Abstract
Some fungal species are opportunistic pathogens that can cause infection in people with compromised immune systems. Activation of caspase-1 and the subsequent secretion of mature interleukin (IL)-1β is a major signaling pathway of the innate immune system, but how yeasts induce caspase-1 activation is unknown. We show here that stimulation of macrophages and dendritic cells with heat-killed Saccharomyces cerevisiae or the purified cell wall components zymosan and mannan induced caspase-1 activation and IL-1β secretion when combined with ATP. Macrophages deficient for the inflammasome adaptor ASC were defective in caspase-1 activation and IL-1β secretion, suggesting involvement of an ASC-dependent inflammasome. Indeed, caspase-1 activation was abrogated in macrophages lacking the NOD-like (NLR) protein Cryopyrin/Nalp3 and in wild type macrophages pretreated with the pannexin-1 inhibitor probenecid. IL-1β secretion further required the Toll-like receptor (TLR) adaptors MyD88 and TRIF, and partially relied on TLR2. We previously showed that bacterial molecules such as lipopolysaccharide (LPS) and peptidoglycan induce activation of caspase-7 through the Cryopyrin inflammasome. Similarly, Cryopyrin and ASC were required for activation of caspase-7 in macrophages stimulated with zymosan or mannan and ATP. These results demonstrate that the conserved fungal components zymosan and mannan require ASC and Cryopyrin for caspase-1 activation and IL-1β secretion and suggest an important role for the Cryopyrin inflammasome during fungal infections.
Pathogen recognition by the innate immune system relies on a limited number of fixed germline-encoded receptors, which have evolved to identify so-called pathogen-associated molecular patterns (PAMPs),2 conserved microbial structures not shared by the host and essential for their survival (1). Examples of PAMPs are LPS from Gram-negative bacteria, peptidoglycan (PGN) from Gram-positive bacteria, and zymosan and mannan from fungi. Several structurally and functionally diverse classes of pattern-recognition receptors (PRRs) exist that induce various host defense pathways, including the Toll-like receptors (TLRs) located in the plasma membrane and intracellular organelles and the more recently identified intracellular family of NOD-like receptors (NLRs) (2).
Previous studies have shown that gain-of-function mutations within the NLR protein Cryopyrin/NALP3 are associated with three autoinflammatory disorders characterized by skin rashes and prolonged episodes of fever in the absence of any apparent infection (3, 4). These hereditary periodic fever syndromes are Muckle-Wells syndrome (MWS), familial cold autoinflammatory syndrome (FACS), and neonatal-onset multisystem inflammatory disease (NOMID), and they are collectively referred to as the Cryopyrin/NALP3-associated periodic syndromes (CAPS). Subsequent studies revealed that the Cryopyrin/Nalp3 plays a crucial role in the assembly of a large (700 kDa) cytosolic protein complex termed the “inflammasome” (5–7). The bipartite adaptor protein ASC bridges the interaction between Cryopyrin/Nalp3 and caspase-1 in the inflammasome; thus allowing the recruitment and autoproteolytic activation of the cysteine protease (2). Activated caspase-1 subsequently mediates the maturation and secretion of the pro-inflammatory cytokines interleukin (IL)-1β and IL-18 (8–10). Interestingly, the Cryopyrin/Nalp3 inflammasome mediates caspase-1 activation in response to a variety of bacterial PAMPs such as LPS and PGN when combined with a second stimulus such as the P2X7 receptor ligand ATP (11–14). Cryopyrin/Nalp3 also mediates caspase-1 activation and IL-1β secretion in macrophages stimulated with viral RNA and ATP (15) or exposed to crystalline substances including uric acid, silica and asbestos (16–18). In contrast, the related NLR protein Ipaf is required for caspase-1 activation in macrophages infected with the intracellular pathogens Salmonella, Legionella, and Shigella (19–21).
Although the roles of specific inflammasomes in response to bacterial and viral PAMPs have been described, the inflammasome complexes that recognize fungal PAMPs to induce caspase-1 activation and IL-1β secretion are unknown. Here we show that heat-killed Saccharomyces cerevisiae and the purified cell wall components zymosan and mannan induced caspase-1 activation and IL-1β secretion from macrophages and dendritic cells upon co-stimulation with ATP. Macrophages deficient for the inflammasome adaptor ASC or the NLR protein Cryopyrin/Nalp3 were defective in zymosan- and mannan-induced caspase-1 activation and IL-1β secretion, whereas TNF-α secretion remained unaffected. Although macrophages lacking the TLR adaptors MyD88 or TRIF still activated caspase-1, zymosan- and mannan-induced secretion of IL-1β was significantly hampered. These results demonstrate that the conserved fungal cell wall components zymosan and mannan require ASC and Cryopyrin for caspase-1 activation and IL-1β secretion and suggest an important role for the Cryopyrin inflammasome during fungal infections.
EXPERIMENTAL PROCEDURES
Mice, Macrophages, and Dendritic Cells
Cryopyrin−/−, Ipaf−/−, ASC−/−, Nod2−/−, RIP2−/−, Myd88−/−, TLR2−/−, and TLR4−/− mice have been described (12, 22). Bone marrow was prepared from the leg bones of 8–20-week-old mice. The legs were dissected, and the bone marrow flushed out. Macrophages were differentiated from bone marrow cells cultured with Iscove's modified Dulbecco's medium (IMDM) supplemented with 30% L929 supernatant containing 10% heat-inactivated fetal bovine serum (Invitrogen), 100 units/ml penicillin, and 100 mg/ml streptomycin at 37 °C in 5% CO2 for 5 days. Bone marrow-derived dendritic cells (BMDCs) were cultured in RPMI supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), 100 units/ml penicillin, 100 mg/ml streptomycin, and 1000 units/ml GM-CSF at 37 °C in 5% CO2 for 7–12 days. Bone marrow-derived macrophages and dendritic cells were then harvested with rubber scrapers and seeded.
Microbial Ligands and Chemicals
Zymosan, depleted zymosan, mannan, heat-killed S. cerevisiae, and ultrapure LPS were purchased from Invivogen and used at a concentration of 10 μg/ml. ATP was from Sigma and used at a final concentration of 3 mm. Probenecid was purchased from Sigma and used at 3 mm.
Immunoblotting
Cells were washed twice with phosphate-buffered saline and scraped in lysis buffer solution (150 mm NaCl, 10 mm Tris, pH 7.4, 5 mm EDTA, 1 mm EGTA, 0.1% Nonidet P-40) supplemented with a protease inhibitor mixture tablet (Roche Applied Science). Samples were clarified, denatured with SDS buffer, and boiled for 5 min. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were immunoblotted with primary antibodies and proteins detected with appropriate secondary anti-rabbit antibody conjugated to horseradish peroxidase followed by enhanced chemiluminescence. Rabbit anti-mouse caspase-1 was a generous gift from Dr. P. Vandenabeele (Ghent University, Belgium). Caspase-7 antibodies were from Cell Signaling Technologies.
Measurements of Cytokines
Mouse cytokines in culture supernatants were measured by enzyme-linked immunoabsorbent assay (R&D Systems) and Luminex assay (Bio-Rad). Data were analyzed with Student's t test. p < 0.05 was considered statistically significant.
RESULTS
Stimulation of Macrophages and Dendritic Cells with Heat-killed S. cerevisiae and ATP Triggers Caspase-1 Activation and IL-1β Secretion
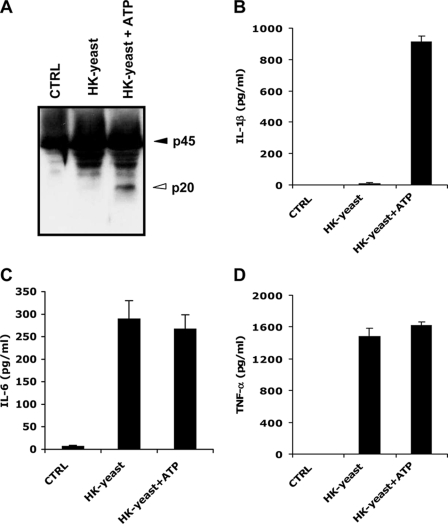
We have previously shown that heat-killed bacteria and ATP activated caspase-1 and secreted significant levels of mature IL-1β (12). To study whether heat-killed yeasts can also induce caspase-1 activation and IL-1β secretion, bone-marrow derived dendritic cells (BMDCs) were stimulated with heat-killed S. cerevisiae for 4 h, the last 30 min of which in the presence of either vehicle control (PBS) or 5 mm ATP. As previously reported for heat-killed bacteria (12), heat-killed Saccharomyces treatment in the absence of ATP did not trigger caspase-1 activation (Fig. 1A) or IL-1β secretion (Fig. 1B) in BMDCs. Dendritic cells nevertheless responded to the heat-killed yeast preparation by inducing significant levels of the caspase-1-independent cytokines IL-6 (Fig. 1C) and TNF-α (Fig. 1D). However, caspase-1 activation and the secretion of significant levels of IL-1β were observed when BMDCs were stimulated with heat-killed yeast and ATP (Fig. 1, A and B). Similar to dendritic cells, caspase-1 activation and IL-1β secretion in BMDMs were only observed upon stimulation with heat-killed yeast and ATP (supplemental Fig. S1, A and B). In contrast, IL-6 and TNF-α were readily secreted in the absence of ATP stimulation (supplemental Fig. S1, C and D). These results demonstrate that, similar to heat-killed bacteria, heat-killed S. cerevisiae preparations contain PAMPs that are capable of inducing caspase-1 activation.
FIGURE 1.
Stimulation of dendritic cells with heat-killed S. cerevisiae lipopolysaccharide and ATP triggers caspase-1 activation and IL-1β secretion. A–D, BMDCs were left untreated (CTRL), or stimulated with heat-killed S. cerevisiae (HK-yeast) for 4 h, the last 30 min in the presence of PBS or 5 mm ATP (HK-yeast+ATP). Cell extracts were immunoblotted for caspase-1 (A), and culture supernatants were analyzed for secreted IL-1β (B), IL-6 (C), and TNF-α (D). Black arrowheads indicate full-length caspase-1 (45 kDa), and white arrowheads mark the large subunits of activated caspase-1 (20 kDa). Cytokine data represent the mean ± S.E. of triplicate wells. All data are representative of at least three independent experiments.
Yeast Zymosan and Mannan Induce Caspase-1 Activation and IL-1β Secretion in ATP-treated Macrophages and Dendritic Cells
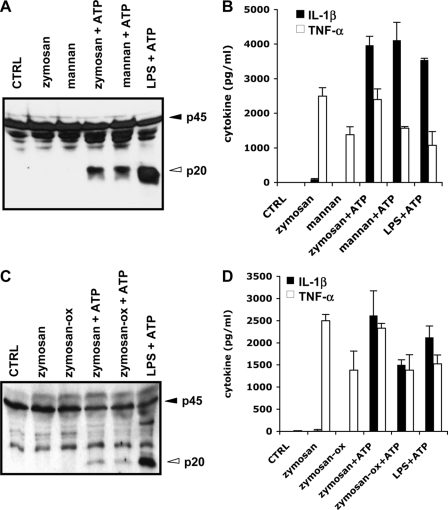
LPS and PGN, the major cell wall components of, respectively, Gram-negative and Gram-positive bacteria, are bacterial PAMPs that potently induce caspase-1 activation and IL-1β secretion from macrophages co-stimulated with ATP (Refs. 11–14 and Fig. 2, A and B). To determine whether the fungal cell wall components zymosan and mannan are capable of inducing caspase-1 activation, BMDMs were stimulated with these fungal PAMPs for 4 h, the last 30 min of which were in the presence of 5 mm ATP. Cell lysates were then prepared and analyzed for caspase-1 activation, whereas culture supernatants were probed for IL-1β content. Both zymosan and mannan potently activated caspase-1 in the presence of ATP (Fig. 2A) and triggered secretion of IL-1β (Fig. 2B). ATP co-stimulation was required for these responses because neither zymosan nor mannan triggered caspase-1 activation or IL-1β secretion alone (Fig. 2, A and B). However, ATP was not required for the secretion of TNF-α from zymosan- or mannan-prestimulated macrophages (Fig. 2B). We sought to confirm these results with NaClO-oxidized zymosan (zymosan-Ox or depleted zymosan), which is devoid of bacterial TLR ligands. Lysates of BMDMs treated with zymosan-Ox and ATP contained levels of active caspase-1 comparable to those found in lysates of zymosan+ATP-treated macrophages (supplemental Fig. S2A). In addition, both zymosan and zymosan-Ox triggered potent IL-1β secretion when combined with ATP (supplemental Fig. S2B). These results were not restricted to macrophages because BMDCs also activated caspase-1 (Fig. 2C) and secreted IL-1β in response to zymosan+ATP or zymosan-Ox+ATP (Fig. 2D). In contrast, TNF-α secretion did not require ATP co-stimulation (Fig. 2D). These results demonstrate that, comparable to bacterial cell wall components, the fungal cell wall components zymosan and mannan can induce caspase-1 activation in macrophages and dendritic cells.
FIGURE 2.
Yeast zymosan and mannan induce caspase-1 activation and IL-1β secretion in ATP-treated macrophages and dendritic cells. A and B, BMDMs were left untreated (CTRL), stimulated with zymosan, mannan, or LPS for 4 h, the last 30 min in the presence of PBS (zymosan, mannan), or 5 mm ATP (zymosan+ATP, mannan+ATP, LPS+ATP). Cell extracts were immunoblotted for caspase-1 (A), and culture supernatants were analyzed for secreted IL-1β and TNF-α (B). C and D, BMDCs were left untreated (CTRL), or stimulated with zymosan, zymosan-Ox, or LPS for 4 h, the last 30 min in the presence of PBS (zymosan, zymosan-Ox) or 5 mm ATP (zymosan+ATP, zymosan-Ox+ATP, LPS+ATP). Cell extracts were immunoblotted for caspase-1 (C), and culture supernatants were analyzed for secreted IL-1β and TNF-α (D). Black arrowheads indicate full-length caspase-1 (45 kDa), and white arrowheads mark the large subunits of activated caspase-1 (20 kDa). Cytokine data represent the mean ± S.E. of triplicate wells. All data are representative of three independent experiments.
The Inflammasome Adaptor ASC Is Required for Zymosan- and Mannan-induced Caspase-1 Activation and IL-1β Secretion
Macrophages deficient for the inflammasome adaptor protein ASC, or the Nod1 and Nod2 adaptor RIP2 were stimulated with zymosan+ATP or mannan+ATP to investigate the molecular mechanism by which zymosan and mannan induce caspase-1 activation and secretion of IL-1β. Macrophages stimulated with LPS+ATP were included as controls. As before, incubation of wild type macrophages with zymosan or mannan and ATP induced caspase-1 activation and secretion of elevated levels of IL-1β in the culture supernatants. Both responses were abolished in ASC-null macrophages (Fig. 3, A and B). The role of ASC was specific because it was dispensable for zymosan- and mannan-induced secretion of TNF-α (Fig. 3C); thus, suggesting a specific role for an ASC-containing inflammasome complex in zymosan- and mannan-induced activation of caspase-1. Similar to what was previously reported for heat-killed bacteria and bacterial PAMPs (2), the Nod1 and Nod2 adaptor RIP2 was not required for zymosan- or mannan-induced caspase-1 activation (Fig. 3A) or cytokine secretion (Fig. 3, B and C).
FIGURE 3.
ASC is required for zymosan- and mannan-induced caspase-1 activation and IL-1β secretion. A–C, BMDMs from wild type, ASC−/−, MyD88−/−, and RIP2−/− mice were left untreated (CTRL), or stimulated with zymosan, mannan, or LPS for 4 h, the last 30 min in the presence of 5 mm ATP. Cell extracts were immunoblotted for caspase-1 (A), and culture supernatants were analyzed for secreted IL-1β (B) and TNF-α (C). Black arrowheads indicate full-length caspase-1 (45 kDa), and white arrowheads mark the large subunits of activated caspase-1 (20 kDa). Cytokine data represent the mean ± S.E. of triplicate wells. All data are representative of three independent experiments.
Cryopyrin Is Required for Zymosan- and Mannan-induced Caspase-1 Activation and IL-1β Secretion
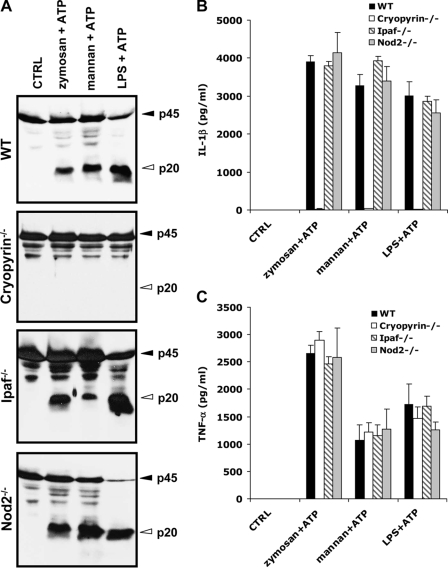
Our results showed that zymosan- and mannan-induced caspase-1 activation required ASC (Fig. 3). This inflammasome adaptor protein is required in both the Cryopyrin and Ipaf inflammasomes. To determine the inflammasome mediating zymosan- and mannan-induced caspase-1 activation, BMDMs from mice deficient for Cryopyrin, Ipaf, or the related NLR family member Nod2 were stimulated with zymosan or mannan for 4 h, the last 30 min of which were in the presence of 5 mm ATP. Caspase-1 was activated in lysates of wild type macrophages stimulated with zymosan or mannan and ATP, but this activation was abolished in Cryopyrin-deficient macrophages (Fig. 4A). Similar results were obtained when the secretion of IL-1β in culture supernatants was analyzed (Fig. 4B). Cryopyrin was specifically required for caspase-1 activation and IL-1β secretion as secretion of TNF-α was unaffected in Cryopyrin-null macrophages (Fig. 4C). Unlike Cryopyrin, the NLRs Ipaf and Nod2 were not required for zymosan- or mannan-induced caspase-1 activation and IL-1β secretion (Fig. 4, A and B).
FIGURE 4.
Cryopyrin is required for zymosan- and mannan-induced caspase-1 activation and IL-1β secretion. A–C, BMDMs from wild type, Cryopyrin−/−, Ipaf−/−, and Nod2−/− mice were left untreated (CTRL), or stimulated with zymosan, mannan, or LPS for 4 h, the last 30 min in the presence of 5 mm ATP. Cell extracts were immunoblotted for caspase-1 (A), and culture supernatants were analyzed for secreted IL-1β (B) and TNF-α (C). Black arrowheads indicate full-length caspase-1 (45 kDa), and white arrowheads mark the large subunits of activated caspase-1 (20 kDa). Cytokine data represent the mean ± S.E. of triplicate wells. All data are representative of at least three independent experiments.
Role of TLRs and Pannexin-1 in Zymosan- and Mannan-induced Inflammasome Activation and IL-1β Secretion
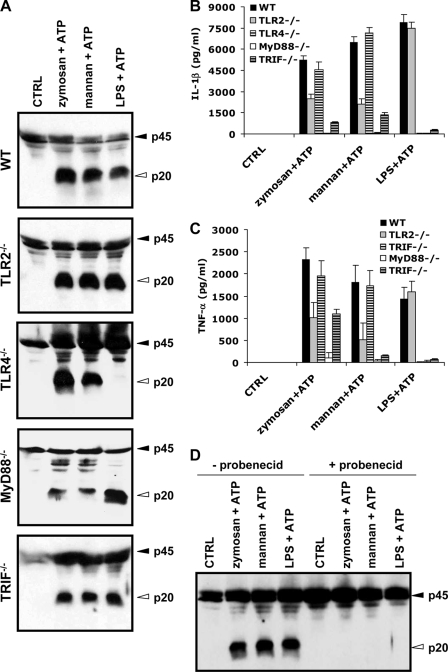
TLRs are known to respond to fungal cell wall components (23, 24). To investigate the roles of TLRs in zymosan- and mannan-induced caspase-1 activation and IL-1β secretion, BMDMs deficient for TLR2, TLR4, or the TLR adaptors MyD88 and TRIF were stimulated with zymosan+ATP or mannan+ATP. Macrophages stimulated with LPS+ATP were included as controls. Both zymosan- and mannan-activated caspase-1 in TLR2−/− and TLR4−/− macrophages (Fig. 5A), although secretion of IL-1β (Fig. 5B) and TNF-α (Fig. 5C) partially depended on TLR2, but not TLR4. In contrast, LPS-induced caspase-1 activation and secretion of IL-1β and TNF-α were completely dependent on TLR4, but not TLR2. We next addressed the roles of MyD88 and TRIF in zymosan- and mannan-induced caspase-1 activation and IL-1β secretion. Zymosan and mannan activated caspase-1 normally in macrophages lacking MyD88 or TRIF (Fig. 5A). However, zymosan-, mannan-, and LPS-induced IL-1β secretion all required MyD88 and partially relied on TRIF (Fig. 5B), likely for the NF-κB-dependent induction of pro-interleukin-1β. This notion was further supported by the observation that TNF-α secretion was also significantly diminished in MyD88−/− and TRIF−/− macrophages (Fig. 5C).
FIGURE 5.
Role of TLRs and pannexin-1 in zymosan- and mannan-induced caspase-1 activation and IL-1β secretion. A–C, BMDMs from wild type, TLR2−/−, TLR4−/−, MyD88−/−, and TRIF−/− mice were left untreated (CTRL), or stimulated with zymosan, mannan, or LPS for 4 h, the last 30 min in the presence of 5 mm ATP. Cell extracts were immunoblotted for caspase-1 (A), and culture supernatants were analyzed for secreted IL-1β (B) and TNF-α (C). D, wild type BMDMs were left untreated (CTRL) or stimulated with zymosan, mannan, or LPS for 4 h. Cells were subsequently incubated with DMSO (−probenecid) or 3 mm probenicid (+probenecid) for 30 min and then stimulated with 5 mm ATP for 30 min. Cell extracts were immunoblotted for caspase-1. Black arrowheads indicate full-length caspase-1 (45 kDa), and white arrowheads mark the large subunits of activated caspase-1 (20 kDa). Cytokine data represent the mean ± S.E. of triplicate wells. All data are representative of at least three independent experiments.
The hemichannel protein pannexin-1 has also been implicated in activation of the Cryopyrin inflammasome by bacterial PAMPs (12, 25, 26). We made use of the previously reported pannexin-1 inhibitor probenecid (27, 28) to assess its role in inflammasome activation by fungal PAMPs. As expected, probenecid prevented caspase-1 activation in LPS+ATP-stimulated macrophages (Fig. 5D). Interestingly, zymosan- and mannan-induced caspase-1 activation was also blocked by probenecid pretreatment (Fig. 5D), suggesting that pannexin-1 is required for activation of the Cryopyrin inflammasome by fungal PAMPs and ATP.
Cryopyrin and ASC Are Required for Zymosan- and Mannan-induced Caspase-7 Activation
We previously showed that bacterial molecules such as LPS and peptidoglycan induce activation of the executioner caspase-7 through the Cryopyrin inflammasome in macrophages co-treated with ATP (29), and this pathway was suggested to be important for LPS-induced endotoxic shock in vivo (30). To analyze whether fungal zymosan and mannan induced caspase-7 activation through the Cryopyrin inflammasome, lysates of wild type, Cryopyrin−/−, and ASC−/− macrophages were analyzed for caspase-7 processing. Similar to caspase-1 (Fig. 2A), processing of caspase-7 was observed only when zymosan- or mannan-prestimulated macrophages were treated with ATP for 30 min (Fig. 6). Caspase-7 processing was abolished in ASC−/− and Cryopyrin−/− macrophages, but not in Ipaf-deficient BMDMs (Fig. 6). These results confirm that zymosan- and mannan-induced caspase-7 activation requires components of the Cryopyrin inflammasome.
FIGURE 6.
Cryopyrin and ASC are required for zymosan- and mannan-induced caspase-7 activation. BMDMs were left untreated (CTRL) or stimulated with zymosan, mannan, or LPS for 4 h, the last 30 min in the presence of PBS (zymosan, mannan) or 5 mm ATP (zymosan+ATP, mannan+ATP, LPS+ATP). Cell extracts were immunoblotted for caspase-7. Black arrowheads indicate full-length caspase-7 (33 kDa), and white arrowheads mark the large subunits of activated caspase-7 (19 kDa). Data shown are representative of three separate experiments.
DISCUSSION
Some fungal species are opportunistic pathogens that can cause infection in people with compromised immune systems. Mannan and zymosan are major components of fungal cell walls and recognition of these fungal cell wall components by a variety of cell-bound and/or soluble receptors including the mannose receptor, TLRs, collectins, and C-type lectins is required for the mammalian immune response (31). However, the role of the intracellular receptors of the NLR family in the innate immune response to fungal mannan and zymosan has not been characterized.
We demonstrated that heat-killed S. cerevisiae and the purified cell wall components zymosan and mannan induced caspase-1 activation and IL-1β secretion from macrophages and dendritic cells when combined with ATP. Zymosan- and mannan-induced caspase-1 activation required the inflammasome components ASC and Cryopyrin, but not the Ipaf inflammasome. Although caspase-1 activation proceeded normally in MyD88−/− and TRIF−/− macrophages, zymosan- and mannan-induced generation of IL-1β required MyD88 and was partially dependent on TRIF and TLR2. In contrast, the NLR protein Nod2 and its adaptor protein RIP2 were dispensable for zymosan- and mannan-induced caspase-1 activation and IL-1β secretion.
Activation of the Cryopyrin inflammasome is believed to involve the generation of a secondary messenger, although the precise nature of this factor remains elusive. Several mechanisms have been suggested including K+ efflux and the generation of reactive oxygen species (ROS) (17, 18, 32, 33), lysosomal destabilization (34), and cytosolic entry of microbial PAMPs through pannexin-1 and destabilized phagosomes (35). Notably, recognition of live Candida albicans by the C- type lectin receptor Dectin-1 was recently proposed to induce activation of the Cryopyrin inflammasome through Syk-induced ROS production, independent of CARD9-mediated NF-κB activation (36). In addition, we demonstrated that zymosan+ATP- and mannan+ATP-induced activation of the Cryopyrin inflammasome was inhibited by the previously reported pannexin-1 inhibitor probenecid (27, 28). This result suggests a role for pannexin-1 in activation of the Cryopyrin inflammasome in cells treated with fungal PAMPs and ATP. Additional studies are required to delineate the signaling pathways that lead to activation of the Cryopyrin inflammasome.
Regardless, our results show that the NLR family member Cryopyrin/Nalp3 should be added to the list of receptors able to sense, directly or indirectly, the presence of fungal cell wall components. In contrast to extracellular receptors such as the mannose receptor, TLRs, collectins, and C-type lectins (31), however, Cryopyrin/Nalp3 is cytosolic and may therefore function as a sensor for the detection of the intracellular presence of fungal components. Hence, its role is specifically targeted to the assembly of caspase-1-activating inflammasome complexes to specifically trigger activation of the leaderless cytokines IL-1β and IL-18, whereas the production of classically secreted cytokines including IL-6 and TNF-α are independent of Cryopyrin/Nalp3. Indeed, the latter cytokines were recently shown to be induced through the extracellular C-type lectin receptor Dectin-1 in macrophages and dendritic cells (37, 38). In conclusion, our findings demonstrate that the Cryopyrin/Nalp3 inflammasome mediates caspase-1 activation in response to the fungal PAMPs zymosan and mannan and suggest a major role for this inflammasome in the innate response to fungal infections.
Supplementary Material
Acknowledgments
We thank Anthony Coyle, John Bertin, Ethan Grant (Millennium Pharmaceuticals), Gabriel Núñez (University of Michigan), Richard Flavell (Yale University), and Shizuo Akira (Osaka University) for a generous supply of mutant mice. We also thank Peter Vandenabeele (Ghent University) for providing the caspase-1 antibody. Conflict-of-interest disclosure: The authors declared no competing interests.
This work was supported, in whole or in part, by National Institutes of Health Grant AR056296. This work was also supported by the American Lebanese Syrian Association Charities (to T.-D. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 ands S2.
- PAMP
- pathogen-associated molecular pattern
- BMDC
- bone marrow-derived dendritic cell
- BMDM
- bone marrow-derived macrophage
- IL
- interleukin
- LPS
- lipopolysaccharide
- NLR
- NOD-like receptor
- TNF
- tumor necrosis factor
- zymosan-Ox
- NaClO-oxidized zymosan
- PBS
- phosphate-buffered saline
- PGN
- peptidoglycan
- TLR
- Toll-like receptor
- ASC
- apoptosis-associated speck-like protein containing a caspase recruitment domain.
REFERENCES
- 1.Janeway C. A., Jr., Medzhitov R. (2002) Annu. Rev. Immunol. 20, 197–216 [DOI] [PubMed] [Google Scholar]
- 2.Kanneganti T. D., Lamkanfi M., Núñez G. (2007) Immunity 27, 549–559 [DOI] [PubMed] [Google Scholar]
- 3.Martinon F., Tschopp J. (2004) Cell 117, 561–574 [DOI] [PubMed] [Google Scholar]
- 4.Pålsson-McDermott E. M., O'Neill L. A. (2004) Immunology 113, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agostini L., Martinon F., Burns K., McDermott M. F., Hawkins P. N., Tschopp J. (2004) Immunity 20, 319–325 [DOI] [PubMed] [Google Scholar]
- 6.Martinon F., Burns K., Tschopp J. (2002) Mol. Cell 10, 417–426 [DOI] [PubMed] [Google Scholar]
- 7.Lamkanfi M., Dixit V. M. (2009) Immunol. Rev. 227, 95–105 [DOI] [PubMed] [Google Scholar]
- 8.Ghayur T., Banerjee S., Hugunin M., Butler D., Herzog L., Carter A., Quintal L., Sekut L., Talanian R., Paskind M., Wong W., Kamen R., Tracey D., Allen H. (1997) Nature 386, 619–623 [DOI] [PubMed] [Google Scholar]
- 9.Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J., Towne E., Tracey D., Wardwell S., Wei F. Y., Wong W., Kamen R., Seshadri T. (1995) Cell 80, 401–411 [DOI] [PubMed] [Google Scholar]
- 10.Kuida K., Lippke J. A., Ku G., Harding M. W., Livingston D. J., Su M. S., Flavell R. A. (1995) Science 267, 2000–2003 [DOI] [PubMed] [Google Scholar]
- 11.Mariathasan S., Weiss D. S., Newton K., McBride J., O'Rourke K., Roose-Girma M., Lee W. P., Weinrauch Y., Monack D. M., Dixit V. M. (2006) Nature 440, 228–232 [DOI] [PubMed] [Google Scholar]
- 12.Kanneganti T. D., Lamkanfi M., Kim Y. G., Chen G., Park J. H., Franchi L., Vandenabeele P., Núñez G. (2007) Immunity 26, 433–443 [DOI] [PubMed] [Google Scholar]
- 13.Sutterwala F. S., Ogura Y., Szczepanik M., Lara-Tejero M., Lichtenberger G. S., Grant E. P., Bertin J., Coyle A. J., Galán J. E., Askenase P. W., Flavell R. A. (2006) Immunity 24, 317–327 [DOI] [PubMed] [Google Scholar]
- 14.Kanneganti T. D., Ozören N., Body-Malapel M., Amer A., Park J. H., Franchi L., Whitfield J., Barchet W., Colonna M., Vandenabeele P., Bertin J., Coyle A., Grant E. P., Akira S., Núñez G. (2006) Nature 440, 233–236 [DOI] [PubMed] [Google Scholar]
- 15.Kanneganti T. D., Body-Malapel M., Amer A., Park J. H., Whitfield J., Franchi L., Taraporewala Z. F., Miller D., Patton J. T., Inohara N., Núñez G. (2006) J. Biol. Chem. 281, 36560–36568 [DOI] [PubMed] [Google Scholar]
- 16.Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. (2008) Nat. Immunol. 9, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassel S. L., Eisenbarth S. C., Iyer S. S., Sadler J. J., Colegio O. R., Tephly L. A., Carter A. B., Rothman P. B., Flavell R. A., Sutterwala F. S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9035–9040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dostert C., Pétrilli V., Van Bruggen R., Steele C., Mossman B. T., Tschopp J. (2008) Science 320, 674–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariathasan S., Newton K., Monack D. M., Vucic D., French D. M., Lee W. P., Roose-Girma M., Erickson S., Dixit V. M. (2004) Nature 430, 213–218 [DOI] [PubMed] [Google Scholar]
- 20.Miao E. A., Alpuche-Aranda C. M., Dors M., Clark A. E., Bader M. W., Miller S. I., Aderem A. (2006) Nat. Immunol. 7, 569–575 [DOI] [PubMed] [Google Scholar]
- 21.Franchi L., Amer A., Body-Malapel M., Kanneganti T. D., Ozören N., Jagirdar R., Inohara N., Vandenabeele P., Bertin J., Coyle A., Grant E. P., Núñez G. (2006) Nat. Immunol. 7, 576–582 [DOI] [PubMed] [Google Scholar]
- 22.Hoshino K., Takeuchi O., Kawai T., Sanjo H., Ogawa T., Takeda Y., Takeda K., Akira S. (1999) J. Immunol. 162, 3749–3752 [PubMed] [Google Scholar]
- 23.Underhill D. M., Ozinsky A., Hajjar A. M., Stevens A., Wilson C. B., Bassetti M., Aderem A. (1999) Nature 401, 811–815 [DOI] [PubMed] [Google Scholar]
- 24.Gantner B. N., Simmons R. M., Canavera S. J., Akira S., Underhill D. M. (2003) J. Exp. Med. 197, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelegrin P., Surprenant A. (2006) EMBO J. 25, 5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelegrin P., Surprenant A. (2007) J. Biol. Chem. 282, 2386–2394 [DOI] [PubMed] [Google Scholar]
- 27.Silverman W. R., de Rivero Vaccari J. P., Locovei S., Qiu F., Carlsson S. K., Scemes E., Keane R. W., Dahl G. (2009) J. Biol. Chem. 284, 18143–18151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverman W., Locovei S., Dahl G. (2008) Am. J. Physiol. Cell Physiol. 295, C761–C767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamkanfi M., Kanneganti T. D., Van Damme P., Vanden Berghe T., Vanoverberghe I., Vandekerckhove J., Vandenabeele P., Gevaert K., Núñez G. (2008) Mol. Cell Proteomics 7, 2350–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamkanfi M., Moreira L. O., Makena P., Spierings D. C., Boyd K., Murray P. J., Green D. R., Kanneganti T. D. (2009) Blood 113, 2742–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown G. D. (2006) Nat. Rev. Immunol. 6, 33–43 [DOI] [PubMed] [Google Scholar]
- 32.Pétrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. (2007) Cell Death Differ. 14, 1583–1589 [DOI] [PubMed] [Google Scholar]
- 33.Franchi L., Kanneganti T. D., Dubyak G. R., Núñez G. (2007) J. Biol. Chem. 282, 18810–18818 [DOI] [PubMed] [Google Scholar]
- 34.Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. (2008) Nat. Immunol. 9, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marina-Garcia N., Franchi L., Kim Y. G., Miller D., McDonald C., Boons G. J., Núñez G. (2008) J. Immunol. 180, 4050–4057 [DOI] [PubMed] [Google Scholar]
- 36.Gross O., Poeck H., Bscheider M., Dostert C., Hannesschläger N., Endres S., Hartmann G., Tardivel A., Schweighoffer E., Tybulewicz V., Mocsai A., Tschopp J., Ruland J. (2009) Nature 459, 433–436 [DOI] [PubMed] [Google Scholar]
- 37.Saijo S., Fujikado N., Furuta T., Chung S. H., Kotaki H., Seki K., Sudo K., Akira S., Adachi Y., Ohno N., Kinjo T., Nakamura K., Kawakami K., Iwakura Y. (2007) Nat. Immunol. 8, 39–46 [DOI] [PubMed] [Google Scholar]
- 38.Taylor P. R., Tsoni S. V., Willment J. A., Dennehy K. M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S., Brown G. D. (2007) Nat. Immunol. 8, 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.