evidence for an inflammatory response in the aorta or renal arteries at the time points studied. Furthermore, it showed that upregulation of many chemokines, receptors, adhesion molecules, and signaling pathways (including CXCL12/SDF-1, CXCR4, VCAM-1, ICAM-1, TGF-β, and 5-LO) preceded the influx of inflammatory cells and induction of perivascular fibrosis. With time, the PA inflammatory microenvironment became more complex with the sustained expression of early expressed chemokines, cytokines, and adhesion molecules and the later appearance of others for which expression was consistent with the increasing and persistent presence of monocytes/macrophages, fibrocytes, and dendritic cells in and around the vessel wall. On removal of the hypoxic stimulus, expression of certain chemokines, chemokine receptors, and adhesion molecules rapidly returned to control levels, findings that preceded the disappearance of inflammatory cells and that were then followed by a reversal of vascular remodeling and PH. We conclude that the development of a complex proinflammatory microenvironment, stimulated by chronic hypoxic exposure, in the PA wall itself is essential for recruitment and retention of circulating inflammatory cells and, ultimately, the development of pulmonary vascular fibrosis and vascular remodeling.
Since it is becoming increasingly clear that whole lung tissue may not accurately reflect specific changes in gene and protein expression in the vessel wall (2, 11, 12, 14, 33, 35, 42, 53, 63), we used LCM to more accurately evaluate PA-specific changes in the mRNA expression of a wide variety of inflammation-related changes during the development of hypoxic PH. Our data demonstrate that hypoxia-induced upregulation of cytokine/chemokine gene expression was largely specific to the PA. For instance, we found that although hypoxia induced upregulation of many genes in the whole lung, this analysis only correctly predicted the vascular upregulation of OPN, CXCL12, CCL2, and IL-6. Important molecules not found to be upregulated in the whole lung array but specifically upregulated at the RNA and/or protein levels were VCAM-1, ICAM, VEGF, ET, CXCR4, CCR2, TGF-β, 5-LO, and FLAP. The findings are thus consistent with and extend the concept proposed by others that whole organ tissue does not provide an accurate assessment of vascular-specific changes (2, 11, 14, 33, 35, 42, 53, 63). Furthermore, whereas other studies in humans and animal models of PH have used LCM to demonstrate PA-specific changes in expression of a single specific cytokine or growth factor of interest (26, 33, 35), the present study analyzed a wide panel of genes, providing a comprehensive analysis of the many changes in inflammatory gene expression that occur during development of hypoxic PH and that based on data in other chronic inflammatory diseases are all involved in the persistence of inflammation and subsequent vascular fibrosis.
Our (13) previous work demonstrated that chronic hypoxia led to a robust and persistent accumulation of circulating leukocytes around the PA. The present study documents that the inflammatory cells accumulating within and around the vessel wall are largely of monocyte origin. The current data thus shed light on the potential cell and molecular mechanisms used for recruitment and retention of these subsets of inflammatory cells in the PA wall. A predominant mononuclear infiltrate has also been reported in the commonly used monocrotaline model of PH (34, 44). Furthermore, in the developing circulation, it has been reported that in the setting of congenital heart disease-associated PH, the presence of high numbers of macrophages/monocytes relative to lymphocytes in the vessel wall correlates with the severity of PH (38). Similarly, it has been shown that PH is more severe in athymic rats lacking lymphocytes (51). In addition, we (13) previously reported that comprised within the monocytic cell population are mesenchymal precursors including fibrocytes. Furthermore, it is known that newly appearing dendritic cells are derived from a monocytic cell subset(s). It is thus clear that the chemokine and cytokine profiles we describe in the vessel wall support recruitment, retention, and likely proliferation and differentiation of monocytes, dendritic cells, and fibrocytes.
We observed that the induction of specific inflammatory genes in the vessel wall is time dependent. Upregulation of factors such as VEGF, CXCL12/SDF-1, OPN, and 5-LO preceded any noticeable accumulation of monocytes in the PA adventitia, whereas other genes, including IL-6 and IL-6R, did not increase apparently until after the appearance of increased numbers of monocytes and dendritic cells in the vessel. In general, we found that as hypoxic PH progressed, as evident by an increasing number of monocytic and dendritic cells and increasing expression of extracellular matrix molecules, both the complexity and magnitude of cytokine gene expression increased. Our findings regarding the early expression of VEGF, as well as of the chemokines CCL2/MCP-1 and CXCL12/SDF-1 and their cognate receptors, CCR2 and CXCR4, are consistent with the expression pattern observed in systemic vessels following angioplasty in the porcine coronary arteries, where a rapid perivascular accumulation of leukocytes is also observed (25). Our findings regarding CXCL12/SDF-1 in this hypoxic model of PH are supported by studies where CXCL12/SDF-1 expression was observed early in ischemic sites, and its expression was directly correlated with the amplitude of hypoxia (3). We also observed early upregulation of VEGF, which has been demonstrated by others to regulate CXCL12/SDF-1 expression and to play a role in the recruitment and retention of inflammatory and progenitor cells (17). Induction of VEGF and CXCL12/SDF-1 by hypoxia may thus provide an effective means of recruitment and retention of circulating cells into the site of injury (8, 17, 36, 58). We also observed that the expression patterns of chemokine receptors CCR2 and CXCR4 paralleled the local expression patterns of their respective ligands CCL2/MCP-1 and CXCL12/SDF-1. In support of this observation are previous studies showing that maximal mRNA levels of CCR2 and CXCR4 coincided with peaks of CCL2/MCP-1 and CXCL12/SDF-1 in perivascular tissue following balloon injury in porcine arteries (25).
The persistence of inflammatory cells in pulmonary adventitia and perivascular spaces observed under sustained hypoxic exposure requires upregulation of specific molecules capable of retaining circulating cells in the local microenvironment. We documented increases in VCAM-1 and ICAM-1 mRNA and protein in the vessel wall, similar to reports in chronically inflamed tissues and in the tumor vasculature (15, 30, 39). Hypoxia and CXCL12/SDF-1 are known to increase VCAM- 1 and integrin expression, and both CXCL12/SDF-1 and VCAM-1 have been shown to be crucial in mediating incorporation of monocytes and progenitor cells into ischemic tissues (52, 55, 62). We also observed early and persistent increases in OPN expression. OPN acts both as a matricellular protein, thereby facilitating adhesion and migration, as well as a soluble cytokine (46, 57). OPN has been shown to induce adhesion, migration, and survival of several cell types including smooth muscle cells and inflammatory cells including monocytes. Importantly, it has been suggested to regulate monocyte/macrophage infiltration during the inflammatory response (7, 46, 57). Besides regulating the acute phase of the inflammatory reaction, OPN appears particularly important in promoting retention of macrophages at sites of chronic inflammation based on numerous studies in OPN −/− mice (46). Thus the present study has identified upregulation of several proteins that could be involved in the prolonged retention of inflammatory cells both in and around the PAs under chronic hypoxic conditions. Importantly, immunohistochemistry demonstrates upregulation of these proteins by cells residing in the adventitia, i.e., most likely fibroblasts. The upregulation of potent adhesive and chemokinetic molecules by adventitial cells would begin to explain the chronic adventitial/perivascular accumulation of inflammatory cells observed in all models of PH and in humans as well.
PH is characterized by increased numbers of collagen-producing fibroblasts and α-smooth muscle actin-expressing myofibroblasts in the vessel wall. In the present study, we examined the expression patterns of factors known to induce fibrocyte and fibroblast differentiation, such as TGF-β1 and ET-1 (22, 40). We found significant increases in TGF-β1 mRNA in the PA and confirmed vessel-specific localization of active TGF-β1 by immunostaining. These observations are consistent with other reports demonstrating that TGF-β1 signaling pathway plays a causative role in hypoxic PH (4, 27). We also observed marked increase in BMP-2 expression. This is potentially interesting because BMPs have been shown to regulate OPN expression and also to exert promigratory and angiogenic effects on smooth muscle cells and endothelial cells, respectively (10, 57). Increases in ET-1 and ET receptor mRNA expression were also observed. ET-1 has been demonstrated to stimulate fibrocyte-fibroblast differentiation (40). Increases in mRNA expression levels of ET-1 and its receptors, ETA and ETB, were observed in a PA-specific manner. We also observed marked upregulation of both 5-LO and FLAP mRNA in the PA wall. This is important not only because of the potent inflammatory effects promoted by the leukotrienes, but also because recent reports propose leukotrienes as potent stimulators of fibrocyte proliferation (56). In addition, we documented increases in PDGF-A and -B and PDGF-Ra and -Rb mRNA in PA tissue from the chronically hypoxic animals. The magnitude of the changes reported here is consistent with that observed in LCM-derived human pulmonary arterial tissue from patients with PH (35). The milieu of growth factors within the PA wall are capable of contributing to local cell proliferation and differentiation as well as contributing to the proliferation and differentiation of monocyte-derived progenitor cells.
IL-6 has been implicated in the pulmonary hypertensive process by a number of studies that demonstrate elevated IL-6 levels in plasma or whole lung tissue (24, 25, 35, 59). Interestingly, we did not observe significant elevation in IL-6 mRNA in PA tissue until late in the course of hypoxia-induced PH. Similarly, increases in IL-6 expression in whole lung tissue were only observed at 21 days of exposure. In the majority of studies regarding IL-6 and PH, the time course of its appearance has not been reported, and whole lung tissue has been used to evaluate its presence. Our findings would suggest its role in hypoxic-induced PH and remodeling to occur at later stages, potentially following the activation of resident cells and their subsequent interactions with recruited monocytes. The persistence of inflammatory cells in the perivascular spaces could promote continued cytokine-induced activation of resident PA cells and promote further inflammatory and angiogenic responses (17, 31). Furthermore, although increases in RANTES and fractalkine expression have been noted in other models of PH and in humans with idiopathic pulmonary arterial hypertension, we did not find upregulation of these factors in the model, which is characterized by robust monocyte/macrophage accumulation (9, 33). These results raise the possibility that the mechanisms controlling initiation and persistence of inflammatory responses in different pulmonary vasculopathies may be species- and/or stimulus-specific and/or be mediated through different cytokine or chemokine signaling pathways.
To further our search for potential factors regulating retention of leukocytes in the vessel wall, we analyzed the spontaneous regression of PH on removal of the hypoxic stimulus. We observed a rapid and often dramatic decline in the mRNA expression of certain inflammatory cytokines that preceded the decline in the number of inflammatory cells, in PA pressure, and in structural remodeling (as defined by extracellular matrix protein expression) on removal from hypoxia. Intriguingly, many of the factors that were induced in the first 24 h of hypoxic exposure, including C5, CXCL12/SDF-1, ICAM-1, TGF-β, and VEGF-A, were also the first to demonstrate significant declines after withdrawal of hypoxic stimulus. Collectively, both the onset and regression data provide potential insights into the molecular mechanisms controlling the recruitment and retention of circulating leukocytes in the PA wall.
In conclusion, we report that sustained hypoxia promotes the development of a PA-specific, proinflammatory microenvironment via time-dependent upregulation of a number of inflammatory mediators and related molecules. This microenvironment is conducive to recruitment and retention of monocytes/fibrocytes and to their differentiation into collagen-producing, α-smooth muscle actin-expressing myofibroblasts. The complexity of this environment would suggest that therapies targeting a single mediator may not be sufficient to cause regression of vascular remodeling and PH. A better understanding of the transcription factors controlling this complex inflammatory network may lead to therapies aimed at disruption of the persistent inflammatory state and thus vascular remodeling.
GRANTS
This work was supported by National Institutes of Health (NIH) Specialized Centers of Clinically Oriented Research (SCCOR) Grant HL-084923-02 and NIH Program Project Grant HL-014985-35.
Supplementary Material
Acknowledgments
We gratefully acknowledge Marcia McGowan for help in preparing the manuscript.
REFERENCES
- 1.Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C, Comhair SA, Xu W, Licina L, Huang L, Anand-Apte B, Yoder MC, Tuder RM, Erzurum SC. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol 172: 615–627, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betsuyaku T, Griffin GL, Watson MA, Senior RM. Laser capture microdissection and real-time reverse transcriptase/polymerase chain reaction of bronchiolar epithelium after bleomycin. Am J Respir Cell Mol Biol 25: 278–284, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10: 858–864, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Chen YF, Feng JA, Li P, Xing D, Zhang Y, Serra R, Ambalavanan N, Majid-Hassan E, Oparil S. Dominant negative mutation of the TGF-β receptor blocks hypoxia-induced pulmonary vascular remodeling. J Appl Physiol 100: 564–571, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol 51: 1527–1538, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Christou H, Morita T, Hsieh CM, Koike H, Arkonac B, Perrella MA, Kourembanas S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res 86: 1224–1229, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Crawford HC, Matrisian LM, Liaw L. Distinct roles of osteopontin in host defense activity and tumor survival during squamous cell carcinoma progression in vivo. Cancer Res 58: 5206–5215, 1998. [PubMed] [Google Scholar]
- 8.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood 104: 3472–3482, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J 22: 358–363, 2003. [DOI] [PubMed] [Google Scholar]
- 10.El-Tanani MK Role of osteopontin in cellular signaling and metastatic phenotype. Front Biosci 13: 4276–4284, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Fink L, Kohlhoff S, Stein MM, Hanze J, Weissmann N, Rose F, Akkayagil E, Manz D, Grimminger F, Seeger W, Bohle RM. cDNA array hybridization after laser-assisted microdissection from nonneoplastic tissue. Am J Pathol 160: 81–90, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink L, Seeger W, Ermert L, Hanze J, Stahl U, Grimminger F, Kummer W, Bohle RM. Real-time quantitative RT-PCR after laser-assisted cell picking. Nat Med 4: 1329–1333, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659–669, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuke S, Betsuyaku T, Nasuhara Y, Morikawa T, Katoh H, Nishimura M. Chemokines in bronchiolar epithelium in the development of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 31: 405–412, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol 17: 368–377, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Gay MA, Gonzalez-Juanatey C, Martin J. Rheumatoid arthritis: a disease associated with accelerated atherogenesis. Semin Arthritis Rheum 35: 8–17, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124: 175–189, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Hall SM, Hislop AA, Wu Z, Haworth SG. Remodelling of the pulmonary arteries during recovery from pulmonary hypertension induced by neonatal hypoxia. J Pathol 203: 575–583, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Herbert C, Hettiaratchi A, Webb DC, Thomas PS, Foster PS, Kumar RK. Suppression of cytokine expression by roflumilast and dexamethasone in a model of chronic asthma. Clin Exp Allergy 38: 847–856, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Herget J, Suggett AJ, Leach E, Barer GR. Resolution of pulmonary hypertension and other features induced by chronic hypoxia in rats during complete and intermittent normoxia. Thorax 33: 468–473, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hislop A, Reid L. Changes in the pulmonary arteries of the rat during recovery from hypoxia-induced pulmonary hypertension. Br J Exp Pathol 58: 653–662, 1977. [PMC free article] [PubMed] [Google Scholar]
- 22.Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem 282: 22910–22920, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Humbert M Update in pulmonary arterial hypertension 2007. Am J Respir Crit Care Med 177: 574–579, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Ito T, Okada T, Miyashita H, Nomoto T, Nonaka-Sarukawa M, Uchibori R, Maeda Y, Urabe M, Mizukami H, Kume A, Takahashi M, Ikeda U, Shimada K, Ozawa K. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res 101: 734–741, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Jabs A, Okamoto E, Vinten-Johansen J, Bauriedel G, Wilcox JN. Sequential patterns of chemokine- and chemokine receptor-synthesis following vessel wall injury in porcine coronary arteries. Atherosclerosis 192: 75–84, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Kwapiszewska G, Wygrecka M, Marsh LM, Schmitt S, Trosser R, Wilhelm J, Helmus K, Eul B, Zakrzewicz A, Ghofrani HA, Schermuly RT, Bohle RM, Grimminger F, Seeger W, Eickelberg O, Fink L, Weissmann N. Fhl-1, a new key protein in pulmonary hypertension. Circulation 118: 1183–1194, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Li P, Oparil S, Novak L, Cao X, Shi W, Lucas J, Chen YF. ANP signaling inhibits TGF-β-induced Smad2 and Smad3 nuclear translocation and extracellular matrix expression in rat pulmonary arterial smooth muscle cells. J Appl Physiol 102: 390–398, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Libby P Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am J Med 121: S21–S31, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Luzzi V, Mahadevappa M, Raja R, Warrington JA, Watson MA. Accurate and reproducible gene expression profiles from laser capture microdissection, transcript amplification, and high density oligonucleotide microarray analysis. J Mol Diagn 5: 9–14, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto H, Hoshi D, Kiire A, Yamanaka H, Kamatani N. Molecular targets of rheumatoid arthritis. Inflamm Allergy Drug Targets 7: 53–66, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121: 335–348, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Peinado VI, Ramirez J, Roca J, Rodriguez-Roisin R, Barbera JA. Identification of vascular progenitor cells in pulmonary arteries of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 34: 257–263, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Perros F, Dorfmuller P, Souza R, Durand-Gasselin I, Godot V, Capel F, Adnot S, Eddahibi S, Mazmanian M, Fadel E, Herve P, Simonneau G, Emilie D, Humbert M. Fractalkine-induced smooth muscle cell proliferation in pulmonary hypertension. Eur Respir J 29: 937–943, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Perros F, Dorfmuller P, Souza R, Durand-Gasselin I, Mussot S, Mazmanian M, Herve P, Emilie D, Simonneau G, Humbert M. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J 29: 462–468, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Perros F, Montani D, Dorfmuller P, Durand-Gasselin I, Tcherakian C, Le Pavec J, Mazmanian M, Fadel E, Mussot S, Mercier O, Herve P, Emilie D, Eddahibi S, Simonneau G, Souza R, Humbert M. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 178: 81–88, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol 28: 299–307, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 43: 25S–32S, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Pinto RF, Higuchi Mde L, Aiello VD. Decreased numbers of T-lymphocytes and predominance of recently recruited macrophages in the walls of peripheral pulmonary arteries from 26 patients with pulmonary hypertension secondary to congenital cardiac shunts. Cardiovasc Pathol 13: 268–275, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Preiss DJ, Sattar N. Vascular cell adhesion molecule-1: a viable therapeutic target for atherosclerosis? Int J Clin Pract 61: 697–701, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep 8: 145–150, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Rabinovitch M Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 118: 2372–2379, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy S, Patel D, Khanna S, Gordillo GM, Biswas S, Friedman A, Sen CK. Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc Natl Acad Sci USA 104: 14472–14477, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Sahara M, Sata M, Morita T, Nakamura K, Hirata Y, Nagai R. Diverse contribution of bone marrow-derived cells to vascular remodeling associated with pulmonary arterial hypertension and arterial neointimal formation. Circulation 115: 509–517, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Sata M Role of circulating vascular progenitors in angiogenesis, vascular healing, and pulmonary hypertension: lessons from animal models. Arterioscler Thromb 26: 1008–1014, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb 27: 2302–2309, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Schober A Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb 28: 1950–1959, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Soehnlein O, Weber C. Myeloid cells in atherosclerosis: initiators and decision shapers. Semin Immunopathol. In press. [DOI] [PubMed]
- 49.Stenmark KR, Davie NJ, Reeves JT, Frid MG. Hypoxia, leukocytes, and the pulmonary circulation. J Appl Physiol 98: 715–721, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, Cool C, Wood K, Parr JE, Boackle SA, Voelkel NF. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med 175: 1280–1289, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tong Q, Zheng L, Lin L, Li B, Wang D, Li D. Hypoxia-induced mitogenic factor promotes vascular adhesion molecule-1 expression via the PI-3K/Akt-NF-kappaB signaling pathway. Am J Respir Cell Mol Biol 35: 444–456, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trogan E, Choudhury RP, Dansky HM, Rong JX, Breslow JL, Fisher EA. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice. Proc Natl Acad Sci USA 99: 2234–2239, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 28: 23–42, vii, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ulyanova T, Scott LM, Priestley GV, Jiang Y, Nakamoto B, Koni PA, Papayannopoulou T. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood 106: 86–94, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vannella KM, McMillan TR, Charbeneau RP, Wilke CA, Thomas PE, Toews GB, Peters-Golden M, Moore BB. Cysteinyl leukotrienes are autocrine and paracrine regulators of fibrocyte function. J Immunol 179: 7883–7890, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev 19: 333–345, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Haider H, Ahmad N, Zhang D, Ashraf M. Evidence for ischemia induced host-derived bone marrow cell mobilization into cardiac allografts. J Mol Cell Cardiol 41: 478–487, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 83: 835–870, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Wright JL, Tai H, Churg A. Cigarette smoke induces persisting increases of vasoactive mediators in pulmonary arteries. Am J Respir Cell Mol Biol 31: 501–509, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Wright JL, Tai H, Churg A. Vasoactive mediators and pulmonary hypertension after cigarette smoke exposure in the guinea pig. J Appl Physiol 100: 672–678, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Yamashita T, Ohneda O, Sakiyama A, Iwata F, Ohneda K, Fujii-Kuriyama Y. The microenvironment for erythropoiesis is regulated by HIF-2alpha through VCAM-1 in endothelial cells. Blood 112: 1482–1492, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Zirlinger M Selection and validation of microarray candidate genes from subregions and subnuclei of the brain. Methods 31: 290–300, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.