Abstract
Mapping protein expression of endothelial cells (EC) in vivo is fundamental to understanding cellular function and may yield new tissue-selective targets. We have developed a monoclonal antibody, MAb J120, to a protein expressed primarily in rat lung and heart endothelium. The antigen was identified as CD34, a marker of hematopoietic stem cells and global marker of endothelial cells in human and mouse tissues. PCR-based cloning identified two CD34 variant proteins, full length and truncated, both of which are expressed on luminal endothelial cell plasma membranes (P) isolated from lung. Truncated CD34 predominated in heart P, and neither variant was detected in P from kidney or liver. CD34 in lung was readily accessible to 125I-J120 inoculated intravenously, and immunohistochemistry showed strong CD34 expression in lung EC. Few microvessels stained in heart and kidney, and no CD34 was detected in vessels of other organs or in lymphatics. We present herein the first complete sequence of a rat CD34 variant and show for the first time that the encoded truncated variant is endogenously expressed on EC in vivo. We also demonstrate that CD34 expression in rat EC, unlike mouse and human, is restricted in its distribution enabling quite specific lung targeting in vivo.
Keywords: immunohistochemistry, immunofluorescence, PCR, SPECT imaging, biodistribution
the cohort of proteins expressed in each particular cell type helps define the cell's function. The molecular fingerprint exhibited by different cells and tissues can provide insights into protein function within a given phenotypic setting. In vitro protein expression, even in cells freshly isolated from tissues and/or grown in culture, can vary considerably from in vivo expression under native conditions found in tissues. Creation of a well-defined molecular atlas is crucial to efforts to identify cell type-specific markers, including disease biomarkers, to use as diagnostic tools and as specific targets for treatment.
The proteomic signature of blood vessels, and especially endothelial cells (EC), has gained a great deal of attention in the search for specific vascular targets as a means of delivering drugs, nanomedicines, and imaging agents to blood vessels (11, 25, 42). Specific delivery of these agents, however, relies on identification of unique targets whose expression is restricted to the desired tissue. While the majority of studies have focused on tumor vessels (for a review see Ref. 6), there is also a need to identify and characterize molecules expressed on normal endothelia, to find reliable endothelial cell markers and to develop good, specific antibodies with which to detect these proteins. While most studies rely on antibodies to proteins such as CD31 and CD34 to detect endothelial cells in vivo and in vitro, not all have been fully characterized in rodent tissues.
Accurately defining the endogenous expression of a given protein is critical to understanding the function of a given protein in vivo. We have used a silica nanoparticle-coating method to isolate the luminal plasma membranes from the rat microvascular endothelium in vivo and have employed a variety of techniques, including mass spectrometry-based proteomics analysis and hybridoma and phage technologies (10, 27, 32, 49), to map the rat EC proteome and to generate a number of monoclonal antibodies (MAbs) to selected protein targets.
In the present study, we describe a new monoclonal antibody, MAb J120, produced by using isolated lung EC surface plasma membrane (P) and classic hybridoma technology. J120 recognizes a protein in the adult rat that is found in lung and heart vasculature but that is accessible to antibody binding primarily in the lung. Our study identifies the antigen as CD34, and we present herein the first full-length rat sequence of a splice variant that encodes a truncated protein. We also show for the first time that the truncated protein is endogenously expressed in the endothelium in vivo. Previous immunohistochemical analyses of adult human (14, 37) and mouse (3) tissues have reported that CD34 apparently is expressed globally on endothelial cells in many, if not all, organs. However, we show here that immunohistochemical detection of vascular CD34 in the rat is quite restricted, occurring primarily in the lung. We also show that CD34 is not expressed in caveolae and that cultivation of aortic and microvascular EC in vitro dramatically alters CD34 expression.
CD34, first identified as an antigen on hematopoietic progenitors, is the most widely used marker of hematopoietic stem cells (HSC) and is used clinically to sort CD34+ cells for engraftment. Despite its clinical importance, the function of CD34 is not clearly understood, and conflicting functions (e.g., proadhesive and antiadhesive) have been reported. In addition, most in vitro functional analyses have focused on cells of lymphoid or myeloid lineages (9, 12, 36), not differentiated EC. The present report is an important first step in defining the distribution of the two CD34 isoforms in the adult vasculature and lays the groundwork for future experiments to identify the function of this molecule in EC and to elucidate the relevance of restricted CD34 expression in the rat endothelium.
METHODS
Animals.
All animal experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Sidney Kimmel Cancer Center (SKCC) and were housed in the SKCC animal care facility. Fischer rats (150–250 g) were obtained from Charles River Laboratories, and BALB/C mice (6–8 wk) were purchased from Jackson Laboratories.
Hybridomas and MAbs.
Luminal plasma membranes (P) isolated from rat lungs using the colloidal silica method (34, 38, 43) were used to immunize BALB/C mice. Production and screening of hybridomas and purification and isotyping of MAb J120 were performed as previously described (Ref. 47 and Supplemental Methods. Supplemental data for this article is available on the AJP-Lung web site.).
Endothelial cell cultivation.
Rat aortic endothelial cells (RAEC) were obtained from VEC Technologies (Rensselaer, NY) and were grown under standard cell culture conditions, using MCDB-131 Complete Medium (VEC Technologies) supplemented with 10% FBS (HyClone, Logan, UT). Rat lung microvascular endothelial cells (RLMVEC) were isolated and maintained as previously described (26). RLMVECs were used in experiments at passage 2 or 3; RAECs were used at passage 3 or 4.
Immunostaining of tissues and cells for microscopy.
Rat tissues were prepared, sectioned, and immunostained as previously described (Ref. 47 and Supplemental Methods). Immunogold staining of tissue for electron microscopy was performed essentially as described (Ref. 33 and Supplemental Methods).
Immunoprecipitation and identification of the antigen recognized by J120.
The protein antigen recognized by J120 was determined using a gel-based liquid chromatography-tandem mass spectrometry (LC-MS/MS) approach in which the immunoprecipitated protein was first resolved by SDS-PAGE. The appropriate band was then excised and subjected to in-gel trypsin digestion, and the resulting peptides were analyzed by LC-MS/MS (Ref. 47 and Supplemental Methods).
cDNA cloning.
Rat CD34 cDNAs were cloned from rat lung RNA using the PCR as previously described (Ref. 47 and Supplemental Methods). Primers were based on the predicted sequence of rat CD34 in the NCBI database and designed to amplify the coding sequence only. Two PCR products of ∼1,100 and 1,300 bp were cloned and sequenced and have been deposited with GenBank under acc. nos. EU448292 and EU448293. The cDNA encoding the extracellular portion of the rat CD34 molecule was further subcloned as two overlapping, partial sequences (see Supplemental Methods). Nucleotides 1–429 (encoding amino acids 1–143) and nucleotides 334–864 (encoding amino acids 112–288) were amplified by PCR. Full-length and partial cDNAs were cloned into the pDEST17 vector using the Gateway System (Invitrogen, Carlsbad, CA) and recombinantly expressed for Western blot analysis (also see Supplemental Methods).
RESULTS
Generation and characterization of new antibody J120.
Luminal EC plasma membranes (P) isolated directly from adult rat lungs in vivo (34, 38, 43) were used to immunize mice for hybridoma production. Parent hybridomas that produced MAbs with a strong immunoreactivity to antigens expressed on rat lung EC were identified by ELISA with lung P. One of these parents, J120 (IgG1, κ), was selected for further study. Western blots of rat lung homogenate (H) and P using J120 showed no signal in H; however, P produced two clear bands, one at ∼62 kDa and a second broad band extending from ∼85 to 93 kDa (Fig. 1A).
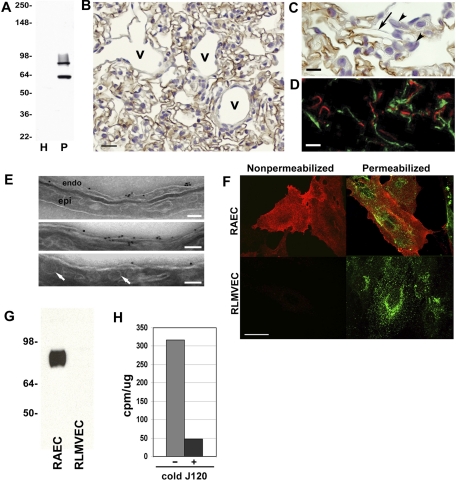
Fig. 1.
J120 immunoreactivity in normal rat lung tissue and subfractions. A: Western blot with J120 of rat normal lung homogenate (H) and luminal endothelial plasma membranes (P) isolated from lung showing 2 immunoreactive protein bands greatly enriched in P over H. Five micrograms of protein were loaded in each lane. Molecular weight markers are to the left. B and C: immunohistochemical analysis of paraffin-embedded sections from normal rat lung. B shows staining of endothelial cells in capillaries. Although staining of small veins (v) is not apparent in this image, heterogeneous CD34 expression was noted in all venules, veins, arterioles, and arteries throughout the lung. C is a higher magnification of lung showing the inconsistent staining in the capillaries; the arrow points to a capillary cut in longitudinal section showing loss of the signal along its length. Note the lack of staining of epithelial cells (arrowheads). D: dual immunofluorescence analysis of normal rat lung cryosections. Sections were immunostained with MAb J120 (red channel) and a goat polyclonal antibody to the lymphatic marker podoplanin (green channel). There is no colocalization of the 2 signals indicating that MAb J120 does not immunostain lymphatic endothelial cells. E: electron micrographs showing immunogold labeling with J120. Binding of J120 was seen on the EC cell surface (endo) but not on epithelial cells (epi) or on caveolae (arrows). Scale bars = 100 nm. F: dual immunofluorescence of rat aortic endothelial cells (RAEC) and rat lung microvascular endothelial cells (RLMVEC) immunostained with J120 (red channel) and caveolin (green channel). Immunostaining of nonpermeabilized cells showed cell surface localization of the J120 antigen on RAEC but not on RLMVEC. Permeabilization of cells to allow caveolin to be stained revealed ample punctate signal in both cell types. The J120 antigen again was present only in the RAEC and did not colocalize with caveolin. G: Western blot analysis of P isolated from cultured RAEC and RLMVEC with J120 showing the presence of the high-molecular-weight signal in RAEC only. H: immunoradiometric assay of J120 binding to cultured RAEC. Cells were incubated for 2 h with 125I-J120 alone or 125I-J120 in the presence of a 100 M excess of unlabeled J120. Results are expressed as cpm/μg of protein extracted from the cells.
Immunohistochemical staining of rat lung showed that EC were stained with J120 (Fig. 1B). Higher magnification (Fig. 1C) showed that the staining pattern was not evenly distributed; some capillaries were not stained, and single capillaries were often not uniformly stained. Heterogeneous staining of larger caliber vessels (i.e., venules, arterioles, veins, and arteries) was also observed throughout the lung, and some immunoreactivity was seen in the connective tissue elements surrounding larger vessels (not shown). J120 did not bind to nonendothelial cells such as epithelium (Fig. 1C), smooth muscle cells, lymphocytes, nerves, or the pleural mesothelium (not shown). When analyzed by dual immunofluorescence on rat lung tissue, J120 did not colocalize with the lymphatic marker podoplanin (Fig. 1D), indicating that J120 specifically labels blood vessel endothelium and not lymphatic EC.
We next looked at the subcellular localization of the J120 antigen on the EC luminal plasma membrane. Immunoelectron microscopy with gold-labeled J120 showed that the protein is present on the EC surface but not in caveolae or epithelial cells (Fig. 1E). These results are consistent with our previous observations that the J120 antigen was not detected in caveolae isolated from P but rather remained in the subfraction of P which had been depleted of caveolae (31). Thus, the antigen is not present or concentrated in caveolae.
Dual immunofluorescence with J120 and a caveolae marker, caveolin, was used to analyze cultured RAEC and RLMVEC. Immunostaining nonpermeabilized cells (to determine cell-surface binding) showed a strong J120 signal on RAEC but not on RLMVEC (Fig. 1F). When immunostaining was repeated on permeabilized cells (to allow the caveolin antibody to access caveolin), there was no colocalization of the J120 signal and the caveolin signal in RAEC, indicating that the J120 antigen was not concentrated in caveolae. In RLMVEC, caveolin was clearly seen, but again there was no J120 signal. The absence of the J120 antigen on RLMVEC was unexpected, given the positive immunohistochemical staining of microvascular EC in sections of rat lung.
To rule out the possibility that, in vitro, an associated protein might be blocking J120 binding to these cells, we isolated P from cultures of RLMVEC and RAEC for analysis by Western blotting. A strong signal was seen in P from RAEC but not RLMVEC (Fig. 1G). We also noted that in RAEC, only the high-molecular-weight signal was seen. Immunoradiometry was also used to try to detect J120 binding. In cultures of RAEC, radiolabeled J120 readily bound to the cells and could be competed with an excess of unlabeled J120 (Fig. 1H); no binding was detected on RLMVEC (not shown).
Expression in other endothelia in vivo.
We next looked at expression of the J120 antigen in other rat tissues by Western blot analysis of H and P from liver, kidney, and heart. Control isolates from rat lung gave the same results as those in Fig. 1A. In the heart samples, no signal was observed in H, and in P, only a single band was observed, which had a slightly faster electrophoretic mobility than the 62-kDa band in lung P. No signals were observed in H or P from kidney and liver (Fig. 2A).
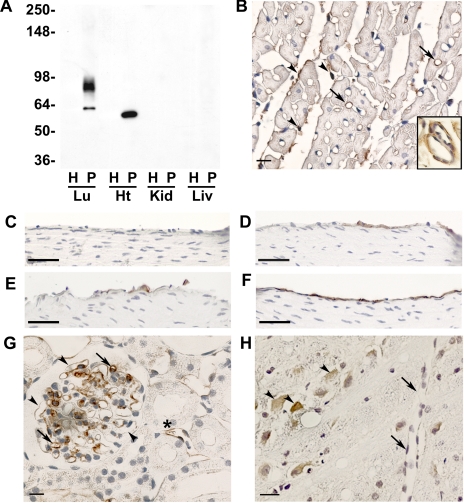
Fig. 2.
J120 immunoreactivity in other normal rat organs. A: Western blot analysis of homogenate and luminal plasma membranes isolated from normal rat lung (Lu), heart (Ht), kidney (Kid), and liver (Liv). Five micrograms of protein were loaded in each lane, and blots were probed with MAb J120. Two bands are seen in lung P, but only one band is apparent in heart and has a slightly faster electrophoretic mobility than the lower-molecular-weight band seen in lung. Molecular weight markers are on the left. B: immunostaining of sections of paraffin-embedded rat heart. Only a few capillaries (arrows) are stained as are fibroblasts and connective tissue elements in the endomysium (arrowheads). Inset shows subendothelial staining in vessels of intermediate size and a lack of staining in the EC. C–F: immunostaining of the aortic arch and the ascending, abdominal, and descending aortas, respectively. Some immunopositive EC are seen in the ascending and abdominal aortas, whereas no staining is seen the EC of the aortic arch. Only in the descending aorta are all cells stained. G: inconsistent staining of capillaries in kidney glomeruli; arrows indicate immunostained capillaries, and arrowheads indicate lack of staining. Some peritubular vessels are also stained (*). H: cerebrum showing J120 immunostaining of neural cells (arrowheads) but not ECs (arrows). Bars in B and G = 10 μm; bars in C–F = 50 μm; bar in H = 20 μm.
Rat heart, kidney, liver, and brain were also analyzed by immunohistochemistry. Heart capillaries were only occasionally stained (less than 2% overall; Fig. 2B), whereas no staining was observed on the EC of larger caliber vessels (Fig. 2B, inset). J120 also reacted with connective tissue elements and many of the fibroblasts in the endomysium between the cardiac muscle fibers (Fig. 2B and inset). J120 staining in various portions of the aorta was heterogeneous. No immunoreactivity was observed on EC in the aortic arch (Fig. 2C), whereas some EC staining was seen in the ascending and abdominal aortas (Fig. 2, D and E), and all EC appeared to be stained in the descending aorta (Fig. 2F). In kidney, capillaries in some of the glomeruli were stained (Fig. 2G), and the staining was heterogeneous. Staining of some podocytes was also evident. Outside of the glomeruli, J120 immunoreactivity in the kidney was occasionally seen on peritubular vessels. None of the vessels in brain were immunostained, but neurons in the cerebrum did express the antigen (Fig. 2H). No liver staining was seen on any endothelial or nonendothelial cell types (data not shown). Vessel staining was also absent from pancreas, spleen, esophagus, stomach, colon, bladder, skin, and skeletal muscle. Occasional staining of connective tissue elements was apparent in skin, pancreas, and skeletal muscle, in the muscular and serosal layers of the esophagus, stomach, colon, and bladder, and in scattered cells throughout the spleen (data not shown).
In vivo immunotargeting.
The accessibility of the antigen to binding by J120 via the blood stream was determined by planar gamma-scintigraphy of rats following intravenous injection of 125I-J120. Five minutes after injection of the antibody, a clear, intense lung image was seen that remained strong through 6 h but was diminished at 24 and 48 h (Fig. 3A). At 4 h, there was an appreciable accumulation in the stomach and a weak signal in the thyroid, which intensified through the 48-h time point. 125I-J120, once bound to the endothelium, likely was not internalized by caveolae [see Fig. 1 and later intravital microscopy (IVM) data] but rather remained on the luminal surface and was subjected to dehalogenation known to occur in blood (39). The appearance of 125I signals in thyroid and stomach is classically observed after creating free-circulating 125I in the blood. No accumulation of 125I-J120 was seen in other organs during the 48-h time course. The lack of signal in the space occupied by the heart between the apex of the right and left upper lung lobes is quite apparent.
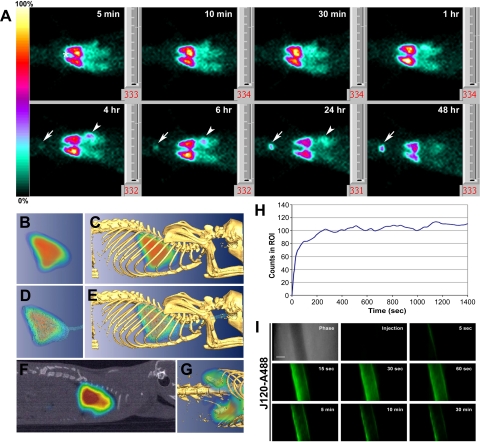
Fig. 3.
In vivo imaging of organ and vessel immunotargeting with labeled MAb J120. A: planar gamma-scintigraphic images acquired at 5, 10, and 30 min, and 1, 4, 6, 24, and 48 h after rats were injected via the tail vein with 125I-J120 (30 μCi at 6 μCi/μg). By 5 min, the antibody has clearly accumulated in the lungs; and at 4 h, new signals in the thyroid (arrow) and stomach (arrowhead) become apparent. No signals were seen in other organs, including heart (*). B–G: tomo graphic scans obtained 30 min after intravenous injection of 30 μCi of 125I-J120 MAb using CT-SPECT. C, E, G: images after fusion of CT isosurface with SPECT. B: volumetric 3-D image of the nuclear signal accumulated in the lung. C: uptake in the lung (from B) correlated with anatomic information from skeleton. D: uptake in the lung (from B) correlated with anatomic information from the thoracic cavity and trachea. E: images from B–D combined. F: sagittal section of CT-SPECT fusion confirms localization of 125I-J120 in thoracic cavity. G: 3-D CT-SPECT fusion showing amplified signal for uptake in kidneys after delineation of lung signal. H: time-course profile (dynamic acquisition) after administration of the 125I-J120 antibody (30-s time frames). Note that the maximum level of uptake is achieved within the first 4 min after injection and remains fairly constant afterwards. I: high-magnification intravital fluorescence microscopy of solitary lung microvessel. Athymic nude mice with implanted rat lung tissue were injected through the tail vein with fluorophore-labeled J120 (J120-A488). The top left image shows a phase-contrast image of a microvessel without other vessels nearby. Fluorescence images were acquired at the postinjection times indicated. Clear endothelial cell-surface binding with J120 is observed, but at no time was extravasation seen. Fading of the J120 signal is due to photobleaching of the fluorophore conjugated to the antibody during the continuous imaging of the microvessel.
To visualize antibody targeting more precisely, we used high-resolution pinhole single photon-emission-computed tomography (SPECT) imaging combined with X-ray-computed tomography (CT). SPECT-CT fusion images showed strong signals, as expected, in lung (Fig. 3, B–F). Accumulation of radiolabeled J120 in the kidney was also detected when the signal was significantly amplified (Fig. 2G).
In another experiment, the course of antibody uptake in the lung was measured in 30-s time frames over a period of 25 min following injection (Fig. 3H). Consistent with the earlier planar gamma-scintigraphy images, lung targeting was quite rapid, with J120 uptake reaching a maximum, stable level within the first 4 min after intravenous inoculation.
We next used IVM to visualize, in real time, lung EC targeting and processing of J120. Nude mice were fitted with dorsal skin fold chambers containing engrafted rat lung tissue, which maintains its vascular identity and permits dynamic imaging of possible caveolar transcytosis in vivo as reported previously (31). Fluorophore-conjugated J120 was intravenously injected, and a single microvessel was imaged at high magnification for a maximum of 30 min (Fig. 3I). Within 5 s, antibody binding to the vascular wall could be seen. J120 remained bound to the endothelium and was not transported across the vessel wall into the underlying tissue. This lack of J120 transport across the microvascular endothelium in lung contrasts sharply with our recent results in the same system showing specific, rapid, and active transcytosis of an antibody to aminopeptidase P, which is concentrated in caveolae (31). Thus our results here are consistent with our observations that the J120 antigen does not concentrate in endothelial caveolae, the flask-like membrane invaginations that mediate transendothelial transport (for review see Ref. 5).
Biodistribution analysis was used to quantify organ uptake in rats that received intravenous injections of 125I-J120 or 125I-IgG1. Slightly more than 29% of the injected dose per gram of tissue (ID/g) was measured in lung 24 h after intravenous injection (Fig. 4A). The only other organs in which the J120 signal was higher than control IgG were heart (1.7% vs. 0.4%), kidney (1.2% vs. 0.3%), and colon (0.8% vs. 0.2%). Although EC staining was not observed in the sections of colon that we examined, the small accumulation of J120 in the colon would suggest the presence of at least some immunopositive vessels.
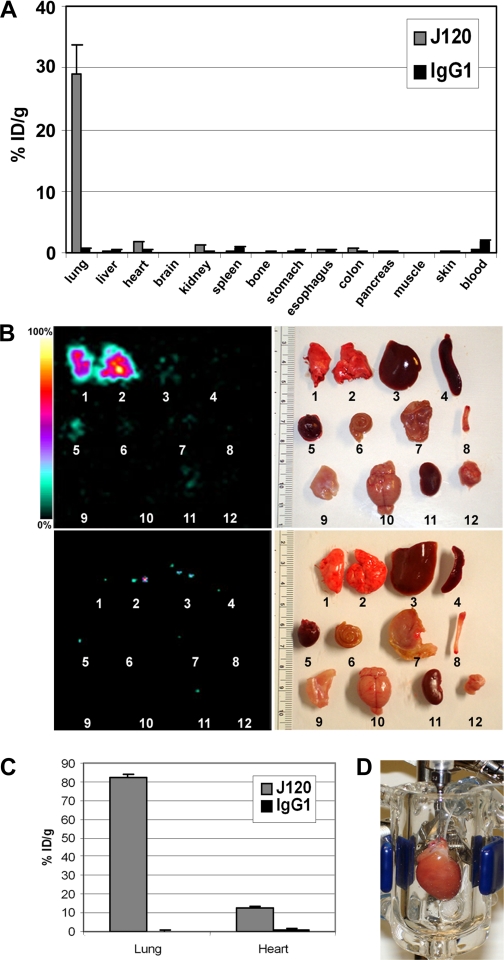
Fig. 4.
Imaging and quantifying radiolabeled J120 uptake in individual organs. Rats were injected via the tail vein with 125I-J120 (30 μg at 6 μCi/μg). Organs and blood samples were harvested 24 h later and analyzed by static gamma-scintigraphic imaging and by directly measuring radioactivity in extracts prepared from each excised organ. Tissue uptake in A and C is expressed as percent injected dose per gram of tissue (%ID/g). A: biodistribution of radiolabeled J120 (gray bars) or isotype-matched control 125I-IgG1 (black bars) measured from the organs indicated at 24-h postinjection. B: static gamma-scintigraphic images (left) and light images (right) of the organs used in the biodistribution study. Organs at top are from an animal injected with 125I-J120; organs at bottom are from an animal injected with isotype-matched control 125I-IgG1. C: quantification of 125I-J120 (gray bars) or isotype-matched control 125I-IgG1 (black bars) uptake in perfused, isolated rat lungs and hearts. D: isolated rat heart undergoing perfusion. Isolated lungs were perfused in a similar manner (not shown).
Gamma-scintigraphy of the excised organs also showed that the majority of 125I-J120 was found in lung tissue, whereas small amounts could be seen in heart, kidney, and liver (Fig. 4B). All targeting indices demonstrated a significant and specific uptake of 125I-J120 in the lungs, with a mean tissue targeting index of 72.8, a tissue selectivity index of 230.4, and 48.5-fold more J120 in the lung compared with the isotype-matched control antibody (Table 1).
Table 1.
Biodistribution and organ targeting indices of 125I-J120
|
%ID/g |
SOA | TTI | TSI | ||
|---|---|---|---|---|---|
| J120 | mIgG1 | ||||
| Lung | 29.1 | 0.6 | 48.5 | 72.8 | 230.4 |
| Liver | 0.2 | 0.4 | 0.5 | 0.5 | 2.4 |
| Heart | 1.7 | 0.4 | 4.3 | 4.3 | 20.2 |
| Brain | 0 | 0 | 0.0 | 0.0 | 0.0 |
| Kidney | 1.2 | 0.3 | 4.0 | 3.0 | 19.0 |
| Spleen | 0.2 | 0.8 | 0.3 | 0.5 | 1.2 |
| Bone | 0.1 | 0.2 | 0.5 | 0.3 | 2.4 |
| Stomach | 0.3 | 0.5 | 0.6 | 0.8 | 2.9 |
| Esophagus | 0.4 | 0.3 | 1.3 | 1.0 | 6.3 |
| Colon | 0.8 | 0.2 | 4.0 | 2.0 | 19.0 |
| Pancreas | 0.3 | 0.2 | 1.5 | 0.8 | 7.1 |
| Muscle | 0.1 | 0.1 | 1.0 | 0.3 | 4.8 |
| Skin | 0.2 | 0.3 | 0.7 | 0.5 | 3.2 |
| Blood | 0.4 | 1.9 | 0.2 | 1.0 | 1.0 |
SOA, specific organ ammumulation (ratio of J120 to control IgG in each organ; TTI, tissue targeting index (antibody in tissue/g of tissue/antibody in blood/g of blood); TSI, tissue selectivity index (TTI for J120/TTI for control IgG.
The rather modest immunotargeting of heart was surprising given the nearly equivalent protein signal detected in lung and heart P by Western blot analysis (Fig. 2A). Because intravenously injected antibody must first pass through the lung vasculature, it is possible that so much antibody was extracted in the lung that the blood concentration of 125I-J120 dropped sufficiently to limit binding to other organs. We therefore perfused isolated hearts and lungs with 125I-J120 to more directly compare binding under equivalent conditions (Fig. 4, C and D). The isotype-matched control IgG showed minimal uptake in either organ (<1%), whereas in lungs, as expected, a large amount of J120, 82.2% ID/g, was bound. The heart, however, bound only 12.4% ID/g (Fig. 4C), suggesting that the protein detected in heart P by Western blotting is not readily accessible to antibody binding in vivo. These data agree with our biodistribution analysis at 1-h postinjection showing 71.6% ID/g uptake in the lungs.
Identifying and cloning of two isoforms of the J120 antigen.
To identify the protein recognized by J120, we used the antibody to immunoprecipitate the antigen from rat lung P. When the immune complexes were resolved by SDS-PAGE, the low-molecular-weight form of the antigen was obscured by the presence of J120 heavy chain, and only the high-molecular-weight form of the antigen could be clearly identified. The gel band was excised, and MS/MS analysis identified the protein as CD34. Five unique CD34 peptides were obtained (Fig. 5A) covering 13.2% of the protein (Fig. 5B). A typical MS spectrum is shown in Fig. 5C.
Fig. 5.
Mass spectrometry (MS) analysis of the protein recognized by MAb J120. Proteins extracted from rat lung P by selective detergent solubilization were resolved by SDS-PAGE, and the gel slice containing the target antigen was analyzed by LC-MS/MS. A: unique proteolytic peptides of rat CD34 (middle) identified by MS/MS. The primary amino acid positions are listed in the lefthand column; the charge status of each identified peptide is listed in the righthand column. B: CD34 protein coverage by MS analysis. Peptides (highlighted in blue) covering 13.2% of the rat CD34 (gi27680487) amino acid sequence were identified in the excised gel band containing the target antigen. C: a typical MS/MS spectrum, GEELMQILCKK.
Identification of the J120 antigen as CD34 was somewhat surprising given the published reports that this protein is widely expressed on the endothelium of blood vessels in human and mouse adult (3, 14, 18, 24, 37) and fetal (14, 50, 51) tissues. To confirm the identity of the protein target for MAb J120, we used RT-PCR to clone the CD34 coding sequence from rat lung RNA for recombinant expression. The PCR reaction yielded two products, one at ∼1,100 kb and one at ∼1,300 kb, both of which were cloned.
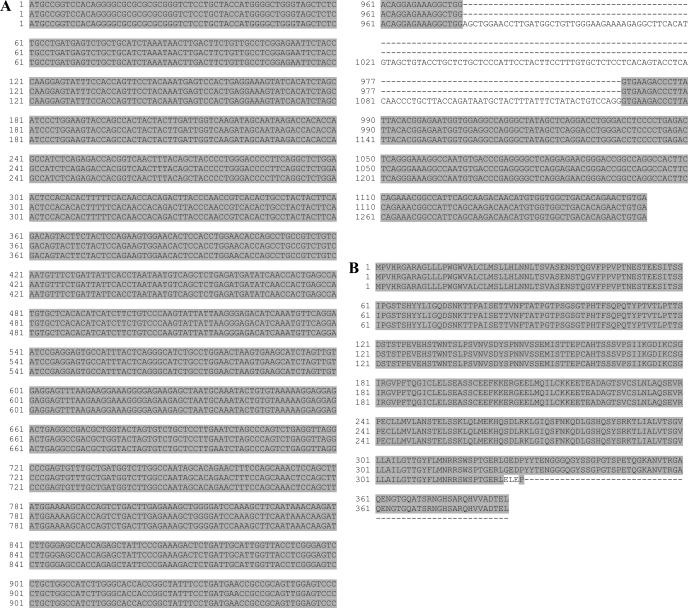
The smaller clone, hereafter referred to as variant 1, consisted of 1,161 nucleotides and was identical to the predicted rat CD34 sequence in the NCBI database (gi27680487). The larger clone, hereafter referred to as variant 2, consisted of 1,312 nucleotides, and, with the exception of an insert of 151 bases at nucleotide 962, aligned with the smaller clone, thus representing a variant of rat CD34 (Fig. 6A). Both variants were also cloned from heart and were identical in sequence with clones isolated from lung (data not shown). The sequences of both variants have been deposited with GenBank under the acc. nos. EU448292 (variant 1) and EU448293 (variant 2).
Fig. 6.
Alignment of the nucleotide and deduced amino acid sequences of the rat CD34 variants. A: alignment of the rat CD34 coding sequences. Top lines: predicted sequences from the NCBI database; middle lines: rat CD34 variant 1; bottom lines: rat CD34 variant 2. B: alignment of the deduced rat CD34 amino acid sequences. Top lines: rat CD34 from the predicted sequences in the NCBI database; middle lines: rat CD34 variant 1; bottom lines: rat CD34 variant 2. The truncation in variant 2 results from an inframe stop codon in the alternate 8th exon.
Both human and mouse CD34 are encoded at the genomic level by eight exons that give rise to two known variants (30, 45) resulting from the use of alternate eighth exons (for a review see Ref. 15). Variant 1 contained exons 1–8 and encoded a full-length protein, whereas in variant 2 an alternate exon (also known as exon X) was inserted between exons 7 and 8. This insert introduced an in-frame stop codon and resulted in a truncated protein lacking most of the cytoplasmic domain. Alignment of the rat CD34 variants with the rat genomic sequence showed the same eight-exon organization as well as the alternate exon usage, which gave rise to the second CD34 variant (Supplemental Fig. S1). Translation of both cDNAs reveals that variant 1 did indeed encode full-length CD34, whereas variant 2 encoded a truncated protein lacking most of the cytoplasmic domain (Fig. 6B). These results are in accord with the work of Omori et al. (35) who partially cloned two variants of the cytoplasmic domain of rat CD34.
When both rat CD34 variants were recombinantly expressed in bacteria and subjected to Western blotting, J120 bound to both isoforms (Fig. 7A) but did not bind to recombinant rat podocalyxin, another member of the CD34 protein family (not shown). These data confirm the specificity of J120 for CD34 and also demonstrated that the epitope to which the antibody binds is resident in the protein core and not dependent on posttranslational modifications. Both variant 1 and variant 2 run at a slower electrophoretic mobility than predicted from their deduced amino acid sequence. This phenomenon has also been seen when analyzing recombinant podocalyxin (20, 47).
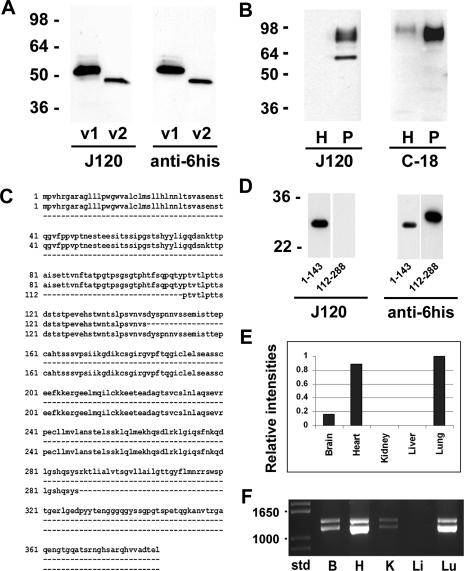
Fig. 7.
Expression of CD34 isoforms. A: Western blot analysis of recombinantly expressed rat CD34 variant 1 (v1) and variant 2 (v2). Left blot was probed with MAb J120; right blot was probed with anti-6his. Both recombinant proteins run at a slower electrophoretic mobility than predicted from their deduced amino acid sequence. B: Western blot analysis of normal rat lung homogenates (H) and endothelial cell plasma membrane (P). The blot shown at left was probed with MAb J120; the blot at right was probed with a polyclonal antibody to the cytoplasmic tail of CD34. Two bands were seen in the blot probed with J120, whereas only the higher-molecular-weight signal was seen in the blot probed with the antibody to the cytoplasmic domain, indicating that the lower-molecular-weight band represents rat CD34 variant 2. C: alignment of the full-length amino acid sequence of rat CD34 (top lines) with the cloned partial sequences of the extracellular domain encompassing amino acids 1–143 (middle lines) and amino acids 112–288 (bottom lines). D: Western blot analysis of recombinantly expressed partial amino acid sequences 1–143 and 112–288. The Western blot strips at left were probed with J120; strips at right were probed with anti-6his to detect the his tag on the amino termini of the recombinantly expressed protein fragments. E: QPCR analysis of CD34 mRNA in rat brain, heart, kidney, liver, and lung. The intensity of the normalized signal from rat lung was arbitrarily set at a value of 1; the intensities in the other organs are relative to the lung value. F: RT-PCR analysis of CD34 mRNA showing the presence of variant 1 (bottom band) and variant 2 (top band) in rat brain (B), heart (H), kidney (K), and lung (Lu) but not in liver (Li). Molecular weight standards are shown at left.
We wished to determine if the two proteins seen in Western blots of lung P might arise from the two variants. Blots of lung H and P were probed with J120 or with a polyclonal antibody to the CD34 cytoplasmic domain (Santa Cruz, C-18). Both J120 and the C-18 antibody bound to the higher-molecular-weight protein band(s), but only J120 bound to the 62-kDa protein (Fig. 7B). It was not possible to determine if the high-molecular-weight band(s) were composed entirely of posttranslationally modified full-length CD34, but our results are consistent with the idea that the low-molecular-weight band is composed primarily (if not completely) of the truncated form. The predicted difference in molecular weight between the unmodified, recombinant full-length and truncated proteins is ∼5.9 kDa (Fig. 7A), yet Figs. 1, 2, and 6 show that the difference between the variants expressed in vivo is 20 kDa or more. The apparent absence of full-length CD34 in the lower-molecular-weight band might suggest that it is not an intermediate form and that the truncated variant (or at least a portion of it) undergoes a very different type of posttranslational modification.
Our results demonstrate that J120 binds to the protein core of the extracellular domain of the rat CD34 molecule. To further define the region to which J120 binds, the extracellular domain was subcloned into two overlapping partial cDNAs, one encoding amino acids 1–143 and the other encoding amino acids 112–288 (Fig. 7C). When these molecules were recombinantly expressed and analyzed by Western blotting, J120 bound only to the partial protein containing amino acids 1–143, whereas the anti-6his antibody bound to both protein fragments (Fig. 7D). These results indicate that the epitope to which J120 binds resides within the first 111 amino acids in the rat CD34 molecule.
To examine CD34 RNA expression levels in various rat organs, we used real-time, quantitative PCR (QPCR). Normalized QPCR data correlated well with the results of the multiorgan Western blot (Fig. 7E). Signals were strongest in both lung and heart, whereas no signals were seen in kidney and liver. Brain, which had not previously been analyzed by Western blotting, gave a relatively weak signal. The QPCR data were also consistent with our proteomics analysis of P isolated from lung, heart, kidney, and liver, in which multiple CD34 peptides were detected in samples from lung and heart but not from liver and kidney (data not shown).
We resolved the PCR products on an agarose gel to analyze the relative expression of the two rat CD34 variants. Both variants were present in lung and heart RNA (Fig. 7F). Surprisingly, there was a strong band corresponding to variant 1 in heart, even though there was little or no variant 1 protein in heart H or P. Weak signals corresponding to variants 1 and 2 were also present in brain and kidney, and no signals were observed in liver. It should be noted that the RNA used in these assays was isolated from whole rat organs. Therefore, nonendothelial CD34-positive cells observed by J120 immunostaining in heart, brain, and kidney could also contribute to the QPCR signals and to the PCR products seen in Fig. 7, C and D.
DISCUSSION
In the present study, we describe MAb J120, which was used to characterize and identify a protein expressed primarily in the endothelium of adult rat lung and heart. The antigen was identified as CD34, which, along with podocalyxin and endoglycan, comprise the CD34 protein family. The vast majority of studies on CD34 have focused on its presence in the hematopoietic system (for review, see Refs. 15, 16, 21, 23), where this antigen is known to mark nonquiescent cells involved in migration or proliferation, but is absent in terminally differentiated lineages (8, 19). Only a handful of studies have looked at vascular expression of CD34, where it has been reported to be widely expressed on the endothelium of blood vessels in normal human and mouse adult (3, 14, 18, 24, 37) and fetal (14, 50, 51) tissues, as well as on lymphatic endothelium in humans (13, 41).
Our results show that the CD34 expression in the rat vasculature is restricted primarily to lung and heart and is accessible to antibody binding and immunohistochemical detection almost exclusively in the lung. The intensity of immunostaining in lung blood vessels is greatest in capillaries but is patchy in nature with some capillaries expressing little or no protein. Heterogeneous staining was noted as well in larger vessels of all types and calibers. Muller et al. (28, 29) also observed that CD34 immunostaining was stronger in capillary EC of human lung tissues than in arteries, veins, arterioles, or venules, but did not report any discontinuity of expression in tissue microvessels. Pusztaszeri et al. (37), however, did not find any differences between CD34 levels in capillaries and larger vessels in normal human lung. CD34 has also been detected in lymphatic EC (LEC) of normal human skin (41). Fiedler et al. (13), however, found that CD34 was expressed by LEC in human colon, breast, lung, and skin tumor tissues, but not in the corresponding normal tissues. In our studies, we observed that in normal rat lung, as in normal human lung tissue, CD34 was not detected on LECs. However, it should be noted that mouse tumor LECs do not express CD34 (13), suggesting that the behavior of rodent lymphatics may not always reflect corresponding biological processes in human tissues.
Like lung EC, aortic EC showed variable immunostaining by J120, with many EC not staining at all. Yet we found that all cultured RAEC expressed at least some detectable CD34. Conversely, none of the cultured RLMVEC expressed detectable CD34 even though many EC in the lung microvasculature were robustly stained with J120. The loss of expression of EC proteins in vitro is somewhat common, and loss of CD34 expression in human EC has been reported (for example, see Refs. 28 and 48). In these studies, the researchers observed that in primary cultures of EC from human umbilical veins (HUVEC), lung (HPMEC), and skin (HDMEC), 10–60% of cultivated cells retained CD34 expression (28, 48), whereas no cells in cultures of EC lines were positive (48). We have previously demonstrated that 41% of the proteins detected in vivo are not present on EC growing in vitro (10). Here we add to those findings and report a loss of CD34 expression upon in vitro cultivation of RLMVECs.
We also compared CD34 expression in rat and mouse at the RNA level using RT-PCR analyses and observed PCR products representing both variants in brain, heart, kidney, and lung, but not in the liver samples. Although it might be expected that the expression pattern of the CD34 protein would likewise be similar in the two species, this appears not to be the case. Baumhueter et al. (3) have reported that CD34 is globally expressed in the mouse vasculature, including liver. The difference between their results and ours may not be due solely to species variations but possibly due to the specificity of the antibodies used. We have demonstrated that MAb J120 recognizes an epitope resident in the protein core. The polyclonal antibody used by Baumhueter et al. (3) was raised against a eukaryotically expressed recombinant protein and may have contained a subset of antibodies against a carbohydrate epitope(s) expressed on CD34 and other vascular proteins. As an example, the carbohydrate epitope recognized by MAb MECA79 is known to be expressed on several endothelial glycoproteins, including CD34 (4, 17, 44). Use of an antibody that recognizes a shared carbohydrate epitope may also explain the results of Acevedo et al. (1). These authors used MAb 30B3 to immunoprecipitate a single 85-kDa protein from rat lung EC membranes and identify it as CD34. Immunohistochemical analysis of rat kidney with this antibody, in contrast with our results, showed immunostaining of most, if not all, glomerular and peritubular vessels. When lysates of isolated glomeruli were analyzed by Western blotting with 30B3, a main immunoreactive band was seen at 95 kDa accompanied by fainter bands at 54 and 47 kDa, but none of these proteins was confirmed to be CD34. Since MAb 30B3 immunostains podocytes and mesangial cells more strongly than EC, it is likely that the majority of their Western blot signal(s) arose primarily from protein(s) expressed by these cells. Although we, too, observed immunostaining of extravascular glomerular cells, the absence of J120 signals in our Western blots of kidney H and P is apparently due to the methods in which the samples were prepared. The colloidal silica used to isolate EC membranes from the kidney and other rat organs was perfused via the blood vasculature and did not come into contact with extravascular cells, so membranes from cells such as podocytes and mesangial cells were not present in P. In our study, only occasional EC immunostaining was observed in the kidney, and the amount of CD34 present in samples of P and whole kidney H analyzed by Western blotting was apparently below the level of detection. This is borne out by the fact that accumulation of 125I-J120 in the kidneys following intravenous injection could be detected by SPECT imaging only when the signal was significantly amplified.
Although the full-length protein cores of rat (386 amino acids) and human (385 amino acids) CD34 vary in length by only one amino acid, the apparent molecular weights of the two proteins are quite different. We observed that endogenous, full-length rat CD34 appeared on Western blots as a broad band of 85–93 kDa, whereas human CD34 is reported to be ∼110 kDa (for example, see Refs. 14 and 23). The core of mouse CD34 is also of a similar size (382 amino acids), but Krause et al. (22) found that the molecular weight of the protein endogenously expressed in NIH/3T3 and PA6 cells was 100 kDa. They also showed that recombinant full-length CD34 was ∼105 kDa when expressed in CHO-K1 cells and 110 kDa when expressed in M1 leukemia cells. It is apparent, then, that variations in the reported molecular weights of CD34 are due to differences in the extensive posttranslational modifications of the protein not only between species but between different cell lines within a species as well.
Fina et al. (14) previously reported a lack of correlation between CD34 mRNA and protein expression in cultured EC and concluded that the protein is either downregulated or processed into a form that is unreactive with CD34 antibodies. It is also possible that epitope masking by interacting protein(s) prevents immune detection of CD34 in tissues and cultured cells and contributes to the patchy immunostaining of vessels observed in rat lung tissue. Likewise, it may explain why the relatively robust expression of CD34 seen in Western blots of heart P (where potential blocking proteins are dissociated) did not translate into strong immunostaining of heart vessels or greater accumulation of radiolabeled J120. It should be noted that in heart P, the truncated form of CD34 was predominant. Although transfection experiments with mast cells have shown that the truncated form can be expressed on the cell surface (9), it may be possible that in EC, truncated CD34 is inaccessible to antibody binding.
We observed that abundance at the RNA level did not always correlate with protein abundance. This is especially noticeable in heart where the variant 2 protein predominates even though there is a strong variant 1 RT-PCR signal. The mechanisms that control expression of each variant are unknown. The wide variance and the patchy distribution of CD34 suggest that different regulatory mechanisms may affect different vascular beds and even neighboring EC of the same vascular bed. Differences in regulation may also extend to different EC cultivated in vitro. As mentioned above, we have noted a loss of CD34 expression in primary RLMVEC cultures. This phenomenon has also been reported to occur in primary cultures of human microvascular EC and in HUVECs (28, 48). However, when we analyzed cultured RAECs by immunofluorescence, the percentage of immunopositive cells was greater than would have been predicted from immunohistochemistry. This may represent an actual upregulation of CD34 expression by RAEC when cultured in vitro. Alternately, it may reflect a situation similar to that seen in rat heart where the antigen is present but not detectable by tissue immunohistochemistry, possibly due to epitope masking by interacting protein(s). Upon in vitro cultivation, these proteins may be dissociated or downregulated, thus allowing J120 to bind to the cell surface. While much useful information can be gained from cultured EC, results from these experiments must be viewed in the context of the changes in gene expression brought on by in vitro cultivation.
Little is known about the functional role of CD34. It has been proposed to inhibit differentiation and promote proliferation in HSCs and to affect adhesion (for review, see Ref. 15). The function of CD34 may vary with each isoform. Transfection experiments conducted in vitro by Fackler et al. (12) demonstrated that full-length CD34 inhibited hematopoietic cell differentiation, whereas the truncated form did not. Using a mast cell aggregation assay, Drew, et al. (9) showed that the truncated form was more effective at inhibiting aggregation than the full-length protein. Vascular CD34 can mediate l-selectin-dependent lymphocyte rolling (36), but as one of four glycoproteins that bind l-selectin (2, 40), other molecules could presumably substitute for CD34. Indeed, CD34 knockout mice are mostly normal, suffering only minor hematopoietic defects (7, 46). Suzuki et al. (46) showed that in CD34-deficient and wild-type mice, there was no difference in lymphocyte rolling or lymphocyte homing. But, they did demonstrate that following inhalation of a model allergen (ovalbumin), there was a substantial deficit in leukocyte trafficking to the lung. We have shown herein that accessible CD34 expression in the rat vasculature is restricted primarily to the lungs, and this constant exposure to the circulating blood cells should be considered in defining its mechanism of action. While retention of accessible CD34 expression in the rat lung might suggest a vital function for this protein in maintaining normal lung physiology, it remains to be determined if this is truly the case.
In the present study, we have used MAb J120 to characterize CD34, whose expression and immunodetection in adult rat tissues, unlike in mouse and human, is restricted primarily to lung. We also present the first complete sequencing of the second rat CD34 variant. Previous transfection studies with cells grown in vitro have shown that human variant 2 can be expressed at the cell surface, and we show here that the rat variant 2 is naturally expressed in vivo. Our results also show that whereas CD34 was found in both lung and heart P, the pattern of expression of the variants differed. In hematopoietic lineages, truncated and full-length CD34 possess different functional properties. Although it would seem logical to assume that variants 1 and 2 would also serve different purposes in vascular EC, there are no studies that address this question. The majority of studies regarding CD34 have focused on hematopoietic cells, but its characterization and function(s) in the vasculature have yet to be fully explored. Herein we provide critical initial characterization towards this ultimate goal.
GRANTS
This work was supported by National Institutes of Health Grants P01-CA-104898, R24-CA-095893, R01-HL-074063, R01-HL-052766, and R01-HL-058216 (to J. E. Schnitzer).
Supplementary Material
Acknowledgments
We acknowledge the excellent technical assistance of Lorraine Guiney, Jody Gonzales, Kally Pascua, Michelle Gonzalez, and Alexina Wempren, and we thank Kerri Hebard-Massey for help in preparing the manuscript.
REFERENCES
- 1.Acevedo LM, Londono I, Oubaha M, Ghitescu L, Bendayan M. Glomerular CD34 expression in short- and long-term diabetes. J Histochem Cytochem 56: 605–614, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumheter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, Lasky LA. Binding of l-selectin to the vascular sialomucin CD34. Science 262: 436–438, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Baumhueter S, Dybdal N, Kyle C, Lasky LA. Global vascular expression of murine CD34, a sialomucin-like endothelial ligand for l-selectin. Blood 84: 2554–2565, 1994. [PubMed] [Google Scholar]
- 4.Berg EL, Robinson MK, Warnock RA, Butcher EC. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol 114: 343–349, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carver LA, Schnitzer JE. Multiple functions and clinical uses of caveolae in endothelium. In: Endothelial Biomedicine, edited by Aird WC. New York: Cambridge University Press, 2007, p. 664–678.
- 6.Carver LA, Schnitzer JE. Proteomic mapping of endothelium and vascular targeting in vivo. In: Endothelial Biomedicine, edited by Aird WC. New York: Cambridge University Press, 2007, p. 881–897.
- 7.Cheng J, Baumhueter S, Cacalano G, Carver-Moore K, Thibodeaux H, Thomas R, Broxmeyer HE, Cooper S, Hague N, Moore M, Lasky LA. Hematopoietic defects in mice lacking the sialomucin CD34. Blood 87: 479–490, 1996. [PubMed] [Google Scholar]
- 8.Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol 133: 157–165, 1984. [PubMed] [Google Scholar]
- 9.Drew E, Merzaban JS, Seo W, Ziltener HJ, McNagny KM. CD34 and CD43 inhibit mast cell adhesion and are required for optimal mast cell reconstitution. Immunity 22: 43–57, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, Oh P, Schnitzer JE. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nature Biotechnol 22: 985–992, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Dvorak HF, Nagy JA, Dvorak AM. Structure of solid tumors and their vasculature: implications for therapy with monoclonal antibodies. Cancer Cells 3: 77–85, 1991. [PubMed] [Google Scholar]
- 12.Fackler MJ, Krause DS, Smith OM, Civin CI, May WS. Full-length but not truncated CD34 inhibits hematopoietic cell differentiation of M1 cells. Blood 85: 3040–3047, 1995. [PubMed] [Google Scholar]
- 13.Fiedler U, Christian S, Koidl S, Kerjaschki D, Emmett MS, Bates DO, Christofori G, Augustin HG. The sialomucin CD34 is a marker of lymphatic endothelial cells in human tumors. Am J Pathol 168: 1045–1053, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood 75: 2417–2426, 1990. [PubMed] [Google Scholar]
- 15.Furness SG, McNagny K. Beyond mere markers: functions for CD34 family of sialomucins in hematopoiesis. Immunol Res 34: 13–32, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Gangenahalli GU, Singh VK, Verma YK, Gupta P, Sharma RK, Chandra R, Luthra PM. Hematopoietic stem cell antigen CD34: role in adhesion or homing. Stem Cells Dev 15: 305–313, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Imai Y, Singer MS, Fennie C, Lasky LA, Rosen SD. Identification of a carbohydrate-based endothelial ligand for a lymphocyte homing receptor. J Cell Biol 113: 1213–1221, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalaria RN, Kroon SN. Expression of leukocyte antigen CD34 by brain capillaries in Alzheimer's disease and neurologically normal subjects. Acta Neuropathol 84: 606–612, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Katz FE, Tindle R, Sutherland DR, Greaves MF. Identification of a membrane glycoprotein associated with haemopoietic progenitor cells. Leuk Res 9: 191–198, 1985. [DOI] [PubMed] [Google Scholar]
- 20.Kershaw DB, Thomas PE, Wharram BL, Goyal M, Wiggins JE, Whiteside CI, Wiggins RC. Molecular cloning, expression, and characterization of podocalyxin-like protein 1 from rabbit as a transmembrane protein of glomerular podocytes and vascular endothelium. J Biol Chem 270: 29439–29446, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood 87: 1–13, 1996. [PubMed] [Google Scholar]
- 22.Krause DS, Ito T, Fackler MJ, Smith OM, Collector MI, Sharkis SJ, May WS. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood 84: 691–701, 1994. [PubMed] [Google Scholar]
- 23.Lanza F, Healy L, Sutherland DR. Structural and functional features of the CD34 antigen: an update. J Biol Regul Homeost Agents 15: 1–13, 2001. [PubMed] [Google Scholar]
- 24.Lin G, Finger E, Gutierrez-Ramos JC. Expression of CD34 in endothelial cells, hematopoietic progenitors and nervous cells in fetal and adult mouse tissues. Eur J Immunol 25: 1508–1516, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay MA Target discovery. Nature Rev 2: 831–838, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Magee JC, Stone AE, Oldham KT, Guice KS. Isolation, culture, and characterization of rat lung microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 267: L433–L441, 1994. [DOI] [PubMed] [Google Scholar]
- 27.McIntosh DP, Tan XY, Oh P, Schnitzer JE. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: a pathway to overcome cell barriers to drug and gene delivery. Proc Natl Acad Sci USA 99: 1996–2001, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller AM, Hermanns MI, Skrzynski C, Nesslinger M, Muller KM, Kirkpatrick CJ. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol 72: 221–229, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Muller AM, Nesslinger M, Skipka G, Muller KM. Expression of CD34 in pulmonary endothelial cells in vivo. Pathobiology 70: 11–17, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Komano H, Nakauchi H. Two alternative forms of cDNA encoding CD34. Exp Hematol 21: 236–242, 1993. [PubMed] [Google Scholar]
- 31.Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotechnol 25: 327–337, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature 429: 629–635, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol 141: 101–114, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh P, Schnitzer JE. Isolation and subfractionation of plasma membranes to purify caveolae separately from glycosyl-phosphatidylinositol-anchored protein microdomains. In: Cell Biology: A Laboratory Handbook, edited by Celis J. Orlando, FL: Academic Press, 1998, p. 34–45.
- 35.Omori N, Omori M, Evarts RP, Teramoto T, Miller MJ, Hoang TN, Thorgeirsson SS. Partial cloning of rat CD34 cDNA and expression during stem cell-dependent liver regeneration in the adult rat. Hepatology 26: 720–727, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Puri KD, Finger EB, Gaudernack G, Springer TA. Sialomucin CD34 is the major l-selectin ligand in human tonsil high endothelial venules. J Cell Biol 131: 261–270, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem 54: 385–395, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol Heart Circ Physiol 285: H1720–H1729, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Russell J, O'Donoghue JA, Finn R, Koziorowski J, Ruan S, Humm JL, Ling CC. Iodination of annexin V for imaging apoptosis. J Nucl Med 43: 671–677, 2002. [PubMed] [Google Scholar]
- 40.Satomaa T, Renkonen O, Helin J, Kirveskari J, Makitie A, Renkonen R. O-glycans on human high endothelial CD34 putatively participating in l-selectin recognition. Blood 99: 2609–2611, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Sauter B, Foedinger D, Sterniczky B, Wolff K, Rappersberger K. Immunoelectron microscopic characterization of human dermal lymphatic microvascular endothelial cells. Differential expression of CD31, CD34, and type IV collagen with lymphatic endothelial cells vs blood capillary endothelial cells in normal human skin, lymphangioma, and hemangioma in situ. J Histochem Cytochem 46: 165–176, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Schnitzer JE Vascular targeting as a strategy for cancer therapy. N Engl J Med 339: 472–474, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Schnitzer JE, McIntosh DP, Dvorak AM, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science 269: 1435–1439, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Streeter PR, Rouse BT, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol 107: 1853–1862, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suda J, Sudo T, Ito M, Ohno N, Yamaguchi Y, Suda T. Two types of murine CD34 mRNA generated by alternative splicing. Blood 79: 2288–2295, 1992. [PubMed] [Google Scholar]
- 46.Suzuki A, Andrew DP, Gonzalo JA, Fukumoto M, Spellberg J, Hashiyama M, Takimoto H, Gerwin N, Webb I, Molineux G, Amakawa R, Tada Y, Wakeham A, Brown J, McNiece I, Ley K, Butcher EC, Suda T, Gutierrez-Ramos JC, Mak TW. CD34-deficient mice have reduced eosinophil accumulation after allergen exposure and show a novel crossreactive 90-kD protein. Blood 87: 3550–3562, 1996. [PubMed] [Google Scholar]
- 47.Testa JE, Chrastina A, Li Y, Oh P, Schnitzer JE. Ubiquitous yet distinct expression of podocalyxin on vascular surfaces in normal and tumor tissues in the rat. J Vasc Res 46: 311–324, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unger RE, Krump-Konvalinkova V, Peters K, Kirkpatrick CJ. In vitro expression of the endothelial phenotype: comparative study of primary isolated cells and cell lines, including the novel cell line HPMEC-ST1.6R. Microvasc Res 64: 384–397, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Valadon P, Garnett JD, Testa JE, Bauerle M, Oh P, Schnitzer JE. Screening phage display libraries for organ-specific vascular immunotargeting in vivo. Proc Natl Acad Sci USA 103: 407–412, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood HB, May G, Healy L, Enver T, Morriss-Kay GM. CD34 expression patterns during early mouse development are related to modes of blood vessel formation and reveal additional sites of hematopoiesis. Blood 90: 2300–2311, 1997. [PubMed] [Google Scholar]
- 51.Young PE, Baumhueter S, Lasky LA. The sialomucin CD34 is expressed on hematopoietic cells and blood vessels during murine development. Blood 85: 96–105, 1995. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.