Abstract
Sre1, the fission yeast sterol regulatory element-binding protein (SREBP), is an endoplasmic reticulum (ER) membrane-bound transcription factor that is a principal regulator of hypoxic gene expression. Under low oxygen, Sre1 is cleaved from its inactive ER precursor form to generate an active nuclear transcription factor that up-regulates genes required for low oxygen growth. To maintain a constant supply of Sre1, Sre1 precursor synthesis must be regulated to replenish Sre1 precursor lost to proteolytic cleavage under low oxygen. In this study, we investigated the mechanisms controlling Sre1 precursor levels. We found that positive feedback regulation at the sre1+ promoter increases the synthesis of the Sre1 precursor under low oxygen and that this regulation is required for maximal Sre1 activation and target gene expression. We also demonstrate that the Sre1 precursor is rapidly degraded by the proteasome in the absence of its binding partner Scp1, which is required for oxygen-regulated Sre1 cleavage. Degradation of Sre1 in the absence of Scp1 requires the ER-associated degradation (ERAD) components Ubc7, an E2 ubiquitin conjugating enzyme, and Hrd1, an E3 ubiquitin ligase. We conclude that positive feedback regulation to up-regulate Sre1 precursor synthesis under low oxygen is essential for Sre1 function and propose that excess Sre1 precursor is removed by ERAD to ensure complex formation between Sre1 and its binding partner Scp1. Thus, Sre1 is a new example of an endogenous ERAD substrate, establishing fission yeast as an organism for the study of this important degradative pathway.
Activation of low oxygen gene expression in Schizosaccharomyces pombe is primarily controlled by the Sre1 transcription factor. Sre1 is the fission yeast homolog of the mammalian sterol regulatory element-binding protein (SREBP)4 (1). SREBPs are ER-membrane bound, basic helix-loop-helix transcription factors that control expression of genes required for uptake and synthesis of cholesterol under sterol limiting conditions (2). SREBP forms a tight complex with its binding partner Scap (SREBP cleavage activating protein). When sterol levels are low, Scap escorts SREBP to the Golgi where SREBP is sequentially cleaved by Site 1 and Site 2 proteases to release the N-terminal transcription factor domain (3). Sterols regulate access of SREBP to the Golgi-localized proteases by controlling entrance of SREBP-Scap into ER transport vesicles (4). Following SREBP cleavage in the Golgi, Scap is thought to recycle to the ER and bind to newly synthesized SREBP to participate in additional rounds of cleavage (2).
In fission yeast, Sre1 is proteolytically cleaved and activated in response to low oxygen (1). Under low oxygen conditions, oxygen-dependent ergosterol synthesis is inhibited, leading to Sre1 cleavage and release of the N-terminal transcription factor domain (Sre1N). In addition, oxygen also controls the degradation of Sre1N. In the presence of oxygen, the prolyl hydroxylase family member Ofd1 accelerates the turnover of Sre1N (5). In the absence of oxygen, Ofd1 is inhibited leading to rapid accumulation of cleaved Sre1N and expression of Sre1 target genes. Sre1 activates expression of genes in pathways involved in non-respiratory oxygen consumption required for low oxygen growth (6).
Like mammalian SREBP, activation of Sre1 also requires binding to Scap, called Scp1 in fission yeast (1). Scap contains a sterol sensing domain in transmembrane domains 2–6 that is required for sterol-regulated cleavage of SREBPs (2). Interestingly, in both yeast and mammals, the SREBP precursor is reduced in the absence of Scap (1, 7). The mechanism by which the SREBP precursor is regulated in the absence of Scap is unknown.
Both luminal and membrane-bound ER proteins are subject to quality control and misfolded proteins are degraded by a process termed ER-associated protein degradation (ERAD) (8). ERAD involves recognition and polyubiquitination of misfolded protein substrates, extraction from the ER, and degradation by the proteasome (9–11). In Saccharomyces cerevisiae, the ERAD pathway involves two ubiquitin ligases, Hrd1p and Doa10p, both of which contain cytosolic RING finger domains (12). Hrd1p is required for degradation of protein substrates with misfolded ER-luminal or membrane domains and Doa10p is required for degradation of proteins with misfolded cytosolic domains (13, 14). Both the Hrd1p and Doa10p E3 ubiquitin ligases can catalyze the polyubiquitination of substrates through the ubiquitin-conjugating enzyme Ubc7p (15). In the sterol pathway, S. cerevisiae Hrd1 and the mammalian E3 ligase gp78 catalyze the ubiquitination and degradation of the sterol biosynthetic enzyme HMG-CoA reductase in sterol-overloaded cells (9, 16). In addition, gp78 mediates sterol-regulated degradation of Insig-1, a central regulator of cholesterol homeostasis (17). Therefore, ERAD functions in the physiological regulated degradation of proteins as well as degradation of misfolded proteins.
Our previous studies revealed that oxygen controls Sre1 proteolytic activation and nuclear Sre1 protein stability to regulate Sre1-dependent target gene expression (1, 5). However, to achieve sustained activation of Sre1 target genes under low oxygen, cleaved Sre1N must be continually produced from the Sre1 precursor. Here, we investigated the mechanisms regulating Sre1 precursor levels independent of oxygen-regulated proteolytic cleavage. We found that positive feedback regulation of the sre1+ promoter is necessary to maintain Sre1 precursor levels and is required for maximal Sre1 activation and function. We also demonstrate that Sre1 precursor levels are dependent upon Scp1. In the absence of Scp1, the Sre1 precursor is rapidly degraded by the proteasome in a mechanism requiring the ERAD components, Ubc7 and Hrd1. These results demonstrate that positive feedback is required for Sre1 pathway function and suggest that Sre1 is an endogenous substrate for ER-associated degradation.
EXPERIMENTAL PROCEDURES
Materials
Yeast extract was obtained from BD Biosciences; amino acids from Sigma; horseradish peroxidase-conjugated affinity purified donkey anti-rabbit and anti-mouse immunoglobulin G (IgG) from Jackson ImmunoResearch; oligonucleotides from Integrated DNA Technologies; cobalt chloride and cycloheximide from Sigma; proteasome inhibitor II ((benzyloxycarbonyl)-Leu-Leu-phenylalaninal) from Calbiochem.
Yeast Strains and Culture
Wild-type haploid S. pombe KGY425 (h−, his3-D1, leu1-32, ura4-D18, ade6-M210) and KGY461 (h+, his3-D1, leu1-32, ura4-D18, ade6-M210) were obtained from the American Type Culture Collection (18). S. pombe strains scp1Δ::ura4+, Nmt*-scp1+, ubc7Δ::kanMX6 (SPBP16F5.04), hrd1Δ::kanMX6 (SPBC17D11.02c), and doa10Δ::kanMX6 (SPBC14F5.07) were generated from wild-type haploid yeast (KGY425 or KGY461) by homologous recombination using established techniques (19). The sre1Δ::kanMX6 and scp1Δ::kanMX6 strains were described previously (1). hrd1–13xMyc:kanMX6 was tagged at the C terminus by homologous recombination in scp1Δ::ura4+ haploid yeast using established techniques (18). The resulting strain scp1Δ::ura4+ hrd1–13xMyc:kanMX6 was then crossed to ubc7Δ:kanMX6. The mts3-1 strain was a kind gift from Colin Gordon (Medical Research Council, UK).
Strain sre1-MP (MP, mutant promoter) contains mutated Sre1 DNA binding sequences SRE2 and SRE3 in the sre1+ promoter to prevent positive feedback regulation by Sre1 (6). The sequence of SRE2 was changed from 5′-ATCACCCCAT-3′ to 5′-ATATACCATA-3′ and the sequence of SRE3 was changed from 5′-GTCAGTCCAC-3′ to 5′-GTATATCATA-3′. To generate sre1-MP base pairs −550 to −450 upstream of the sre1+ open reading frame were replaced with ura4+ by homologous recombination. This strain was subsequently transformed with a 850-bp PCR product containing sequences directly upstream of the sre1+ open reading frame amplified using oligonucleotides SRE2 + 3F and SRE2 + 3R and the SRE2*SRE3* plasmid template described previously (6). Transformants were selected on minimal medium containing 5-fluoroorotic acid to counterselect for expression of the ura4+ gene product (20). Transformants were screened by PCR and confirmed by sequencing. Standard genetic techniques were used to generate the scp1Δ mts3-1, scp1Δ ubc7Δ, scp1Δ hrd1Δ, and scp1Δ doa10Δ strains (21).
Yeast strains were grown to exponential phase at 30 °C (unless otherwise indicated) in yeast extract plus supplements (YES) (225 μg/ml each of histidine, leucine, adenine, lysine, and uracil) or in Edinburgh minimal medium where indicated using standard techniques (1). Anaerobic growth conditions were maintained using an In vivo2 400 work station (Biotrace, Inc.) as described previously (1, 6). For plate assays, yeast grown on YES (5 × 103 cells) were 5-fold serially diluted on YES agar or YES agar containing 1.6 mm CoCl2 and were grown for 3–6 days.
Plasmids
pCaMV-Sre1, which expresses sre1+ from the constitutive cauliflower mosaic virus (CaMV) promoter was generated in three steps. First, sre1+ was reverse transcribed from S. pombe RNA and cloned into pSL1180 (GE Healthcare Bioscience) using primers 5′-GATCGCGGCCGCATGCAAGCTCAATTCCGTCAGTT-3′ and 5′-GATCGTCGAC(T)18-3′ to yield PJE426. Next, this plasmid was digested with XhoI and the 1.6-kb XhoI sre1+ fragment was cloned into the XhoI-SalI restriction sites of pCaMV-Sre1N plasmid previously described to yield PJE569 (1). This plasmid was then digested with BamHI-PstI and the sre1+ fragment was cloned into BamHI-PstI-digested pSLF101 to yield pCaMV-Sre1 (pBT102) (22).
Northern and Western Blotting
Total RNA isolation and Northern blot analysis as well as probes for sre1+, hem13+, and tub1+ have been described previously (1, 5). Whole cell yeast extract preparation, membrane protein extraction, Western blot analysis, anti-Sre1 IgG polyclonal antibody, and anti-Scp1 monoclonal antibodies 8G4C11, 1G1D6, and 7B4A3 have been described (1, 23).
For protein half-life analysis, the indicated yeast strains were grown in the presence or absence of oxygen to exponential phase where indicated and then shifted to aerobic conditions in the presence of cycloheximide (100 μg/ml) (Sigma). Samples were collected, and whole cell lysates (40 μg) were subjected to Western blot analysis. Blots were developed using the HyGLO HP Detection Kit (Denville Scientific) or the SuperSignal® West Dura Extended Duration Substrate (Pierce), exposed to film and imaged using the Versadoc Imaging System (Bio-Rad). Quantification was performed using Quantity One Software (Bio-Rad). Each half-life analysis figure shows results representative of at least three independent experiments.
Immunoprecipitation
Yeast cells (5 × 107) were lysed with glass beads in TAP lysis buffer (6 mm Na2HPO4, 4 mm NaH2PO4, 1% (w/v) digitonin (Calbiochem), 150 mm NaCl, 4 mm EDTA, 50 mm sodium fluoride, and 0.3 mm Na3VO4) and protease inhibitors. Cleared lysates were incubated with anti-Myc 9E10 monoclonal antibody (Santa Cruz Biotechnology) and Protein G-Sepharose (GE Healthcare) overnight at 4 °C. Bound fraction was washed three times with TAP lysis buffer, and bound and unbound fractions were subjected to immunoblot analysis.
RESULTS
Positive Feedback Regulation Is Required for Maximal Sre1 Activation and Function
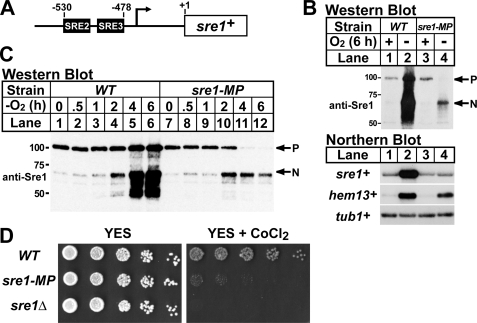
The amount of Sre1 precursor is controlled by rates of synthesis, oxygen-regulated proteolytic cleavage, and degradation. Previously, we showed that Sre1 binds to its own promoter and up-regulates expression of sre1+ mRNA in a positive feedback mechanism (1, 5, 6). To determine the contribution of this positive feedback to Sre1 precursor levels and activity of the Sre1 pathway, we mutated two Sre1 binding sites (SRE2 and SRE3) in the sre1+ promoter required for positive feedback regulation and designated this strain sre1-MP (mutant promoter) (Fig. 1A) (5, 6). To test if mutations in SRE2 and SRE3 blocked the low oxygen increase in sre1+ mRNA, we grew wild-type and sre1-MP cells in the presence or absence of oxygen for 6 h. Wild-type cells grown in the absence of oxygen showed a dramatic increase in Sre1N and a large increase in the sre1+ mRNA (Fig. 1B, lanes 1 and 2). In contrast, sre1+ mRNA did not increase in sre1-MP cells in the absence of oxygen (Fig. 1B, lanes 3 and 4). Sre1 cleavage was significantly reduced and expression of hem13+, a known Sre1 target gene, was also reduced in sre1-MP cells compared with wild-type cells grown in the absence of oxygen (Fig. 1B) (6). sre1+ mRNA and Sre1 precursor levels were similar in wild-type and sre1-MP cells in the presence of oxygen, indicating that the SRE2 and SRE3 elements were not required for synthesis of the sre1+ mRNA or Sre1 precursor in normoxic conditions (Fig. 1B, lanes 1 and 3). Importantly, Sre1 precursor was significantly reduced in sre1-MP cells in the absence of oxygen (Fig. 1B, lanes 2 and 4), suggesting that increased sre1+ mRNA expression under low oxygen is required to maintain a supply of Sre1 precursor.
FIGURE 1.
Positive feedback regulation at the sre1+ promoter is required for maximal Sre1 activation and growth. A, diagram of the sre1+ promoter. The Sre1 regulatory elements (SREs) are shown in black. B, wild-type (WT) and sre1-MP cells were grown in rich medium ± oxygen for 6 h. Whole cell lysates were subjected to Western blot analysis with an antibody directed against the N terminus of Sre1 (anti-Sre1 IgG). P and N denote the precursor and Sre1N nuclear forms, respectively. Total RNA (5 μg) was subjected to Northern analysis with the indicated 32P-labeled probes. α-Tubulin (tub1+) mRNA served as a loading control. C, wild-type and sre1-MP cells were grown for increasing times −oxygen. Whole cell lysates were analyzed as in B. D, 5-fold serial dilutions of wild-type, sre1-MP, and sre1Δ cells were plated YES or YES containing 1.6 mm CoCl2.
To examine the kinetics of Sre1 precursor depletion in sre1-MP cells, wild-type and sre1-MP cells were grown in the absence of oxygen for increasing times. As shown previously, in wild-type cells Sre1 cleavage was maximal at 4–6 h and Sre1 precursor increased under low oxygen (Fig. 1C, lanes 1–6) (1). However, in sre1-MP cells Sre1 precursor decreased over time and was depleted by 4 h of low oxygen growth (Fig. 1C, lanes 7–12). Additionally, accumulation of Sre1N was maximal at 2 h and then decreased at 4 and 6 h mirroring the depletion of Sre1 precursor (Fig. 1C, lanes 7–12). Together, these results indicate that positive feedback regulation of the sre1+ promoter is required for maximal Sre1 activation and target gene expression under low oxygen.
Our previous studies showed that Sre1 cleavage is activated by cobalt chloride, a hypoxia mimetic that inhibits fungal sterol synthesis (24), and that sre1+ is required for growth on cobalt chloride containing medium. To test if positive feedback regulation at the sre1+ promoter is physiologically required for Sre1 function, we assayed growth of wild-type, sre1-MP, or sre1Δ cells on rich medium containing cobalt chloride. sre1Δ cells showed wild-type growth on rich medium, but failed to grow on medium containing cobalt chloride (Fig. 1D) (24). sre1-MP cells grew similar to wild-type cells on rich medium. However, sre1-MP cells displayed a dramatic growth defect on medium containing cobalt chloride consistent with reduced Sre1 function (Fig. 1D). Collectively, these results demonstrate that positive feedback regulation of sre1+ is required for maximal Sre1 activation and Sre1 pathway function.
Scp1 Is Required for Sre1 Stability
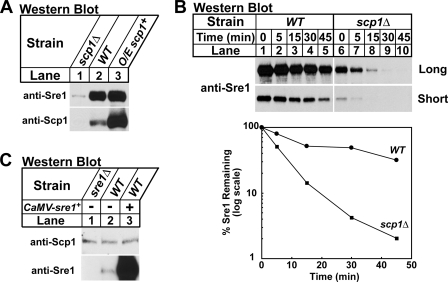
Having established that positive feedback regulation is required to maintain the Sre1 precursor supply, we next investigated how Sre1 precursor degradation is regulated. For these experiments, cells were grown in the presence of oxygen to inhibit Sre1 proteolytic cleavage. In the ER, Sre1 forms a stable complex with Scp1 (1). In the absence of Scp1, Sre1 precursor was reduced (Fig. 2A, lanes 1 and 2). To test whether the reduction in Sre1 precursor was due to decreased Sre1 stability, we performed a cycloheximide chase experiment to measure Sre1 half-life in wild-type and scp1Δ cells. Wild-type and scp1Δ cells were treated with cycloheximide to inhibit protein translation and whole cell lysates were collected after increasing time. In wild-type cells, Sre1 precursor half-life was 15–30 min (Fig. 2B, short exposure). However, in scp1Δ cells Sre1 degradation was accelerated (Fig. 2B, long exposure). These results indicate that Scp1 is required for Sre1 precursor stability in addition to its role in Sre1 proteolytic cleavage. Interestingly, overexpression of scp1+ only slightly increased Sre1 precursor (Fig. 2A, lanes 2 and 3), suggesting that excess Scp1 has little additional effect on steady-state levels of Sre1.
FIGURE 2.
Scp1 is required for Sre1 precursor stability. A, scp1Δ, wild-type (WT), and cells overexpressing scp1+ from the thiamine repressible nmt* promoter were grown in Edinburgh minimal medium in the absence of thiamine. Whole cell extracts or urea-solubilized membranes were subjected to Western blot analysis with anti-Sre1 IgG or a mixture of anti-Scp1 IgG monoclonal antibodies 8G4C11, 1G1D6, and 7B4A3, respectively. B, wild-type and scp1Δ cells were grown in YES, at t = 0 cycloheximide (100 μg/ml) was added and samples were collected at the indicated times. Whole cell lysates were subjected to Western blot analysis with anti-Sre1 IgG. Images for wild-type and scp1Δ cells were from the same film and both long and short exposure times are shown. The percentage of Sre1 precursor remaining relative to t = 0 is quantified below. C, sre1Δ and wild-type cells containing an empty vector or a plasmid expressing Sre1 from the constitutive CaMV promoter were grown in Edinburgh minimal medium. Whole cell extracts or urea-solubilized membranes were analyzed as in A.
To test if Sre1 in turn controls the stability of Scp1, we measured Scp1 in sre1Δ cells, wild-type cells, and cells overexpressing sre1+. We detected no change in Scp1 levels among these three samples (Fig. 2C), and cycloheximide chase experiments revealed that Scp1 is a long lived protein whose half-life is not dependent upon Sre1 (t½ > 120 min, data not shown). Together, these results demonstrate that Sre1 precursor protein stability is dependent on Scp1 and that Scp1 is a stable protein under these conditions.
Degradation of Sre1 Requires the Proteasome
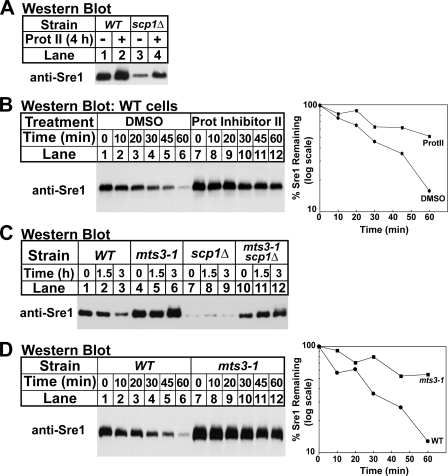
Because Sre1 is unstable in the absence of Scp1, we next sought to determine the mechanism by which Sre1 is degraded. To test whether Sre1 degradation in scp1Δ cells requires the proteasome, wild-type and scp1Δ cells were treated with a proteasome inhibitor for 4 h. Treatment with the proteasome inhibitor increased Sre1 precursor levels in scp1Δ cells (Fig. 3A, lanes 3 and 4). Interestingly, treatment with proteasome inhibitor also slightly increased the Sre1 precursor in wild-type cells (Fig. 3A, lanes 1 and 2), indicating that Sre1 precursor is subject to proteasomal regulation in wild-type cells. To test if the increase in Sre1 precursor in wild-type cells is due to increased protein stability, we performed a cycloheximide chase experiment using wild-type cells treated with dimethyl sulfoxide (vehicle) or proteasome inhibitor for 4 h. Treatment with proteasome inhibitor increased the stability of the Sre1 precursor compared with the vehicle only control (Fig. 3B), demonstrating that pharmacological inhibition of the proteasome increases Sre1 stability.
FIGURE 3.
Degradation of Sre1 requires the proteasome. A, wild-type (WT) and scp1Δ cells were grown in the presence of dimethyl sulfoxide (DMSO) or Proteasome Inhibitor II (200 μm) for 4 h. B, wild-type cells were grown in the presence of dimethyl sulfoxide or Proteasome Inhibitor II (200 μm) for 4 h. At t = 0, cycloheximide (100 μg/ml) was added, and samples were collected at the indicated times. The percentage of Sre1 precursor remaining relative to t = 0 is quantified to the right. C, wild-type, mts3-1, scp1Δ, and mts3-1 scp1Δ cells were grown overnight at 25 °C. At t = 0, cells were shifted to 35.5 °C and samples were collected at the indicated times. D, wild-type and mts3-1 cells were grown overnight at 25 °C. Cells were then shifted to 35.5 °C for 1.5 h. At t = 0, cycloheximide (100 μg/ml) was added, and samples were collected at the indicated times. The percentage of Sre1 precursor remaining relative to t = 0 is quantified to the right. In each experiment, cells were grown in YES and whole cell lysates were subjected to Western blot analysis with anti-Sre1 IgG.
To test genetically if the proteasome is required for Sre1 degradation, we examined the level of Sre1 precursor in mts3-1 cells that have a temperature-sensitive mutation in a regulatory subunit of the proteasome (25). Wild-type, mts3-1, scp1Δ, and mts3-1 scp1Δ cells were grown for increasing times at the non-permissive temperature (35.5 °C) and samples were collected for Western blotting. At t = 0, mts3-1 scp1Δ cells had more Sre1 precursor than scp1Δ cells (Fig. 3C, lanes 7 and 10), suggesting that at the permissive temperature the proteasome was partially inhibited in these cells and Sre1 was stabilized. Growth at 35.5 °C further increased Sre1 precursor in mts3-1 scp1Δ cells, but not in scp1Δ cells (Fig. 3C, lanes 7–12), demonstrating that inhibition of the proteasome restored Sre1 precursor in the absence of Scp1. In addition, growth of mts3-1 cells containing scp1+ also increased the Sre1 precursor (Fig. 3C, lanes 4–6). Cycloheximide chase experiments showed that the Sre1 precursor was stabilized in mts3-1 cells (Fig. 3D). Collectively, these results demonstrate that degradation of the Sre1 precursor requires the proteasome.
The E2 Ubiquitin-conjugating Enzyme Ubc7 Is Required for Sre1 Degradation
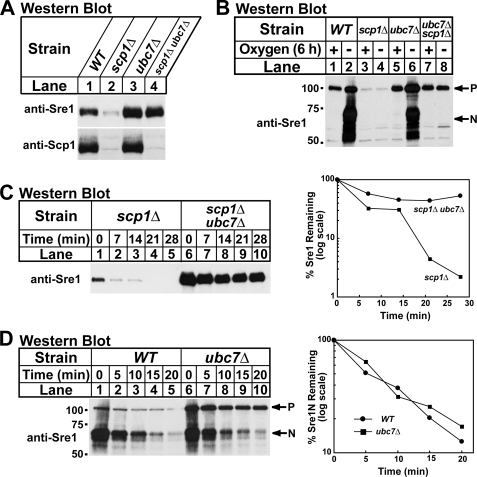
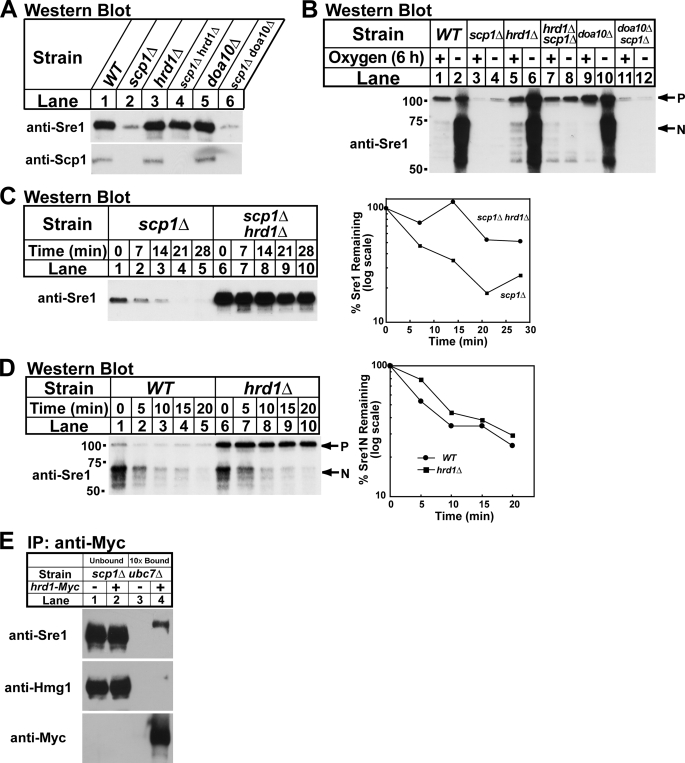
Based on the fact that Sre1 is an ER-membrane protein, its stability decreased in the absence of Scp1 (Fig. 2), and its turnover required the proteasome (Fig. 3), we hypothesized that components of the ERAD pathway may be required for Sre1 degradation. Interestingly, ubc7+, the S. pombe homolog of the S. cerevisiae UBC7, which codes for an E2 ubiquitin-conjugating enzyme, was identified as a Sre1-dependent target gene by microarray analysis (6, 26). Because other Sre1 target genes regulate Sre1, we investigated whether Ubc7 is required for Sre1 degradation (5). We generated a scp1Δ ubc7Δ strain and assayed Sre1 precursor by Western blotting. In scp1Δ cells the Sre1 precursor was decreased, but deletion of ubc7+ restored the Sre1 precursor (Fig. 4A, lanes 2 and 4). Importantly, despite equivalent amounts of Sre1 precursor in wild-type and scp1Δ ubc7Δ cells, Sre1N was not detectable in the absence of oxygen confirming that Scp1 is required for Sre1 cleavage in addition to Sre1 stability (Fig. 4B, lanes 1–2 and 7–8). Cells lacking ubc7+ displayed wild-type levels of Sre1 cleavage in the absence of oxygen (Fig. 4B, lanes 5 and 6).
FIGURE 4.
The E2 ubiquitin-conjugating enzyme Ubc7 is required for Sre1 degradation. A, indicated yeast strains were cultured in YES. Whole cell extracts or urea-solubilized membranes were subjected to Western blot analysis with anti-Sre1 IgG or a mixture of anti-Scp1 IgG monoclonal antibodies 8G4C11, 1G1D6, and 7B4A3, respectively. B, indicated yeast strains were grown in YES ± oxygen for 6 h. C, scp1Δ and scp1Δ ubc7Δ cells were grown in YES + oxygen. At t = 0 cycloheximide (100 μg/ml) was added, and samples were collected at the indicated times. The percentage of the Sre1 precursor remaining relative to t = 0 is quantified to the right. D, wild-type (WT) and ubc7Δ cells were grown in YES in the absence of oxygen for 6 h. At t = 0, cycloheximide (100 μg/ml) was added, the cultures were shifted to the presence of oxygen, and samples were collected at the indicated times. The percentage of Sre1N remaining relative to t = 0 is quantified to the right. Whole cell lysates were subjected to Western blot analysis with anti-Sre1 IgG. P and N denote the precursor and Sre1N nuclear forms, respectively.
To determine whether the increase in Sre1 precursor in scp1Δ ubc7Δ cells was due to increased protein stability, we performed a cycloheximide chase experiment using scp1Δ and scp1Δ ubc7Δ cells. Deletion of ubc7+ increased the half-life of the Sre1 precursor in scp1Δ cells, indicating that Ubc7 is required for rapid degradation of Sre1 in the absence of Scp1 (Fig. 4C). To determine whether the stabilization of Sre1 was specific to the precursor form of Sre1, we measured the half-life of Sre1N in wild-type and ubc7Δ cells. To measure the half-life of Sre1N, cells were grown in the absence of oxygen for 6 h to generate Sre1N by proteolytic cleavage. Cells were then shifted to the presence of oxygen to block Sre1 precursor cleavage, cycloheximide was then added, and whole cell lysates were collected after increasing times. Sre1N showed a similar rate of turnover in both wild-type and ubc7Δ cells (Fig. 4D), indicating that the function of Ubc7 in Sre1 degradation is specific for the Sre1 precursor. Scp1 levels were equal in wild-type and ubc7Δ cells, indicating that Scp1 is not targeted for degradation by the Ubc7-dependent pathway (Fig. 4A, lanes 1 and 3, lower panel). Taken together, these results demonstrate that the E2 ubiquitin-conjugating enzyme Ubc7 is required for Sre1 precursor degradation.
The E3 Ubiquitin Ligase Hrd1 Is Required for Sre1 Degradation
Two E3 ubiquitin ligases, Doa10 and Hrd1, catalyze polyubiquitination of ER protein substrates through Ubc7 in S. cerevisiae (15). Sequence data base searches identified homologs of HRD1 and DOA10 in S. pombe (27). To test if either Doa10 or Hrd1 are required for Sre1 degradation in scp1Δ cells, we generated scp1Δ doa10Δ and scp1Δ hrd1Δ yeast strains and assayed the Sre1 precursor. Deletion of hrd1+ increased the Sre1 precursor in scp1Δ cells, whereas deletion of doa10+ had no effect (Fig. 5A, lanes 2, 4, and 6). Consistent with the results in Fig. 4B, restoration of Sre1 precursor in scp1Δ hrd1Δ cells did not bypass the requirement of Scp1 for Sre1 cleavage under low oxygen (Fig. 5B, lanes 7 and 8).
FIGURE 5.
The E3 ubiquitin ligase Hrd1 is required for Sre1 degradation. A, indicated yeast strains were cultured in YES. Whole cell extracts or urea-solubilized membranes were subjected to Western blot analysis with anti-Sre1 IgG or a mixture of anti-Scp1 IgG monoclonal antibodies 8G4C11, 1G1D6, and 7B4A3, respectively. B, indicated yeast strains were grown in YES ± oxygen for 6 h. P and N denote the precursor and Sre1N nuclear forms, respectively. C, scp1Δ and scp1Δ hrd1Δ cells were grown in YES + oxygen. At t = 0, cycloheximide (100 μg/ml) was added, and samples were collected at the indicated times. The percentage of Sre1 precursor remaining relative to t = 0 is quantified to the right. D, wild-type (WT) and hrd1Δ cells were grown in YES in the absence of oxygen for 6 h. At t = 0, cycloheximide (100 μg/ml) was added, the cultures were shifted to the presence of oxygen, and samples were collected at the indicated times. The percentage of Sre1N remaining relative to t = 0 is quantified to the right. Whole cell lysates were subjected to Western blot analysis with anti-Sre1 IgG. E, cells (5 × 107) were harvested and detergent-solubilized extracts were subjected to immunoprecipitation (IP) with anti-Myc 9E10 IgG. Unbound and bound fractions were immunoblotted using the indicated antibodies. Bound fractions were 10× overloaded relative to unbound.
To determine whether Hrd1 is required for stability of Sre1 in scp1Δ cells, Sre1 degradation was measured in a cycloheximide chase experiment using scp1Δ and hrd1Δ scp1Δ cells. Sre1 was rapidly degraded in scp1Δ cells (Fig. 5C, lanes 1–5), and deletion of hrd1+ stabilized Sre1 (Fig. 5C, lanes 6–10). Hrd1 specifically functions to degrade the Sre1 precursor insomuch as deletion of hrd1+ had no effect on the degradation of Sre1N (Fig. 5D). Last, hrd1Δ and doa10Δ cells showed equal levels of Scp1 compared with wild-type cells indicating that Hrd1 and Doa10 do not control Scp1 levels (Fig. 5A, lower panel).
E3 ubiquitin ligases impart specificity to the ubiquitin-proteasome system by binding and targeting substrate proteins for degradation (10). To test whether Hrd1 forms a complex with the Sre1 precursor, we performed an immunoprecipitation experiment in hrd1-Myc scp1Δ ubc7Δ cells. In the absence of Scp1, Sre1 precursor should be targeted for ERAD by Hrd1, but ubiquitination and degradation should be blocked due to deletion of Ubc7. Hrd1-Myc immunoisolated from detergent cell extracts specifically formed a complex with the Sre1 precursor, but not the stable ER membrane protein HMG-CoA reductase, Hmg1 (Fig. 5E, lanes 3 and 4). Taken together, these results indicate that the E3 ubiquitin ligase Hrd1 is required to degrade the Sre1 precursor in the absence of Scp1.
Blocking Sre1 Precursor Degradation Does Not Bypass Need for Positive Feedback Regulation
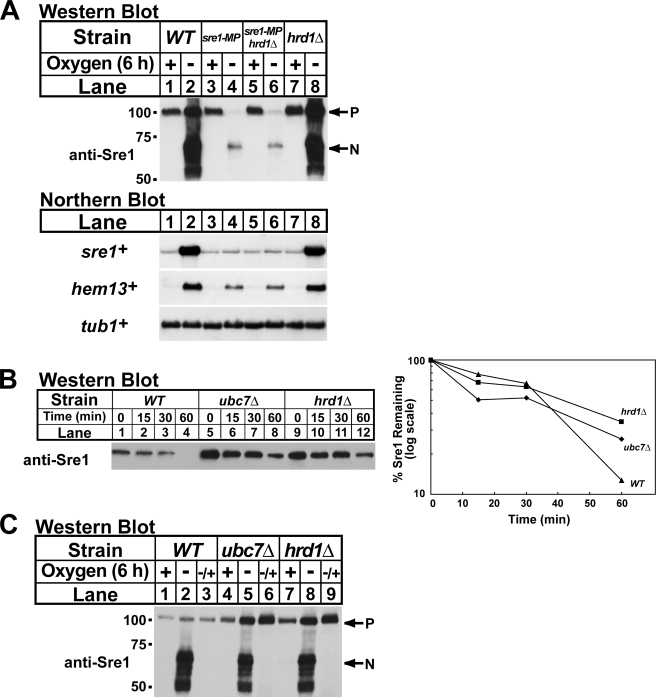
Sre1 precursor supply is controlled by new synthesis and Ubc7-Hrd1-dependent degradation. To evaluate the contribution of the degradative pathway to Sre1 cleavage, we tested whether blocking degradation of the Sre1 precursor would result in increased Sre1 cleavage in cells lacking positive feedback regulation of Sre1. To test this, we generated a sre1-MP hrd1Δ strain to simultaneously inhibit positive feedback regulation and degradation of the Sre1 precursor. We grew wild-type, sre1-MP, sre1-MP hrd1Δ, and hrd1Δ cells in the presence and absence of oxygen for 6 h and harvested samples for Western blot and Northern blot analysis. Under low oxygen, sre1-MP cells showed decreased Sre1 precursor, cleaved Sre1, and hem13+ mRNA compared with wild-type cells (Fig. 6A, lanes 1–4). Interestingly, deletion of hrd1+ in sre1-MP cells had no effect on Sre1 cleavage or hem13+ mRNA under low oxygen conditions (Fig. 6A, lanes 1–6). Consistent with these results, deletion of hrd1+ did not rescue growth of sre1-MP cells on medium containing cobalt chloride (data not shown). Thus, inhibition of Sre1 precursor degradation does not result in increased Sre1 cleavage and is not sufficient to bypass the requirement for positive feedback regulation of sre1+.
FIGURE 6.
Abrogation of Sre1 degradation does not bypass the need for positive feedback regulation. A, wild-type (WT), sre1-MP, sre1-MP hrd1Δ, and hrd1Δ cells were grown in YES ± oxygen for 6 h. Total RNA (5 μg) was subjected to Northern analysis with the indicated 32P-labeled probes. α-Tubulin (tub1+) mRNA served as a loading control. P and N denote the precursor and Sre1N nuclear forms, respectively. B, the indicated yeast strains were grown in YES in the presence of oxygen. At t = 0, cycloheximide (100 μg/ml) was added, and samples were collected at the indicated times. The percentage of Sre1 precursor remaining relative to t = 0 is quantified to the right. C, wild-type, ubc7Δ and hrd1Δ cells were grown in YES either ± oxygen for 6 h or −oxygen for 6 h and then shifted to +oxygen for 1 h (−/+). Whole cell lysates were subjected to Western blot analysis with anti-Sre1 IgG.
Ubc7-Hrd1 Degrade Sre1 Precursor in Wild-type Cells
Our data indicate that Ubc7 and Hrd1 function to degrade the Sre1 precursor that is not bound to Scp1 (Figs. 2–5). Based on these findings, we hypothesized that the Ubc7-Hrd1 degradative pathway functions to remove Sre1 produced in excess of available Scp1. To determine the contribution of Ubc7 and Hrd1 to Sre1 precursor degradation when Scp1 is present, we performed a cycloheximide chase experiment in wild-type, ubc7Δ, and hrd1Δ cells in the presence of oxygen. Cells lacking Ubc7 or Hrd1 showed elevated Sre1 precursor at t = 0 (Fig. 6B). However, the half-life of the Sre1 precursor was similar in all three strains (20–30 min), indicating that only a minor fraction of Sre1 precursor is degraded by ERAD in wild-type cells in the presence of oxygen. The continued turnover of Sre1 precursor in ubc7Δ and hrd1Δ cells suggests that Sre1 is proteolytically activated to produce Sre1N at a basal rate in the presence of oxygen.
To confirm that Ubc7-Hrd1 also function to degrade Sre1 not bound to Scp1 in wild-type cells, we created a situation in which Sre1 synthesis would exceed available Scp1. Under low oxygen, cells up-regulate sre1+ transcription through positive feedback regulation to maintain a supply of Sre1 precursor for proteolytic cleavage (Fig. 1). When oxygen is reintroduced, Sre1 cleavage is blocked (5), the Sre1-Scp1 complex accumulates, and the amount of free Scp1 available to bind to the newly synthesized Sre1 precursor is reduced. Thus, under this reoxygenated condition we expected that the elevated rate of Sre1 synthesis would exceed the amount of available Scp1.
For this experiment, we grew wild-type, ubc7Δ, and hrd1Δ cells for 6 h in the presence of oxygen, the absence of oxygen, or the absence of oxygen for 6 h and then plus oxygen for 1 h. In wild-type cells, Sre1 was proteolytically activated under low oxygen and the Sre1 precursor increased slightly (Fig. 6C, lanes 1 and 2). After shifting back to the presence of oxygen, Sre1N disappeared indicating that Sre1 cleavage was inhibited under these conditions (Fig. 6C, lane 3). In ubc7Δ and hrd1Δ cells, the Sre1 precursor was elevated in the presence of oxygen and these cells accumulated higher levels of Sre1 precursor under low oxygen (Fig. 6C, lanes 4, 5, 7, and 8). Importantly, both ubc7Δ and hrd1Δ cells accumulated high levels of Sre1 precursor relative to wild-type cells when shifted back to the presence of oxygen for 1 h (Fig. 6C, lanes 3, 6, and 9). These results indicate that Ubc7 and Hrd1 function to degrade the Sre1 precursor in wild-type cells. The requirement for Ubc7 and Hrd1 is apparent upon reoxygenation when the elevated synthesis of Sre1 exceeds the amount of Scp1 available to bind newly synthesized Sre1.
DISCUSSION
Under low oxygen, Sre1-dependent target gene expression increases due to proteolytic cleavage of the Sre1 precursor and stabilization of nuclear Sre1N (1, 5). However, to achieve sustained activation of Sre1 target genes Sre1N must be continually produced from the Sre1 precursor by proteolytic cleavage. Here, we investigated the mechanisms by which the Sre1 precursor is regulated independent of oxygen-regulated proteolytic cleavage. Previously, we showed that Sre1 up-regulates its own expression by a positive feedback mechanism (5, 6). Using a yeast strain containing engineered mutations in the sre1+ promoter, we found here that positive feedback regulation of Sre1 synthesis is required for sustained activation of Sre1N under low oxygen and growth of cells on cobalt chloride medium (Fig. 1). These data highlight the importance of this positive feedback regulation for Sre1 pathway function.
We also investigated mechanisms regulating Sre1 precursor degradation. We found that in the absence of the Sre1-binding partner Scp1, the Sre1 precursor is rapidly degraded by the proteasome (Figs. 2 and 3). Moreover, this degradation requires Ubc7 and Hrd1, known components of the ERAD pathway, and the Sre1 precursor formed a specific complex with Hrd1 (Figs. 4 and 5) (12). Finally, we showed that Sre1 is an endogenous substrate for the Ubc7-Hrd1 pathway in wild-type cells (Fig. 6). These data suggest that Sre1 is a substrate for the E3 ubiquitin ligase Hrd1. However, to date we have been unable to detect Sre1 ubiquitinylation, leaving alternate explanations for the Hrd1-dependent degradation of Sre1.
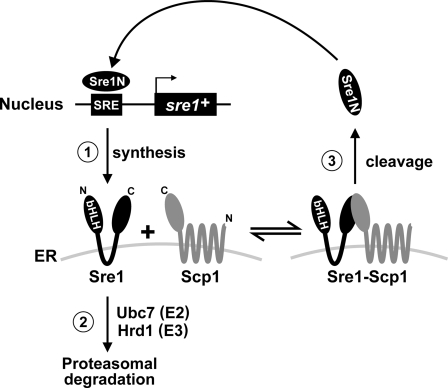
Our data support a model in which there are three primary ways to regulate Sre1 precursor (Fig. 7): 1) synthesis of the Sre1 precursor is controlled by positive feedback regulation; 2) the E2 ubiquitin-conjugating enzyme Ubc7 and the E3 ubiquitin ligase Hrd1 mediate the proteasomal degradation of Sre1 precursor not bound to Scp1; and 3) the Sre1 precursor is subject to Scp1-dependent cleavage under low oxygen to produce Sre1N (1, 6). In the ER, the Sre1 precursor exists as either free Sre1 or Sre1-Scp1 complex (Fig. 7, middle). Under low oxygen, Sre1 bound to Scp1 is proteolytically cleaved to release Sre1N, the N-terminal transcription factor domain. Sre1N enters the nucleus and up-regulates sre1+ expression by a positive feedback mechanism that results in increased synthesis of the Sre1 precursor and sustained cleavage under low oxygen (Fig. 1).
FIGURE 7.
Model for Sre1 precursor regulation. Sre1 precursor is regulated by a balance of synthesis, degradation, and proteolytic cleavage. Under low oxygen, Scp1 facilitates Sre1 precursor proteolytic cleavage. The released N-terminal basic helix-loop-helix (bHLH) transcription factor domain Sre1N up-regulates expression of sre1+ mRNA by a positive feedback mechanism to replenish Sre1 precursor depleted by cleavage. In the ER, Sre1 precursor molecules not complexed to Scp1 are subject to ER-associated degradation via Ubc7, Hrd1, and the proteasome.
In this study, we also demonstrated that Sre1 is highly unstable in the absence of Scp1 and is degraded by the proteasome via the Ubc7-Hrd1 pathway (Figs. 2–5). Based on these data, we hypothesize that free Sre1 is recognized as a misfolded, orphan subunit and is degraded by an ERAD-like mechanism requiring Ubc7 and Hrd1 (Fig. 7, step 2) (8, 12). Similar degradative mechanisms control hetero-oligomeric complex formation of the acetylcholine receptor, immunoglobulins, major histocompatibility complex class I molecules, and the yeast SNARE Ufe1 (8, 28). By interacting with Scp1, Sre1 is no longer recognized as misfolded and is protected from proteasomal destruction. Importantly, the rapid degradation of free Sre1 ensures that Sre1 exists in a complex with Scp1, thereby guaranteeing that Sre1 cleavage is regulated by Scp1 and oxygen supply. Given that the Sre1 precursor is a physiological substrate for ER quality control, our studies establish fission yeast as a new organism for the dissection of this important degradative pathway.
Like fission yeast Sre1, mammalian SREBP precursor is also reduced in the absence of Scap, likely due to decreased protein stability (7). It is unknown whether an ERAD-like mechanism also controls this degradation. Interestingly, overexpression of the ER-associated ubiquitin ligase TRC8 decreased expression of the SREBP target genes encoding HMG-CoA reductase and fatty-acid synthase in HEK293 cells, implicating TRC8 in regulation of SREBP activity (29). Additional studies are required to delineate the pathways regulating SREBP protein stability in mammalian cells and the potential role for TRC8 in this process.
Acknowledgments
We thank Espenshade Lab members and Susan Michaelis for experimental suggestions and for reviewing the manuscript. We also kindly thank Colin Gordon (Medical Research Council, UK) for providing the mts3-1 strain.
This work was supported, in whole or in part, by National Institutes of Health Grant HL-077588.
- SREBP
- sterol regulatory element-binding protein
- Scap
- SREBP cleavage activating protein
- ER
- endoplasmic reticulum
- ERAD
- endoplasmic reticulum-associated degradation
- SRE
- Sre1 regulatory element
- YES
- yeast extract plus supplements
- CaMV
- cauliflower mosaic virus
- MP
- mutant promoter
- HMG-CoA
- 3-hydroxy-3-methylglutaryl-coenzyme A.
REFERENCES
- 1.Hughes A. L., Todd B. L., Espenshade P. J. (2005) Cell 120, 831–842 [DOI] [PubMed] [Google Scholar]
- 2.Espenshade P. J., Hughes A. L. (2007) Annu. Rev. Genet. 41, 401–427 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein J. L., DeBose-Boyd R. A., Brown M. S. (2006) Cell 124, 35–46 [DOI] [PubMed] [Google Scholar]
- 4.Nohturfft A., Yabe D., Goldstein J. L., Brown M. S., Espenshade P. J. (2000) Cell 102, 315–323 [DOI] [PubMed] [Google Scholar]
- 5.Hughes B. T., Espenshade P. J. (2008) EMBO J. 27, 1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todd B. L., Stewart E. V., Burg J. S., Hughes A. L., Espenshade P. J. (2006) Mol. Cell Biol. 26, 2817–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawson R. B., DeBose-Boyd R., Goldstein J. L., Brown M. S. (1999) J. Biol. Chem. 274, 28549–28556 [DOI] [PubMed] [Google Scholar]
- 8.Ellgaard L., Helenius A. (2003) Nat. Rev. Mol. Cell Biol. 4, 181–191 [DOI] [PubMed] [Google Scholar]
- 9.Hampton R. Y. (2002) Curr. Opin. Cell Biol. 14, 476–482 [DOI] [PubMed] [Google Scholar]
- 10.Meusser B., Hirsch C., Jarosch E., Sommer T. (2005) Nat. Cell Biol. 7, 766–772 [DOI] [PubMed] [Google Scholar]
- 11.Sayeed A., Ng D. T. (2005) Crit. Rev. Biochem. Mol. Biol. 40, 75–91 [DOI] [PubMed] [Google Scholar]
- 12.Nakatsukasa K., Brodsky J. L. (2008) Traffic 9, 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho P., Goder V., Rapoport T. A. (2006) Cell 126, 361–373 [DOI] [PubMed] [Google Scholar]
- 14.Huyer G., Piluek W. F., Fansler Z., Kreft S. G., Hochstrasser M., Brodsky J. L., Michaelis S. (2004) J. Biol. Chem. 279, 38369–38378 [DOI] [PubMed] [Google Scholar]
- 15.Kostova Z., Tsai Y. C., Weissman A. M. (2007) Semin. Cell Dev. Biol. 18, 770–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song B. L., Javitt N. B., DeBose-Boyd R. A. (2005) Cell Metab. 1, 179–189 [DOI] [PubMed] [Google Scholar]
- 17.Lee J. N., Song B., DeBose-Boyd R. A., Ye J. (2006) J. Biol. Chem. 281, 39308–39315 [DOI] [PubMed] [Google Scholar]
- 18.Burke J. D., Gould K. L. (1994) Mol. Gen. Genet. 242, 169–176 [DOI] [PubMed] [Google Scholar]
- 19.Bähler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. (1998) Yeast 14, 943–951 [DOI] [PubMed] [Google Scholar]
- 20.Boeke J. D., Trueheart J., Natsoulis G., Fink G. R. (1987) Methods Enzymol. 154, 164–175 [DOI] [PubMed] [Google Scholar]
- 21.Moreno S., Klar A., Nurse P. (1991) Methods Enzymol. 194, 795–823 [DOI] [PubMed] [Google Scholar]
- 22.Forsburg S. L. (1993) Nucleic Acids Res. 21, 2955–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes A. L., Stewart E. V., Espenshade P. J. (2008) J. Lipid Res. 49, 2001–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H., Bien C. M., Hughes A. L., Espenshade P. J., Kwon-Chung K. J., Chang Y. C. (2007) Mol. Microbiol. 65, 1018–1033 [DOI] [PubMed] [Google Scholar]
- 25.Gordon C., McGurk G., Wallace M., Hastie N. D. (1996) J. Biol. Chem. 271, 5704–5711 [DOI] [PubMed] [Google Scholar]
- 26.Nielsen I. S., Nielsen O., Murray J. M., Thon G. (2002) Eukaryot. Cell 1, 613–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson R., Locher M., Hochstrasser M. (2001) Genes Dev. 15, 2660–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun S., Jentsch S. (2007) EMBO Rep. 8, 1176–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brauweiler A., Lorick K. L., Lee J. P., Tsai Y. C., Chan D., Weissman A. M., Drabkin H. A., Gemmill R. M. (2007) Oncogene 26, 2263–2271 [DOI] [PubMed] [Google Scholar]