Abstract
Obese adipose tissue (AT) is associated with chronic inflammation, and we hypothesized that the keratinocyte-derived chemokine (KC), the mouse ortholog of human interleukin-8, plays a role in obesity-mediated AT inflammation and the subsequent manifestation of insulin resistance. KC expression is increased in the AT and plasma of genetically (ob/ob) and high fat diet-induced obese mouse models, and this increase may be mediated by the elevated leptin and tumor necrosis factor-α levels associated with obesity. Obesity-induced KC expression occurs primarily in stromal vascular cells and not in adipocytes, and it is high in preadipocytes and decreases during adipogenesis. Although KC has no effect on adipogenesis, it induces adipocyte expression of inflammatory factors and the insulin resistance mediator, suppressor of cytokine signaling 3. Using chimeric mice deficient in the KC receptor CXCR2 in their bone marrow, we show that the lack of CXCR2 in hematopoietic cells is sufficient to protect from adipose and skeletal muscle macrophage recruitment and development of insulin resistance in diet-induced obese mice. These studies suggest that KC and its receptor CXCR2 are potential targets for the development of new therapeutic approaches for treatment of obesity-related insulin resistance, type II diabetes, and related cardiovascular diseases.
Obesity is characterized by systemic low grade inflammation that appears to contribute to the genesis of insulin resistance (IR),3 type 2 diabetes, and increased risk for cardiovascular diseases (reviewed in Ref. 1). Furthermore, adipose tissue (AT) produces a variety of inflammatory factors, and its excessive development in obesity is associated with accumulation of AT macrophages (ATMs) (1), whose recruitment and proinflammatory activation are required for the development of IR in obese mice (reviewed in Ref. 2). An important question concerning ATMs is/are the trigger(s) driving the recruitment of these cells in obesity.
Efforts at identifying factors that attract and recruit ATMs have mostly focused on the CC chemokine MCP-1 (monocyte chemoattractant protein-1) and its receptor CCR2. These studies have led to contradicting results with several publications showing that MCP-1 and CCR2 are important for ATM recruitment and the subsequent development of IR (3–5), whereas others show no involvement of this chemokine and its receptor in these processes (6–8). Furthermore, the studies that claim a role for MCP-1 and CCR2 in ATM recruitment and IR show that deficiency of the ligand or the receptor did not result in normalization of ATM content, indicating that other factors also participate in ATM recruitment. These findings suggest that the precise role of the MCP-1/CCR2 axis in ATM recruitment and IR is unclear, and that other chemokines and their receptors could also play a role in these processes. One such chemokine is interleukin 8 (IL-8), the prototypical CXC chemokine known to recruit and activate monocytes and to attract polymorphonuclear leukocytes to sites of inflammation (9). IL-8 is elevated in plasma of obese subjects (10, 11) and correlates with adiposity and insulin sensitivity, suggesting an involvement of this chemokine in obesity-related health complications (12–14). Additionally, IL-8 is implicated in the pathogenesis of atherosclerosis and cardiovascular disease, two obesity-associated disorders (15). Finally, IL-8 is an angiogenic factor, and angiogenesis is a hallmark of AT expansion in obesity (16). Although these findings suggest an important role for IL-8 in AT biology and pathology, little is known regarding the mechanism of regulation of IL-8 in obesity and its role in AT biology and pathology. This is probably due, in part, to the absence of suitable animal models because mice and rats do not have a clear-cut IL-8 ortholog (17).
Although rodent keratinocyte-derived chemokine (KC) shows the highest homology with human growth-related oncogene (GRO-α), it appears to be the closest equivalent to IL-8, as judged by its pattern of expression and putative function (18). Monocytes express the KC receptor (CXCR2), and KC triggers monocyte arrest on early atherosclerotic endothelium, one of the first steps in the invasion of tissues by macrophages (19). Interaction of monocyte CXCR2 with its ligand KC leads to up-regulation of α4β1 integrin affinity and firm adhesion to the endothelium (19). Furthermore, both KC and its receptor play a central role in macrophage infiltration and accumulation in atherosclerotic lesions in mice (20, 21). However, no information is currently available regarding the role of KC in macrophage recruitment in obese AT or its role in AT biology and pathology.
In this study, we show that KC expression is elevated in AT and plasma of genetically (ob/ob) and diet-induced obese (DIO) mice, probably as the result of increased leptin and tumor necrosis factor α (TNF-α) levels associated with obesity. We also show that obesity-induced KC is mostly derived from nonadipocyte sources in AT and that KC does not affect adipocyte differentiation but does increase pro-inflammatory cytokine expression in adipocytes. Finally, we show in a DIO model in chimeric mice lacking CXCR2 on their macrophages that the KC receptor plays an important role in macrophage accumulation in adipose and skeletal muscle tissue and subsequent development of IR.
EXPERIMENTAL PROCEDURES
Animal Studies
Animal studies were approved by our Institutional Animal Care and Use Committee and the Animal Research Committee, in accordance with Public Health Policy regarding the use and care of laboratory animals. Genetically obese mice deficient for leptin (C57BL/6J-Lepob; ob/ob), their respective lean counterparts (C57BL/6J WT), and CXCR2-deficient mice (C.129S2(B6)-Il8rbtm1MwmJ) and their appropriate WT controls (Balb/cJ) were from The Jackson Laboratories, Bar Harbor, ME. The p55−/−, p75−/−, and p55p75−/− mice were kindly provided by G. S Hotamisligil (Harvard School of Public Health). In some experiments, C57BL/6J WT mice were injected intraperitoneally with 4 μg of recombinant murine TNF-α (Genzyme Diagnostics, Cambridge, MA) or 10 μg of leptin (R & D Systems). Control animals were injected with 100 μl of saline. Three hours later, AT was removed and processed for the preparation of total RNA. For the DIO model, C57BL/6J (8-week-old) male or bone marrow transplanted male mice (see below) were placed for 16 weeks on HFD (D12492, 60% fat, Research Diets, New Brunswick, NJ).
AT digestion and cell fractionation, bone marrow transplantations, and glucose and insulin tolerance tests were performed as described previously (22, 23). Details are available as supplemental material.
Cell Culture Studies
3T3-L1 mouse embryo fibroblasts were obtained from the American Type Culture Collection (Manassas, VA). These cells were cultured and differentiated into adipocytes as described previously (24). In some experiments, total RNA and conditioned media were harvested from the differentiating cells at different time points to measure KC mRNA and protein expression, respectively. In other experiments, cells were treated with saline (control) or KC (10 or 100 ng/ml; PeproTech, Rocky Hill, NJ) either initially before starting the differentiation process (at pre-confluent and confluent stage) or chronically throughout the whole differentiation process, where KC was added every 3 days when culture media were changed, beginning at the pre-confluent stage. Differentiated 3T3-L1 cells were fixed by incubation with 10% neutral buffered formalin for 20 min and stained with Oil Red O as described previously (25). The dye was extracted from the cells with isopropyl alcohol, and the absorbance was measured spectrophotometrically at 510 nm. For gene expression studies, mature 3T3-L1 adipocytes (day 11) were treated either with saline (control) or KC (10 or 100 ng/ml) for 24 h before harvesting total RNA.
AT Staining and Measurements
F4/80 immunohistochemistry was carried out on 6-μm deparaffinized sections of mouse fat pads. Details are available as supplemental material.
Adipocyte diameters were measured using the SPOT software (Diagnostic Instruments, Inc). Five microscopic photographs were taken from the AT sections of each mouse. Adipocyte diameters were measured from three random fields (25 cells/field) from each photograph. Crown-like structures were quantified as described previously (23). Details are available as supplemental material.
KC Enzyme-linked Immunosorbent Assay
Concentrations of KC in plasma and conditioned cell culture medium were determined by using an enzyme-linked immunosorbent assay kit (Quantikine, R & D Systems).
Statistical Analysis
Statistical significance between two groups was determined by the Student's t test. Comparisons among several groups were performed by analysis of variance, and statistical significance was calculated using the Dunnett's multiple comparison test.
RESULTS
KC Expression Is Elevated in Both Genetically and Diet- induced Obese Mice and Is Modulated by Leptin and TNF-α
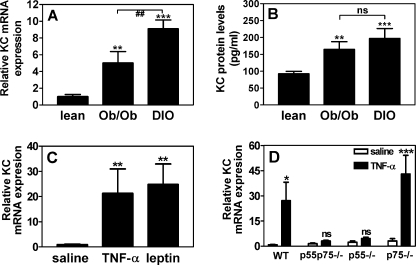
Although KC is the functional analog of human IL-8, which is increased in human obesity (10, 11), no information is available regarding the expression of KC in obese mice. We therefore measured KC expression in lean, genetically obese (ob/ob), and DIO mice (Fig. 1). KC mRNA levels in epididymal AT from the genetically obese ob/ob and DIO mice are 5- and 9-fold higher, respectively (Fig. 1A), when compared with their lean counterparts. In parallel, KC antigen in the plasma was elevated by 1.8-fold in ob/ob and 2.1-fold in DIO mice (Fig. 1B). Because obesity is associated with hyperleptinemia and elevated levels of TNF-α (26), we determined whether either TNF-α or leptin regulates the expression of KC in vivo. Lean mice, injected with TNF-α or leptin, showed an increase in AT KC mRNA levels by 21- or 25-fold, respectively (Fig. 1C). The regulatory effect of leptin on KC expression is also apparent from Fig. 1, A and B; even though the leptin-deficient ob/ob mice have a higher body weight (58.1 ± 2.5 g) than the DIO mice (48.6 ± 3.6 g), KC mRNA and protein levels are less pronounced, confirming a role for leptin in KC regulation. However, the same data show that leptin is only partially responsible for the increased KC expression in obese mice, as is evidenced by the KC increase that remains in the leptin-deficient ob/ob mice. To further assess the contribution of TNF-α to the observed increase in KC expression, we analyzed adipose tissue KC mRNA levels in wild-type and TNF-α receptor-deficient (p55−/−, p75−/−, and p55p75−/−) lean mice, injected with either saline or TNF-α. Fig. 1D shows that TNF-α injection results in an induction of KC mRNA levels in adipose tissue of wild-type and p75−/− mice, but not in p55−/− and p55p75−/− mice, confirming that TNF-α is a major inducer of KC expression in adipose tissue and that the p55 TNF-α receptor is crucial for this induction. Together, these results show that AT KC mRNA expression and plasma levels of KC are significantly increased in both genetically and diet-induced obese mice, probably as the result of the high levels of leptin and TNF-α associated with obesity.
FIGURE 1.
Changes in KC expression in genetically and diet-induced obese mice. KC mRNA levels in epididymal white adipose tissue (A) and KC protein levels in serum (B) from lean, ob/ob, and DIO mice. **, p < 0.005; ***, p < 0.001 versus lean; ##, p < 0.005; ns, not significant. n = 6, mean ± S.D. C, relative KC mRNA expression in adipose tissue from wild-type C57BL/6 mice 3 h after intraperitoneal injection with saline (control), TNF-α (4 μg), or leptin (10 μg). **, p < 0.005 versus saline, n = 6. Mean ± S.D. D, relative KC mRNA expression in adipose tissue from wild-type, p55−/−, p75−/−, and p55p75−/− mice 3 h after intraperitoneal injection with saline (control) or TNF-α (4 μg). *, p < 0.05; ***, p < 0.001; ns, not significant versus saline, n = 4, mean ± S.D.
Obesity-induced KC Is Mostly Derived from Nonadipocyte Sources in AT
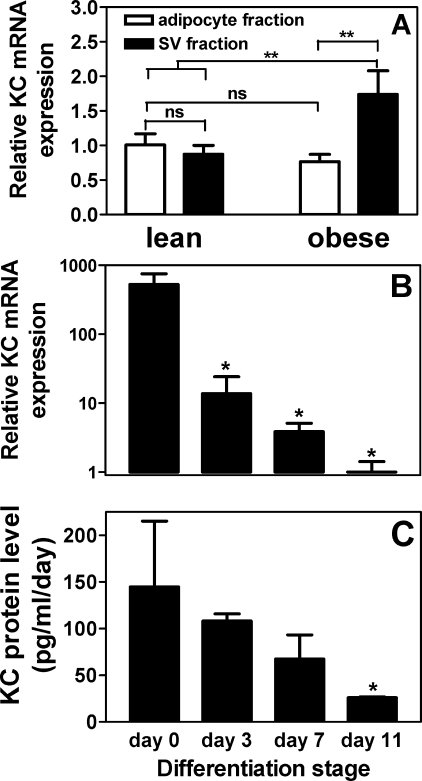
Next, we investigated the cellular sources responsible for KC expression in obese and lean AT. Cell fractionation experiments were performed to determine relative KC mRNA levels in both adipocyte and stromal vascular (SV) fractions of AT from lean and obese (ob/ob) mice. As shown in Fig. 2A, KC mRNA levels were similar in both adipocytes and SV cells from lean AT, suggesting that both fractions are sources of KC. However, in ob/ob mice, although KC expression in the adipocyte fraction was similar to that observed for lean adipocytes, its expression in the SV fraction was significantly increased compared with lean mice. This suggests that the increase in KC in the AT of obese mice is primarily due to increased expression from cells within the SV fraction and not from adipocytes.
FIGURE 2.
KC expression in adipose fractions and during in vitro adipogenesis. A, KC mRNA levels in adipocyte and SV fractions of epididymal white adipose tissue of lean and obese mice. ns, not significant; **, p < 0.005. n = 6, mean ± S.D. B, changes in KC mRNA levels during adipogenesis. At day 0, confluent 3T3-L1 preadipocytes were induced to differentiate, and by day 11 the majority of cells had converted into mature adipocytes. C, changes in KC protein expression in conditioned media from the same cells as shown in B. *, p < 0.05 versus day 0. n = 3, mean ± S.D.
KC Is Highly Expressed in Preadipocytes and Decreases during Adipogenesis
The AT SV fraction consists of multiple cell types (e.g. endothelial cells, fibroblasts, preadipocytes, and macrophages) (27). To analyze the expression of KC in preadipocytes and during adipocyte differentiation, we studied an in vitro model of adipogenesis using 3T3-L1 cells. KC gene expression was high in 3T3-L1 preadipocytes (day 0), and this expression decreased during adipocyte differentiation, to a level in mature adipocytes (day 11) that was ∼500-fold lower than in preadipocytes (Fig. 2B). A parallel decrease was observed in KC protein expression in conditioned media from differentiating 3T3-L1 adipocytes (Fig. 2C). For example, preadipocytes (day 0) express 145 pg/ml/day, and this decreased to 26 pg/ml/day in mature adipocytes (day 11). Together, these in vitro results show that KC is highly expressed in preadipocytes and decreases during adipogenesis.
KC Does Not Affect Adipogenesis but Increases Inflammatory Cytokine/Chemokine Gene Expression in Adipocytes
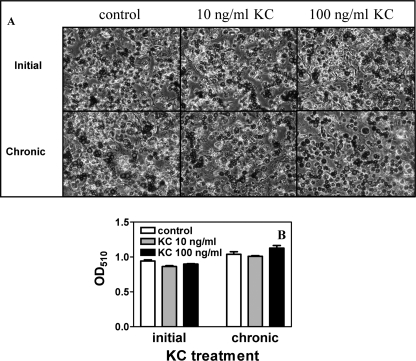
Several studies have shown that the expression of cytokines/chemokines, including IL-1, IL-6, TNF-α, and MCP-1, decreases during adipogenesis, similar to what we observe for KC (Fig. 2, B and C). These cytokines/chemokines inhibit adipocyte differentiation (28–31), raising the possibility that KC may also inhibit adipogenesis. We therefore analyzed the effect of exogenous KC on 3T3-L1 differentiation. Neither initial (acute, 1 day) nor chronic treatment (i.e. 11 days) of 3T3-L1 cells with 10 or 100 ng/ml KC inhibited adipogenesis as revealed by the lack of difference in the appearance and extent of Oil Red O staining and quantification of lipid accumulation of the differentiated cells (Fig. 3).
FIGURE 3.
Effect of KC on 3T3-L1 adipogenesis. 3T3-L1 cells were treated with KC before the cells became confluent (initial) or during the entire differentiation time course (chronic) where KC was supplemented to the culture media every 3 days during media change. A, bright field photographs of the differentiated cells after Oil Red O staining. B, quantification of the lipid content of the cells after differentiation by quantifying Oil Red O uptake. n = 3. Mean ± S.D.
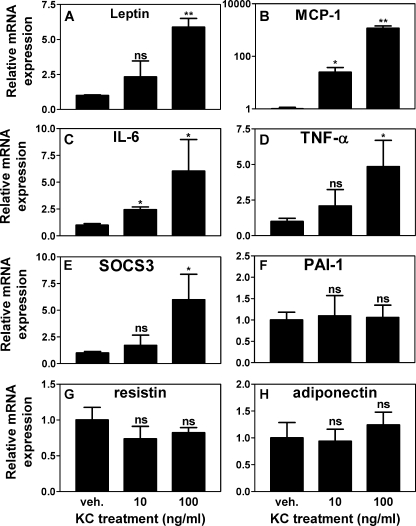
Although KC does not seem to affect adipogenesis (Fig. 3), and its expression is not increased in adipocytes in obese AT (Fig. 2A), we hypothesized that increased KC in obese AT by SV cells (e.g. preadipocytes) could affect the expression of inflammatory genes in neighboring adipocytes. As shown in Fig. 4, KC treatment of adipocytes (10 or 100 ng/ml for 24 h) resulted in a dose-dependent increase in expression of the inflammatory adipokines leptin, MCP-1, IL-6, and TNF-α but no change in the adipokines PAI-1 resistin and adiponectin. Interestingly, KC also induced the expression of SOCS3, a well established mediator of insulin resistance (32). These results suggest that the obesity-induced increase in AT KC expression may contribute to adipose inflammation via the induction of proinflammatory cytokines/chemokines in adipocytes. These changes together with increased SOCS3 expression may ultimately lead to the development of insulin resistance.
FIGURE 4.
Induction of inflammatory factors in adipocytes by KC treatment. Mature 3T3-L1 adipocytes were treated with 10 ng/ml vehicle (veh.) or 100 ng/ml KC for 24 h. Levels of mRNA for leptin (A), MCP-1 (B), IL-6 (C), TNF-α (D), SOCS3 (E), PAI-1 (F), resistin (G), and adiponectin (H) were measured using real time quantitative RT-PCR. ns indicates not significant; *, p < 0.05; **, p < 0.005 versus vehicle. n = 3, mean ± S.D.
Lack of the KC Receptor CXCR2 on Bone Marrow-derived Cells Results in Reduced Infiltration of Macrophages into the Adipose Tissues and Skeletal Muscle
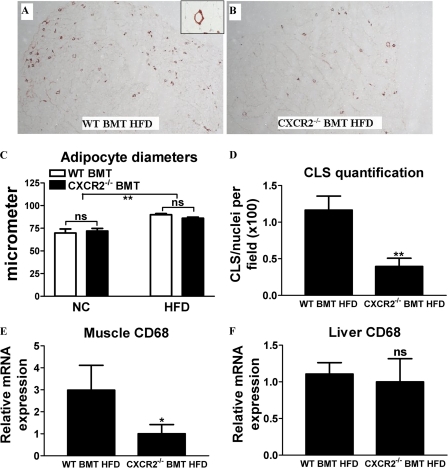
Because both KC and its receptor play a central role in macrophage infiltration and accumulation in atherosclerotic lesions in mice (20, 21), we hypothesized that the KC/CXCR2 axis could play an important role in adipose tissue macrophage infiltration associated with obesity. We therefore studied chimeric mice deficient in the KC receptor CXCR2 in their bone marrow (BM) and, as a result, in all BM-derived cells (e.g. macrophages). Animals that received wild-type BM were used as a control group. Half of the animals of each group received a normal chow (NC) diet (10% fat) and the other half a HFD (60% fat). Mice fed the HFD gained more weight than the NC control group; this was not influenced by the BM genotype. After 8 weeks of diet, body weight of control WT BMT mice was 34.8 ± 0.6 g at HFD versus 28.1 ± 1.0 g at NC (p < 0.001, mean ± S.E., n = 5). The body weights of CXCR2−/− BMT mice were 35.1 ± 0.4 g at HFD versus 28.3 ± 1.3 at NC (p < 0.001, mean ± S.E., n = 5). Average weekly food intake was stable throughout the study in both NC and HFD groups and was not different between BM genotypes (data not shown). No significant differences were observed between the different groups when comparing the white blood cell count and differential (supplemental Table I). AT sections stained for the macrophage marker F4/80 indicated that less macrophages infiltrated the AT of the HFD-fed CXCR2−/− BMT mice compared with HFD-fed WT BMT mice (Fig. 5, A and B). We obtained similar results when staining for MAC2 (data not shown). Macrophages were rarely observed in AT from the lean NC-fed mice (data not shown). The infiltrated macrophages formed crown-like structures around presumably dead adipocytes as described previously (Fig. 5A, inset) (33). Quantification of the adipocyte diameters as a measure of adipocyte size showed a 20–30% increase in adipocyte size in HFD- versus NC-fed mice but no differences between genotypes (Fig. 5C). Quantification of the number of crown-like structures per field as a measure of macrophage infiltration of the AT showed a 66% reduction in the HFD-fed CXCR2−/− BMT mice compared with their WT counterparts (Fig. 5D). We and others have shown previously that obesity is also associated with increased macrophage content in skeletal muscle (34–37). Therefore, we measured muscle mRNA levels of the macrophage marker CD68. As shown in Fig. 5E, the expression of this marker is 3-fold lower in HFD-fed CXCR2−/− BMT mice compared with their WT counterparts. Because liver contains its own specialized resident macrophages (i.e. Kupffer cells), we analyzed whether the lack of CXCR2 on BM-derived cells affected macrophage content in the liver. As shown in Fig. 5F, CD68 expression levels in livers of HFD-fed CXCR2−/− BMT mice did not differ from that of their WT counterparts. Together, these results demonstrate that although the lack of the KC receptor CXCR2 on BM-derived cells has no effect on weight gain and adipocyte size in a DIO mouse model, it results in a dramatic reduction of macrophage infiltration of obese adipose and muscle tissue, confirming a role for the KC/CXCR2 nexus in macrophage infiltration.
FIGURE 5.
Adipose and muscle tissue macrophage infiltration is decreased in mice lacking the KC receptor CXCR2 on bone marrow-derived cells. Immunohistochemical staining for the macrophage marker F4/80 in epiWAT sections of HFD-fed mice that received WT bone marrow (A) and CXCR2−/− bone marrow (B). The inset in A shows an adipocyte surrounded by macrophages, a so-called crown-like structure. C, quantification of adipocyte diameters as a measurement of adipocyte size in epididymal white adipose tissue of WT and CXCR2−/− BMT mice fed either NC or HFD. ns indicates not significant, **, p < 0.005. Mean ± S.D. D, quantification of crown-like structures (CLS) as a measurement of ATM infiltration in HFD-fed WT and CXCR2−/− BMT mice. E, relative mRNA levels for the macrophage marker CD68 in quadriceps muscles; F, liver as determined by quantitative PCR. *, p < 0.05; **, p < 0.005 versus WT BMT HFD. n = 5, mean ± S.D.
Lack of the KC Receptor CXCR2 on BM-derived Cells Results in Protection against HFD-induced IR
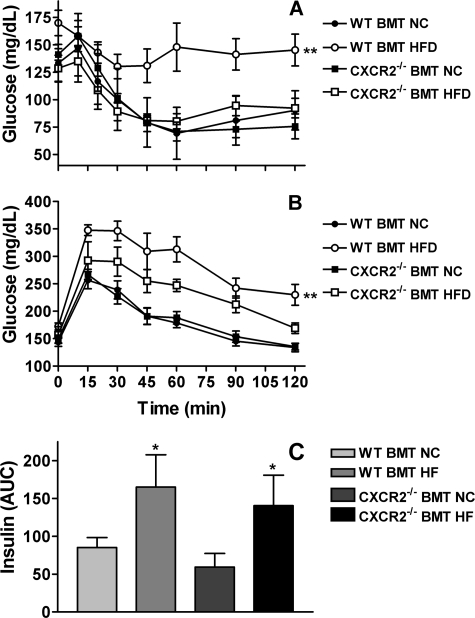
As it has now been well established that macrophage recruitment and proinflammatory activation are required for the development of IR in obese mice (1), we performed insulin and glucose tolerance tests to evaluate the insulin sensitivity in both WT and CXCR2−/− BMT mice fed either NC or HFD. Fasting glucose levels increased with HFD in WT BMT mice (171.6 ± 5.3 mg/dl in HFD-fed versus 141.0 ± 7.2 mg/dl in NC-fed, p < 0.05, mean ± S.E., n = 5) but not in CXCR2−/− BMT mice (146.1 ± 11.1 mg/dl in HFD-fed versus 147.5 ± 5.6 mg/dl in NC-fed, mean ± S.E., n = 5). Furthermore, fasting insulin levels increased with HFD in both WT and CXCR2−/− BMT mice (3.85 ± 0.61 μg/liter in HFD-fed versus 1.64 ± 0.18 μg/liter in NC-fed WT BMT mice and 3.02 ± 0.77 μg/liter in HFD-fed versus 1.07 ± 0.30 μg/liter in NC-fed CXCR2−/− BMT mice, p < 0.05, mean ± S.E., n = 5), but there were no significant differences between BM genotypes. As shown in Fig. 6A, injection of insulin in NC-fed mice resulted in a similar drop in blood glucose levels regardless of BM genotype. However, HFD-fed WT BMT mice were less responsive to insulin and only showed a slight drop in blood glucose level. In contrast, the HFD-fed CXCR2−/− BMT mice behaved like the NC-fed mice and showed a large drop in blood glucose levels after insulin administration.
FIGURE 6.
CXCR2−/− BMT mice are protected against HFD-induced insulin resistance. In vivo glucose homeostasis in NC- or HFD-fed WT or CXCR2−/− BMT mice as determined by insulin tolerance test (A) or GTT (B). **, p < 0.005 versus CXCR2−/− BMT HFD. n = 5, mean ± S.D. C, insulin secretion during GTT presented as area under curve (AUC) of plasma insulin levels measured at regular intervals. *, p < 0.05 versus NC-fed. n = 5, mean ± S.D.
We also performed a glucose tolerance test (GTT) and made similar observations (Fig. 6B). HFD-induced obesity led to glucose intolerance in WT BMT mice, whereas CXCR2−/− BMT mice were partially protected. Finally, the glucose-induced insulin secretion during the GTT was increased by high fat feeding, but no significant differences were observed between BM genotypes (Fig. 6C). Together, these results indicate that CXCR2−/− BMT mice are partially protected against HFD-induced IR.
DISCUSSION
The present study provides novel information on the expression and regulation of KC in AT of obese mice, and it demonstrates a potential role for KC and its receptor CXCR2 in the inflammation, macrophage recruitment, and insulin resistance associated with obesity.
Our studies demonstrating elevated KC expression in AT and plasma of obese mice are consistent with what has been reported for IL-8 expression in human obesity (38). Elevated KC in AT of obese mice appeared to be derived primarily from stromal/vascular cells, likely to be preadipocytes and/or invading macrophages, again consistent with IL-8 expression in human obesity (38). In obese individuals the AT becomes the largest endocrine organ, and adipose-derived proteins have systemic effects on other organs (2). Thus, increased adipose KC expression may contribute both to local inflammation and to systemic increase in KC and therefore may also contribute to the accelerated atherosclerosis associated with obesity.
Bone marrow transplantation from CXCR2-deficient mice demonstrates that the lack of CXCR2 on bone marrow-derived cells results in a dramatic reduction of obese adipose and muscle tissue macrophage infiltration. Furthermore, the lack of CXCR2 on bone marrow transplanted cells also resulted in significant protection against HFD-induced IR, thereby identifying a crucial role for this chemokine receptor not only in the inflammation but also the subsequent manifestation of IR in obesity. It should be noted that CXCR2 affinity is associated with the presence of a conserved Glu-Leu-Arg (ELR) motif in chemokines (39). Thus, absence of CXCR2 will not only disrupt KC signaling but also the other ELR+ members of the CXC chemokine family. We therefore cannot exclude the possibility that the in vivo effects observed in the CXCR2−/− BMT mice are because of disruption of signaling through multiple chemokines and not just KC alone.
While this manuscript was undergoing revisions, Chavey et al. (40) published a study showing that like KC another ligand of CXCR2, called CXCL5, is also expressed in white adipose tissue, is increased in obesity, and is regulated by TNF-α. Based on immunoselective isolation of cell fractions, they suggest that the major contributors of CXCL5 in adipose tissue are macrophages present in the stromal vascular fraction. However, the use of IL-6 and MCP-1 as specific markers for macrophages in the selection process is misleading because these proteins are also highly expressed in preadipocytes (29, 41). Therefore, the relative contribution of macrophages and preadipocytes to both CXCL5 and KC expression by the stromal vascular fraction deserves further study. Similar to our observations of protection from insulin resistance in CXCR2−/− BMT mice, Chavey et al. (40) show that whole-body CXCR2 knock-out mice are also protected against obesity-induced IR. Although we did not observe any significant differences in fasting insulin levels between BM genotypes, Chavey et al. (40) noted an unexpected increase in fasting insulin levels in their whole-body CXCR2−/− mice. This suggests that the systemic genetic deletion of CXCR2 could also affect other pathways, and it may be that the observed increase in insulin sensitivity merely reflects the higher insulin levels in these whole-body knock-outs. Chavey et al. (40) suggest that increased CXCL5 expression by adipose tissue directly promotes IR by negative modulation of the insulin signaling pathway through binding to the CXCR2 receptor in distant muscle cells. However, we also observed protection against obesity-induced IR in our CXCR2−/− BMT mice, and these animals are only deficient for the receptor in their bone marrow-derived cells (e.g. macrophages), but still have an intact CXCR2 receptor on their muscle cells. Based on these data, we would therefore argue that the protection for obesity-induced IR in CXCR2−/− mice is not related to the observation that CXCL5 treatment causes IR in muscle cells, but more likely to the known role of CXCR2 and its ligands in inflammation, as is evidenced by our data on adipose and muscle tissue macrophage infiltration in CXCR2−/− BMT mice.
Increasing evidence suggest that the SOCS family of proteins play central mechanistic roles in the development of insulin resistance. SOCS3 binds the insulin receptor and prevent its binding to insulin receptor substrate-1 (IRS-1), thus inhibiting IRS-1 phosphorylation and downstream insulin signaling (32). SOCS3 also inhibits insulin signaling by targeting both IRS-1 and IRS-2 for proteosomal degradation (32). Our studies showing that KC can directly induce SOCS3 expression in adipocytes provides an additional mechanistic link by which the KC/CXCR2 axis may lead to insulin resistance in the setting of obesity.
While preparing this manuscript, a study was published showing that neutrophils transiently infiltrate intra-abdominal adipose tissue early in the course of high fat feeding (42). Between days 3 and 7 after commencing high fat feeding, neutrophils were present within the parenchyma of the adipose tissue in-between adipocytes but were absent at later time points (42). Because KC is a potent chemoattractant for neutrophils (43), it is not unlikely that KC and CXCR2 play an important role in this transient neutrophil infiltration of adipose tissue. However, because we did not harvest tissues at such early time points during the high fat feeding of our CXCR2−/− BMT mice, we could not draw any conclusions regarding a potential role for CXCR2 in this transient neutrophil infiltration. This will be a subject of future investigations.
Several independent studies have demonstrated that proinflammatory cytokines (e.g. TNF-α, IL-1, and IL-6) and chemokines (e.g. MCP-1, MIP-1α, and IL-8) can inhibit adipogenesis by blocking stem cell commitment and differentiation as well as induce dedifferentiation of mature adipocytes (28–31). However, in our study, KC did not affect adipogenesis nor did it induce dedifferentiation of mature adipocytes as determined by Oil Red O staining, and by the demonstration that KC treatment did not change the expression of the adipogenesis markers adipsin and lipoprotein lipase (data not shown). Similarly, CXCL5 also did not affect adipogenesis (40). Thus, KC and CXCL5 appear to be unique with respect to their effects on adipogenesis. The mechanistic nature of this difference warrants further investigation.
In summary, this study demonstrates that expression of the chemokine KC, the functional IL-8 analog in rodents, is increased in plasma and adipose tissue of obese mice and that one or more cell types in the stromal vascular cell population in the adipose tissues (i.e. nonadipocytes) are responsible for this increased expression. We show that KC can induce the expression of inflammatory factors and SOCS3 in adipocytes and that the absence of its receptor CXCR2 on bone marrow-derived cells leads to a reduction of macrophage infiltration of obese adipose and muscle tissue and protection from obesity-induced insulin resistance. These studies suggest that KC and its receptor CXCR2 are potential targets for the development of new therapeutic approaches for treatment of obesity-related insulin resistance and type II diabetes and related cardiovascular diseases.
Supplementary Material
Acknowledgment
Work performed in the laboratory of Dr. D. J. Loskutoff was supported by National Institutes of Health Grant HL075736.
This work was supported, in whole or in part, by National Institutes of Health Grant R01HL071146 (to F. S.). This work was also supported by Scientist Development Grant 0635408N from the American Heart Association and an INSERM junior researchers temporary contract (to J. G. N.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Table I.
- IR
- insulin resistance
- AT
- adipose tissue
- KC
- keratinocyte-derived chemokine
- IL
- interleukin
- DIO
- diet-induced obese
- TNF-α
- tumor necrosis factor-α
- SOCS3
- suppressor of cytokine signaling 3
- ATM
- AT macrophage
- WT
- wild type
- NC
- normal chow
- BM
- bone marrow
- GTT
- glucose tolerance test
- SV
- stromal vascular
- IRS
- insulin receptor substrate
- HFD
- high fat diet
- BMT
- bone marrow transplantation.
REFERENCES
- 1.Pradhan A. (2007) Nutr. Rev. 65, S152–156 [DOI] [PubMed] [Google Scholar]
- 2.de Luca C., Olefsky J. M. (2008) FEBS Lett. 582, 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., Kasuga M. (2006) J. Clin. Invest. 116, 1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisberg S. P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K., Charo I., Leibel R. L., Ferrante A. W., Jr. (2006) J. Clin. Invest. 116, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamei N., Tobe K., Suzuki R., Ohsugi M., Watanabe T., Kubota N., Ohtsuka-Kowatari N., Kumagai K., Sakamoto K., Kobayashi M., Yamauchi T., Ueki K., Oishi Y., Nishimura S., Manabe I., Hashimoto H., Ohnishi Y., Ogata H., Tokuyama K., Tsunoda M., Ide T., Murakami K., Nagai R., Kadowaki T. (2006) J. Biol. Chem. 281, 26602–26614 [DOI] [PubMed] [Google Scholar]
- 6.Chen A., Mumick S., Zhang C., Lamb J., Dai H., Weingarth D., Mudgett J., Chen H., MacNeil D. J., Reitman M. L., Qian S. (2005) Obes. Res. 13, 1311–1320 [DOI] [PubMed] [Google Scholar]
- 7.Inouye K. E., Shi H., Howard J. K., Daly C. H., Lord G. M., Rollins B. J., Flier J. S. (2007) Diabetes 56, 2242–2250 [DOI] [PubMed] [Google Scholar]
- 8.Kirk E. A., Sagawa Z. K., McDonald T. O., O'Brien K. D., Heinecke J. W. (2008) Diabetes 57, 1254–1261 [DOI] [PubMed] [Google Scholar]
- 9.Remick D. G. (2005) Crit. Care Med. 33, S466–467 [DOI] [PubMed] [Google Scholar]
- 10.Straczkowski M., Dzienis-Straczkowska S., Stêpieñ A., Kowalska I., Szelachowska M., Kinalska I. (2002) J. Clin. Endocrinol. Metab. 87, 4602–4606 [DOI] [PubMed] [Google Scholar]
- 11.Kim C. S., Park H. S., Kawada T., Kim J. H., Lim D., Hubbard N. E., Kwon B. S., Erickson K. L., Yu R. (2006) Int. J. Obes. 30, 1347–1355 [DOI] [PubMed] [Google Scholar]
- 12.Bruun J. M., Verdich C., Toubro S., Astrup A., Richelsen B. (2003) Eur. J. Endocrinol. 148, 535–542 [DOI] [PubMed] [Google Scholar]
- 13.Straczkowski M., Kowalska I., Nikolajuk A., Dzienis-Straczkowska S., Szelachowska M., Kinalska I. (2003) Cardiovasc. Diabetol. 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herder C., Haastert B., Müller-Scholze S., Koenig W., Thorand B., Holle R., Wichmann H. E., Scherbaum W. A., Martin S., Kolb H. (2005) Diabetes 54, S11–17 [DOI] [PubMed] [Google Scholar]
- 15.Boisvert W. A., Curtiss L. K., Terkeltaub R. A. (2000) Immunol. Res. 21, 129–137 [DOI] [PubMed] [Google Scholar]
- 16.Trayhurn P., Wood I. S. (2004) Br. J. Nutr. 92, 347–355 [DOI] [PubMed] [Google Scholar]
- 17.Modi W. S., Yoshimura T. (1999) Mol. Biol. Evol. 16, 180–193 [DOI] [PubMed] [Google Scholar]
- 18.Watanabe K., Iida M., Takaishi K., Suzuki T., Hamada Y., Iizuka Y., Tsurufuji S. (1993) Eur. J. Biochem. 214, 267–270 [DOI] [PubMed] [Google Scholar]
- 19.Huo Y., Weber C., Forlow S. B., Sperandio M., Thatte J., Mack M., Jung S., Littman D. R., Ley K. (2001) J. Clin. Invest. 108, 1307–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boisvert W. A., Santiago R., Curtiss L. K., Terkeltaub R. A. (1998) J. Clin. Invest. 101, 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boisvert W. A., Rose D. M., Johnson K. A., Fuentes M. E., Lira S. A., Curtiss L. K., Terkeltaub R. A. (2006) Am. J. Pathol. 168, 1385–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007) J. Clin. Invest. 117, 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solinas G., Vilcu C., Neels J. G., Bandyopadhyay G. K., Luo J. L., Naugler W., Grivennikov S., Wynshaw-Boris A., Scadeng M., Olefsky J. M., Karin M. (2007) Cell Metab. 6, 386–397 [DOI] [PubMed] [Google Scholar]
- 24.Green H., Kehinde O. (1975) Cell 5, 19–27 [DOI] [PubMed] [Google Scholar]
- 25.Ding S. T., McNeel R. L., Mersmann H. J. (2002) In Vitro Cell Dev. Biol. Anim. 38, 352–357 [DOI] [PubMed] [Google Scholar]
- 26.Eckel R. H., Grundy S. M., Zimmet P. Z. (2005) Lancet 365, 1415–1428 [DOI] [PubMed] [Google Scholar]
- 27.Schäffler A., Schölmerich J., Buechler C. (2007) Nat. Clin. Pract. Endocrinol. Metab. 3, 345–354 [DOI] [PubMed] [Google Scholar]
- 28.Gerhardt C. C., Romero I. A., Cancello R., Camoin L., Strosberg A. D. (2001) Mol. Cell. Endocrinol. 175, 81–92 [DOI] [PubMed] [Google Scholar]
- 29.Sartipy P., Loskutoff D. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 7265–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing H., Northrop J. P., Grove J. R., Kilpatrick K. E., Su J. L., Ringold G. M. (1997) Endocrinology 138, 2776–2783 [DOI] [PubMed] [Google Scholar]
- 31.Ohsumi J., Sakakibara S., Yamaguchi J., Miyadai K., Yoshioka S., Fujiwara T., Horikoshi H., Serizawa N. (1994) Endocrinology 135, 2279–2282 [DOI] [PubMed] [Google Scholar]
- 32.Howard J. K., Flier J. S. (2006) Trends Endocrinol. Metab. 17, 365–371 [DOI] [PubMed] [Google Scholar]
- 33.Strissel K. J., Stancheva Z., Miyoshi H., Perfield J. W., 2nd, DeFuria J., Jick Z., Greenberg A. S., Obin M. S. (2007) Diabetes 56, 2910–2918 [DOI] [PubMed] [Google Scholar]
- 34.Hevener A. L., Olefsky J. M., Reichart D., Nguyen M. T., Bandyopadyhay G., Leung H. Y., Watt M. J., Benner C., Febbraio M. A., Nguyen A. K., Folian B., Subramaniam S., Gonzalez F. J., Glass C. K., Ricote M. (2007) J. Clin. Invest. 117, 1658–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen M. T., Favelyukis S., Nguyen A. K., Reichart D., Scott P. A., Jenn A., Liu-Bryan R., Glass C. K., Neels J. G., Olefsky J. M. (2007) J. Biol. Chem. 282, 35279–35292 [DOI] [PubMed] [Google Scholar]
- 36.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr. (2003) J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patsouris D., Li P. P., Thapar D., Chapman J., Olefsky J. M., Neels J. G. (2008) Cell Metab. 8, 301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruun J. M., Lihn A. S., Madan A. K., Pedersen S. B., Schiøtt K. M., Fain J. N., Richelsen B. (2004) Am. J. Physiol. Endocrinol. Metab. 286, E8–13 [DOI] [PubMed] [Google Scholar]
- 39.Addison C. L., Daniel T. O., Burdick M. D., Liu H., Ehlert J. E., Xue Y. Y., Buechi L., Walz A., Richmond A., Strieter R. M. (2000) J. Immunol. 165, 5269–5277 [DOI] [PubMed] [Google Scholar]
- 40.Chavey C., Lazennec G., Lagarrigue S., Clapé C., Iankova I., Teyssier J., Annicotte J. S., Schmidt J., Mataki C., Yamamoto H., Sanches R., Guma A., Stich V., Vitkova M., Jardin-Watelet B., Renard E., Strieter R., Tuthill A., Hotamisligil G. S., Vidal-Puig A., Zorzano A., Langin D., Fajas L. (2009) Cell Metab. 9, 339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harkins J. M., Moustaid-Moussa N., Chung Y. J., Penner K. M., Pestka J. J., North C. M., Claycombe K. J. (2004) J. Nutr. 134, 2673–2677 [DOI] [PubMed] [Google Scholar]
- 42.Elgazar-Carmon V., Rudich A., Hadad N., Levy R. (2008) J. Lipid Res. 49, 1894–1903 [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi Y. (2006) Crit. Rev. Immunol. 26, 307–316 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.