Abstract
Transfer RNA (N2,N2-guanine)-dimethyltransferase (Trm1) catalyzes N2,N2-dimethylguanine formation at position 26 (m22G26) in tRNA. In the reaction, N2-guanine at position 26 (m2G26) is generated as an intermediate. The trm1 genes are found only in archaea and eukaryotes, although it has been reported that Aquifex aeolicus, a hyper-thermophilic eubacterium, has a putative trm1 gene. To confirm whether A. aeolicus Trm1 has tRNA methyltransferase activity, we purified recombinant Trm1 protein. In vitro methyl transfer assay revealed that the protein has a strong tRNA methyltransferase activity. We confirmed that this gene product is expressed in living A. aeolicus cells and that the enzymatic activity exists in cell extract. By preparing 22 tRNA transcripts and testing their methyl group acceptance activities, it was demonstrated that this Trm1 protein has a novel tRNA specificity. Mass spectrometry analysis revealed that it catalyzes methyl transfers not only to G26 but also to G27 in substrate tRNA. Furthermore, it was confirmed that native tRNACys has an m22G26m2G27 or m22G26m22G27 sequence, demonstrating that these modifications occur in living cells. Kinetic studies reveal that the m2G26 formation is faster than the m2G27 formation and that disruption of the G27-C43 base pair accelerates velocity of the G27 modification. Moreover, we prepared an additional 22 mutant tRNA transcripts and clarified that the recognition sites exist in the T-arm structure. This long distance recognition results in multisite recognition by the enzyme.
To date, more than 100 modified nucleosides have been identified in various RNA species with the majority of them having been found in tRNA molecules (1, 2). All of the modified nucleosides are introduced into tRNA by specific tRNA modification enzymes or guide RNA modification systems during the post-transcriptional process. Among them, N2,N2-dimethylguanine at position 26 (m22G26) in tRNA is generated by tRNA (N2,N2-guanine)-dimethyltransferase (tRNA (m22G26) methyltransferase; TrMet (m2,2G26); EC 2.1.1.32) (2, 3). This enzyme catalyzes the methyl transfer reaction from S-adenosyl-l-methionine (AdoMet)2 to the N2 atom of G26, which is located at the junction between the D-arm and the anticodon arm in tRNA. In the reaction, two molecules of AdoMet are consumed, and N2-methylguanine at position 26 (m2G26) is generated as an intermediate (4). Thus, in some cases, this enzyme catalyzes transfer of only one methyl group and functions as a tRNA (m2G26) methyltransferase (5). The enzymatic activity was initially detected in rat liver (6, 7) and then found in various organisms (8–12). The most highly purified enzyme from a native source was obtained from Tetrahymena pyriformis (4). The responsible gene was first determined to be trm1 from Saccharomyces cerevisiae (13, 14) and then experimentally identified from various eukaryotes (Schizosaccharomyces pombe (15), Caenorhabditis elegans (16), and human (17)) and archaea (Pyrococcus furiosus (18), Pyrococcus horikoshii (19), and Haloferax volcanii (20)), as is consistent with the distribution of the m22G26 (or m2G26) modification in tRNA.
The m2G and m22G modifications in tRNA can be found not only at position 26 but also at positions 6, 7, 9, 10, 18, and 27 in various organisms (21). However, only m22G10 formation enzymes (the Trm11-Trm112 complex in yeast (22) and Trm-m22G10 enzyme in Pyrococcus abyssi (23, 24)) have been identified thus far. These tRNA (m22G10) methyltransferases are structurally different from tRNA (m22G26) methyltransferase (Trm1). Recently, we determined the x-ray crystal structure of P. horikoshii Trm1 (19); this protein includes N-terminal and C-terminal domains with class I methyltransferase (25) and novel folds, respectively. In contrast, amino acid sequence alignment, computational modeling, and deletion mutant experiments have revealed that the Trm-m22G10 enzyme contains a THUMP domain in the N-terminal region and a catalytic domain with a class I methyltransferase fold in the C-terminal portion (26).
For the past several years, we have studied RNA modification enzymes from Aquifex aeolicus (27–31). A. aeolicus is a hyper-thermophilic eubacterium, which grows at close to 95 °C. The 16 S rRNA gene has been analyzed from the perspective of molecular evolution, and it has been suggested that this bacterium is the earliest diverging eubacterium (32), although there is debate concerning the diverging point of this bacterium (33, 34). The complete genome has been determined, and a putative trm1 gene was found (35). In this study, we have focused on this eubacterial trm1 gene product. We demonstrate that this gene product is genuinely expressed in A. aeolicus cells and has a novel tRNA methyltransferase activity, which is completely different from eukaryotic and archaeal Trm1 enzymes reported thus far. Moreover, we report a long distance recognition mechanism of this enzyme. Abbreviations of the modified nucleosides in this paper are listed in supplemental Table 1.
EXPERIMENTAL PROCEDURES
Materials
[methyl-14C]AdoMet (1.95 GBq/mmol) and [methyl-3H]AdoMet (2.47 TBq/mmol) were purchased from ICN. Cold AdoMet was obtained from Sigma. DE52 is a product of Whatman. CM-Toyopearl 650M was from Tosoh. DNA oligomers were bought from Invitrogen, and T7 RNA polymerase was from Toyobo. Other chemical reagents were of analytical grade.
Culture of A. aeolicus
The culture source of A. aeolicus and 100 ml of culture medium in controlled gas (H2/CO2 (v/v, 4:1) mixed gas pressurized by air to 2 atm) were kindly provided by Dr. Harald Huber (Universitat Regensburg, Germany). The culture was performed at 85 °C for 24 h.
Measurement of Enzymatic Activity
The standard assay used during the purification was measured incorporation of 14C-methyl groups from [methyl-14C]AdoMet to A. aeolicus tRNAHis transcript; 0.1 μm enzyme, 11 μm transcript, and 38 μm [methyl-14C]AdoMet in 45 μl of buffer A (50 mm Tris-HCl (pH 7.5), 5 mm MgCl2, 6 mm 2-mercaptoethanol, and 50 mm KCl) were incubated for 15 min at 55 °C. An aliquot (30 μl) was then used for the filter assay. RNA transcripts were prepared as reported previously (29).
If discrimination between the m2G and m22G formation activities was necessary, we employed two-dimensional TLC (36). The 14C-methylated RNA was dissolved in 5 μl of 50 mm ammonium acetate (pH 5.0) and digested with 2.5 units of nuclease P1, and then 2 μl of standard nucleotides containing 0.05 A260 unit each of pA, pG, pC, and pU was added. 2 μl of the sample was spotted onto a thin layer plate (Merck code number 1.05565, cellulose F) and separated using the following solvent systems: first dimension, isobutyric acid/concentrated ammonia/water, 66:1:33, v/v; second dimension, isopropyl alcohol/HCl/water, 70:15:15, v/v. Incorporation of 14C-methyl groups was monitored with a Fuji Photo Film BAS2000 imaging analyzer. Standard nucleotides were marked by UV 260 nm irradiation.
To visualize the methyl transfer reaction, we used 10% PAGE (7 m urea) and an imaging analyzer system as described previously (28, 29). Briefly, tRNA (0.1 A260 units) was incubated with 0.1 μm Trm1 and 38 μm [methyl-14C]AdoMet for 10 min at 55 °C in 30 μl of buffer A and then loaded onto a 10% polyacrylamide gel (7 m urea). The gel was stained with methylene blue and dried. The incorporation of 14C-methyl groups into tRNA was monitored with a Fuji Photo Film BAS2000 imaging analyzer.
Measurements of Kinetic Parameters for tRNATyr Transcripts
First, we carried out time course experiments at 55 °C with 0.1 μm Trm1, 11 μm tRNA transcript, and 38 μm [methyl-14C]AdoMet in 210 μl of buffer A. The aliquots (30 μl each) were taken at appropriate times (0, 2, 5, 7.5, 10, and 15 min) and formations of 14C-pm2G and 14C-pm22G monitored by two-dimensional TLC. Under these conditions, only pm2G linearly increased for the first 10 min, and m22G formation was barely observable; pm22G content was less than 5% of the pm2G content in the sample at 10 min. Kinetic parameters for tRNATyr transcripts were determined by incorporation of 3H-methyl groups into tRNA. Both Km and Vmax values for tRNATyr transcripts were small, and activity measurement with low concentrations of enzyme and tRNA transcripts was very difficult. Therefore, we determined approximate kinetic parameters under unusual conditions as follows: 0.1 μm Trm1, 40 μm [methyl-3H]AdoMet, and various concentrations (0.06, 0.1, 0.15, 0.3, 0.6, 0.9, and 1.5 μm) of tRNATyr transcript were incubated at 55 °C for 10 min, and then the filter assay was performed.
Purification of Native tRNACys by Solid-phase DNA Probe Column Chromatography
3′-Biotin-labeled DNA oligomer (5′-TGC AGT CCC CTG CCT AAC CGC TC-biotin 3′) was used as a probe. Because we expected the G26 in the native tRNACys to be modified to m22G26, the nucleotide at position 11 in the DNA was designed as T instead of C to form an m22G26-T11 base pair. 100 μl of slurry of streptavidin-Sepharose high performance resin (GE Healthcare) was poured into a 1.5-ml Amicon Ultrafree-MC tube (0.22 μm), and then the buffer was removed by centrifugation at 500 × g for 10 s. The resin was equilibrated by addition of 400 μl of 20 mm Tris-HCl (pH 7.5), and the buffer was removed by centrifugation at 500 × g for 10 s. This treatment was repeated two times. 0.8 A260 unit of DNA probe dissolved in 400 μl of 20 mm Tris-HCl (pH 7.5) was poured into the tube, mixed with the resin, incubated for 10 min at room temperature, and then the buffer removed by centrifugation at 500 × g for 10 s. The DNA immobilized on the resin in the tube, 2× hybridization buffer (40 mm Tris-HCl (pH 7.5), 1.8 m tetramethylammonium chloride, 200 μm EDTA), and 10.0 A260 units of A. aeolicus total RNA dissolved in 200 μl of water were preincubated at 69 °C for 5 min. The resin, total RNA (200 μl), and 200 μl of 2× hybridization buffer were mixed and then incubated at 69 °C for 5 min. The hybridization was performed by cooling from 69 to 65 °C for 4 min, and incubation was at 65 °C for 5 min. Unbound RNA was removed by centrifugation at 500 × g for 10 s, and then the resin was washed with 400 μl of 20 mm Tris-HCl (pH 7.5). This treatment was repeated five times. The elution of tRNACys was performed as follows. The resin and 400 μl of the elution buffer (20 mm Tris-HCl (pH 7.5)) were preincubated at 65 °C for 5 min, mixed, incubated at 65 °C for 5 min, and then quickly centrifuged at 500 × g for 10 s. The eluted RNA was collected by ethanol precipitation. In this study, tRNACys was further purified by 10% PAGE (7 m urea). Detailed procedures to the other tRNA cases will be published.3
Mass Spectrometry
Transfer RNACys transcript (50 μg) was methylated with 0.5 μm Trm1 and 670 μm nonradioisotope-labeled AdoMet for 12 h at 55 °C in 100 μl of buffer A. The RNA was purified by 10% PAGE (7 m urea). The RNA was visualized by UV (254 nm) irradiation on a thin layer plate (Funacell P-254, Japan), excised, and extracted with 400 μl of gel elution buffer (0.5 m ammonium acetate, 10 mm MgCl2, 1 mm EDTA, and 0.1% SDS). The extracted sample was passed through a Steradisc 13 filter unit (0.2 μm, Kurabo, Japan), and the RNA was recovered by ethanol precipitation. About 0.05 A260 units of methylated tRNACys transcript was used for total nucleoside analysis, and about 200 fmol of the transcript and native tRNACys were digested with RNase A or RNase T1 for RNA fragment analysis. Mass spectrometric analysis was carried out as described previously (37–41) with the following modifications. For total nucleoside analysis, we used yeast total tRNA as control for assignment of modified nucleosides. For the analyses of RNase A or RNase T1 digests, we employed a linear ion trap-orbitrap hybrid mass spectrometer (LTQ Orbitrap XL, Thermo Fisher Scientific) equipped with a custom-made nanospray ion source and a splitless nano HPLC system (DiNa, KYA Technologies). The concentration of RNase T1 was adjusted to 5 units/μl. The digests mixed with TEAA (triethylamine acetate, pH 7.0) were loaded onto a nano-LC trap column (C18, inner diameter 0.5 × 1.0 mm), desalted, and then concentrated with 0.1 m TEAA (pH 7.0). RNA fragments were eluted from the trap column and directly injected into a C18 capillary column (HiQ Sil; 3-μm C18, 100 Å pore size; inner diameter 0.1 × 100 mm, KYA Technologies). The chromatography was carried out in the same condition as described (37), and the eluent was sprayed from a sprayer tip attached to the capillary column. The ionization voltage was set to −1.9 kV, and ions were scanned in the negative polarity mode.
The following sections are described in the supplemental material: construction of A. aeolicus Trm1 expression system in Escherichia coli; purification of the recombinant Trm1 protein, and Western blotting.
RESULTS
Putative trm1 Gene Is Encoded in the A. aeolicus Genome, and the Gene Product Has tRNA Methyltransferase Activity
In 1998, the complete genome sequence of A. aeolicus was determined, and the existence of a putative trm1 gene was reported (35). All trm1 gene products experimentally analyzed thus far have a tRNA (m22G26) methyltransferase activity. This enzyme activity is only found in eukaryotes and archaea, consistent with the distribution of the trm1 genes. Thus, although A. aeolicus belongs to the eubacteria, a putative trm1 gene is encoded. We compared the amino acid sequence of the A. aeolicus trm1 gene product with those of experimentally identified tRNA (m22G26) methyltransferases (Trm1 proteins) (Fig. 1). As shown in Fig. 1, the A. aeolicus trm1 gene product has many of the amino acid residues conserved among the Trm1 proteins. In fact, during the course of this study, we determined the crystal structures of P. horikoshii Trm1 (19). In the study, it was found that two phenylalanine residues (corresponding to Phe-27 and Phe-134 in A. aeolicus Trm1) form a pocket, which is predicted to be a part of the G26-binding site (19). Furthermore, an aspartic acid (corresponding to Asp-132 in A. aeolicus Trm1) was expected to be a catalytic center. These Trm1-specific amino acid residues are conserved in A. aeolicus Trm1 in addition to the amino acid sequence motifs conserved among methyltransferases (42). Thus, the amino acid sequence alignment strongly suggested the A. aeolicus trm1 gene product to be a tRNA (m22G26) methyltransferase.
FIGURE 1.
Amino acid sequence alignment of Trm1 proteins. The alignment was generated by ClustalW 1.83 (59) and ESPript (60) programs. Amino acid sequences of Trm1 proteins experimentally identified are compared. Numbering of amino acid residues is based on the sequence of the A. aeolicus Trm1. Conserved and semi-conserved residues are boxed and highlighted, respectively. Secondary structure of P. horikoshii Trm1 is shown at the top of the alignment; arrows and coils represent β-sheets and helices, respectively. Asterisks and a reverse triangle show two Phe (Phe-27 and Phe-134) and Asp-132 residues, respectively.
To investigate whether the A. aeolicus putative trm1 gene product has tRNA methyltransferase activity, we performed PCR cloning and attempted expression in E. coli. Although the expression level in E. coli was very low (10 μg of recombinant protein/1 liter of culture), we could detect weak but clear m22G formation activity in the yeast tRNAPhe transcript in the supernatant of crude extract (data not shown). However, the expression level was so low as to make purification of the enzyme difficult. To improve the yield of the recombinant protein, the coding sequence of the N terminus was optimized for translation in E. coli (see supplemental material). This alteration did not cause any changes in amino acid sequence. The expression level was dramatically increased (2 mg of recombinant protein/1 liter of culture), and we could purify the recombinant protein as shown in Fig. 2. It is noteworthy that this purified Trm1 fraction does not contain any nucleic acids. In the case of purification of P. furiosus Trm1, it has been reported that RNA bound tightly to the recombinant protein, and that RNase A treatment is effective for removal of the RNA (18). We also observed contamination by RNA in the A. aeolicus Trm1 fraction during the purification steps; however, we could separate the protein and RNA by repeated ion-exchange chromatography (see supplemental material). The enzyme assay revealed that the purified Trm1 protein has strong tRNA methyltransferase activity (data not shown).
FIGURE 2.

Purified A. aeolicus Trm1 protein. Three μg of purified A. aeolicus Trm1 was analyzed by 15% SDS-PAGE. The gel was stained with Coomassie Brilliant Blue. Mobility of standard markers is shown at the left.
trm1 Gene Product Is Expressed in Living A. aeolicus Cells, and m22G Formation Activity Can Be Detected in Cell Extract
Although we could show the tRNA methyltransferase activity of the recombinant Trm1 protein, an important question remained, namely whether there was expression of the putative trm1 gene in living A. aeolicus cells. To analyze the expression of the Trm1 protein in A. aeolicus cells, we prepared an anti-A. aeolicus Trm1 polyclonal antibody and performed Western blotting analysis (Fig. 3A). As shown in Fig. 3A, a single band corresponding to the Trm1 protein was clearly detected, demonstrating that the trm1 gene is really expressed in living A. aeolicus cells. Furthermore, we examined whether m2G and m22G formation activity could be observed in the cell extract. In this experiment, we used an E. coli tRNA mixture as the substrate. Because the E. coli tRNA mixture is already modified by tRNA modification enzymes of E. coli cells, limited methyltransferase activities of the A. aeolicus extract could be detected. The E. coli tRNA mixture, [14C]AdoMet, and A. aeolicus cell extract were incubated at 55 °C overnight, and 14C-methylated nucleotides were analyzed by two-dimensional TLC (Fig. 3B). As shown in Fig. 3B, 14C-pm2G and 14C-pm22G spots could be observed, demonstrating that tRNA (m2G and m22G) methyltransferase activities exist in the A. aeolicus cells. In this experiment, we detected 14C-labeled pm1A, pm6A, pm7G, and pCm, as well as pm2G and pm22G. Of these the pm1A and pm6A were probably derived from tRNA (m1A58) methyltransferase (TrmI) activity (43), because pm6A can be generated from pm1A nonenzymatically. The pm7G was probably generated by tRNA (m7G46) methyltransferase (TrmB) (28, 30, 44), suggesting that G46 in the E. coli tRNA mixture is not completely modified to m7G46 in E. coli cells. The Cm modification seemed to occur mainly at C32, because C32 in native tRNACys is modified to Cm32 as described below. Based on these experimental results, we concluded that the trm1 gene product is expressed in living A. aeolicus cells, and the m2G and m22G formation activities exist in the cell extract.
FIGURE 3.
Western blotting analysis of Trm1 and AdoMet-dependent tRNA methyltransferase activity in A. aeolicus cell extract. A, cell extract (50 μg of proteins) of A. aeolicus was separated on a 15% SDS-polyacrylamide gel (left panel). The gel was stained with Coomassie Brilliant Blue. The Trm1 protein in the cell extract was detected by Western blotting analysis. The SDS-PAGE was performed under the same conditions as described in the left panel, and then electroblotting was performed. The primary antibody (anti-A. aeolicus Trm1 polyclonal antibody) was custom-made. The fluorescence derived from the secondary antibody was monitored on a Typhoon model 9410 system. The band corresponding to the Trm1 is shown by an arrow. B, E. coli tRNA mixture from [14C]AdoMet and A. aeolicus cell extract was incubated at 55 °C overnight, and 14C-methylated nucleotides were analyzed by two-dimensional TLC. Positions of standard markers (pA, pG, pC, and pU) are enclosed by dotted circles.
Recombinant Trm1 Protein Transfers More Than Two Methyl Groups into One tRNA Molecule and Methylates an A. aeolicus tRNATyr Transcript Containing A26
The substrate tRNA specificities of both eukaryotic and archaeal Trm1 proteins have been reported (9–12). Although there are several differences in the tRNA specificity of eukaryote and archaea Trm1 proteins, they all recognize the D-stem and variable region of tRNA. To compare the substrate tRNA specificity of the A. aeolicus Trm1 to those of eukaryotic and archaeal enzymes, we prepared 22 tRNA transcripts (Fig. 4A). Fig. 4B shows the results of the assay at 15-min periods. These results revealed that the tRNA specificity of the A. aeolicus Trm1 is completely different from those of eukaryotic and archaeal Trm1 proteins. For example, H. volcanii tRNAVal (CAC) transcript was well methylated by A. aeolicus Trm1, although the G26 in this tRNA is not modified in living H. volcanii cells (45). Furthermore, to our surprise, A. aeolicus tRNATyr transcript was well methylated; the nucleotide at position 26 in this tRNA is not G but A. Moreover, we found that more than two methyl groups were incorporated into the A. aeolicus tRNACys transcript by the time course experiment (data not shown). We checked the template DNA sequences for in vitro transcription and repeated the time course experiments. However, we could not find any mistakes in these experiments. Thus, we found an unexpected tRNA specificity of the A. aeolicus Trm1; however, we could not rationally explain the mechanism through these experiments.
FIGURE 4.
Methyl transfer activity of A. aeolicus Trm1. A, nucleotide sequences of tested tRNA transcripts are compared. Positions from 8 to 48 are shown. We prepared full-length transcripts; sequences of the aminoacyl stem and T-arm are abbreviated by dotted lines. Yeast tRNAPhe (G26U) is a mutant transcript, in which G26 is substituted with U. B, methyl group incorporation into tRNA transcripts and E. coli tRNA mixture at 15-min periods are shown by bars. This graph does not represent the initial velocities of the m2G26 formation, because m22G26, m2G27, and/or m22G27 formations are included in some cases (for example, A. aeolicus tRNACys and tRNATyr transcripts) as described in the text. Overnight incubation increases m22G via m2G in all methylated tRNAs.
In Vitro Experiment Revealed That A. aeolicus Trm1 Methylates Not Only G26 but Also G27 in tRNA
A. aeolicus tRNATyr transcript was well methylated, although this tRNA contains A26 instead of G26. This result suggested that A. aeolicus Trm1 methylates a nucleotide(s) at another position in addition to the G26. Initially, we suspected methylation of G10 in the D-stem. However, the A. aeolicus tRNAGln transcript was not methylated at all, and this tRNA has G10 and A26 (Fig. 4). Thus, the additional modification site(s) did not seem to be G10. Furthermore, comparison of substrate tRNA sequences did not throw up a key sequence that enabled us to solve this puzzle.
These experimental results prompted us to employ LC/MS analysis of the methylated RNA. A. aeolicus tRNACys transcript was methylated with nonradioisotope-labeled AdoMet and A. aeolicus Trm1. The methylated RNA was completely digested with nuclease P1 and alkaline phosphatase, and its nucleosides were analyzed (Fig. 5A). As shown in Fig. 5A, only m2G and m22G were detected as modified nucleosides. Thus, it was confirmed that the modification site(s) is only the N2 atom of the guanine base(s). This is consistent with the results of the 14C-nucleotide analysis on two-dimensional TLC (data not shown). Next, we performed MS/MS analysis of RNase A-digested fragments. The methylated tRNACys was completely digested with RNase A, and its fragments were analyzed on LC/MS (Fig. 5B). Three fragments (m/z = 1194.7, 1201.7, and 1208.7) were eluted on LC. These masses strongly suggested that the fragments were derived from GGGGGACp (m/z = 1187.7) corresponding to the sequence from G26 to C32 in tRNACys; masses of the fragments coincide with the expected masses of mono-, di-, and trimethylated fragments, respectively. The modified positions were analyzed by MS/MS analysis (Fig. 5C). The sequence of the tri-methylated fragment was determined to be m22Gm2GGGGACp. This result clearly shows that the additional methylation site is G27. This is the reason that tRNACys accepts more than two methyl groups per tRNA molecule. Furthermore, this activity explains the methylation of tRNATyr, because tRNATyr has an 26AG27 sequence. Moreover, the ratio of the methylated fragments suggests that there is an order of the modifications at least in the case of the tRNACys transcript. Initially, the m2G modification occurs at G26 and then the second methyl transfer reaction generates m22G26. The third methyl transfer reaction modifies G27 to m2G27. As a result the m22G26m2G27 sequence is generated.
FIGURE 5.
Mass spectrometry analysis of methylated A. aeolicus tRNACys transcript. A, A. aeolicus tRNACys transcript was methylated with nonradioisotope-labeled AdoMet, and then its nucleosides were analyzed by LC/MS. The eluted positions of m2G and m22G, which are determined by another control experiment, are shaded. B, methylated tRNACys transcript was digested with RNase A, and its fragments were analyzed by LC/MS. The calculated m/z values are shown. C, fragments shown in B were analyzed by MS/MS. Nucleotide sequences determined by MS are shown in the panels.
Fraction of Native tRNACys Has an m22G26m22G27 Sequence
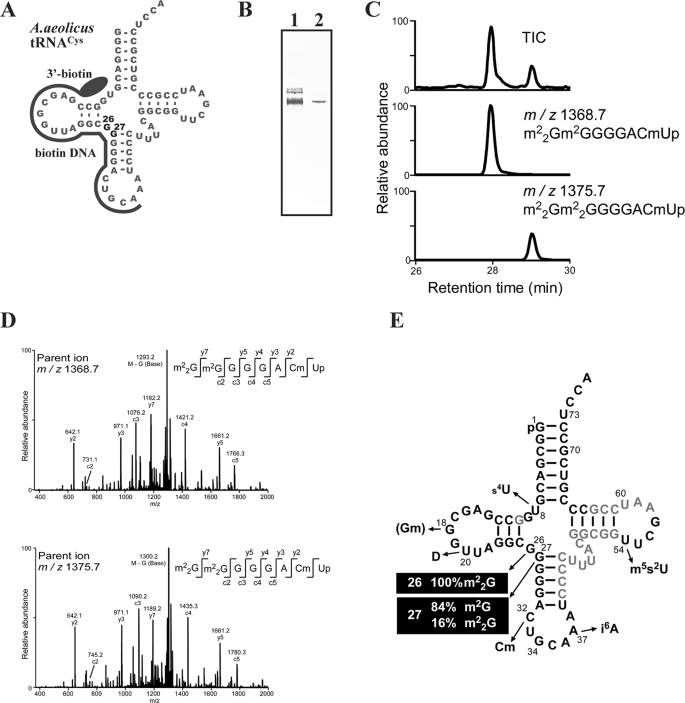
As described above, our experimental results showed that recombinant A. aeolicus Trm1 could modify the N2 atom of G27. However, we suspected that these results were potentially artifactual, occurring under the specific in vitro conditions. Therefore, we undertook purification of native tRNACys from A. aeolicus cells by solid-phase DNA probe column chromatography to ascertain whether there was an in vivo modification. In general, purification of tRNA from thermophiles is very difficult, because the tRNA structure is stabilized by modifications and the melting temperature is very high. In fact, our first approach using a general method (46) failed (data not shown). To overcome this problem, we employed novel solid-phase DNA probe column chromatography.3 Briefly, the major alterations are as follows. The DNA sequence was designed to be complementary to the RNA sequence from the D-loop to the anti-codon loop (Fig. 6A), because the sequence in this region of tRNACys is quite different from those of the other A. aeolicus tRNA species. To elute the RNA efficiently, tetramethylammonium chloride was used as the salt in the hybridization buffer. This approach successfully purified tRNACys (Fig. 6B), although the yield was only 0.09 A260 units from 10.0 A260 units of total RNA. We further purified the tRNACys by 15% PAGE (7 m urea) for LC/MS analysis. The purified tRNACys (around 200 fmol) was digested with RNase A, and the fragments were analyzed (Fig. 6C). Unexpectedly, a fraction of G27 was modified to m22G27 (Fig. 6D). The peak area of the fragments demonstrated that native tRNACys was modified to m22G26m22G27 (16%) through m22G26m2G27 (84%). Thus, a fraction of native tRNACys has an m22G26m22G27 modification.
FIGURE 6.
Purification and modified nucleotide analysis of native tRNACys. A, nucleotide sequence of A. aeolicus tRNACys is depicted as a cloverleaf structure. The 3′-biotin DNA probe region is illustrated. B, purified tRNACys was analyzed by 10% PAGE (7 m urea). The gel was stained with toluidine blue. Lane 1, low molecular weight RNA fraction extracted from A. aeolicus cells (0.03 A260 unit); lane 2, purified tRNACys (0.009 A260 unit). C, MS analysis of the RNase A-digested fragments of native tRNACys. Two fragments derived from nucleotides from G26 to U33 were eluted, for which calculated m/z values and sequences are shown. D, nucleotide sequences of two fragments shown in C were determined by MS/MS analysis. C32 was found to be modified to Cm32 in addition to G26 and G27. E, modified nucleotides in A. aeolicus tRNACys are shown in the cloverleaf structure.
In this experiment, we mainly focused on the modifications of 26GG27; however, we identified several other modifications (s4U8, D20, Cm32, i6A37, and m5s2U54) (Fig. 6E). Among them, the m5s2U54 modification has not been previously found in eubacteria except in Thermus thermophilus (47–50). Furthermore, in our previous study, we reported that the A. aeolicus tRNACys transcript is a very poor substrate for A. aeolicus tRNA (Gm18) methyltransferase (TrmH) (27). As expected, it was confirmed that the Gm18 modification in native tRNACys is hardly detectable. Moreover, A58 was found to be unmodified (not m1A58), suggesting that A. aeolicus TrmI (tRNA (m1A58) methyltransferase (43)) modifies specific tRNA species.
Kinetic Studies with Mutant A. aeolicus tRNATyr Transcripts
Our experimental results suggest that the G26 modification is preferred over G27 by A. aeolicus Trm1. To measure the methyl transfer speed to G26 and G27 separately, we made a mutant tRNATyr transcript (tRNATyr 26GA27), in which the 26AG27 sequence was substituted with a 26GA27 sequence (Fig. 7). The wild-type and mutant transcripts have only one target guanine, G27 or G26, respectively. We performed time course experiments with these transcripts and monitored m2G and m22G formations by TLC. When 0.1 μm enzyme and 11 μm transcript were incubated at 55 °C for 10 min, the formed pm22G was less than 5% the level of pm2G (data not shown). Thus, we confirmed that the first methyl transfer reaction (m2G formation) could be measured within 10 min under these conditions. After these pilot experiments, we measured the kinetic parameters for the transcripts. As shown in Fig. 7B, the G26 modification in the A26G,G27A,C43U mutant is clearly more rapid than the G27 modification in the wild-type tRNA achieved via a decrease of the Km value. Thus, these results suggest that the positions of the guanine bases reflect on the first binding of tRNA. In the tRNATyr A26G,G27A transcript, the base pair between A27 and C43 is probably not formed. Therefore, to clarify the role of this base pair, we made two mutant tRNATyr transcripts, in which the G27-C43 base pair was substituted with an A27-U43 base pair or a G27-U43 base pair. As shown in Fig. 7B, the G27-U43 base pair is preferable to the G27-C43 base pair. This result is in good agreement with the idea that methyl transfer to the N2 atom of G27 requires disruption of the base pair. The kinetic study showed that the disruption of G27-C43 base pair is more effective on the methyl transfer velocity than the position of the target guanine base. In contrast, in the case of G26 methylation, the A27-U43 pair does not have a significant effect.
FIGURE 7.
Kinetic study for tRNATyr variants. A, nucleotide sequence of tRNATyr is depicted as a cloverleaf structure. The mutation positions are highlighted in circles. B, kinetic parameters for the wild-type and mutant tRNATyr transcripts are given. The parameters are average values of three independent experiments.
A. aeolicus Trm1 Recognizes the T-arm Structure in tRNA
To address the tRNA recognition mechanism, we prepared additional 22 tRNATyr mutant transcripts (Fig. 8A). To distinguish between m2G26 and m2G27 formation, the mutants were based on tRNATyr 26GA27 (transcripts 1–11; Fig. 8, B and C) and tRNATyr 26AG27 (transcripts 12–22; Fig. 8, D and E). The 14C-methyl group incorporations from [14C]AdoMet into the transcripts were analyzed by autoradiogram of the gels. We attempted to measure kinetic parameters for these transcripts; however, the second methylation (m22G formation) was observed with low concentration samples especially in the case of the truncated mutants (data not shown). Therefore, we compared the initial velocities (Fig. 8, C and E).
FIGURE 8.
Determination of the recognition sites by truncated tRNA transcripts. A, truncated tRNA transcripts are depicted as cloverleaf-like structures. As described in the text, we prepared two series of transcripts based on the tRNATyr G26A,G27A mutant (B and C) and tRNATyr A26G,A27G (original wild type; D and E). Transcript numbers 1–11 are based on the tRNATyr G26A,G27A mutant, and numbers 12–22 are based on tRNATyr A26G,A27G. B and D, methyl group incorporation was monitored by 10% PAGE (7 m urea). Left panels show the patterns of methylene blue staining, and right panels are autoradiograms of the same gels. The lane numbers correspond to the transcript numbers in A. C and E, initial velocities are compared. This is the average of three independent experiments.
To our surprise, the recognition sites of the A. aeolicus Trm1 are completely different from those of archaeal and eukaryotic Trm1 proteins. When the D-stem was disrupted (transcripts 2 and 13) or the variable region was deleted (transcripts 11 and 22), both m2G26 and m2G27 formations were accelerated, whereas the D-stem and variable region were reported to be essential for archaeal and eukaryotic enzymes (9–12). In contrast, the deletion of the T-arm structure resulted in severe loss of the methyl acceptance activity (transcripts 7 and 18). Thus, the main recognition site(s) of the A. aeolicus Trm1 exists in the T-arm structure. When the 55UC56 sequence was substituted with 55AA56 (transcripts 9 and 20), the methyl acceptance activity was enhanced, demonstrating that the interaction between T- and D-arms is not required. This result is consistent with the result of D-loop deletion (transcripts 3 and 14). Multiple recognition sites seem to exist in the T-arm structure, because the deletion of the T-loop (transcripts 8 and 19) or disruption of the T-stem (transcripts 10 and 21) resulted in severe loss of methyl acceptance activity. Taken together with tRNA specificities (Fig. 4), the ribose-phosphate backbone in the T-arm structure is probably recognized. Furthermore, the L-shaped tRNA structure is probably disrupted in the RNA-Trm1 complex because several truncated transcripts are rapidly modified than the wild type.
Based on these experimental results, we compared the recognition mechanisms of the Trm1 proteins (Fig. 9). A. aeolicus Trm1 recognizes the G26 and G27 bases from the T-arm (Fig. 9A). In contrast, archaeal and eukaryotic Trm1 proteins recognize the G26 base from the D-stem and variable region (Fig. 9B). The distance between the T-arm and G26 (or G27) is longer than the distance between the D-stem, variable region, and G26. These differences from binding site to the target guanine base(s) may decide the multisite or single site recognition of the Trm1 proteins.
FIGURE 9.
Schematic drawing of the target guanine recognition mechanisms of Trm1 proteins. A. aeolicus Trm1 recognizes the G26 and G27 bases from the T-arm region (A). In contrast, archaeal and eukaryotic enzymes recognize only the G26 base from the D-stem and variable region (B). A. aeolicus Trm1 recognizes the target sites distantly spaced as compared with archaeal and eukaryotic enzymes. This long distance target site recognition confers the multisite specificity of A. aeolicus Trm1.
DISCUSSION
In general, trm1 genes have been found only in archaea and eukaryotes. The only exception is the A. aeolicus trm1, a eubacterial gene. In this study, we demonstrate that the A. aeolicus trm1 gene product has a novel tRNA methyltransferase activity, which catalyzes methyl transfer not only to G26 but also to G27. The expression of the gene product in A. aeolicus cells was confirmed by Western blotting analysis, and the tRNA (m2G and m22G) methyltransferase activity was found in the cell extract. Furthermore, modification to m22G27 in addition to m22G26 was confirmed in purified native tRNACys. Moreover, in vitro experiments reveal that the purified Trm1 protein catalyzes methyl transfers to G27 as well as G26. In the reaction, the protein does not require any another subunit and/or guide RNA to recognize G27. These experimental results clearly reveal that A. aeolicus Trm1 catalyzes methyl transfer not only to G26 but also to G27. Thus, A. aeolicus Trm1 is an enzyme with multisite recognition. There are other examples of multisite recognition enzymes. E. coli TruA modifies U38, U39, and U40 to Ψ38, Ψ39, and Ψ40, respectively (51, 52). P. abyssi TrmI catalyzes methyl transfer to both A57 and A58 (53). S. cerevisiae Trm4 is responsible for formation of m5C at positions 34, 40, 48, and 49 (54). Furthermore, it has recently been reported that P. abssi PAB1947 protein (archaeal Trm4) changes site specificity in the presence of PAB1946 protein (archaese) (55). Moreover, S. cerevisiae Trm7 protein catalyzes 2′-O-methylation of nucleotides at positions 32 and 34 (56). Although these examples have been reported, there is little knowledge concerning the multisite specificity mechanism. In this study, we propose a long distance recognition mechanism by A. aeolicus Trm1, whereby differences in the distance between the recognition sites and G26 (or G27) results in multisite or single-site recognition in Trm1 proteins.
To access G27, G27-C43 base pair needs to be disrupted, because the N2 atom of G27 forms a hydrogen bond with C43 in the anticodon stem. In the G27-U43 base pair, the N2 atom does not form a hydrogen bond and probably locates to the tRNA surface. Trm1 protein prefers this tRNA structure; the tRNATyr 43C→U mutant transcript was modified rapidly as compared with the wild-type tRNA transcript. Furthermore, we were able to demonstrate m22G27 formation in native tRNACys. These experimental results suggest that the fourth modification (m22G27 formation) requires disruption of the G27-C43 base pair. This idea is consistent with the substrate RNA recognition mechanism by TruA, a multisite specificity RNA modification enzyme for which it has recently been reported that balance between the flexibility and stability of the substrate RNA is important for substrate recognition (52).
The m22G27 modification is found only in three tRNA species other than A. aeolicus tRNACys, namely bovine liver tRNATyr (57) and human placenta tRNATyr-1 and tRNATyr-2 (58). These tRNA species have a 27GU43 sequence, consistent with our experimental results. For more than 20 years, the m22G27 modification in these tRNA species has been an enigma in the tRNA modification field, because the purified human Trm1 protein does not modify G27 in human tRNATyr transcript (17). Our experimental results in this study provide a possible explanation to solve this puzzle. The human (or bovine) Trm1 protein probably localizes to the nuclear membrane, and the structure of the tRNATyr may be partly disrupted on the nuclear membrane and/or during export to the cytoplasm, and this may result in a chance to modify the G27. In the perturbed tRNA structure, distance between the D-stem, variable region, and target site (G26 or G27) is probably changed. Thus, the m22G27 modification may be explainable through modification by the eukaryote Trm1 protein without assuming a need for the existence of an m22G27-specific enzyme. To prove this hypothesis, further study will be necessary.
In general, modified nucleotides in the three-dimensional core of tRNA stabilize the L-shaped RNA structure. The fourth methylation (m22G27 formation) disrupts the G27-C43 base pair in A. aeolicus tRNACys, because the two methyl groups block the Watson-Crick type hydrogen bond formation with C43. The disruption of a base pair seems to destabilize the tRNA structure. However, in the case of A. aeolicus tRNACys, it may have a stabilizing effect on the structure. The two methyl groups on the N2 atom of G27 can rotate freely and form hydrophobic interactions with G26 and C28, although the precise structure of tRNACys has not been reported. Thus, this hydrophobic interaction may be advantageous to the organism by stabilizing the tRNA to a greater extent than is seen with the formation of the Watson-Crick hydrogen bonds. This may be important for tRNA structure at high temperatures, at which A. aeolicus lives.
Finally, it is worthwhile considering the origin of the A. aeolicus trm1 gene for which there are two hypotheses. The first is that the A. aeolicus trm1 gene is derived from some archaeal gene by horizontal transfer. The other hypothesis is that the A. aeolicus trm1 gene is a remnant from a common ancestor before the divergence of eubacteria and archaea. Our genetic tree analysis suggests that the A. aeolicus trm1 gene more closely resembles the trm1 genes encoded in methane archaea (data not shown). Thus, although the tRNA substrate specificity of A. aeolicus Trm1 is considerably different from those of the archaeal Trm1 proteins reported, the A. aeolicus gene may be derived from archaea. Furthermore, the m22G27 modification may be present in some other archaeal tRNA species. To solve these questions, further study will be necessary.
Supplementary Material
Acknowledgments
We thank the following members of Ehime University: Prof. Yaeta Endo for use of laboratory facilities; Dr. Akira Hirata for preparation of the amino acid sequence alignment; Hiroshi Takeda for molecular cloning of the trm1 gene; Toru Takehara for construction of the vector; and Chikako Iwashita and Takashi Toyooka for technical support in Western blotting analysis.
This work was supported in part by Japan Society for the Promotion of Science Research Fellowship for Young Scientists 20-4827 (to C. T.), Grant-in-aid for Science Research on Priority Areas 20034041 (to H. H.), and Grant-in-aid for Science Research 19350087 (to H. H.) from the Ministry of Education, Science, Sports, and Culture of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Experimental Procedures and Table 1.
- AdoMet
- S-adenosyl-l-methionine
- LC/MS
- liquid chromatography/mass spectrometry
- MS/MS
- tandem mass spectrometry.
REFERENCES
- 1.Rozenski J., Crain P. F., McCloskey J. A. (1999) Nucleic Acids Res. 27, 196–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunin-Horkawicz S., Czerwoniec A., Gajda M. J., Feder M., Grosjean H., Bujnicki J. M. (2006) Nucleic Acids Res. 34, D145–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia G. R., Goodenough-Lashhua D. M. (1998) in Modification and Editing of RNA (Grosjean H., Benne R. eds) pp. 555–560, American Society for Microbiology,Washington, DC [Google Scholar]
- 4.Reinhart M. P., Lewis J. M., Leboy P. S. (1986) Nucleic Acids Res. 14, 1131–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Constantinesco F., Motorin Y., Grosjean H. (1999) J. Mol. Biol. 291, 375–392 [DOI] [PubMed] [Google Scholar]
- 6.Kuchino Y., Nishimura S. (1970) Biochem. Biophys. Res. Commun. 40, 306–313 [DOI] [PubMed] [Google Scholar]
- 7.Glick J. M., Averyhart V. M., Leboy P. S. (1978) Biochim. Biophys. Acta 518, 158–171 [DOI] [PubMed] [Google Scholar]
- 8.Stange N., Beier H. (1987) EMBO J. 6, 2811–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edqvist J., Grosjean H., Stråby K. B. (1992) Nucleic Acids Res. 20, 6575–6581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edqvist J., Blomqvist K., Stråby K. B. (1994) Biochemistry 33, 9546–9551 [DOI] [PubMed] [Google Scholar]
- 11.Grosjean H., Edqvist J., Stråby K. B., Giegé R. (1996) J. Mol. Biol. 255, 67–85 [DOI] [PubMed] [Google Scholar]
- 12.Constantinesco F., Motorin Y., Grosjean H. (1999) Nucleic Acids Res. 27, 1308–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips J. H., Kjellin-Stråby K. (1967) J. Mol. Biol. 26, 509–518 [DOI] [PubMed] [Google Scholar]
- 14.Ellis S. R., Morales M. J., Li J. M., Hopper A. K., Martin N. C. (1986) J. Biol. Chem. 261, 9703–9709 [PubMed] [Google Scholar]
- 15.Niederberger C., Gräub R., Costa A., Desgrès J., Schweingruber M. E. (1999) FEBS Lett. 464, 67–70 [DOI] [PubMed] [Google Scholar]
- 16.Liu J., Zhou G. Q., Stråby K. B. (1999) Gene 226, 73–81 [DOI] [PubMed] [Google Scholar]
- 17.Liu J., Strâby K. B. (2000) Nucleic Acids Res. 28, 3445–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Constantinesco F., Benachenhou N., Motorin Y., Grosjean H. (1998) Nucleic Acids Res. 26, 3753–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ihsanawati, Nishimoto M., Higashijima K., Shirouzu M., Grosjean H., Bessho Y., Yokoyama S. (2008) J. Mol. Biol. 383, 871–884 [DOI] [PubMed] [Google Scholar]
- 20.Grosjean H., Gaspin C., Marck C., Decatur W. A., de Crécy-Lagard V. (2008) BMC Genomics 9, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auffinger P., Westhof E. (1998) in Modification and Editing of RNA (Grosjean H., Benne R. eds) pp. 569–576, American Society for Microbiology, Washington, DC [Google Scholar]
- 22.Purushothaman S. K., Bujnicki J. M., Grosjean H., Lapeyre B. (2005) Mol. Cell. Biol. 25, 4359–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armengaud J., Urbonavicius J., Fernandez B., Chaussinand G., Bujnicki J. M., Grosjean H. (2004) J. Biol. Chem. 279, 37142–371452 [DOI] [PubMed] [Google Scholar]
- 24.Urbonavicius J., Armengaud J., Grosjean H. (2006) J. Mol. Biol. 357, 387–399 [DOI] [PubMed] [Google Scholar]
- 25.Schubert H. L., Blumenthal R. M., Cheng X. (2003) Trends Biochem. Sci. 28, 329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabant G., Auxilien S., Tuszynska I., Locard M., Gajda M. J., Chaussinand G., Fernandez B., Dedieu A., Grosjean H., Golinelli-Pimpaneau B., Bujnicki J. M., Armengaud J. (2006) Nucleic Acids Res. 34, 2483–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hori H., Kubota S., Watanabe K., Kim J. M., Ogasawara T., Sawasaki T., Endo Y. (2003) J. Biol. Chem. 278, 25081–25090 [DOI] [PubMed] [Google Scholar]
- 28.Okamoto H., Watanabe K., Ikeuchi Y., Suzuki T., Endo Y., Hori H. (2004) J. Biol. Chem. 279, 49151–49159 [DOI] [PubMed] [Google Scholar]
- 29.Takeda H., Toyooka T., Ikeuchi Y., Yokobori S., Okadome K., Takano F., Oshima T., Suzuki T., Endo Y., Hori H. (2006) Genes Cells 11, 1353–1365 [DOI] [PubMed] [Google Scholar]
- 30.Tomikawa C., Ochi A., Hori H. (2008) Proteins 71, 1400–1408 [DOI] [PubMed] [Google Scholar]
- 31.Toyooka T., Awai T., Kanai T., Imanaka T., Hori H. (2008) Genes Cells 13, 807–816 [DOI] [PubMed] [Google Scholar]
- 32.Burggraf S., Olsen G. J., Stetter K. O., Woese C. R. (1992) Syst. Appl. Microbiol. 15, 353–356 [DOI] [PubMed] [Google Scholar]
- 33.Griffiths E., Gupta R. S. (2001) Microbiology 147, 2611–2622 [DOI] [PubMed] [Google Scholar]
- 34.Griffiths E., Gupta R. S. (2006) Int. J. Syst. Evol. Microbiol. 56, 99–107 [DOI] [PubMed] [Google Scholar]
- 35.Deckert G., Warren P. V., Gaasterland T., Young W. G., Lenox A. L., Graham D. E., Overbeek R., Snead M. A., Keller M., Aujay M., Huber R., Feldman R. A., Short J. M., Olsen G. J., Swanson R. V. (1998) Nature 392, 353–358 [DOI] [PubMed] [Google Scholar]
- 36.Keith G. (1995) Biochimie 77, 142–144 [DOI] [PubMed] [Google Scholar]
- 37.Suzuki T., Ikeuchi Y., Noma A., Suzuki T., Sakaguchi Y. (2007) Methods Enzymol. 425, 211–229 [DOI] [PubMed] [Google Scholar]
- 38.Ikeuchi Y., Kitahara K., Suzuki T. (2008) EMBO J. 27, 2194–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noma A., Kirino Y., Ikeuchi Y., Suzuki T. (2006) EMBO J. 25, 2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeuchi Y., Shigi N., Kato J., Nishimura A., Suzuki T. (2006) Mol. Cell 21, 97–108 [DOI] [PubMed] [Google Scholar]
- 41.Kaneko T., Suzuki T., Kapushoc S. T., Rubio M. A., Ghazvini J., Watanabe K., Simpson L., Suzuki T. (2003) EMBO J. 22, 657–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bujnicki J. M., Leach R. A., Debski J., Rychlewski L. (2002) J. Mol. Microbiol. Biotechnol. 4, 405–415 [PubMed] [Google Scholar]
- 43.Droogmans L., Roovers M., Bujnicki J. M., Tricot C., Hartsch T., Stalon V., Grosjean H. (2003) Nucleic Acids Res. 31, 2148–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Bie L. G., Roovers M., Oudjama Y., Wattiez R., Tricot C., Stalon V., Droogmans L., Bujnicki J. M. (2003) J. Bacteriol. 185, 3238–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta R. (1984) J. Biol. Chem. 259, 9461–9471 [PubMed] [Google Scholar]
- 46.Tsurui H, Kumazawa Y., Sanokawa R., Watanabe Y., Kuroda T., Wada A., Watanabe K., Shirai T. (1994) Anal. Biochem. 221, 166–172 [DOI] [PubMed] [Google Scholar]
- 47.Watanabe K., Shinma M., Oshima T., Nishimura S. (1976) Biochem. Biophys. Res. Commun. 72, 1137–1144 [DOI] [PubMed] [Google Scholar]
- 48.Shigi N., Sakaguchi Y., Suzuki T., Watanabe K. (2006) J. Biol. Chem. 281, 14296–14306 [DOI] [PubMed] [Google Scholar]
- 49.Shigi N., Suzuki T., Terada T., Shirouzu M., Yokoyama S., Watanabe K. (2006) J. Biol. Chem. 281, 2104–2113 [DOI] [PubMed] [Google Scholar]
- 50.Shigi N., Sakaguchi Y., Asai S., Suzuki T., Watanabe K. (2008) EMBO J. 27, 3267–3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kammen H. O., Marvel C. C., Hardy L., Penhoet E. E. (1988) J. Biol. Chem. 263, 2255–2263 [PubMed] [Google Scholar]
- 52.Hur S., Stroud R. M. (2007) Mol. Cell 26, 189–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roovers M., Wouters J., Bujnicki J. M., Tricot C., Stalon V., Grosjean H., Droogmans L. (2004) Nucleic Acids Res. 32, 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Motorin Y., Grosjean H. (1999) RNA 5, 1105–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Auxilien S., El Khadali F., Rasmussen A., Douthwaite S., Grosjean H. (2007) J. Biol. Chem. 282, 18711–18721 [DOI] [PubMed] [Google Scholar]
- 56.Pintard L., Lecointe F., Bujnicki J. M., Bonnerot C., Grosjean H., Lapeyre B. (2002) EMBO J. 21, 1811–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson G. D., Pirtle I. L., Pirtle R. M. (1985) Arch. Biochem. Biophys. 236, 448–453 [DOI] [PubMed] [Google Scholar]
- 58.van Tol H., Stange N., Gross H. J., Beier H. (1987) EMBO J. 6, 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson J. D., Higgins D. G., Gibson T. J. (1994) Nucleic Acids Res. 22, 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gouet P., Courcelle E., Stuart D. I., Métoz F. (1999) Bioinformatics 15, 305–308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.