Abstract
α1-Antitrypsin (AT) is a major elastase inhibitor within the lung. Oxidation of critical methionine residues in AT generates oxidized AT (Ox-AT), which has a greatly diminished ability to inhibit neutrophil elastase. This process may contribute to the pathogenesis of chronic obstructive pulmonary disease (COPD) by creating a functional deficiency of AT permitting lung destruction. We show here that Ox-AT promotes release of human monocyte chemoattractant protein-1 (MCP-1) and IL-8 from human lung type epithelial cells (A549) and normal human bronchial epithelial (NHBE) cells. Native, cleaved, polymeric AT and secretory leukoproteinase inhibitor (SLPI) and oxidized conformations of cleaved, polymeric AT and SLPI did not have any significant effect on MCP-1 and IL-8 secretion. These findings were supported by the fact that instillation of Ox-AT into murine lungs resulted in an increase in JE (mouse MCP-1) and increased macrophage numbers in the bronchoalveolar lavage fluid. The effect of Ox-AT was dependent on NF-κB and activator protein-1 (AP-1)/JNK. These findings have important implications. They demonstrate that the oxidation of methionines in AT by oxidants released by cigarette smoke or inflammatory cells not only reduces the antielastase lung protection, but also converts AT into a proinflammatory stimulus. Ox-AT generated in the airway interacts directly with epithelial cells to release chemokines IL-8 and MCP-1, which in turn attracts macrophages and neutrophils into the airways. The release of oxidants by these inflammatory cells could oxidize AT, perpetuating the cycle and potentially contributing to the pathogenesis of COPD. Furthermore, these data demonstrate that molecules such as oxidants, antiproteinases, and chemokines, rather than act independently, are likely to interact to cause emphysema.
Keywords: chronic obstructive pulmonary disease, interleukin-8, oxidants
chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide. It is characterized by progressive and largely irreversible airflow limitation due to alveolar destruction (emphysema), small airway narrowing, and chronic bronchitis. COPD is associated with an abnormal pulmonary inflammatory response to noxious particles. Most cases of COPD are triggered by chronic inhalation of cigarette smoke, which leads to the characteristic infiltration of neutrophils, macrophages, and CD8+ T lymphocytes. This inflammatory cell infiltrate persists after smoking cessation and is associated with the severity of disease (3, 25).
α1-Antitrypsin (AT) is a serine proteinase inhibitor produced primarily by hepatocytes, macrophages, and bronchial epithelial cells (9). The normal protein is termed M-AT according to its migration on isoelectric focusing. It is the most abundant proteinase inhibitor within the lung whose main physiological target is neutrophil elastase (9, 35). The crystal structure of AT is characterized by a dominant β-sheet A and an exposed reactive center loop (55). Its inhibitory mechanism relies on cleavage of the methionine-serine P1-P1′ by neutrophil elastase. This initiates a conformational change in the molecule whereby the elastase molecule is transferred to the lower pole of the molecule and inactivated (26, 56). One in two thousand North Europeans are homozygous for the Z (342Glu→Lys) variant of AT. This variant accumulates in the hepatocyte by a process of loop-sheet polymerization whereby the reactive loop of one molecule inserts into β-sheet A of a second and so on to form chains of polymers (34, 37). Polymerization in the liver is associated with cirrhosis, and the accompanying plasma deficiency leaves the lungs exposed to neutrophil elastase resulting in premature emphysema (17, 33, 36, 47, 54). The association of Z-AT with emphysema and the finding that proteinases could cause experimental emphysema in small animals were critical in initiating the proteinase:antiproteinase theory of emphysema (22).
Oxidation of the P1 methionine (methionine 358) or methionine 351 to methionine sulfoxide significantly reduces the ability of AT to inhibit neutrophil elastase (4, 28, 58). Hydrogen peroxide in cigarette smoke and N-chloroamines and hypochlorous acid in neutrophils can oxidize and inactivate AT (46, 53, 58). Thus the oxidation of AT by cigarette smoke or free radicals in vivo could lead to a relative deficiency of elastase inhibitors and has been suggested as a mechanism contributing to the development of emphysema in M-AT individuals (5, 6, 11, 19). Oxidant-mediated inactivation of AT has also been suggested as playing a role in other lung diseases such as cystic fibrosis, adult respiratory distress syndrome, and bronchiectasis (38, 39, 45, 50).
α1-Antitrypsin also appears to have effects on fibroblast proliferation, and recent studies showed that AT has direct and indirect antiapoptotic properties (14, 20, 48, 57, 61, 64). In vivo AT can exist in different conformational forms; native, reactive center loop-cleaved, oxidized, complexed with neutrophil elastase and Z-AT can be found as polymers. The conformational changes can be the result of inflammation, such as cleavage by non-target proteinases and oxidation by reactive oxygen species. While these conformations result in a loss of proteinase inhibitory activity, they can have other biological effects such as chemoattraction, inflammatory cell activation, and cytokine induction. For instance, oxidized AT (Ox-AT) and the cleaved peptide fragment of AT stimulate monocyte activation, and AT-elastase complexes and polymeric AT are chemotactic to neutrophils (1, 13, 16, 21, 27, 29, 36, 41, 42, 44, 52). In this study, we assess the effect of Ox-AT on the production of monocyte chemotactic protein-1 (MCP-1) and IL-8.
MATERIALS AND METHODS
Preparation and characterization of conformations of α1-antitrypsin.
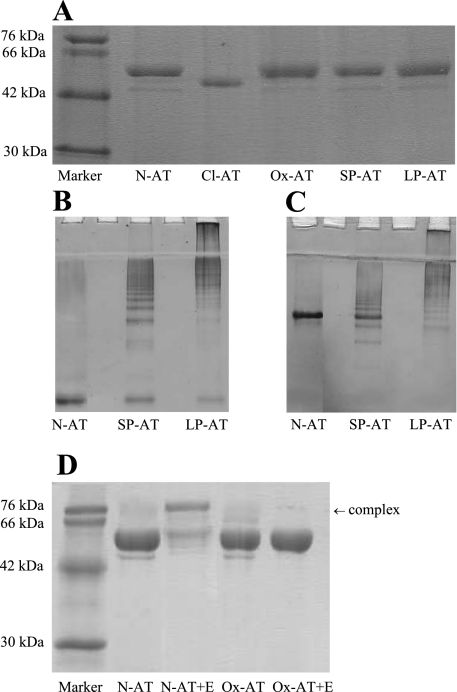
Native M-AT (N-AT) was purified from human plasma by ammonium sulfate fractionation followed by glutathione and anion exchange chromatography. N-AT migrated as a single band on 7.5% (wt/vol) nondenaturing and 12% SDS-PAGE and showed a normal unfolding transition on transverse urea gel PAGE. It was 70–80% active when assessed by titration against bovine α-chymotrypsin. The native protein was then used to prepare the other AT conformations. Reactive loop-cleaved antitrypsin (Cl-AT) was generated by incubation with Staphylococcus aureus V8 protease (Sigma, UK) at 37°C for 2 h (Fig. 1A). The protease was then removed by anion exchange chromatography using a mono-Q column. This is distinct from the cleaved fragment; in this form COOH-terminal segment remains firmly attached to the core of cleaved AT. Long- and short-chain polymeric AT (LP-AT and SP-AT, respectively) were formed by heating native M-AT (2 mg/ml) at 55°C for 40 and 3 h, respectively. These polymers are predicted to have the same loop sheet linkage as Z-AT polymers, thus the same tertiary structure. Polymeric AT was assessed on 7.5% nondenaturing PAGE with and without 8 M urea (Fig. 1, B and C). Native, cleaved, and polymeric AT, secretory leukocyte protease inhibitor (SLPI) (purified recombinant SLPI was obtained from the Dept. of Otolaryngology and Head and Neck Surgery, Malmoe University Hospital, Sweden) was oxidized as previously described (5, 41). Briefly, N-chlorosuccinimide (Sigma, UK) was incubated in a 25 M excess to AT in 0.1 M Tris·HCl (pH 8.0) at room temperature for 30 min. Excess N-chlorosuccinimide was removed and the buffer exchanged to PBS using a centrifugal microconcentrator (Centricon YM30). Ox-AT was tested for the ability to form an SDS-stable complex with neutrophil elastase (Calbiochem). Samples of Ox-AT or N-AT were incubated with neutrophil elastase at a 2:1 molar ratio for 15 min at 37°C. The reaction was stopped by adding SDS sample buffer and boiling. Mixtures were analyzed by 12% SDS-PAGE and stained with Coomassie blue. Ox-AT was confirmed and distinguished from N-AT by its inability to form an SDS-stable complex with human neutrophil elastase (Fig. 1D).
Fig. 1.
Characterization of conformations of α1-antitrypsin (AT). A: 12% (wt/vol) SDS-PAGE. Two micrograms of native AT (N-AT), Staphylococcus aureus V8 protease reactive loop-cleaved AT (Cl-AT), oxidized AT (Ox-AT), short-chain polymers (SP-AT), and long-chain AT polymers (LP-AT), were loaded onto 12% SDS-PAGE, 7.5% (wt/vol) nondenaturing PAGE with (B) and without (C) 8 M urea. Gel demonstrates difference in short- and long-chain polymers. Five micrograms of SP-AT and LP-AT were loaded per lane. Note the small amount of contamination of N-AT in SP-AT. D: 12% (wt/vol) SDS-PAGE demonstrating the difference in N-AT and Ox-AT. N-AT could form an SDS-stable complex with human neutrophil elastase, unlike Ox-AT.
Conformations used in all assays were tested for contaminating LPS by QCL-1000 Chromogenic LAL Endpoint Assay (Cambrex). The heat stable-cleaved conformation of AT was heated at 60°C for 4 h and filtered through a 100-kDa Centricon membrane to ensure complete removal of LPS. The non-heat-stable N-AT was decontaminated using END-X beads (Associates of Cape Cod). All samples used in the subsequent experiments contained less than 10 ng/ml LPS. Protein solutions were snap-frozen in PBS and stored at −80°C until required.
A549 cell culture.
The A549 lung epithelial cell line (American Type Culture Collection) was grown in DMEM supplemented with 4,500 mg of glucose/l, l-glutamine, NaHCO3, and pyridoxine HCL (Sigma), with 5% FCS and 100 units of penicillin and 0.1 mg/ml of streptomycin (Sigma), supplemented DMEM at 37°C, 5% carbon dioxide (CO2) until ∼80% confluence. Cells were trypsinized, and 1 × 105 cells/100 μl media were grown per well in a 96-well plate overnight. The following morning, the media was aspirated and replaced with 100 μl of fresh media for 30 min followed by addition of 15 μl of either N-AT, Ox-AT, SLPI, Ox-SLPI, Cl-AT, Ox-Cl-AT, LP-AT, or Ox-LP-AT to give a final concentration of 0.03, 0.1, or 0.3 mg/ml. TNFα was used as a positive control, and LPS (0.01 μg/ml) was used as a negative control. The cells were cultured for 4, 10, and 24 h. The culture supernatant was collected at each time point and quantified for IL-8, MCP-1, and TNFα by ELISA.
Normal human bronchial epithelial cell culture.
Normal human bronchial epithelial (NHBE) cells (CC-2540, Lonza) were cultured in supplemented DMEM at 37°C and 5% CO2 until ∼80% confluence. Cells were trypsinized, and 1 × 105 cells/100 μl media were grown per well in a 96-well plate overnight. The following morning, the media was aspirated and replaced with 100 μl of fresh media for 30 min followed by addition of 15 μl of N-AT or Ox-AT to give a final concentration of 0.03 or 0.3 mg/ml. The cells were cultured for 4 and 24 h. The culture supernatant was collected at each time point and quantified for IL-8 and MCP-1 by ELISA.
Intratracheal instillation of Native and Ox-AT into murine lungs.
All experiments were approved by the Home Office. This was done as previously described for polymeric AT (36). Female C57BL/6 mice, 8 wk of age, were anesthetized and intubated with a nonpyrogenic 22-gauge cannula (Terumo Medical). Mice received either 40 μl of 2 mg/ml of Ox-AT or N-AT diluted in PBS. A separate group of mice received 40 μl of PBS alone. After 4, 24, and 72 h, cohorts of C57BL/6J mice (at least 6 in each group) were killed. Bronchoalveolar lavage (BAL) was performed with eight aliquots of 0.5 ml of PBS. The BAL samples were centrifuged, and BAL fluid (supernatant) was aliquoted. Protease inhibitor cocktail (Sigma) and PMSF (1 mmol/l) were added, and the samples were stored at −80°C until use. The cell pellet was resuspended, and red blood cells were lysed in 0.15 mol/l NH4Cl, 0.01 mol/l KHCO3, and 1 μmol/l disodium ethylene diaminetetra acetic acid (pH 7.2) for 30 s followed by a 5-min wash (200 g) in 5 ml of PBS. The cell pellet was resuspended in 1 ml of PBS, and the total number of cells was quantified by the mean of two hemocytometer counts. To determine inflammatory cell types in BAL fluid, cytospins were prepared (350 rpm, 3 min, Cytospin 3, Shandon) from the BAL cellular fraction and were stained with Hema 3 (Fisher, Pittsburgh, PA). The proportions of different inflammatory cells were determined by counting at least 200 cells.
Frozen whole lung tissue from mice was homogenized using Tissue Tearor (Biospec Products) in cell lysis buffer (CelLytic, Sigma) on ice. The lung homogenates were centrifuged, and proteinase inhibitor cocktail (Sigma), PMSF (1 mmol/l), and 1 and 10 phenanthroline (1 mmol/l) were added to the supernatants. The supernatants were kept on ice and immediately assessed for the total protein (RC DC protein kit, Bio-Rad).
ELISA for IL-8.
An inhouse ELISA for IL-8 was developed. ELISA plates (Nunc Immunoplate Maxisorb) were coated overnight at 4°C with 1 μg/ml mouse anti-human IL-8 (R&D Systems) in 0.05 M sodium carbonate buffer (pH 9.6). The plates were washed three times with 300 μl/well wash buffer (PBS/0.05% Tween 20) and blocked for at least 1 h at room temperature with PBS containing 1% BSA/0.05% Tween 20. Recombinant human IL-8 standards and cell culture supernatants, 100 μl/well, were incubated for 2 h at room temperature and then washed. Anti-human IL-8 biotin 1:300 (R&D Systems) was added, and the plate was washed after 2 h. Streptavidin-HRP (R&D Systems, 1:1,000) was added, and the plates were incubated in the dark for an additional 2 h. The plates were washed and then incubated with the HRP substrate p-nitrophenyl phosphate (Sigma) for 20 min. The plates were read in a microplate reader at a wavelength of 405 nm (model 680, Bio-Rad). The range of detection of this assay is 50–30,000 pg/ml.
ELISA for human MCP-1.
The concentration of human MCP-1 in culture supernatant was quantified by ELISA using human MCP-1/CCL2, DuoSet, according to the manufacturer's instructions (R&D Systems).
ELISA for mouse KC, JE, and TNFα.
Mouse KC (mouse IL-8), JE (mouse MCP-1), and TNFα concentration in mouse BAL fluid and homogenized lung tissue was quantified by ELISA with respective DuoSets (R&D Systems) according to the manufacturer's instructions. To correct for dilution effects, results were expressed compared with the total protein.
Nuclear protein extraction from cultured cells and lung homogenates.
Nuclear protein extraction was performed according to the manufacturer's instructions (Nuclear Extract Kit, Active Motif, Belgium). Briefly, cells in a 24-well plate (4 × 105 cells/well) were suspended in ice-cold PBS/phosphatase inhibitor solution by scraping and centrifuging at 1,000 rpm for 10 min at 4°C. Lung homogenates prepared as above were centrifuged at 10,000 g for 10 min 2× at 4°C. The cell pellets were resuspended in 50 μl 1× hypotonic buffer and incubated on ice for 15 min to swell the cell membrane. Five microliters of detergent was then added to the suspension to lyse the cell membrane. The nuclei were recruited into the pellet by centrifuging at 14,000 g for 2 min. To extract the nuclear protein, the pellet was resuspended in 50 μl of complete lysis buffer (containing DTT and proteinase inhibitor cocktail) and incubated on ice for 30 min. The nuclear protein was isolated from the supernatant after centrifuging at 14,000 g for 10 min.
Protein assay.
The protein level was assessed using the RC DC protein kit (Bio-Rad).
ELISA for NF-κB activation.
NF-κB activation was assessed using the TransAM NF-κB kit according to the manufacturer's instructions (Active Motif, Belgium). Briefly, in the TransAM NF-κB kit, an oligonucleotide that contained the NF-κB consensus site (5′-gggattttcc-3′) has been immobilized onto a 96-well plate. The active form of NF-κB can bind to this site. The NF-κB active site on nuclear cell extract (4 μg) from cultured cells and homogenized lung tissue was detected through use of NF-κB antibody p50 (1:1,000) followed by addition of a secondary antibody conjugated to HRP (1:1,000). Active NF-κB was quantified by reading absorbance at 450 nm (model 680, Bio-Rad).
ELISA for AP-1 activation.
Activator protein-1 (AP-1) activation was assessed using the TransAM AP-1 c-Jun kit according to the manufacturer's instructions (Active Motif). Briefly, in the TransAM AP-1 kit, the oligonucleotide containing the TPA responsive element (5′-cgctttgatgagtcagccggaa-3′) has been immobilized on a 96-well plate. The AP-1 dimers in the nuclear cell extracts bind specifically to this oligonucleotide. The AP-1 active site on nuclear cell extract (4 μg) from cultured cells and homogenized lung tissue was detected through use of AP-1 antibody c-Jun (1:500) followed by addition of a secondary antibody conjugated to HRP (1:1,000). Active AP-1 was quantified by reading absorbance at 450 nm (model 680, Bio-Rad).
Inhibition of NF-κB, p38 MAPK, and JNK.
Bay11-7082, an inhibitor of NF-κB, SB-203580, an inhibitor of p38 MAPK, and SP-600125, an inhibitor of JNK, were dissolved in 10% DMSO and added to cells at a final concentration of 10 μM. Previously published reports indicated that 10–50 μM inhibitor do not have any significant cytotoxic effects (30, 32, 60). We also evaluated the cytotoxic effect of each inhibitor using A549 cells at 4, 10, and 24 h of culture using Trypan blue assay. We found that there was no significant cytotoxic effect of any of the inhibitors (10 μM) on cell proliferation (data not shown).
Effect of Ox-AT on NF-κB and AP-1 activity.
A549 cells were preincubated with either Bay11-7082 (10 μM), SB-203580 (10 μM), or SP-600125 (10 μM). Ox-AT (0.3 mg/ml) was added 20 min later. Nuclear proteins were extracted from the cells after 1.5 h of treatment, and 4 μg of protein was used for NF-κB and AP-1 assays.
JNK activity.
SP-600125 (10 μM), an inhibitor of JNK, was added to the cell culture media of A549 cells 20 min before treatment with 0.3 mg/ml Ox-AT. The stimulated cells in a 96-well plate were directly fixed in situ for the JNK assay 1 h after treatment with Ox-AT. Antibody to phosphorylated JNK and antibody to total JNK (FACE JNK kit, Active Motif) were used separately for the detection of activated JNK and total JNK. A standardized JNK level was obtained by subtracting from background (PBS) and expressed as optical density (OD) 450 nm (JNK level)/OD 595 nm (cell number) (model 680, Bio-Rad).
Western blot analysis.
Proteins from NHBE cells were extracted using protein lysis buffer (10 mM Tris·HCl, pH 7.8, 1.5 mM EDTA, pH 8.0, 10 mM KCl, 1 mM sodium orthovanadate, 0.05% Nonidet P-40, 1 mM PMSF) and equalized for loading. Ten micrograms of protein from NHBE cells or 50 μg from homogenized lung tissue was electrophoresed on a 12% SDS-PAGE and then electroblotted onto nitrocellulose membranes (Schleicher and Schuell, Biosciences). The blots were blocked with PBS/T containing 5% nonfat dry milk for 1 h at room temperature. Western blot for activated or phosphorylated JNK (phospho-JNK) was performed by incubating with a primary antibody, phospho-JNK (1 mg/ml, that detects endogenous human, mouse, and rat Thr183/Tyr185 of JNK1), and phosphorylated and nonphosphorylated JNK (total-JNK, 0.2 mg/ml, R&D Systems) at 1:500 in PBS/T with 5% nonfat dry milk for 1 h at room temperature. The blots were washed three times with PBS/T and incubated with HRP-conjugated swine anti-rabbit IgG at 1:1,000 (Dako). The signals were visualized by an enhanced chemiluminescence (ECL) system (Amersham). Densitometric semiquantitative analysis of Western blot bands was performed using Image Processing and Analysis in Java (ImageJ), version 1.41j (http://www.ansci.wisc.edu/equine/parrish/index.html). The density of each protein band was corrected for background and expressed in arbitrary units.
Relative effect of JNK and NF-κB inhibitors on Ox-AT induced IL-8 and MCP-1.
A549 cells (4 × 105 cells/well) were cultured in a 24-well plate overnight. The following morning, cells were incubated in fresh media for 20 min. Cells were preincubated for 20 min with either Bay11-7082 (10 μM) or SP-600125 (10 μM) or in combination of Bay11-7082 (10 μM) and SP-600125 (10 μM). Ox-AT (0.3 mg/ml) was added 20 min later. Nuclear proteins were extracted from the cells after a 1.5-h treatment, and 4 μg of protein was used to quantify the activity of NF-κB and AP-1 by reading absorbance at 450 nm (model 680, Bio-Rad). For quantification of Ox-AT-induced IL-8 and MCP-1 production in the presence of the individual inhibitors or in combination, A549 cells were cultured overnight and preincubated for 20 min with either Bay11-7082 (10 μM) or SP-600125 (10 μM) or a combination of Bay11-7082 (10 μM) and SP-600125 (10 μM). Ox-AT (0.3 mg/ml) was added 20 min later. Cells were cultured for 24 h, and subsequently cultured supernatant was collected and quantified for IL-8 and MCP-1 production, as detailed previously.
Statistical analysis.
All statistical analysis was performed using SigmaStat and SPSS software (version 12.0.1, for Windows). The statistical significance was set at P < 0.05. Data are presented as means (SE).
RESULTS
Effect of conformations of AT on the production of IL-8 and MCP-1 from A549 cells.
A549 cells were treated for 4, 10, and 24 h separately with Ox-AT, N-AT, Cl-AT, LP-AT, and SP-AT at different final concentrations: 0.03, 0.1, or 0.3 mg/ml. LPS 0.01 μg/ml (LPS 0.01) and PBS were negative controls. This LPS dose was chosen as the conformations of AT had a contaminating dose of less than 1 ng/ml LPS. TNFα was used as a positive control.
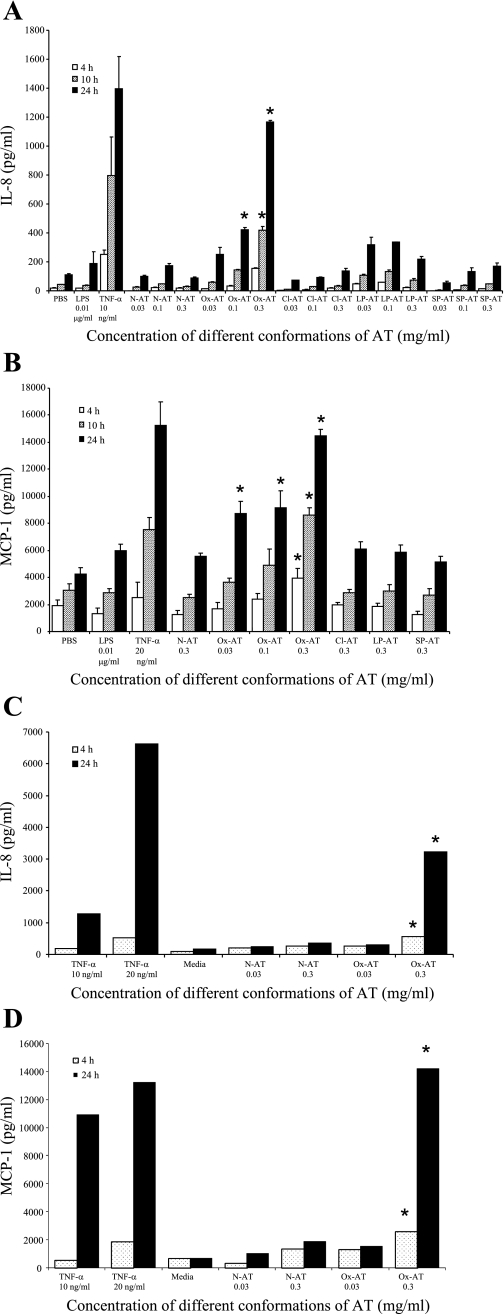
Ox-AT significantly induced the production of IL-8 in A549 cells in a time- and dose-dependent manner [Ox-AT 0.1, 24 h, IL-8 (means SE) = 424(14) pg/ml vs. PBS 24 h, IL-8 = 110(8) pg/ml, P = 0.029; Ox-AT 0.3, 10 h, IL-8 = 418(26) pg/ml vs. PBS 10 h, IL-8 = 43(3) pg/ml, P = 0.049; Ox-AT 0.3, 24 h, IL-8 = 1,168(9) pg/ml vs. PBS 24 h, P = 0.008] (Fig. 2A). N-AT, Cl-AT, LP-AT, and SP-AT had no significant effect on IL-8 production. A low concentration of LPS (0.01 μg/ml) did not affect IL-8 secretion.
Fig. 2.
A: effect of different conformations of AT on production of IL-8 from A549 cells. Ox-AT significantly induced IL-8 production in A549 cells (Ox-AT 0.1 mg/ml for 24 h, P = 0.029; Ox-AT 0.3 mg/ml for 10 h, P = 0.029; for 24 h, P = 0.008 vs. PBS). N-AT, Cl-AT, LP-AT, and SP-AT had no significant effect on IL-8 production. TNFα (10 ng/ml) was used as a positive control. LPS (0.01 μg/ml) and PBS were negative controls. The LPS (10 ng/ml) control was assessed as the samples of AT had contamination of 1 ng/ml LPS. This contamination of LPS had no effect on IL-8 production. Data are means (SE) from at least 5 experiments, *P < 0.05. B: effect of different conformations of AT on production of MCP-1 from A549 cells. Ox-AT significantly induced MCP-1 production in A549 cells (Ox-AT 0.3 mg/ml for 4, 10, and 24 h, P < 0.05 vs. PBS; Ox-AT 0.1 and 0.03 mg/ml for 24 h, P < 0.05 vs. PBS). N-AT, Cl-AT, SP-AT, and LP-AT had no significant effect on MCP-1 production. LPS (10 ng/ml) and PBS were negative controls. TNFα (20 ng/ml) was used as a positive control. Data are means (SE) from at least 5 experiments, *P < 0.05. C: effect of different conformations of AT on production of IL-8 from normal human bronchial epithelial (NHBE) cells. Ox-AT (0.3 mg/ml) significantly induced IL-8 production in NHBE cells compared with N-AT (0.3 mg/ml) at both 4 and 24 h (Ox-AT 0.3 mg/ml at 4 h, P = 0.039; Ox-AT 0.3 mg/ml at 24 h, P = 0.032). There was no significant increase in IL-8 production with 0.03 mg/ml Ox-AT compared with 0.03 mg/ml N-AT at 4 or 24 h. TNFα was used as a positive control. Culture media was used as a negative control. Data are representative of 3 independent experiments. *P < 0.05. D: effect of different conformations of AT on production of MCP-1 from NHBE cells. Ox-AT (0.3 mg/ml) significantly induced MCP-1 production in NHBE cells compared with N-AT (0.3 mg/ml) at 4 and at 24 h (Ox-AT 0.3 mg/ml at 4 h, P = 0.017; Ox-AT 0.3 mg/ml at 24 h, P = 0.014). TNFα was used as a positive control. Culture media was used as a negative control. Data are representative of 3 independent experiments, *P < 0.05.
Ox-AT significantly induced the production of MCP-1 in A549 cells [Ox-AT 0.03, 24 h, MCP-1 = 8,725(894) pg/ml vs. PBS 24 h, MCP-1 = 4,225(470) pg/ml, P = 0.041; Ox-AT 0.1, 24 h, MCP-1 = 9,167(1,229) pg/ml vs. PBS 24 h, P = 0.035; Ox-AT 0.3, 4 h, MCP-1 = 3,925(711) pg/ml vs. PBS 4 h, MCP-1 = 1,900(424) pg/ml, P = 0.044; Ox-AT 0.3, 10 h, MCP-1 = 8,608(519) pg/ml vs. PBS 10 h, MCP-1 = 3,075(459) pg/ml, P = 0.028; Ox-AT 0.3, 24 h, MCP-1 = 14,500(424) pg/ml vs. PBS 24 h, P = 0.005]. N-AT, Cl-AT, LP-AT, and SP-AT had no significant effect on MCP-1 production. A low concentration of LPS (0.01 μg/ml) had no effect on MCP-1 production (Fig. 2B).
MCP-1 and IL-8 can be produced in response to TNFα; therefore, we also measured this cytokine in the supernatant. There was no significant TNFα secretion in response to Ox-AT (data not shown).
Effect of different conformations of AT on the production of IL-8 and MCP-1 from NHBE cells.
NHBE cells were treated for 4 and 24 h separately with Ox-AT or N-AT at different final concentrations of 0.03 or 0.3 mg/ml. Ox-AT significantly induced the production of IL-8 in NHBE cells in a time- and dose-dependent manner compared with N-AT (Ox-AT 0.3 mg/ml, at 4 h, IL-8 = 565.9 pg/ml, P = 0.039; Ox-AT 0.3, 24 h, IL-8 = 3,223.7 pg/ml, P = 0.032). N-AT (0.03 or 0.3 mg/ml) had no significant effect on IL-8 production at 4 or 24 h. The proinflammatory cytokine TNFα (10 or 20 ng/ml) was used as a positive control, and culture media was used as a negative control (Fig. 2C).
Ox-AT significantly induced MCP-1 production in NHBE cells in time- and dose-dependent manner compared with N-AT (0.03 mg/ml) at 4 and 24 h (Ox-AT 0.3 mg/ml at 4 h, MCP-1 = 2,557.4 pg/ml, P = 0.017; Ox-AT 0.3 mg/ml at 24 h, MCP-1 = 14,213.6 pg/ml, P = 0.014). There was no significant increase in MCP-1 production with 0.03 mg/ml Ox-AT compared with 0.03 mg/ml N-AT at 4 or 24 h. TNFα was used as a positive control. Culture media was used as a negative control (Fig. 2D).
Effect of oxidized SLPI and oxidized cleaved and polymeric AT on the production of IL-8 and MCP-1 from A549 cells.
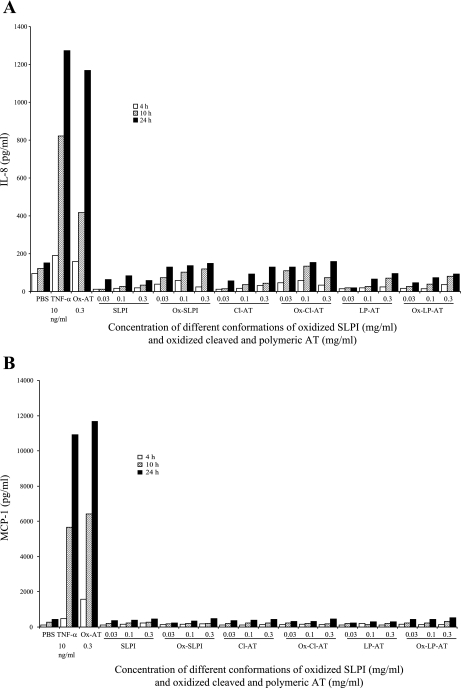
To assess whether the effect seen with Ox-AT was specific or a function of oxidized proteins in general, oxidized SLPI, Ox-Cl-AT, and Ox-LP-AT at final concentrations of 0.03, 0.1, or 0.3 mg/ml were added to A549 cells. The supernatant was removed at 4, 10, and 24 h and quantified by ELISA. None of these oxidized proteins had any significant effect on the production of IL-8 or MCP-1 (Fig. 3, A and B).
Fig. 3.
A: effect of oxidized secretory leukocyte protease inhibitor (SLPI), oxidized cleaved AT, and polymeric AT on production of IL-8 from A549 cells. There was no significant increase in IL-8 production due to treatment with Ox-SLPI, Ox-Cl-AT, or Ox-LP-AT compared with PBS. TNFα (10 ng/ml) and Ox-AT (0.3 mg/ml) were used as positive controls. PBS was the negative control. Data are representative of 3 independent experiments. B: effect of Ox-SLPI, oxidized cleaved, and polymeric AT on production of MCP-1 from A549 cells. There was no significant increase in MCP-1 production due to treatment with Ox-SLPI, Ox-Cl-AT, or Ox-LP-AT compared with PBS. TNFα (10 ng/ml) and Ox-AT (0.3 mg/ml) were used as positive controls. PBS was negative control. Data are representative of 3 independent experiments.
Effect of Ox-AT on NF-κB p50 activation in A549 cells.
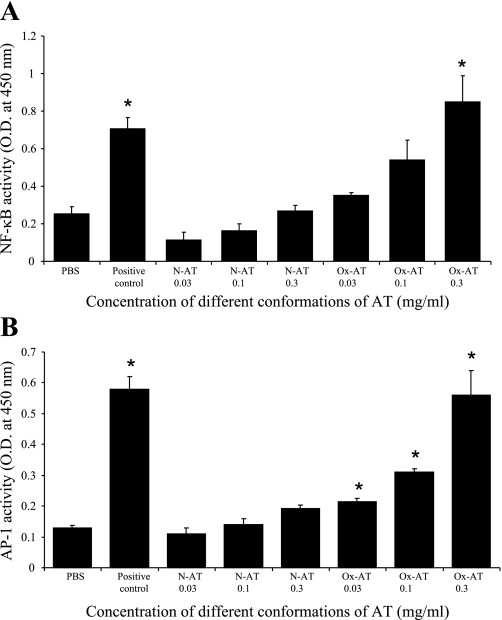
Ox-AT increased the activity of NF-κB significantly in a concentration-dependent manner compared with PBS (P = 0.014). N-AT had no effect on the activity of NF-κB (Fig. 4A). The positive control was Jurkat cells with high endogenous NF-κB p50 activity.
Fig. 4.
A: effect of Ox-AT on induction of NF-κB activity from A549 cells. There was a significant increase in NF-κB activity with 0.3 mg/ml Ox-AT compared with PBS (P = 0.014). The positive control was Jurkat cells with high endogenous NF-κB activity (P = 0.004 compared with PBS). Data are means (SE) from 3 separate experiments, *P < 0.05. B: effect of Ox-AT on induction of AP-1 activity from A549 cells. There was a significant increase in AP-1 activity induced by Ox-AT compared with PBS-treated cells (0.03 mg/ml Ox-AT, P = 0.024; 0.1 mg/ml Ox-AT, P < 0.001; 0.3 mg/ml Ox-AT, P = 0.005). The positive control was K562 cells with high endogenous AP-1 activity (P < 0.001 compared with PBS). Data are means (SE) from 3 separate experiments, *P < 0.05.
Effect of Ox-AT on AP-1 activation in A549 cells.
Ox-AT increased the activity of AP-1 significantly in a concentration-dependent manner compared with PBS (0.03 mg/ml Ox-AT, P = 0.024; 0.1 mg/ml Ox-AT, P < 0.001; 0.3 mg/ml Ox-AT, P = 0.005). N-AT had no significant effect on the activity of AP-1 (Fig. 4B). The positive control was K562 cells with high endogenous AP-1 activity.
Effect of SP-600125 (JNK inhibitor) on the activation of JNK in A549 cells treated with Ox-AT.
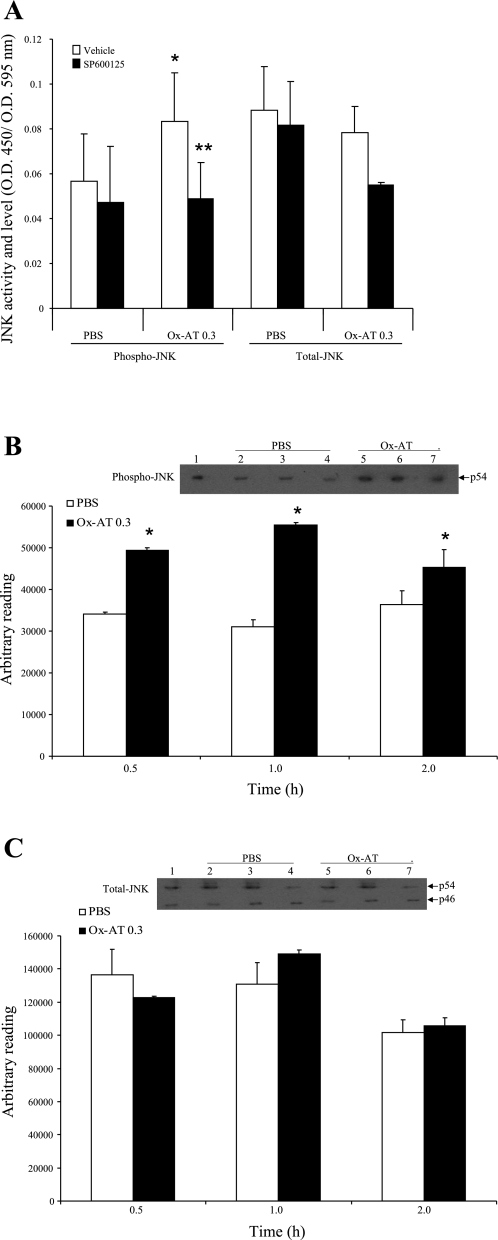
Ox-AT significantly increased the level of activated JNK [Ox-AT, OD450/OD595 = 0.084(0.021) vs. PBS, OD450/OD595 = 0.057(0.021), P = 0.001] but had no effect on total JNK (P = 0.7). The ability of Ox-AT to induce JNK activation was significantly blocked by the JNK inhibitor SP-600125: OD450/OD595 = 0.049(0.016), P = 0.016 vs. Ox-AT. SP-600125 had no effect on the total level of JNK in cells of both PBS group and Ox-AT group (P = 0.109 and 0.171, respectively) (Fig. 5A).
Fig. 5.
A: effect of inhibitor of JNK on activation of JNK in A549 cells treated with Ox-AT. Ox-AT treatment increased level of activated JNK but not total JNK significantly compared with PBS (*P < 0.001, P = 0.7, respectively). SP-600125 significantly inhibited this effect of Ox-AT on activation of JNK (**P = 0.016). SP-600125 did not have any significant effect on total level of JNK in cells of both PBS group and Ox-AT (P = 0.109 and 0.171, respectively). Data are means (SE) from 4 separate experiments, *, **P < 0.05. B: Western blot analysis of NHBE cell lysate for phosphorylated JNK (phospho-JNK). Western blot analysis shows that p54 JNK isoform was expressed highly in Ox-AT (0.3 mg/ml)-treated cells compared with PBS-treated cells, which was further verified by densitometric analysis of each band using ImageJ software. Densitometric results show level of p54 phospho-JNK is significantly higher and peaking at 1 h in Ox-AT-treated cells compared with PBS-treated cells (0.5 h P < 0.001; 1 h P < 0.001; 2 h P = 0.043). Lane 1, positive control A431 cell lysate; lane 2, 0.5 h PBS; lane 3, 1 h PBS; lane 4, 2 h PBS; lane 5, 0.5 h Ox-AT; lane 6, 1 h Ox-AT; lane 7, 2 h Ox-AT. Gel is representative of 3 independent experiments. Data are means (SE) of 3 independent experiments, *P < 0.05. C: Western blot analysis of NHBE cell lysate for total JNK. Western blot analysis for total JNK shows total (phosphorylated and nonphosphorylated) JNK ranging in size between 46 and 54 kDa. Densitometric analysis of both bands from each sample (expressed cumulatively) using ImageJ software demonstrated that total JNK was expressed in comparable levels in both Ox-AT (0.3 mg/ml)- and PBS-treated cells. Densitometric analysis of bands showed there was no significant difference between Ox-AT- and PBS-treated cells at 0.5, 1, or 2 h. Lane 1, positive control A431 cell lysate; lane 2, 0.5 h PBS; lane 3, 1 h PBS; lane 4, 2 h PBS; lane 5, 0.5 h Ox-AT; lane 6, 1 h Ox-AT; lane 7, 2 h Ox-AT. Gel is representative from 3 independent experiments. Data are means (SE) of 3 independent experiments.
Western blot analysis of NHBE cell lysate for phospho-JNK.
Western blot analysis of NHBE cells demonstrated that phospho-JNK is induced by Ox-AT (0.3 mg/ml) compared with PBS-treated cells at 0.5, 1, and 2 h (Fig. 5B). Densitometric analysis using ImageJ software showed the level of phospho-JNK was significantly higher, peaking at 1 h in Ox-AT-treated cells compared with PBS-treated cells (0.5 h, P < 0.001; 1 h, P < 0.001; and 2 h, P = 0.043) (Fig. 5B). Western blot analysis for total JNK showed that Ox-AT had no significant effect on total JNK (Fig. 5C).
Effect of inhibitors of NF-κB, p38 MAPK, and JNK on the induction of IL-8 in A549 cells treated with Ox-AT.
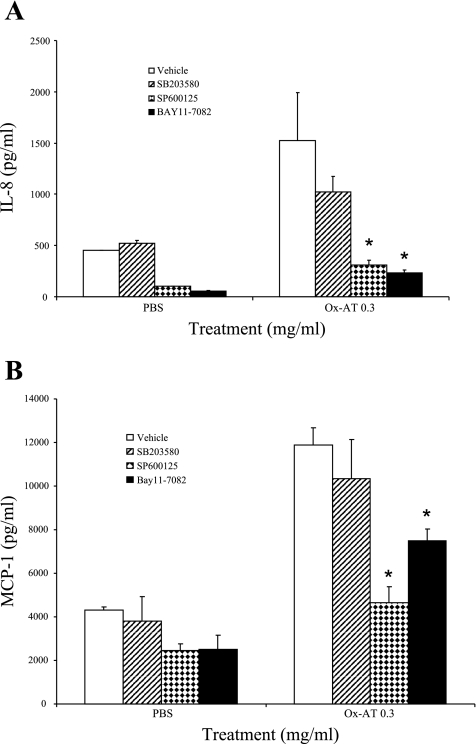
Bay11-7082, an inhibitor of NF-κB, SB-203580, an inhibitor of p38 MAPK, and SP-600125, an inhibitor of JNK, were separately added to the cell culture media of A549 cells 20 min before treatment with 0.3 mg/ml Ox-AT. Ox-AT-induced IL-8 production [IL-8 = 1526.133(270.03)] was significantly reduced in the presence of the NF-κB inhibitor [Ox-AT + Bay11-7082, IL-8 = 231(33) pg/ml vs. Ox-AT (P = 0.023)] and the JNK inhibitor [Ox-AT + SP-600125, IL-8 = 311(47) pg/ml vs. Ox-AT (P = 0.027)]. There was no significant reduction in Ox-AT-induced IL-8 production in the presence of the MAPK inhibitor (Ox-AT + SB203580, P = 0.379) (Fig. 6A).
Fig. 6.
A: effect of inhibitors of NF-κB, p38 MAPK, and JNK on induction of IL-8 and MCP-1 treated with Ox-AT. Ox-AT (0.3 mg/ml) significantly induced IL-8 production in A549 cells (P < 0.001), but this was significantly reduced by Bay11-7082 (P = 0.023) and SP-600125 (P = 0.027). SB-203580 had no significant effect on IL-8 production. Data are means (SE) from 4 separate experiments, *P < 0.05. B: SP-600125 (10 μM) significantly inhibited MCP-1 production in A549 cells (P = 0.003 vs. Ox-AT). Bay11-7082 (10 μM) also had an inhibitory effect on MCP-1 production (P < 0.05 vs. Ox-AT), but SB-203580 had no significant effect on MCP-1 production. Data are means (SE) from 4 separate experiments, *P < 0.05.
Effect of inhibitors of NF-κB, p38 MAPK, and JNK on the induction of MCP-1 in A549 cells treated with Ox-AT.
Under the same experimental conditions, as above, Ox-AT-induced MCP-1 production [MCP-1 = 11,188(780)] was significantly reduced in the presence of the NF-κB inhibitor [Ox-AT + Bay11-7082, MCP-1 = 7,502(524) pg/ml vs. Ox-AT, P = 0.049] and the JNK inhibitor [Ox-AT + SP-600125, MCP-1 = 4,658(727) pg/ml vs. Ox-AT, P = 0.003]. There was no significant effect on Ox-AT-induced MCP-1 production in the presence of the MAPK inhibitor (Ox-AT + SB-203580) (Fig. 6B).
Effect of inhibitors of NF-κB and JNK on Ox-AT-induced AP-1 and NF-κB activity.
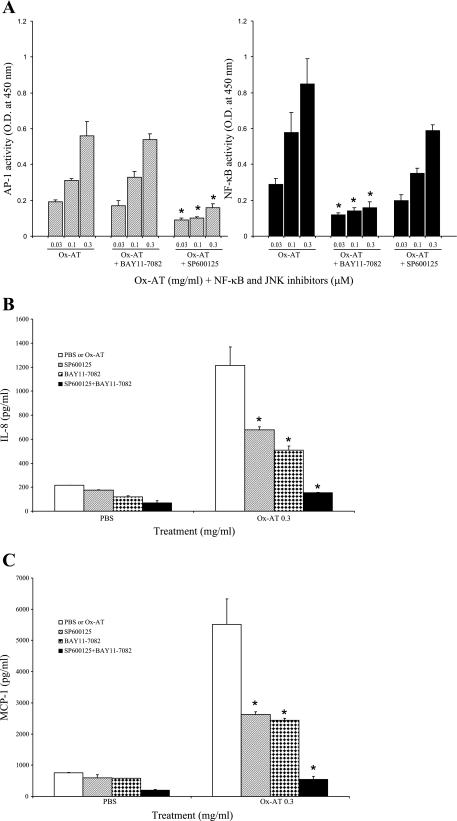
A549 cells were treated with either the NF-κB inhibitor (Bay11-7082, 10 μM) or the JNK inhibitor (SP-5600125, 10 μM) dissolved in 10% DMSO followed by 0.03, 0.1, or 0.3 mg/ml Ox-AT (Fig. 7). The pharmacological inhibitor of JNK (SP-600125) is a small molecule that exhibits high specificity for JNK, which results in inhibition of JNK-induced AP-1 activation (31, 40); therefore, we used the inhibitor SP-600125 to study Ox-AT-induced AP-1 activity. Ox-AT induced AP-1 activity in a dose-dependent manner. The Ox-AT-induced AP-1 activity was significantly inhibited in the presence of SP-600125 (0.03 mg/ml Ox-AT, P = 0.007; 0.1 mg/ml Ox-AT, P < 0.001; and 0.3 mg/ml Ox-AT, P = 0.006). However, Bay11-7082 had no effect on AP-1 activity. Ox-AT induced NF-κB activity in a dose-dependent manner. Bay11-7082 significantly inhibited Ox-AT-induced NF-κB activity (0.03 mg/ml Ox-AT, P < 0.001; 0.1 mg/ml Ox-AT, P = 0.018; and 0.3 mg/ml Ox-AT, P = 0.007). There was no significant difference in NF-κB activity after addition of the JNK (AP-1 pathway inhibitor) inhibitor.
Fig. 7.
A: effect of inhibitors of NF-κB and JNK on Ox-AT-induced AP-1 and NF-κB activity. Ox-AT-induced AP-1 activity in the presence of SP-600125 was significantly inhibited (0.03 mg/ml Ox-AT, P = 0.007; 0.1 mg/ml Ox-AT, P < 0.001; 0.3 mg/ml Ox-AT, P = 0.006). However, in the presence of Bay11-7082, AP-1 activity was not affected. Ox-AT-induced NF-κB activity in the presence of Bay11-7082 was significantly inhibited (0.03 mg/ml Ox-AT, P < 0.001; 0.1 mg/ml Ox-AT, P = 0.018; 0.3 mg/ml Ox-AT, P = 0.007). SP-600125 did not have any significant effect on Ox-AT-induced NF-κB activity. B: relative effect of NF-κB and JNK inhibitors on induction of IL-8 treated with Ox-AT. There was a significant inhibition of Ox-AT (0.3 mg/ml)-induced IL-8 production in the presence of SP-600125 alone (P = 0.021) and Bay11-7082 alone (P = 0.01), with 46.9% and 58.9% reduction, respectively. Ox-AT (0.3 mg/ml)-induced IL-8 production was almost completely abolished (91.2% reduction) by the combination of SP-600125 and Bay11-7082 (P = 0.003). C: relative effect of NF-κB and JNK inhibitors on induction of MCP-1 by Ox-AT. There was a significant inhibition of Ox-AT (0.3 mg/ml)-induced MCP-1 production in the presence of SP-600125 alone (P = 0.024) and Bay11-7082 alone (P = 0.019), with 55.4% and 59.2% reduction, respectively. Ox-AT (0.3 mg/ml)-induced MCP-1 production was almost completely abolished (92.2% reduction) by the combination of SP-600125 and Bay11-7082 (P = 0.004). *P < 0.05.
Relative effect of NF-κB and JNK inhibitors on the induction of IL-8 treated with Ox-AT.
Ox-AT-induced IL-8 production [IL-8 = 1,212.6 (153.7)] was significantly reduced in the presence of SP-600125 (10 μM) alone [IL-8 = 678.5(25.2), P = 0.021] and Bay11-7082 (10 μM) alone [IL-8 = 508.5(33.7), P = 0.01], with 46.9% and 58.9% reduction, respectively. Ox-AT-induced IL-8 production was almost completely abolished (91.2%) in the presence of both SP-600125 and Bay11-7082 [IL-8 = 153.13(2.4), P = 0.003]. These data show that the combination of both inhibitors (SP-600125 and Bay11-7082) acts additively to reduce IL-8 production.
Effect of inhibitors of NF-κB and JNK on the induction of MCP-1 treated with Ox-AT.
Ox-AT-induced MCP-1 production [MCP-1 = 5,518.65(810.5)] was significantly reduced in the presence of SP-600125 (10 μM) alone [MCP-1 = 2,929.57(85.59), P = 0.024] and Bay11-7082 (10 μM) alone [MCP-1 = 2,443.1(64.4), P = 0.019], with a 55.4% and 59.2% reduction, respectively. Ox-AT-induced MCP-1 production was significantly reduced (92.2%) in the presence of both SP-600125 and Bay11-7082 [MCP-1 = 550.73(99.16), P = 0.004]. The data show that the combination of both inhibitors (SP-600125 and Bay11-7082) acts additively to reduce MCP-1 production.
Effect of intratracheal instillation of Ox-AT and N-AT in murine lungs.
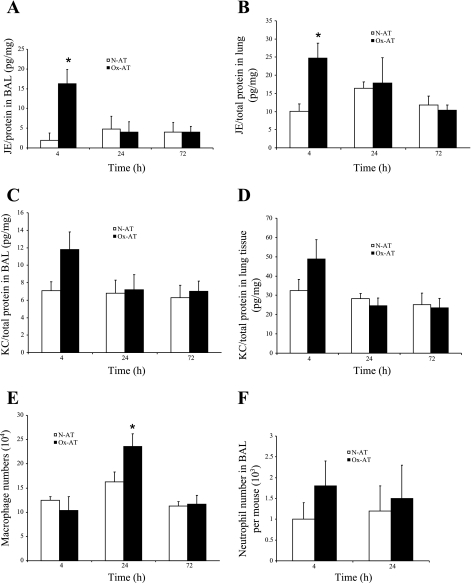
Intratracheal instillation of Ox-AT resulted in increased JE in the airway and in the lung tissue. In the BAL fluid, 4 h after Ox-AT, there was a significant increase in JE/total protein 16.2(3.6) pg/mg vs. N-AT 2.9(1.9) pg/mg, P = 0.037. Concentrations of JE had returned to baseline by 24 h, and there was no significant difference; Ox-AT 4(2.6) pg/mg vs. N-AT 4.8(3.2) pg/mg, P = 0.85 (Fig. 8A).
Fig. 8.
Effect of intratracheal instillation of Ox-AT and N-AT on lung cytokines and inflammatory cells. A: Ox-AT can significantly induce JE production in BAL. After 4-h Ox-AT group, JE/total protein 16.2(5.6) pg/mg vs. N-AT 2.9(1.9) pg/mg, P = 0.037; 24-h Ox-AT 4(2.6) pg/mg vs. N-AT 4.8 (3.2) pg/mg, P = 0.85. *P < 0.05. B: in homogenized lung tissue, 4-h Ox-AT group, JE/total protein 24.7(4.1) pg/mg vs. N-AT 10.1(2) pg/mg, P = 0.05; 24-h Ox-AT 17.9(6.9) pg/mg vs. N-AT 16.4(1.75) pg/mg, P = 0.84. C: Ox-AT did not significantly affect KC production in BAL fluid. In BAL, 4-h Ox-AT, KC/total protein 11.8(2.1) pg/mg vs. N-AT 7.1(1) pg/mg, P = 0.11; 24-h Ox-AT 7.2(1.7) pg/mg vs. N-AT 6.8 (1.5) pg/mg, P = 0.43. D: in homogenized lung tissue after 4-h Ox-AT, KC/total protein 48.9(10) pg/mg vs. N-AT 32.5(5.8) pg/mg, P = 0.10; 24-h Ox-AT 24.7(4) pg/mg vs. N-AT 28.2(2.7) pg/mg, P = 0.31. E: there was an increase in number of macrophages in BAL fluid after intratracheal instillation of Ox-AT. The macrophages in BAL 24-h Ox-AT group 23.6(2.8) × 104 vs. N-AT 16.3(0.7) × 104, P = 0.03; but 4-h Ox-AT 12.5(2) × 104 vs. 10.4(2.6) × 104, P = 0.53. F: there was an increase in number of neutrophil influx in BAL fluid after instillation of Ox-AT compared with N-AT. However, Ox-AT did not have any statistically significant effect on number of neutrophils in BAL. At 4-h Ox-AT group, 1.8(0.6) × 104 neutrophils vs. N-AT 1(0.4) × 104, P = 0.19; 24-h Ox-AT 1.5(0.8) × 104 vs. N-AT 1.2(0.4) × 104, P = 0.24.
There was also a significant increase in JE in homogenized lung tissue 4 h after instillation of Ox-AT. JE/total protein was 24.7(4.1) pg/mg vs. N-AT 10.1(2) pg/mg, P = 0.05. After 24-h Ox-AT treatment, there was no significant change; 17.9(6.9) pg/mg vs. N-AT 16.4(1.75) pg/mg, P = 0.84 (Fig. 8B).
There was a trend towards increased KC production in the airway and in lung tissue, which was not statistically significant. In the BAL fluid, after 4 h, Ox-AT KC/total protein 11.8(2.1) pg/mg vs. N-AT 7.1(1) pg/mg, P = 0.11; 24 h, Ox-AT 7.2(1.7) pg/mg vs. N-AT 6.8 (1.5) pg/mg, P = 0.43 (Fig. 8C). In the homogenized lung tissue after 4 h, Ox-AT KC/total protein 48.9 (10) pg/mg vs. N-AT 32.5(5.8) pg/mg, P = 0.10; 24 h, Ox-AT 24.7(4) pg/mg vs. N-AT 28.2(2.7) pg/mg, P = 0.31 (Fig. 8D). There was no effect of Ox-AT on secretion of TNFα in BAL fluid or lung tissue.
Effect on BAL cell profile.
Intratracheal instillation of Ox-AT was found to significantly induce macrophage influx after 24 h; Ox-AT 23.6(2.8) × 104 vs. N-AT 16.3(0.7) × 104, P = 0.03 (Fig. 8E). Ox-AT did not have any significant effect on the number of neutrophils in BAL. At 4 h, Ox-AT group 1.8(0.6) × 104 neutrophils vs. N-AT 1(0.4) × 104, P = 0.19; 24 h, Ox-AT 1.5(0.8) × 104 vs. N-AT 1.2(0.4) × 104, P = 0.24 (Fig. 8F).
Effect of Ox-AT on NF-κB p50 activity in lung tissue.
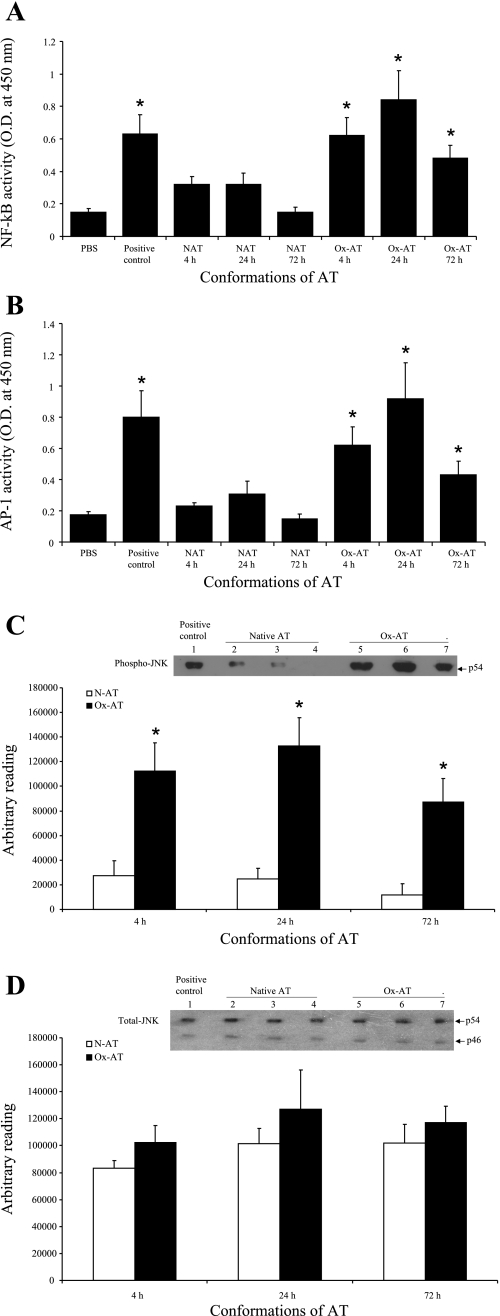
Following intratracheal instillation of Ox-AT, NF-κB was found to be significantly increased in lung homogenates in a time-dependent manner compared with PBS (4 h, P = 0.028; 24 h, P = 0.029; and 72 h, P = 0.037). Intratracheal N-AT had no significant effect on the activity of NF-κB in lung homogenates (Fig. 9A).
Fig. 9.
A: effect of Ox-AT on induction of NF-κB activity in lung tissue. There was a significant increase in NF-κB activity following intratracheal Ox-AT compared with PBS (4 h, P = 0.028; 24 h, P = 0.029; 72 h, P = 0.037). Intratracheal N-AT did not have any significant effect on NF-κB activity. The positive control was Jurkat cells with high endogenous NF-κB activity (P = 0.032 compared with PBS). Data are means (SE) from 3 separate experiments. *P ≤ 0.05. B: effect of Ox-AT on induction of AP-1 activity in lung tissue. There was a significant increase in AP-1 activity following intratracheal Ox-AT compared with PBS (4 h, P = 0.019; 24 h, P = 0.033; 72 h, P = 0.049). Intratracheal N-AT did not have any significant effect on AP-1 activity. The positive control was K562 cells with high endogenous AP-1 activity (P = 0.022 compared with PBS). Data are means (SE) from 3 separate experiments. *P ≤ 0.05. C: Western blot analysis of homogenized lung tissue for phosphorylated JNK. The p54 JNK isoform was expressed highly in lung homogenates from Ox-AT-treated mice compared with lung homogenates from N-AT-treated mice. Densitometric analysis of each band using ImageJ software demonstrated the level of p54 phospho-JNK was significantly higher and peaking at 24 h in lung homogenates from Ox-AT-treated mice compared with lung homogenates from N-AT-treated mice (4 h, P = 0.031; 24 h, P = 0.012; 72 h, P = 0.023). Lane 1, positive control A431 cell lysate; lane 2, 4 h N-AT; lane 3, 24 h N-AT; lane 4, 72 h N-AT; lane 5, 2 h Ox-AT; lane 6, 24 h Ox-AT; lane 7, 72 h Ox-AT. Gel is representative of 3 independent experiments. Data are means (SE) of 3 independent experiments. *P ≤ 0.05. D: Western blot analysis of homogenized lung tissues for total JNK. Western blot analysis for total JNK shows total (phosphorylated and nonphosphorylated) JNK ranging in size between 46 and 54 kDa. Densitometric analysis of both bands from each sample (expressed cumulatively) using ImageJ software demonstrated total JNK was expressed in comparable levels in lung homogenates from both Ox-AT- and N-AT-treated mice. Densitometric analysis of bands showed there was no significant difference in total JNK in lung homogenates from Ox-AT- and N-AT-treated mice at 4, 24, or 72 h. Lane 1, positive control A431 cell lysate; lane 2, 4 h N-AT; lane 3, 24 h N-AT; lane 4, 72 h N-AT; lane 5, 4 h Ox-AT; lane 6, 24 h Ox-AT; lane 7, 72 h Ox-AT. Gel is representative of 3 independent experiments. Data are means (SE) of 3 independent experiments.
Effect of Ox-AT on AP-1 activity in lung tissue.
Intratracheal Ox-AT resulted in a significantly increased activity of AP-1 in lung homogenates in a time-dependent manner compared with PBS (4 h, P = 0.019; 24 h, P = 0.033; and 72 h, P = 0.049). Intratracheal N-AT had no significant effect on the activity of AP-1 in lung homogenates (Fig. 9B).
Western blot analysis of lung tissue for JNK.
Western blot analysis of homogenized lung tissue demonstrated that phospho-JNK was induced by Ox-AT compared with N-AT at 4 h, 24 h, and 72 h (Fig. 9C). Densitometric analysis using ImageJ software showed that the level of phospho-JNK was significantly higher in lung homogenates from Ox-AT-treated mice, peaking at 24 h compared with lung homogenates from N-AT-treated mice (4 h, P = 0.031; 24 h, P = 0.012; and 72 h, P = 0.023) (Fig. 9C). There was no significant difference between the amount of total JNK detected from lung homogenates from Ox-AT- and N-AT-treated mice at 4, 24, and 72 h (Fig. 9D).
DISCUSSION
AT is the most abundant proteinase inhibitor within the lung whose main physiological target is neutrophil elastase. Studies have demonstrated the importance of AT in protecting lung tissues against proteolytic damage from neutrophil elastase, and individuals with severe AT deficiency are highly susceptible to early-onset emphysema. The P1 methionine, which determines the specificity and inhibitory activity of AT, is the most readily oxidized amino acid of proteins. Oxidation of the P1 methionine significantly reduces the ability of AT to inhibit neutrophil elastase. It can be oxidized by cigarette smoke-derived oxidants or those produced in vivo such as peroxide, hydroxyl radicals, chloramines, inducible nitric oxide, and peroxynitrite (4, 28, 58). Polymorphonuclear leukocytes and macrophages secrete large quantities of oxidants at sites of inflammation and have been shown to oxidize AT and thus potentially disturb the proteinase-antiproteinase equilibrium (58).
Ox-AT has been found in inflammatory exudates at levels of ∼5–10% that of total AT (63), and AT recovered from BAL fluid in smokers is 40% less active compared with nonsmokers due to oxidation of the P1 methionine to methionine sulfoxide (11). The oxidation of AT by cigarette smoke or free radicals in vivo has been proposed as a mechanism by which elastin and thus alveolar destruction occurs in COPD (5, 6, 11, 19).
In this study, we found that Ox-AT significantly induces the production of IL-8 and MCP-1 from a lung epithelial cell line (A549 cells) in a time- and dose-dependent manner. TNFα secretion was not affected by Ox-AT. None of the other AT conformations, such as native, cleaved, or polymerized AT had any significant effect on IL-8 or MCP-1 production. We also confirmed that Ox-AT induced production of both IL-8 and MCP-1 in primary NHBE cells.
To address the question of whether the ability of Ox-AT to induce IL-8 and MCP-1 was unique or simply a function of oxidized proteins, we repeated the experiment with Ox-SLPI, Ox-Cl-AT, and Ox-LP-AT. We found that none of these oxidized proteins was able to stimulate IL-8 or MCP-1 secretion from A549 cells.
Our study in A549 cells and NHBE cells demonstrated that Ox-AT increased NF-κB and AP-1 activity and promoted activation of JNK. Furthermore, inhibition of the NF-κB and the JNK/AP-1 pathways inhibited production of IL-8 and MCP-1. Both pathways appear to act independently and equally to mediate the effects of Ox-AT.
To assess the relevance of the cell data, we instilled Ox-AT and N-AT into C57BL/6 murine lungs. Instillation of Ox-AT resulted in an increase in JE (murine equivalent of MCP-1) in the BAL fluid and lung tissue. Furthermore, there was an increase in macrophage numbers in BAL fluid. Although there was a trend of an increase in murine KC and neutrophils in BAL fluid after instillation of Ox-AT, this was not statistically significant. This effect appears to be specific for Ox-AT as there was no significant effect on BAL chemokines after instillation of the native protein or as previously demonstrated after intratracheal instillation of polymerized AT (36).
Further in vivo analysis demonstrated that Ox-AT significantly increased both NF-κB and AP-1 activity assessed in lung homogenates, peaking at 24 h after instillation of Ox-AT. Furthermore, Ox-AT promoted activation of JNK. These results suggest involvement of both NF-κB and AP-1 pathways in mediating effects of Ox-AT in vivo and provide strong support for our cell data.
COPD is characterized by an infiltration of neutrophils, macrophages, and CD8+ T lymphocytes (3). The exact processes that lead to this inflammatory response are not clear. However, CC and CXC chemokines and their receptors such as TNFα, IL-8, MIP-1α, and MCP-1 have been shown to play a role in COPD (2, 7, 8, 12, 15, 28, 23, 24, 43, 59). The important role of these molecules has been suggested by the finding that mice deficient in CCR5 or CCR6 are partially protected from the development of pulmonary emphysema (7).
IL-8 is a member of the CXC subfamily of chemokines and is a potent neutrophil chemoattractant that has been implicated in the development of COPD (12, 18, 23, 24, 43, 69). It can be produced by a variety of cell types, including human bronchial epithelial cell monocyte/macrophages, T cells, neutrophils, fibroblasts, and endothelial cells in response to proinflammatory stimuli such as IL-1, TNFα, LPS, and viruses. IL-8 binds to two seven-transmembrane, G protein-coupled receptors, CXCR1 and CXCR2, as well as to the nonsignaling Duffy antigen on red blood cells. An excess of IL-8 has been reported in the sputum and BAL from individuals with COPD (23, 24, 59). The bronchiolar epithelium and alveolar type II cells are rich sources of IL-8, and constitutive and stimulated release of IL-8 from human bronchial epithelial cells from COPD patients is greater than that released from control cells (18, 51, 62).
MCP-1 belongs to the CC chemokine family and functions as a chemoattractant for mononuclear phagocytes, CD45RO+ T lymphocytes, B cells, and NK cells. Increased MCP-1 and its receptor, CCR2, in the lung have been positively associated with leukocyte infiltration in COPD (10, 18).
Our findings suggest that the oxidation of methionines in AT by oxidants in cigarette smoke or those released from inflammatory cells not only reduces the effective antielastase protection in the lung but also converts AT into a proinflammatory stimulus. Ox-AT generated in the airway interacts directly with epithelial cells to release chemokines. These chemokines attract macrophages into the airways. Our data also demonstrate that macrophages can be attracted into the airways without being preceded by neutrophil influx. Although our findings are not the sole mechanism for the development of COPD, they further support the role of the macrophage in the pathophysiology of lung inflammation in COPD. Release of oxidants by these inflammatory cells could oxidize newly synthesized AT, which has diffused into the airways and would perpetuate the cycle. This process may be amplified by Ox-AT induction of MCP-1 synthesis from monocytes (41). These pathways may be one explanation as to why inflammation persists after smoking cessation in COPD (49).
Furthermore, these data demonstrate how common paradigms and mediators in COPD, namely proteinase-antiproteinase and oxidant-antioxidant balance, chemokines, and inflammatory cells, interlink to cause COPD.
GRANTS
This work was funded by Alpha One Foundation, Wellcome Trust, Action Medical Research, Zoega Foundation, Swedish Research council, MAS Foundation, and Lundstrom Foundation. This work was supported by the Cambridge National Institute for Health Research Biomedical Research Centre.
REFERENCES
- 1.Banda MJ, Rice AG, Griffin GL, Senior RM. The inhibitory complex of human alpha 1-proteinase inhibitor and human leukocyte elastase is a neutrophil chemoattractant. J Exp Med 167: 1608–1615, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes PJ Cytokine modulators as novel therapies for airway disease. Eur Respir J, Suppl 34: 67s–77s, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 22: 672–688, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Beatty K, Bieth J, Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem 255: 3931–3934, 1980. [PubMed] [Google Scholar]
- 5.Beatty K, Matheson N, Travis J. Kinetic and chemical evidence for the inability of oxidized alpha 1-proteinase inhibitor to protect lung elastin from elastolytic degradation. Hoppe Seylers Z Physiol Chem 365: 731–736, 1984. [DOI] [PubMed] [Google Scholar]
- 6.Beatty K, Robertie P, Senior RM, Travis J. Determination of oxidized alpha-1-proteinase inhibitor in serum. J Lab Clin Med 100: 186–192, 1982. [PubMed] [Google Scholar]
- 7.Bracke KR, D'Hulst AI, Maes T, Moerloose KB, Demedts IK, Lebecque S, Joos GF, Brusselle GG. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol 177: 4350–4359, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Brandsma CA, Hylkema MN, Luinge MA, Geerlings M, Klok PA, Cassee FR, Timens W, Postma DS, Kerstjens HA. Nitrogen dioxide exposure attenuates cigarette smoke-induced cytokine production in mice. Inhal Toxicol 20: 183–189, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Brantly M, Nukiwa T, Crystal RG. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med 84: 13–31, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Capelli A, Di Stefano A, Gnemmi I, Balbo P, Cerutti CG, Balbi B, Lusuardi M, Donner CF. Increased MCP-1 and MIP-1beta in bronchoalveolar lavage fluid of chronic bronchitics. Eur Respir J 14: 160–165, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Carp H, Miller F, Hoidal JR, Janoff A. Potential mechanism of emphysema: alpha 1-proteinase inhibitor recovered from lungs of cigarette smokers contains oxidized methionine and has decreased elastase inhibitory capacity. Proc Natl Acad Sci USA 79: 2041–2045, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung KF Cytokines in chronic obstructive pulmonary disease. Eur Respir J, Suppl 34: 50s–59s, 2001. [PubMed] [Google Scholar]
- 13.Dabbagh K, Laurent GJ, Shock A, Leoni P, Papakrivopoulou J, Chambers RC. Alpha-1-antitrypsin stimulates fibroblast proliferation and procollagen production and activates classical MAP kinase signalling pathways. J Cell Physiol 186: 73–81, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Daemen MA, Heemskerk VH, van't Veer C, Denecker G, Wolfs TG, Vandenabeele P, Buurman WA. Functional protection by acute phase proteins alpha(1)-acid glycoprotein and alpha(1)-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation 102: 1420–1426, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly LE, Barnes PJ. Chemokine receptors as therapeutic targets in chronic obstructive pulmonary disease. Trends Pharmacol Sci 27: 546–553, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Elliott PR, Bilton D, Lomas DA. Lung polymers in Z alpha1-antitrypsin deficiency-related emphysema. Am J Respir Cell Mol Biol 18: 670–674, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha 1-antitrypsin deficiency. N Engl J Med 314: 736–739, 1986. [DOI] [PubMed] [Google Scholar]
- 18.Fuke S, Betsuyaku T, Nasuhara Y, Morikawa T, Katoh H, Nishimura M. Chemokines in bronchiolar epithelium in the development of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 31: 405–412, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Gadek JE, Fells GA, Crystal RG. Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science 206: 1315–1316, 1979. [DOI] [PubMed] [Google Scholar]
- 20.Ginzberg HH, Shannon PT, Suzuki T, Hong O, Vachon E, Moraes T, Abreu MT, Cherepanov V, Wang X, Chow CW, Downey GP. Leukocyte elastase induces epithelial apoptosis: role of mitochondial permeability changes and Akt. Am J Physiol Gastrointest Liver Physiol 287: G286–G298, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Graziadei I, Kahler CM, Wiedermann CJ, Vogel W. The acute-phase protein alpha 1-antitrypsin inhibits transferrin-receptor binding and proliferation of human skin fibroblasts. Biochim Biophys Acta 1401: 170–176, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Gross P, Tolker E, Babyak MA, Kaschak M. Experimental lung cancer in hamsters. Repetitive intratracheal applications of two carcinogenic hydrocarbons. Arch Environ Health 11: 59–65, 1965. [DOI] [PubMed] [Google Scholar]
- 23.Hill AT, Bayley DL, Campbell EJ, Hill SL, Stockley RA. Airways inflammation in chronic bronchitis: the effects of smoking and alpha1-antitrypsin deficiency. Eur Respir J 15: 886–890, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Hill AT, Campbell EJ, Bayley DL, Hill SL, Stockley RA. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with alpha(1)-antitrypsin deficiency (PiZ). Am J Respir Crit Care Med 160: 1968–1975, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature 407: 923–926, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Janciauskiene S, Lindgren S. Human monocyte activation by cleaved form of alpha-1-antitrypsin involvement of the phagocytic pathway. Eur J Biochem 265: 875–882, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Johnson D, Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem 254: 4022–4026, 1979. [PubMed] [Google Scholar]
- 29.Joslin G, Griffin GL, August AM, Adams S, Fallon RJ, Senior RM, Perlmutter DH. The serpin-enzyme complex (SEC) receptor mediates the neutrophil chemotactic effect of alpha-1 antitrypsin-elastase complexes and amyloid-beta peptide. J Clin Invest 90: 1150–1154, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamthong PJ, Wu M. Inhibitor of nuclear factor-kappaB induction by cAMP antagonizes interleukin-1-induced human macrophage-colony-stimulating-factor expression. Biochem J 356: 525–530, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol 9: 240–246, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Koch A, Giembycz M, Stirling RG, Lim S, Adcock I, Wassermann K, Erdmann E, Chung KF. Effect of smoking on MAP kinase-induced modulation of IL-8 in human alveolar macrophages. Eur Respir J 23: 805–812, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Laurell CB, Eriksson S. The electrophoretic α1-globulin pattern of serum in α1-antitrypsin deficiency. Scand J Clin Lab Invest 15: 132–140, 1963. [Google Scholar]
- 34.Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature 357: 605–607, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Lomas DA, Mahadeva R. Alpha1-antitrypsin polymerization and the serpinopathies: pathobiology and prospects for therapy. J Clin Invest 110: 1585–1590, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahadeva R, Atkinson C, Li Z, Stewart S, Janciauskiene S, Kelley DG, Parmar J, Pitman R, Shapiro SD, Lomas DA. Polymers of Z alpha1-antitrypsin co-localize with neutrophils in emphysematous alveoli and are chemotactic in vivo. Am J Pathol 166: 377–386, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahadeva R, Chang WS, Dafforn TR, Oakley DJ, Foreman RC, Calvin J, Wight DG, Lomas DA. Heteropolymerization of S, I, and Z alpha1-antitrypsin and liver cirrhosis. J Clin Invest 103: 999–1006, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McElvaney NG, Hubbard RC, Birrer P, Chernick MS, Caplan DB, Frank MM, Crystal RG. Aerosol alpha 1-antitrypsin treatment for cystic fibrosis. Lancet 337: 392–394, 1991. [DOI] [PubMed] [Google Scholar]
- 39.McGuire WW, Spragg RG, Cohen AB, Cochrane CG. Studies on the pathogenesis of the adult respiratory distress syndrome. J Clin Invest 69: 543–553, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta 1333: F85–F104, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Moraga F, Janciauskiene S. Activation of primary human monocytes by the oxidized form of alpha1-antitrypsin. J Biol Chem 275: 7693–7700, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Moraga F, Lindgren S, Janciaskiene S. Effects of noninhibitory alpha-1-antitrypsin on primary human monocyte activation in vitro. Arch Biochem Biophys 386: 221–226, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Morrison D, Strieter RM, Donnelly SC, Burdick MD, Kunkel SL, MacNee W. Neutrophil chemokines in bronchoalveolar lavage fluid and leukocyte-conditioned medium from nonsmokers and smokers. Eur Respir J 12: 1067–1072, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Mulgrew AT, Taggart CC, Lawless MW, Greene CM, Brantly ML, O'Neill SJ, McElvaney NG. Z alpha1-antitrypsin polymerizes in the lung and acts as a neutrophil chemoattractant. Chest 125: 1952–1957, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Ogden BE, Murphy SA, Saunders GC, Pathak D, Johnson JD. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis 130: 817–821, 1984. [DOI] [PubMed] [Google Scholar]
- 46.Ossanna PJ, Test ST, Matheson NR, Regiani S, Weiss SJ. Oxidative regulation of neutrophil elastase-alpha-1-proteinase inhibitor interactions. J Clin Invest 77: 1939–1951, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perlmutter DH Liver injury in alpha1-antitrypsin deficiency: an aggregated protein induces mitochondrial injury. J Clin Invest 110: 1579–1583, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrache I, Fijalkowska I, Medler TR, Skirball J, Cruz P, Zhen L, Petrache HI, Flotte TR, Tuder RM. alpha-1 Antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol 169: 1155–1166, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Retamales I, Elliott WM, Meshi B, Coxson HO, Pare PD, Sciurba FC, Rogers RM, Hayashi S, Hogg JC. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med 164: 469–473, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol 75: 2419–2424, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Schulz C, Wolf K, Harth M, Kratzel K, Kunz-Schughart L, Pfeifer M. Expression and release of interleukin-8 by human bronchial epithelial cells from patients with chronic obstructive pulmonary disease, smokers, and never-smokers. Respiration 70: 254–261, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Scott GK, Tse CA. Modulation of cell proliferation by protein proteinase inhibitors. A new analytical approach. Biol Chem Hoppe Seyler 369, Suppl: 131–135, 1988. [PubMed] [Google Scholar]
- 53.Scott LJ, Russell GI, Nixon NB, Dawes PT, Mattey DL. Oxidation of alpha1-proteinase inhibitor by the myeloperoxidase-hydrogen peroxidase system promotes binding to immunoglobulin A. Biochem Biophys Res Commun 255: 562–567, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Sharp HL, Bridges RA, Krivit W, Freier EF. Cirrhosis associated with alpha-1-antitrypsin deficiency: a previously unrecognized inherited disorder. J Clin Invest 73: 934–939, 1969. [PubMed] [Google Scholar]
- 55.Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O'Donnell E, Salvesen GS, Travis J, Whisstock JC. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem 276: 33293–33296, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Stratikos E, Gettins PG. Major proteinase movement upon stable serpin-proteinase complex formation. Proc Natl Acad Sci USA 94: 453–458, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki T, Moraes TJ, Vachon E, Ginzberg HH, Huang TT, Matthay MA, Hollenberg MD, Marshall J, McCulloch CA, Abreu MT, Chow CW, Downey GP. Proteinase-activated receptor-1 mediates elastase-induced apoptosis of human lung epithelial cells. Am J Respir Cell Mol Biol 33: 231–247, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taggart C, Cervantes-Laurean D, Kim G, McElvaney NG, Wehr N, Moss J, Levine RL. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem 275: 27258–27265, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Tanino M, Betsuyaku T, Takeyabu K, Tanino Y, Yamaguchi E, Miyamoto K, Nishimura M. Increased levels of interleukin-8 in BAL fluid from smokers susceptible to pulmonary emphysema. Thorax 57: 405–411, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uchida C, Gee E, Ispanovic E, Haas TL. JNK as a positive regulator of angiogenic potential in endothelial cells. Cell Biol Int 32: 769–776, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Van Molle W, Libert C, Fiers W, Brouckaert P. Alpha 1-acid glycoprotein and alpha 1-antitrypsin inhibit TNF-induced but not anti-Fas-induced apoptosis of hepatocytes in mice. J Immunol 159: 3555–3564, 1997. [PubMed] [Google Scholar]
- 62.Witherden IR, Vanden Bon EJ, Goldstraw P, Ratcliffe C, Pastorino U, Tetley TD. Primary human alveolar type II epithelial cell chemokine release: effects of cigarette smoke and neutrophil elastase. Am J Respir Cell Mol Biol 30: 500–509, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Wong PS, Travis J. Isolation and properties of oxidized alpha-1-proteinase inhibitor from human rheumatoid synovial fluid. Biochem Biophys Res Commun 96: 1449–1454, 1980. [DOI] [PubMed] [Google Scholar]
- 64.Yang JJ, Kettritz R, Falk RJ, Jennette JC, Gaido ML. Apoptosis of endothelial cells induced by the neutrophil serine proteases proteinase 3 and elastase. Am J Pathol 149: 1617–1626, 1996. [PMC free article] [PubMed] [Google Scholar]