Abstract
CAG repeats form stable hairpin structures, which are believed to be responsible for CAG repeat expansions associated with certain human neurological diseases. Human cells possess an accurate DNA hairpin repair system that prevents expansion of disease-associated CAG repeats. Based on transgenic animal studies, it is suggested that (CAG)n expansion is caused by abnormal binding of the MutSβ mismatch recognition protein to (CAG)n hairpins, leading to hijacking mismatch repair function during (CAG)n hairpin repair. We demonstrate here that MutSβ displays identical biochemical and biophysical activities (including ATP-provoked conformational change, ATPase, ATP binding, and ADP binding) when interacting with a (CAG)n hairpin and a mismatch. More importantly, our in vitro functional hairpin repair assays reveal that excess MutSβ does not inhibit (CAG)n hairpin repair in HeLa nuclear extracts. Evidence presented here provides a novel view as to whether or not MutSβ is involved in CAG repeat instability in humans.
Expansion of trinucleotide repeats (TNRs)3 causes hereditary neurological disorders such as Huntington disease and myotonic dystrophy, whose clinical symptoms are directly linked to expansion of CAG and CTG repeats, respectively (1–3). The precise mechanisms by which TNR expansion occurs and the factors that promote it are not fully understood. It has been proposed that CAG and CTG repeats form thermostable hairpins that include A-A and T-T mispairs in the hairpin stem (4, 5). Therefore, cellular mechanisms that process DNA hairpin/loop structures and/or A-A or T-T mispairs may influence TNR stability.
Recent studies have identified and characterized a DNA hairpin repair (HPR) system in human cells that promotes CAG/CTG repeat stability (6, 7). The mechanism of human HPR involves incision and removal of CAG/CTG repeat hairpins in a nick-directed and proliferating cell nuclear antigen-dependent manner, followed by DNA resynthesis using the continuous strand as a template (6). In addition to human HPR, the human mismatch repair (MMR) system is well known for its role in stabilizing simple repetitive sequences called microsatellites, which are prone to forming small loops or insertion/deletion (ID) mispairs. In human cells, MutSα (MSH2–MSH6) and MutSβ (MSH2–MSH3) both bind to 1–2-nt ID mispairs, but MutSβ has higher affinity for these small loops (8). Defects in MMR genes cause microsatellite instability and predisposition to cancer (9), demonstrating that MMR is essential for genetic stability in human cells. Surprisingly, genetic studies in mice suggest that MutSβ promotes (CAG)n expansion and TNR instability. These studies show that expansion of a heterologous (CAG)n tract occurs in wild type and MSH6−/− mice but that expansion of the (CAG)n tract is suppressed in MSH2−/− and MSH3−/− mice (10, 11). Recently, Owens et al. (11) reported that binding to a (CAG)n hairpin influences the protein conformation, nucleotide binding, and hydrolysis activities of MutSβ so that they are different from what has been reported for MutSα during mismatch recognition. It is therefore hypothesized that (CAG)n hairpins, through their ability to alter the biochemical properties of MutSβ, hijack the MMR process, leading to CAG repeat expansion instead of CAG hairpin removal (11). However, it is not clear why MMR, a major genome maintenance system, would promote TNR instability instead of TNR stability. We, therefore, have developed a novel functional assay and examined the validity of this hypothesis. Our results reveal that MutSβ displays normal biochemical activities when binding to CAG hairpins and does not inhibit (CAG)n hairpin repair. The observations presented here provide novel thoughts on whether or not or how MutSβ is involved in CAG repeat instability in human cells.
EXPERIMENTAL PROCEDURES
Preparation of CAG/CTG Hairpin Substrates
Oligonucleotide duplexes containing (5′-CAG-3′)35/(3′-GTC-5′)35, (5′-CTG-3′)35/(3′-GAC-5′)35, (5′-CAG-3′)10/(3′-GTC-5′)10, or (5′-CTG-3′)10/(3′-GAC-5′)10 were cloned into EcoRI and HindIII sites of bacterial phage M13mp18-UKY replication form (RF) DNA (13) to create M13mp18-UKY derivatives M13mp18-UKY-(CAG)35, M13mp18-UKY-(CTG)35, M13mp18- UKY-(CAG)10, or M13mp18-UKY-(CTG)10, respectively. Individual derivatives were confirmed by DNA sequencing. To obtain a DNA heteroduplex containing a (CAG)25 hairpin in the complementary (C) strand, M13mp18-UKY-(CTG)35 RF DNA was first linearized with BglI and PvuI and then hybridized with M13mp18-UKY-(CTG)10 single-stranded viral (V) DNA. This hybridization forms a heteroduplex containing a (CAG)25 hairpin in the C strand and a 29-nucleotide gap 5′ to the hairpin. This substrate was designated 5′ C-(CAG)25, meaning that it contains a (CAG)25 hairpin in the C strand and a 29-nt single-strand gap 5′ to the heterology (see Fig. 1, top right diagram). Conversely, substrate 5′ V-(CTG)25 has a (CTG)25 hairpin in the V strand and a 29-nt gap 5′ to the hairpin (see Fig. 1, top left diagram) and was derived from hybridization of M13mp18-UKY-(CTG)35 viral single-stranded DNA and M13mp18-UKY-(CTG)10 RF double-stranded DNA digested with BglI and PvuI.
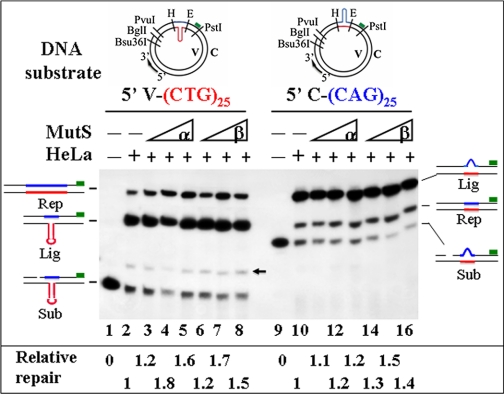
FIGURE 1.
MutSβ does not inhibit (CTG)25 or (CAG)25 HPR. HPR assays were performed in a 40-μl reaction containing 200 ng of DNA substrate and 100 μg of HeLa nuclear extract in the presence or absence of MutSα or MutSβ, as indicated. After incubation for 30 min at 37 °C, DNA products were purified, digested with Bsu36I and PstI, and fractionated by electrophoresis followed by Southern blot analysis using a 32P-labeled probe (green box) that anneals to the 3′-end of the Bsu36I-PstI fragment on the C strand. Where indicated, 0.4, 2.0, and 4.0 μg of MutSα or MutSβ were preincubated with DNA substrates prior to assembling the complete reaction. The structures of the reaction products are indicated schematically to the left or right of each panel and are described under “Results.” Relative repair was determined by densitometry of the autoradiograph and is indicated at the bottom of the figure. Red and blue typefaces or lines indicate CTG and CAG repeats, respectively. Rep, Lig, and Sub stand for repair products, unrepaired gap-ligated substrates, and unreacted substrates, respectively. The arrow points to a minor species derived from ligation of the unremoved 29-nt BglI-PvuI fragment to the 5′-end of the 5′-V-(CTG)25 substrate. H and E stand for HindIII and EcoRI, respectively.
Cell Culture, Nuclear Extract, and Protein Preparations
HeLa S3 cells were cultured in RPMI 1640 with 5% fetal bovine serum (Hyclone) and 4 mm glutamine at 37 °C in a 5% CO2 atmosphere. Nuclear extracts were prepared as described previously (13). MutSα and MutSβ proteins were expressed in insect cells, purified to near homogeneity, and examined for MMR activity as described (14).
CAG/CTG Hairpin Repair Assay
DNA HPR assays were performed in a 40-μl reaction containing 200 ng of DNA substrate, 100 μg of HeLa nuclear extract, 20 mm Tris-HCl (pH 7.6), 110 mm KCl, 5 mm MgCl2, 1.5 mm ATP, 1 mm glutathione, and 0.1 mm dNTP in the presence or absence of MutSα or MutSβ. After incubation for 30 min at 37 °C, DNA products were purified, digested with Bsu36I and PstI, and separated on a 6% denaturing polyacrylamide gel followed by electro-transferring to nylon membrane. The membrane was probed with a 32P-end-labeled oligonucleotide specifically annealing to the 3′-end of the Bsu36I-PstI fragment in the C strand to score for conversion of 35 CAG/CTG repeats to 10 CAG/CTG repeats or vice versa. Repair products, as well as unrepaired molecules, were visualized by exposing to x-ray film.
Gel Mobility Shift Analysis
Gel-shift assays were performed in 20-μl reactions containing 10 mm HEPES-KOH (pH 7.5), 110 mm KCl, 1 mm EDTA, 1 mm dithiothreitol, 32P-labeled oligonucleotide duplexes, and MutSβ in the presence of 10-fold excess amount of unlabeled oligonucleotide homoduplex. The reactions were incubated on ice for 20 min followed by the addition of 5 μl of 50% (w/v) sucrose. Samples were loaded on and separated by electrophoresis through a 6% non-denaturing polyacrylamide gel in buffer containing 50 mm Tris borate (pH 7.5) and 1 mm EDTA. The buffer was recirculated during electrophoresis. The gel was dried and analyzed by a Storm PhosphorImager (GE Healthcare).
Nucleotide UV Cross-linking and ATPase Analyses
The nucleotide cross-linking assays were performed essentially as described (8, 15). Reactions were assembled and incubated on ice in nucleotide binding buffer containing 50 mm Tris-HCl (pH 8.0), 110 mm NaCl, 2 mm dithiothreitol, 100 mg/ml bovine serum albumin, 0.5 mm EDTA, and 5% glycerol in the presence or absence of 5 mm MgCl2. Where specified, DNA heteroduplex or homoduplex was added 10 min prior to the addition of nucleotide. MutSβ was mixed with [α-32P]ATP, [α-32P]ADP, or [γ-32P]ATP and incubated for 10 min. Samples were then subjected to 10 min of UV cross-linking (Stratalinker) followed immediately by fractionation by 8% SDS-PAGE gel. Radiolabeled bands were quantified using a PhosphorImager. [α-32P]ADP was generated by incubating [α-32P]ATP with hexokinase and purified as described (15). ATPase activity of MutSβ was assayed in 20-μl reactions containing 50 mm Tris-HCl (pH 8.0), 110 mm NaCl, 0.5 mm EDTA, 5 mm MgCl2, and 0.05–100 μm [γ-32P]ATP. After incubation at 37 °C for 10 min, the reactions were terminated and fractionated through a 20% denaturing polyacrylamide gel. 32P-containing species were detected by a PhosphorImager.
RESULTS
MutSβ Does Not Inhibit CAG/CTG Hairpin Repair
Repair of DNA hairpins formed within CAG and CTG TNRs has recently been characterized in human cells (6, 7). The HPR system removes (CAG)n or (CTG)n hairpins by incisions in a nick-directed, proliferating cell nuclear antigen-dependent, and error-free manner (6). To determine whether MutSβ hijacks (CAG)n HPR, a functional in vitro assay was used to examine the catalytic competence of MutSβ in repair of a (CAG)25 hairpin in the gapped strand and a (CTG)25 hairpin in the non-gapped strand by HeLa nuclear extracts. In this assay, the DNA substrate is incubated with HeLa nuclear extracts in the presence or absence of excess exogenous human MutSβ. Because HPR is always targeted to the nicked/gapped DNA strand (6, 7), repair can be readily scored by monitoring changes in the length of the nicked/gapped strand of the DNA substrate using a 32P-labeled probe (6) (Fig. 1).
As expected, both substrates were efficiently repaired by HeLa nuclear extracts, with the repair being targeted in the gapped strand. Incubation of HeLa nuclear extracts with the (CTG)25 substrate, whose hairpin is located in the continuous strand (Fig. 1, left panel), yielded two major novel bands (Fig. 1, lane 2), i.e. the repair product (top band, 19%) and the unrepaired but gap-filled and/or gapped-ligated substrate (middle band). The repair product is 75 nt longer than the gap-ligated substrate, indicating that the continuous strand was used as a template for repair DNA synthesis. Similarly, processing of substrate C-(CAG)25 (containing a hairpin in the gapped strand) by HeLa extracts generated a repair product (lane 10, middle band, 22%) that is 75 nt shorter than the gap-ligated unrepaired substrate (lane 10, top band), consistent with the notion that HPR is targeted to the nicked/gapped strand (6, 7). It is worth mentioning that the repair product, which no longer contains a (CAG)25 hairpin in the C strand, migrates slower than the original substrate; this is because the (CAG)25 hairpin-containing PstI-PvuI fragment (i.e. the size of the original substrate shown in Fig. 1, lanes 9–16) is 32 nt shorter than the PstI-Bsu36I fragment without a (CAG)25 hairpin (the size of the repair product).
Surprisingly, when excess exogenous human MutSβ, which is very active in repair of insertion/deletion mispairs in a defined MMR system (data not shown and Ref. 14), was preincubated with the DNA substrate prior to assembling the complete reaction, there was no reduction or inhibition of either (CAG)25 or (CTG)25 HPR. Instead, the repair was 1.1–1.7-fold higher in the presence of MutSβ (Fig. 1, lanes 6–8 for the CTG hairpin and lanes 14–16 for the CAG hairpin). This surprising result suggests that MutSβ facilitates (CAG)n and (CTG)n HPR, likely through interactions with these hairpins. In addition, the extent of repair did not decrease when the DNA substrate was incubated with MutSβ and HeLa nuclear extract at the same time (data not shown). Similar results were also obtained with MutSα (Fig. 1, lanes 3–5 and 11–13). These observations show that neither MutSα nor MutSβ inhibits (CAG)n or (CTG)n HPR in this in vitro assay.
MutSβ Binds CAG/CTG Hairpins and ID Mispairs in Similar Manners
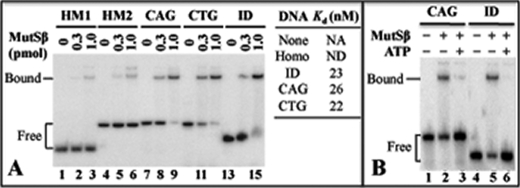
To determine whether MutSβ interacts with (CAG)n and (CTG)n hairpins, electrophoretic mobility shift analysis was performed using purified MutSβ and a (CAG)13 hairpin (11) and a (CTG)13 hairpin substrates. As shown in Fig. 2A, MutSβ binds both (CAG)13 (lane 9) and (CTG)13 (lane 12) hairpins with a Kd of 26 and 22, respectively, which is similar to the Kd (23) for an ID substrate (lane 15). It is known that mismatch binding by MutSα leads to an ATP-provoked conformational change that allows the protein to be released from the DNA (16–18). However, little is known about the MutSβ activities during its mismatch recognition. To determine whether hairpin binding alters MutSβ biophysical properties as proposed (11), gel-shift analysis was performed in the presence of ATP. As shown in Fig. 2B, ATP inhibits both the MutSβ-ID and the MutSβ-(CAG)13 hairpin interactions (lanes 3 and 6), suggesting that MutSβ undergoes an ATP-induced conformational change whether it is bound to a (CAG)13 hairpin or to an ID mispair.
FIGURE 2.
MutSβ binds to CAG- and CTG hairpins as it does to an ID mispair. Gel-shift analysis was performed as described (8) using 0.3 or 1 pmol of MutSβ, 1 pmol of the (CAG)13 hairpin substrate as described in Ref (11), or other indicated DNA duplexes. A, MutSβ binds specifically to a (CAG)13 hairpin (CAG), a (CTG)13 hairpin (CTG), or a GT-dinucleotide ID mispair (ID) as compared with a homoduplex (Homo) containing random sequences (HM1) and a (CAG/CTG)13-containing homoduplex (HM2). NA, not applicable; ND, not detectable. B, ATP inhibits the MutSβ-CAG-hairpin interaction.
MutSβ Exhibits Identical Nucleotide Binding and ATPase Activities When Interacting with Hairpin and ID Heteroduplexes
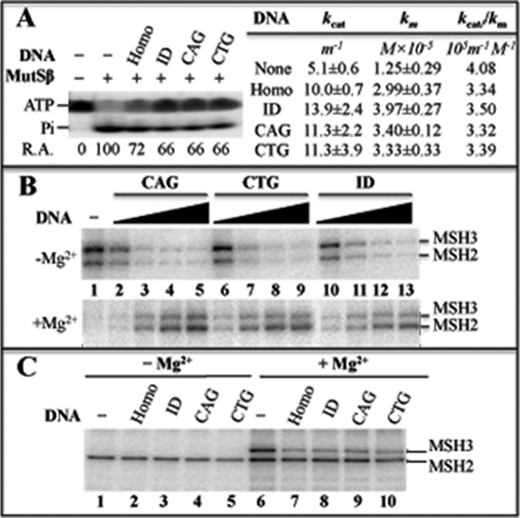
All MutS proteins possess a weak ATPase activity and a nucleotide (ATP and ADP) binding activity (9). Previous studies have shown that binding of MutSα to a mismatch enhances its ATPase and ATP binding activities (17, 18). Interestingly, the MutSβ-ID interaction reduces MutSβ ATPase activity (8). We therefore examined the effects of ID and hairpin heteroduplexes on MutSβ ATPase. As shown in Fig. 3A, MutSβ displays an identical reduction in ATPase activity (from 100% in the absence of DNA to 66% in the presence of heteroduplexes) regardless of its interaction with a (CAG)13 hairpin, a (CTG)13 hairpin, or an ID heteroduplex (compare lane 2 with lanes 4–6). Kinetic studies revealed that although the kcat value (13.9 min−1) for the ID substrate is higher than that (11.3 min−1) for the CAG or CTG hairpin substrate (Fig. 3A), which appears to be in agreement with the data reported previously (11), the catalytic efficiencies, kcat/kM, for the individual DNA substrates used are almost the same, 3.32 for CAG hairpin, 3.39 for CTG hairpin, and 3.5 for ID heteroduplex (Fig. 3A), suggesting that the reduction in MutSβ ATPase activity induced by DNA is not specific or unique to the (CAG)13 or (CTG)13 hairpin structure.
FIGURE 3.
Analysis of MutSβ nucleotide binding and hydrolysis activities. A, ATPase activity. [γ-32P]ATP was incubated with MutSβ (0.2 μm) and 5 mm MgCl2 for 10 min in the presence or absence of the indicated DNA substrates, and samples were electrophoresed in an 20% SDS-PAGE gel as described (8). Relative ATPase activity (R.A.) was determined by dividing the amount of 32P-phosphate (Pi) with the amount of 32P-phosphate in the reaction without DNA and multiplying by 100. ATPase assays were also performed by incubating MutSβ with 4.0 pmol of individual DNA substrates and varying concentrations of ATP. The resulting data were fit to the Michaelis-Menten equation. kcat and kM values and standard deviations were calculated from three independent experiments. Homo, a perfect matched oligonucleotide duplex DNA; CAG, a (CAG)13 hairpin; CTG, a (CTG)13 hairpin; ID, a GT-dinucleotide insertion/deletion mispair. m−1 and M−1 stand for min−1 and molarity−1, respectively. B and C, nucleotide binding activity. MutSβ (0.2 μm) was incubated with either [γ-32P]ATP (B) or [α-32P]ADP (C) in the presence or absence of DNA duplexes and 5 mm MgCl2, as indicated, followed by UV cross-linking and SDS-PAGE (8).
MutSβ nucleotide binding affinity was determined by performing UV cross-linking experiments (8, 15). The results show that all DNA heteroduplexes, including a CAG hairpin and an ID mispair, inhibit binding of MutSβ to ATP by 60% in the absence of Mg2+ (Fig. 3B, upper panel, also see quantitative data in supplemental Table 1). In the presence of Mg2+, DNA substrates no longer inhibit MutSβ-ATP interactions (Fig. 3B, lower panel), leading to an enhanced (2–3-fold) ATP binding (see supplemental Table 1). This is consistent with the fact that DNA substrates inhibit MutSβ ATPase activity (Fig. 3A) (8). Fig. 3C shows similar analysis for ADP. Again, the type of DNA substrates has no effects on ADP binding, but Mg2+ stimulates binding of MutSβ to ADP, particularly the MSH3 subunit (Fig. 3C, compare lanes 6–10 with lanes 1–5, also see supplemental Table 1), which differs from MutSα and its MSH6 subunit (8, 15). These data strongly suggest that binding to a (CAG)n or a (CTG)n hairpin does not alter the nucleotide binding and ATPase activities of MutSβ, which are associated with its function in MMR.
DISCUSSION
A previous study (11) reported that “CAG-hairpin binding inhibits the ATPase activity of Msh2–Msh3 and alters both nucleotide (ADP and ATP) affinity and binding interfaces between protein and DNA.” These alterations are considered “critical functional defects in the Msh2–Msh3-CAG hairpin complex that could misdirect the DNA repair process,” i.e. “the aberrant enzymatic and/or structural properties of the Msh2–Msh3-hairpin DNA complex may divert the repair process to other non-MMR pathway, leading to expansion instead of repair” (11). However, the results presented here demonstrate that MutSβ exhibits identical biochemical and biophysical activities, including nucleotide binding and hydrolysis (Fig. 3), and ATP-induced conformational change and protein translocation/sliding when MutSβ interacts with its favored ID mispair or a CAG/CTG hairpin (Fig. 2B). More convincingly, our functional in vitro HPR assays reveal that excess MutSβ does not inhibit CAG/CTG hairpin removal (Fig. 1). Therefore, binding to CAG hairpins does not alter MutSβ MMR activities and has no inhibitory roles in CAG HPR.
Although the discrepancy between these studies requires further investigations, we did identify the following differences: (i) the previous study was performed with a recombinant His-tagged MutSβ, whereas the present study was performed with a preparation of MutSβ that lacks an epitope tag and (ii) the MutSβ protein used in the present study is active in a functional MMR assay (data not shown and Ref. 14), but the MutSβ protein used in the previous study was not tested for its MMR function. These factors may have contributed to the difference in these studies. We also found that data were analyzed differently in these two studies. For example, kcat and kcat/kM were used to evaluate MutSβ ATPase activity in the previous and current studies, respectively. Despite the fact that both studies show different kcat values for MutSβ ATPase activity when incubating with different DNA substrates, a much smaller difference was pronounced when kcat/kM values were used. A good example is that although Owens et al. (11) observed a kcat value of 6.3 ± 0.2 and 5.0 ± 0.2 min−1 for a homoduplex and a CAG hairpin, respectively, the kcat/kM values for both substrates are essentially the same (1.9 × 105 min−1 m−1), indicating that there is little difference in MutSβ ATPase activity when the protein interacts with these DNA substrates, a conclusion of the current study. It is worth mentioning that although kcat is frequently used to express enzyme activity, the term kcat/kM, referred to as the catalytic efficiency, is often employed as a specificity constant to compare the relative rates of the same enzyme reacting with different substrates (19–22). We found that the latter is very useful to determine MutSβ ATPase activities because the kcat/kM values accurately reflect the observed ATP hydrolysis when MutSβ was incubated with different DNA substrates (Fig. 3A).
We also realize that differential interpretations of the existing data contribute to the distinct conclusions in these two studies. Both studies have shown inhibition of the MutSβ ATPase activity by DNA substrates, which completely differs from the well documented property of MutSα or Escherichia coli MutS, whose ATPase activity is stimulated by DNA substrates (23–25). As a result, MutSβ was thought to have altered its activities when interacting with a CAG hairpin in the hijacking model (11, 12). Our recent studies (8) have revealed significant differences in the biochemical functions between MutSα and MutSβ during recognition and interaction with base-base and ID mismatches. For example, MutSβ binds ADP with higher affinity than MutSα, and DNA substrates partially inhibit MutSβ ATPase activity but stimulate MutSα ATPase activity (8). A more recent study by Owens et al. (26) also revealed some of these distinct properties between MutSα and MutSβ. Taken together, we believe that the distinct properties of MutSβ from MutSα are specific for its recognition of ID heteroduplexes (8) but did not result from its binding to CAG hairpins (Figs. 2 and 3 in this study). Therefore, it is not appropriate to use MutSα properties to interpret MutSβ behaviors.
In summary, both our previous studies and the data presented here support a notion that binding of (CAG)n hairpin by MutSβ does not interfere with (CAG)n HPR in vitro. These observations strongly suggest that the hijacking model (11, 12) may not be practical for the involvement of MutSβ in CAG repeat instability shown in transgenic mice (10, 11). Our results presented here raise many questions on this issue. Does the transgenic mouse model of CAG repeats truly reflect CAG repeat expansion in human cells, i.e. does MutSβ indeed promote CAG repeat expansions in humans? If it does, why and how does such a microsatellite stabilization system promote microsatellite (i.e. CAG repeats) instability? A recent study by Lin et al. (27) suggests that MutSβ may influence CAG repeat instability via transcription; however, the mechanism is unclear. Therefore, thorough investigations are required to elucidate the mechanism of TNR expansions in specific human diseases, as well as the potential in vivo role of MutSβ or other DNA repair proteins in this process.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants GM072756 and CA115942 (to G.-M. L.) and CA104333 (to L. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
- TNR
- trinucleotide repeat
- HPR
- DNA hairpin repair
- MMR
- mismatch repair
- ID
- insertion/deletion mismatch
- nt
- nucleotide
- RF
- replication form
- V
- viral
- C
- complementary.
REFERENCES
- 1.Lahue R. S., Slater D. L. (2003) Front. Biosci. 8, s653–665 [DOI] [PubMed] [Google Scholar]
- 2.Mirkin S. M. (2007) Nature 447, 932–940 [DOI] [PubMed] [Google Scholar]
- 3.Pearson C. E., Nichol Edamura K., Cleary J. D. (2005) Nat. Rev. Genet. 6, 729–742 [DOI] [PubMed] [Google Scholar]
- 4.Gacy A. M., Goellner G., Juraniæ N., Macura S., McMurray C. T. (1995) Cell 81, 533–540 [DOI] [PubMed] [Google Scholar]
- 5.Pearson C. E., Tam M., Wang Y. H., Montgomery S. E., Dar A. C., Cleary J. D., Nichol K. (2002) Nucleic Acids Res. 30, 4534–4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou C., Chan N., Gu L., Li G. M. (2009) Nat. Struct. Mol. Biol., doi:10.1038/nsmb.1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panigrahi G. B., Lau R., Montgomery S. E., Leonard M. R., Pearson C. E. ( 2005) Nat. Struct. Mol. Biol. 12, 654–662 [DOI] [PubMed] [Google Scholar]
- 8.Tian L., Gu L., Li G. M. (2009) J. Biol. Chem. 284, 11557–11562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li G. M. (2008) Cell Res. 18, 85–98 [DOI] [PubMed] [Google Scholar]
- 10.Manley K., Shirley T. L., Flaherty L., Messer A. (1999) Nat. Genet. 23, 471–473 [DOI] [PubMed] [Google Scholar]
- 11.Owen B. A., Yang Z., Lai M., Gajec M, Badger J. D., 2nd, Hayes J. J., Edelmann W., Kucherlapati R., Wilson T. M., McMurray C. T. (2005) Nat. Struct. Mol. Biol. 12, 663–670 [DOI] [PubMed] [Google Scholar]
- 12.McMurray C. T. (2008) DNA Repair 7, 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo S., Presnell S. R., Yuan F., Zhang Y., Gu L., Li G. M. (2004) J. Biol. Chem. 279, 16912–16917 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Yuan F., Presnell S. R., Tian K., Gao Y., Tomkinson A. E., Gu L., Li G. M. (2005) Cell 122, 693–705 [DOI] [PubMed] [Google Scholar]
- 15.Mazur D. J., Mendillo M. L., Kolodner R. D. (2006) Mol. Cell 22, 39–49 [DOI] [PubMed] [Google Scholar]
- 16.Drummond J. T., Li G. M., Longley M. J., Modrich P. (1995) Science 268, 1909–1912 [DOI] [PubMed] [Google Scholar]
- 17.Gradia S., Subramanian D., Wilson T., Acharya S., Makhov A., Griffith J., Fishel R. (1999) Mol. Cell 3, 255–261 [DOI] [PubMed] [Google Scholar]
- 18.Mendillo M. L., Mazur D. J., Kolodner R. D. (2005) J. Biol. Chem. 280, 22245–22257 [DOI] [PubMed] [Google Scholar]
- 19.Eisenthal R., Danson M. J., Hough D. W. (2007) Trends Biotechnol. 25, 247–249 [DOI] [PubMed] [Google Scholar]
- 20.Johnson K. A. (1992) in The Enzymes (Sigman D. S. ed) Vol. 20, pp, 1–61, Academic Press, Orlando, FL [Google Scholar]
- 21.Radzicka A., Wolfenden R. (1995) Science 267, 90–93 [DOI] [PubMed] [Google Scholar]
- 22.Takamatsu S., Kato R., Kuramitsu S. (1996) Nucleic Acids Res. 24, 640–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjornson K. P., Allen D. J., Modrich P. (2000) Biochemistry 39, 3176–3183 [DOI] [PubMed] [Google Scholar]
- 24.Bowers J., Sokolsky T., Quach T., Alani E. (1999) J. Biol. Chem. 274, 16115–16125 [DOI] [PubMed] [Google Scholar]
- 25.Gradia S., Acharya S., Fishel R. (2000) J. Biol. Chem. 275, 3922–3930 [DOI] [PubMed] [Google Scholar]
- 26.Owen B. A., Lang W. H., McMurray C. T. (2009) Nat. Struct. Mol. Biol. 16, 550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y., Dion V., Wilson J. H. (2006) Nat. Struct. Mol. Biol. 13, 179–180 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.