Abstract
We have investigated in detail the role of intra-organelle Ca2+ content during induction of apoptosis by the oxidant menadione while changing and monitoring the Ca2+ load of endoplasmic reticulum (ER), mitochondria, and acidic organelles. Menadione causes production of reactive oxygen species, induction of oxidative stress, and subsequently apoptosis. In both pancreatic acinar and pancreatic tumor AR42J cells, menadione was found to induce repetitive cytosolic Ca2+ responses because of the release of Ca2+ from both ER and acidic stores. Ca2+ responses to menadione were accompanied by elevation of Ca2+ in mitochondria, mitochondrial depolarization, and mitochondrial permeability transition pore (mPTP) opening. Emptying of both the ER and acidic Ca2+ stores did not necessarily prevent menadione-induced apoptosis. High mitochondrial Ca2+ at the time of menadione application was the major factor determining cell fate. However, if mitochondria were prevented from loading with Ca2+ with 10 μm RU360, then caspase-9 activation did not occur irrespective of the content of other Ca2+ stores. These results were confirmed by ratiometric measurements of intramitochondrial Ca2+ with pericam. We conclude that elevated Ca2+ in mitochondria is the crucial factor in determining whether cells undergo oxidative stress-induced apoptosis.
Apoptosis, a mechanism of programmed cell death, usually occurs through intrinsic or extrinsic apoptotic pathways. The caspases involved in apoptosis can be split into two groups, the initiator caspases such as caspase-9 and effector caspases such as caspase-3. Effector caspases are activated by initiator caspases and mediate many of the morphological cellular changes associated with apoptosis (1).
Calcium is an important signaling ion involved in the regulation of many physiological as well as pathological cellular responses (2). In the pancreas, we have shown that Ca2+ signals elicit enzyme secretion (3), apoptosis (4–6), and pathological intracellular activation of digestive enzymes (7). As such, there must be mechanisms in place by which the cell can differentiate between apoptotic and non-apoptotic Ca2+ signals.
The spatiotemporal pattern of calcium signaling is crucial for the specificity of cellular responses. For example, repetitive cytosolic calcium spikes confined to the apical region of the pancreatic acinar cell are elicited by physiological stimulation with acetylcholine (ACh) or cholecystokinin (CCK) and result in physiological secretion of zymogen granules (8, 9). However, a sustained global increase in free cytosolic Ca2+ induced by supramaximal stimulation with CCK, which resembles prolonged hyperstimulation of pancreatic acinar cells in the pathophysiology of acute pancreatitis, can lead to premature activation of digestive enzymes and vacuole formation within the cell (10–12). Alternatively, global repetitive calcium spikes induced in the pancreatic acinar cell in response to oxidant stress can lead to induction of the mitochondrial permeability transition pore (mPTP)4 and apoptosis (4, 5, 13).
To understand the role of calcium in apoptosis, several investigators have examined the influence of intracellular stores on the molding of calcium signals that lead to cell death (14–16). It has been well established in a range of cell types that the endoplasmic reticulum (ER) is the major intracellular calcium store required for induction of apoptosis. Pinton et al. (17) have shown that decreasing ER Ca2+ concentration with tBuBHQ increased HeLa cell survival in response to oxidant stress induced by ceramide. Scorrano and Korsmeyer (18) also observed that double knock-out Bax and Bak (pro-apoptotic proteins) mouse fibroblasts displayed a reduced resting concentration of ER Ca2+ compared with wild type and were resistant to induction of apoptosis by various stimulants, including ceramide. These important studies strongly suggest that the concentration of Ca2+ in the ER is a critical determinant of cellular susceptibility to apoptotic stimuli in the cell types studied.
A key event in early apoptosis is permeabilization of the mitochondrial membrane. The mPTP is a pore whose molecular composition is still debated (19). Activation of an open pore state can result in swelling of the mitochondrial matrix and release of the apoptogenic proteins from the intermembrane space (20).
One important activator of the mPTP is Ca2+ (20–22), a function which implicates Ca2+ in the initiation of apoptosis (23, 24). Once Ca2+ is released from the ER into the cytoplasm, mitochondria take up part of the released Ca2+ to prevent propagation of large calcium waves (25–27). This influx is followed by calcium efflux from the mitochondria back into the cytosol (28, 29). An increase in mitochondrial Ca2+ concentration in response to physiological stimuli induces increased activity of the mitochondrial respiratory chain and the synthesis of ATP to meet with increasing energy demands on the cell. When mitochondria are exposed to a pathological overload of calcium, opening of the mPTP is triggered, leading to mitochondrial dysfunction and eventually cell death. The mechanism through which calcium can trigger mPTP opening is still unclear and may involve cyclophilin D (30) and voltage-dependent anion channel (31). The mitochondria are endowed with selective and efficient calcium uptake (a calcium-selective uniporter) and release mechanisms (Ca2+/Na exchanger, Ca2+/H+ exchanger, and mPTP) (16, 29, 32, 33).
Oxidant stress is a well known inducer of apoptosis in several cell types (34) and is thought to play an important role in the pathogenesis of acute pancreatitis (35). We have used the quinone compound menadione to induce oxidative stress in the pancreatic acinar cell. Menadione is metabolized by flavoprotein reductase to semiquinone and then is oxidized back to quinone, resulting in generation of superoxide anion radicals, hydrogen peroxide, and other reactive oxygen species (ROS) (36). In vivo, menadione causes depolarization and swelling of the mitochondria (37). In pancreatic acinar cells, treatment with menadione not only produces an increase in ROS, but has also been found to evoke cytosolic Ca2+ responses, mPTP opening, activation of caspases and apoptotic cell death (4, 5). When cells were pretreated with the calcium chelator BAPTA-AM, menadione was unable to induce apoptosis, indicating that oxidant stress-induced apoptosis in the pancreatic acinar cell is highly calcium-dependent. Here we show that in pancreatic acinar cells, oxidative stress-induced apoptosis is strongly dependent on the Ca2+ concentration within mitochondria at the time of ROS production.
EXPERIMENTAL PROCEDURES
Pancreatic Acinar Cell Preparation
Male CD1 mice were sacrificed by cervical dislocation (in accordance with the Animal (Scientific Procedure) Act, 1986), and the pancreas was excised. Single or small clusters of acinar cells were isolated as previously described (38). The isolated cells were washed by centrifugation in a standard buffer solution (140 mm NaCl, 1.13 mm MgCl2, 1 mm CaCl2, 4.7 mm KCl, 10 mm glucose, 10 mm HEPES, pH 7.2). All experiments were performed at room temperature (23–25 °C), and cells were used within 3–4 h after isolation.
Tissue Culture
AR42J cells were maintained in RPMI 1640 plus 10 mm HEPES, 10% fetal calf serum, 2.5 μg/ml fungizone, and 5 μg/ml gentamycin, at 37 °C 5% CO2 in 25-cm3 flasks. Cells were detached using trypsin (5% trypsin + 0.53 mm EDTA), and split 1 in 10 once a week. For microscopy, cells were seeded into 5-cm3 glass bottom plates and allowed to attach for 24 h. Cell differentiation was then induced by incubation with 50 nm dexamethazone for 24–48 h.
Ca2+ Measurements
To measure changes in cytosolic Ca2+, isolated pancreatic acinar cells were loaded with Fluo-4-AM (2.5 μm) at room temperature for 30 min. The cells were then washed and resuspended in calcium-free buffer solution. Measurements were conducted as described earlier (8, 38). AR42J cells were loaded with 10 μm Fluo-4, AM for 40 min at 37 °C. Cells were washed and bathed in standard buffer solution. Fluorescence was imaged over time using a Leica confocal microscope (Leica Microsystems, Wetzlar, Germany) with an Ar/ArKr laser at 488 nm and a 488/543/637 dichroic mirror). Dual staining measurements were performed using a line by line protocol, which effectively excludes the possibility of a cross-talk between indicators (39). Ca2+ measurements using fluorescent indicators have been discussed in detail previously (Refs. 4, 40, for review see Ref. 41).
To measure changes in mitochondrial Ca2+, cells were loaded with Rhod2, AM (5 μm) for 40 min at 37 °C. Cells were then washed and resuspended in calcium-free buffer solution. Fluorescence was imaged over time using confocal microscopy (excitation 543 nm, emission >560 nm) as described previously (8). Mitochondrial Ca2+ changes were also monitored in cells transfected with the ratiometric probe 2mt8 mitochondrial calcium-sensitive pericam (42).
Lipofectamine 2000 Transfection of AR42J Cells
The cells were transfected with 1 μg of DNA after complex formation with Lipofectamine 2000 (Invitrogen) using a ratio of 1:3 according to the manufacturer's protocol. Medium was then removed after 24 h and replaced with fresh medium containing 50 nm dexamethazone and incubated for a further 24 h. Cells were imaged on Olympus IX71 inverted microscope using a TILL-Photonic Polychrome V monochromator.
Amaxa Nucleofection of Isolated Pancreatic Acinar Cells
Acinar cells were isolated in standard buffer solution as described earlier under “Pancreatic Acinar Cell Preparation” with addition of MEM amino acids (Invitrogen), 0.005% trypsin inhibitor (Sigma), chick embryo extract (US Biological), and 100 units/ml Pen/Strep/Fungizone (Biosera, Ringmer) adjusted to pH 7.3. Cells were resuspended in 100 μl of Nucleofection solution and transfected according to the manufacturer's protocol (Amaxa, Cologne, Germany). Cells were incubated at 30 °C for 20 h to allow expression of pericam. Fluorescence was measured using an Olympus IX71 inverted microscope with TILL-Photonic Polychrome V monochromator (excitation 415 & 470 nm, emission >500 nm).
Measurements of Changes in Mitochondrial Membrane Potential
Isolated pancreatic acinar cells were loaded with 100 nm TMRM at 37 °C for 20 min. Cells were then washed and resuspended in calcium-free buffer solution. AR42J cells were loaded with 25 nm TMRM at 37 °C for 5 min and then washed with standard buffer solution. Confocal microscopy was used to image fluorescence (excitation 543 nm, emission 560–700 nm).
Depleting Intracellular Ca2+ Stores
To deplete ER calcium stores in pancreatic acinar cells SERCA inhibitor, thapsigargin (TG) was applied for fast (with 10 μm) or slow depletion (usually 30 nm followed by 10 μm thapsigargin). In AR42J cells slow depletion was achieved by application of 200 nm followed by 10 μm thapsigargin.
Measurements of Apoptosis
Apoptosis was assessed by measuring caspase activation. Isolated pancreatic acinar cells were washed and suspended in calcium-free buffer solution (140 mm NaCl, 1.13 mm MgCl2, 4.7 mm KCl, 10 mm glucose, 2 mm EDTA, 10 mm HEPES, pH 7.2). Cells were then loaded with fluorescent indictor-linked substrates for activated caspase-9 (10 μm, Z-LEHD-R110), caspase-8 (10 μm, Z-IETD-R110), or general caspases (10 μm, R110-aspartic acid amide) at room temperature for 20 min. After loading, cells were washed and resuspended in standard or calcium-free buffer. The isolated cells were then placed on a confocal microscope stage, and fluorescence was imaged over time (excitation 488 nm, emission 505–543 nm).
Chemicals
Lipofectamine 2000, caspase substrates, and fluorescent dyes were from Invitrogen and thapsigargin from TOCRIS Biosciences (Bristol, UK). All other chemicals were from Sigma.
Statistics
Data are presented as means ± S.E. of the mean of whole cell fluorescence. The one-way analysis of variance test was used for statistical comparison between control and treatment groups. p < 0.05 was considered significant.
RESULTS
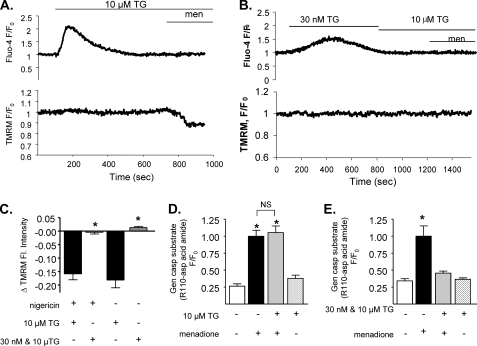
Our previous data strongly indicate that Ca2+ is required for menadione-induced apoptosis in pancreatic acinar cells (4–6). However, the role of intracellular Ca2+ stores in the process has not been assessed. The ER is the major intracellular Ca2+ store and has been suggested to play a major role in the induction of apoptosis in several cell types; therefore, the involvement of ER Ca2+ store in menadione-induced apoptosis was examined first. To deplete ER Ca2+ stores, isolated pancreatic acinar cells were treated with thapsigargin, a SERCA pump inhibitor. Cells were dual-loaded with the cytosolic Ca2+ indicator Fluo-4-AM and the mitochondrial membrane potential indicator TMRM and then treated with a high dose of thapsigargin (10 μm) in the absence of external Ca2+ (43). Fig. 1 A shows that 10 μm thapsigargin caused, as expected, a sharp and transient increase in cytosolic Ca2+; when the cells were then challenged with menadione, mitochondria membrane depolarized. The extent of depolarization caused by menadione under these conditions was only about ∼20% of that induced by treatment with the uncoupler CCCP. However, if the cells were treated with a low dose of thapsigargin (30 nm) to allow a slow emptying of ER Ca2+ stores (38) (Fig. 1B), a smaller and more gradual increase in cytosolic Ca2+ was observed. 30 nm thapsigargin did indeed cause a complete depletion of ER Ca2+, since a subsequent addition of 10 μm thapsigargin did not further increase cytosolic [Ca2+]. Mitochondria need a high level of cytosolic Ca2+ to load effectively (15) and therefore should be prevented by slow emptying Ca2+ stores. When menadione was added after thapsigargin, no mitochondrial depolarization was observed. Fig. 1C shows a summary of mitochondrial depolarization induced by menadione after a high dose of thapsigargin as compared with a low dose (Δfluorescence = −0.182 ± 0.029, n = 8 compared with = 0.013 ± 0.005, n = 5, p < 0.00012). Addition of nigericin to deplete acidic store (44) did not change the outcome of thapsigargin experiments (Fig. 1C). Thus mitochondrial depolarization is only dependent on mitochondrial Ca2+ and not on the ER or acidic store content of Ca2+. Activation of apoptosis was then assessed by measuring caspase activation (using a fluorescent substrate) under conditions used in Fig. 1, A and C. A similar increase in relative fluorescence was observed (1.055 ± 0.099, n = 16 and 1.0 ± 0.088, n = 14, p > 0.69, Fig. 1D) regardless of the treatment of cells with or without 10 μm thapsigargin (Fig. 1D). A high concentration of thapsigargin alone was not able to induce caspase activation (fluorescence 0.264 ± 0.035, n = 13 for control and 0.380 ± 0.045, n = 14 for 10 μm thapsigargin only, p = 0.05). Of importance, in cells pretreated with 30 nm followed by 10 μm thapsigargin, menadione caused only minor caspase activation compared with cells treated with menadione alone, despite ER stores being empty (Fig. 1, B and C). In fact, fluorescence of the caspase substrate was 0.342 ± 0.031 (n = 16) in control, untreated cells, 1.0 ± 0.153 (n = 18) in cells treated with menadione only and 0.457 ± 0.028 (n = 17) in cells treated with 30 nm + 10 μm thapsigargin + menadione. Fluorescence levels in cells treated only with 30 nm + 10 μm thapsigargin were indistinguishable 0.365 ± 0.022, n = 15, from those of controls (Fig. 1E, p < 0.0019 for menadione versus 30 nm + 10 μm thapsigargin + menadione). Together, these results demonstrate that menadione induces apoptosis by mechanisms not directly dependent on ER Ca2+ and suggests that other mechanisms determine whether menadione induces apoptosis in pancreatic acinar cells.
FIGURE 1.
A sharp increase in cytosolic calcium is required for induction of apoptosis in acinar cells. Isolated mouse pancreatic acinar cells were loaded with 100 nm TMRM-AM and 2.5 μm Fluo-4-AM (A and B). Fluorescence was measured over time before and after treatment with 30 μm menadione in the presence of 10 μm thapsigargin (A) or 30 nm + 10 μm thapsigargin (B). Changes of TMRM fluorescence after menadione treatment in different calcium store depletion conditions were compared (C). Separate groups of isolated cells were incubated with general caspase substrate, and cells were treated with 10 μm thapsigargin (D) or 30 nm and subsequent 10 μm thapsigargin (E) before application of 30 μm menadione (mean ± S.E., *, p < 0.05).
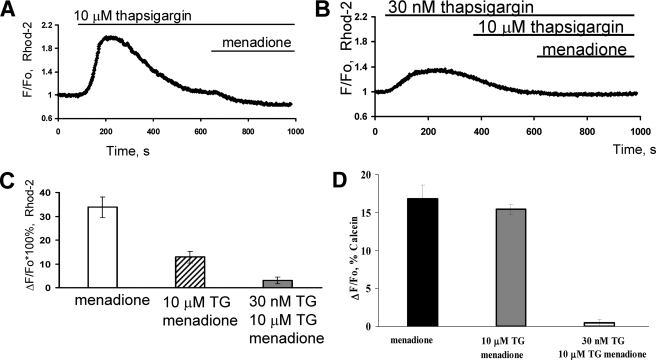
Mitochondria are known to play a significant role in calcium signaling during induction of apoptosis. Therefore, we studied mitochondrial Ca2+ levels in pancreatic acinar cells to determine the role of mitochondria in oxidative stress-induced apoptosis. Cells were loaded with the mitochondria-specific Ca2+ indicator Rhod-2. When cells were treated with 10 μm thapsigargin, there was a large, sharp, and transient increase in mitochondrial Ca2+ concentration (Fig. 2A). The level of Ca2+ within mitochondria remained elevated for more than 10 min after thapsigargin application. Indeed when menadione was applied ∼10 min after thapsigargin, a decrease in Rhod-2 fluorescence was observed, which indicates an efflux of Ca2+ from the mitochondria (Fig. 2A, n = 6). However, in cells treated with 30 nm thapsigargin (followed by 10 μm thapsigargin) the amplitude of the mitochondrial Ca2+ transient was much lower. Crucially, no efflux of Ca2+ from mitochondria in response to menadione was observed (Fig. 2B, n = 5). When the amplitudes of menadione-induced Ca2+ responses were compared, the decrease in Rhod-2 fluorescence was significantly greater (p < 0.0063) in cells treated with 10 μm thapsigargin (12.8%±2.4, n = 6) as compared with cells treated sequentially with 30 nm followed by 10 μm thapsigargin (3.0%±1.3, n = 5) (Fig. 2C).
FIGURE 2.
Menadione can induce release of calcium from mitochondria of acinar cells due to mPTP. Isolated mouse pancreatic acinar cells were loaded with Rhod 2-AM (A–C) or Calcein AM (D). Fluorescence was measured over time before and after treatment with menadione in the presence of 10 μm thapsigargin (A) or 30 nm thapsigargin followed by 10 μm thapsigargin (B). A comparison of amplitudes of changes of Rhod 2 fluorescence after menadione treatment under A and B conditions is shown in C (mean ± S.E., *, p < 0.05). D, fluorescence of calcein was quenched with CoCl2 in all intracellular organelles except mitochondria. Menadione induces mPTP as shown previously (8). Pretreatment for 10 min with high dose (10 μm) thapsigargin did not change the mPTP opening in response to menadione. However, pretreatment with low dose (30 nm) and subsequent high (10 μm) thapsigargin prevented mPTP induction by menadione (mean ± S.E., *, p < 0.05).
To show induction of the mPTP, we used the fluorescent probe calcein loaded together with cobalt (8), measuring calcein fluorescence exclusively from mitochondria under these conditions (Fig. 2D). Menadione induced mPTP and subsequently reduced fluorescence (16.8 + 1.8%, n = 5) of calcein to allow either calcein efflux or Co2+ influx as shown in pancreatic acinar cells previously (8). Pretreatment with high dose (10 μm) thapsigargin for 10 min did not change significantly the mPTP opening in response to menadione (15.4 + 0.7, n = 5, p > 0.1). However, pretreatment with low dose (30 nm) and subsequent high (10 μm) thapsigargin effectively abolished mPTP induction by menadione (0.5 + 0.2, n = 5, p < 0.05, Fig. 2D).
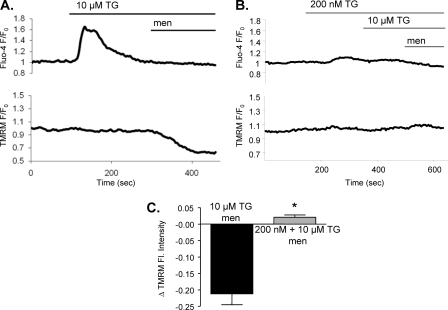
To confirm the Rhod-2 results shown above we transfected cells with mitochondria-specific Ca2+ sensitive pericam (41). The results obtained with the mitochondrial pericam were essentially similar to those with Rhod-2. However, transfection with pericam is highly inefficient in freshly isolated acinar cells and the number of positive cells extremely low. To bypass these problems, we have used the AR42J cell line. First, experiments depicted in Fig. 1 were repeated using AR42J cells loaded with Fluo4 and TMRM (Fig. 3 and supplemental Fig. S1A). Effects of the low and high doses of thapsigargin on cytosolic Ca2+ and TMRM fluorescence reported above for the freshly isolated acinar cells were also observed in AR42J cells. In particular, as shown in Fig. 3, A and C addition of 10 μm thapsigargin induced a sharp and large increase in cytosolic Ca2+ concentration and after menadione application, a substantial mitochondrial membrane depolarization (Δfluorescence = −0.212 ± 0.033, n = 6) (Fig. 3, A and C). The other protocol (200 nm followed by 10 μm of thapsigargin) caused a small and slow cytosolic Ca2+ increase and no depolarization as opposed to fast Ca2+ release data (n = 6, Fig. 3, B and C).
FIGURE 3.
AR42J cells also require sharp increases in cytosolic calcium for induction of apoptosis. AR42J cells were loaded with 25 nm TMRM-AM and 10 μm Fluo-4-AM (A and B). Fluorescence was measured over time before and after application of 30 μm menadione in cells pretreated with 10 μm thapsigargin (A, n = 6) or 200 nm thapsigargin followed by 10 μm thapsigargin (B, n = 6). Changes in TMRM fluorescence after menadione treatment were compared in C (mean ± S.E., *, p < 0.05).
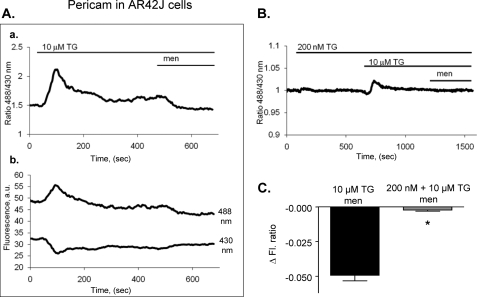
As shown in Fig. 4A, a high dose of thapsigargin showed a large, sharp and transient influx of Ca2+ into the mitochondria. When cells were then treated with menadione, a substantial decrease in mitochondrial Ca2+ was observed (Fig. 4A). Vice versa, when cells were first exposed to low dose of thapsigargin (before high dose), much smaller and slower Ca2+ influx was observed, and the Ca2+ release in response to menadione was practically abolished (Fig. 4B). On average, the change in ratio of pericam fluorescence shows a more significant decrease in fluorescence of cells treated with menadione in the presence of a high dose of thapsigargin (Δratio fluorescence −0.081 ± 0.021, n = 8) as compared with the cells challenged with low dose followed by high dose of thapsigargin (Δratio fluorescence −0.005 ± 0.006, n = 12, p < 0.0002, Fig. 4C). These experiments were also performed in pancreatic acinar cells expressing pericam using a nucleofection technique with similar results (supplemental Fig. S1, B and C). The change in ratio of pericam fluorescence after menadione shows a noticeable decrease in the presence of a high dose of thapsigargin (Δratio fluorescence −0.081 ± 0.021, n = 8) as compared with the absence of change after low dose followed by high dose of thapsigargin (Δratio fluorescence −0.005 ± 0.006, n = 12, p < 0.0002).
FIGURE 4.
Menadione can induce release of calcium from mitochondria of AR42J cells (pericam measurements). AR42J cells were transfected with fluorescent mitochondrial ratiometric calcium pericam. Fluorescence was measured over time before and after menadione in cells pretreated for 10 min with 10 μm thapsigargin (A) or for 10 min with 200 nm and subsequently with 10 μm thapsigargin (B). The ratio of pericam fluorescence is shown in Aa and B, while original traces of pericam fluorescence (488 nm and 430 nm excitation) are shown in Ab. The change in fluorescence after menadione treatment under each condition (A and B) was compared (C) (mean ± S.E., n = 8–11 per group, *, p < 0.05).
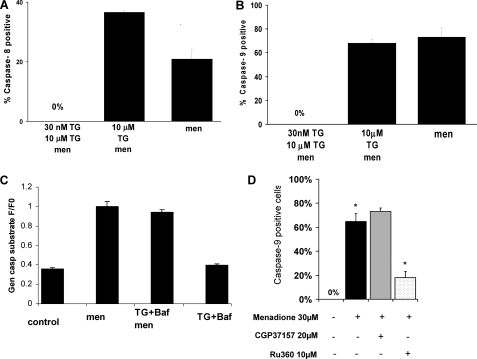
Our next goal was to investigate activation of the initiator caspases-8 and -9 under different Ca2+ store emptying conditions (Fig. 5, A and B). Menadione has induced activation of caspase-9 in the majority of cells (73 + 7% of cells, Fig. 5B) and activation of caspase-8 (21 + 3% of cells, Fig. 5A) in some cells, as we have reported previously (5). Emptying of ER with 10 μm thapsigargin did not significantly alter the activation of caspase-9 by menadione (68 + 3% of cells) but increased activation of caspase-8 (36 + 1% of cells). However, slow emptying of ER with 30 nm thapsigargin and subsequent 10 μm thapsigargin completely blocked activation of both caspase-8 and -9 (0 cells in both cases).
FIGURE 5.
Caspase-8 and -9 activation by menadione with different ways of emptying calcium stores in pancreatic acinar cells is shown. Caspase-8 (A) and -9 (B) activation by menadione when calcium content of ER was reduced slowly (30 nm thapsigargin followed by 10 μm thapsigargin) or quickly (10 μm thapsigargin) or with 100 nm Bafilomycin A1. Bars represent percent of apoptotic cells. C shows general caspase substrate controls in the presence of thapsigargin and Bafilomycin A1. D shows that caspase-9 activation is significantly inhibited by the mitochondrial uniporter inhibitor Ru360.
Previously we have reported lysosomal-related apoptosis induced by menadione in a proportion of pancreatic acinar cells (5). Bafilomycin A1 is a known inhibitor vacuolar H+ ATPase and has been extensively used to disrupt the acidic gradient (45) and deplete acidic Ca2+ stores (43). However, Bafilomycin A1 did not block activation of caspases by menadione or menadione after TG (Fig. 5C) (4, 5).
To confirm our conclusion that high concentrations of Ca2+ in mitochondria are required for the induction of apoptosis by menadione, we have used two pharmacological agents to modulate mitochondrial Ca2+: CGP37157, an inhibitor of mitochondrial Na+/Ca2+ exchanger to increase mitochondrial matrix Ca2+ and Ru360, an inhibitor of mitochondrial Ca2+ uptake to reduce mitochondrial matrix Ca2+. Menadione induced activation of caspase-9 in 64.7 + 6.7% cells (n = 5) as compared with control (0%, n = 6). CGP37157 insignificantly increased an already high percentage of apoptotic cells after menadione (73.1 + 2.8%, n = 4). However, caspase-9 activation was substantially inhibited by mitochondrial uniporter inhibitor Ru360 (18 + 5.2%, n = 4, p < 0.05, Fig. 5D).
DISCUSSION
In this study, mouse pancreatic acinar cells and AR42J cells were used to investigate the role of different Ca2+ stores in oxidant-induced apoptosis. Previous studies have shown that menadione induces apoptosis in both acinar and AR42J cells (4, 46). Inhibition of apoptosis by ROS scavengers (6) confirms that menadione-induced apoptosis is highly dependent on oxidants. In AR42J cells, a study has shown oxidative stress caused by glucose/glucose oxidase induces apoptosis, implicating caspase-3 (47). Treatment with the oxidant menadione has been shown to activate within 30 min both caspase-9 and -8 and subsequently caspase-3 (4, 5) because of menadione-induced ROS production and calcium spikes in both acinar and AR42J cells. The crucial role of Ca2+ in menadione-induced apoptosis has been also demonstrated previously by inhibition of the induction of apoptosis with strong cytosolic Ca2+ chelator BAPTA (4). Freshly isolated mouse pancreatic acinar cells are the ideal model for in vitro studies of the role of different organelles in physiological and pathological calcium signaling (39, 40, 43). Indeed, these cells maintain the typical morphological characteristics of acinar cell in the intact organ, i.e. high basal-to-apical polarity. However, freshly isolated pancreatic acinar cells are difficult to transfect. For this reason, we have mainly used the rat exocrine pancreatic tumor cell line AR42J, which maintains many of the characteristics of the normal cells, while easy to transfect.
We have investigated here the contribution of Ca2+ content in ER, acidic stores, and mitochondria in the regulation of menadione-induced apoptosis. Pancreatic acinar cells are highly polarized with the granular region containing two separate Ca2+ stores: extensions of the ER connected to the main ER store in the basal region and acidic Ca2+ stores (43). Disruption of acidic gradient, which has been shown to prevent Ca2+ release from acidic store (43, 44), did not affect induction of apoptosis by menadione. In fact emptying of both Ca2+ stores, ER with high doses of thapsigargin and acidic store with Bafilomycin A1 failed to block menadione-induced apoptosis (Fig. 5C). Menadione could induce Ca2+ entry into the cell, as we have shown previously (4); however, our experiments in this study were conducted in the nominal free calcium solution; therefore, we will not discuss this aspect here. Having ruled out both the ER and acidic stores as major Ca2+ players in the induction of oxidant-induced apoptosis we have to conclude that the mitochondria themselves could act as a temporary Ca2+ store.
In acinar cells, in contrast to ACh-evoked Ca2+ release, menadione-induced Ca2+ response is accompanied by partial mitochondrial depolarization (4). This effect has been also replicated in AR42J cells. We have also shown that reduction in mitochondrial membrane potential in acinar cells is due to activation of the mPTP. These data have been confirmed in experiments with the mPTP inhibitor bongkrekic acid, because it completely eliminated menadione-stimulated mitochondrial depolarization (4, 5). Cytoplasmic calcium spikes induced by menadione leads to Ca2+ uptake into the mitochondria followed by mPTP opening (4), which requires both high Ca2+ in mitochondria and oxidants, which in turn is followed by a subsequent Ca2+ release from the mitochondria (Figs. 2–4).
To investigate the role of mitochondrial Ca2+ in the induction of apoptosis, we have used two independent experimental approaches: the mitochondrial Ca2+-sensitive probe Rhod-2 and the Ca2+-sensitive mitochondria-targeted GFP-based indicator 2mt8 ratiometric pericam (42). High doses of TG have been routinely used by our laboratory to completely empty ER in pancreatic acinar cells (43). Treatment with high doses of TG resulted in Ca2+-loading of mitochondria in both acinar and AR42J cells (Figs. 2 and 3) as revealed by either Rhod 2 or pericam. This effect reflects the ability of mitochondria to accumulate Ca2+ released from the stores (26). In both acinar and AR42J cells, subsequent addition of menadione (after TG) resulted in a decrease in mitochondrial Ca2+, indicating that menadione can stimulate the release of Ca2+ from the mitochondria. This suggests that after a high dose of thapsigargin, mitochondria can hold residual Ca2+ for a very long time (tens of minutes). It is at present unclear why mitochondria do not efficiently release this residual Ca2+ load, despite cytosolic Ca2+ concentration returning to basal levels. One possibility is that after massive Ca2+ uptake, part of this Ca2+ forms precipitates with phosphate in the matrix that slowly dissolve (and thus continue to supply Ca2+). Indeed when the ER was slowly emptied (using low doses of TG), the overall Ca2+ uptake by mitochondria was reduced, and in particular the prolonged increase of mitochondrial Ca2+ was abolished. Under these latter conditions, menadione-dependent mitochondrial depolarization and mPTP opening were prevented (Fig. 3B). These observations suggest that mitochondrial depolarization and mPTP are closely linked. The data here presented are consistent with previous observations suggesting that activation of mitochondria-dependent apoptosis requires a double hit, one by Ca2+ and the second by another toxic agent, e.g. in our experiments ROS production by menadione. Accordingly, a Ca2+ rise within mitochondria alone (without depolarization) is beneficial, ROS production is weakly toxic, but a strong synergism for apoptosis is provided by the simultaneous combination, of ROS production and mitochondrial Ca2+ elevation. In other words, mitochondria function as “coincidence detectors” capable of translating into an apoptotic signal the production of ROS only if their matrix Ca2+ is elevated (17, 22, 48). We have confirmed our observations also pharmacologically, i.e. inhibitor of mitochondrial uniporter Ru360 substantially inhibited apoptosis induction measured with caspase-9 substrate (Fig. 5D). Inhibitor of Na/Ca2+ exchanger CGP37157 only slightly increased the percentage of apoptotic cells; however, we have to take into account that the rate of menadione-induced apoptosis was already very high.
Our data show that the crucial calcium requirement for ROS-induced apoptosis is the elevated Ca2+ in mitochondria. By regulating mitochondrial Ca2+, irrespective of endoplasmic reticulum Ca2+ (or acidic store content), it is possible to influence cell fate, i.e. potentiate or inhibit apoptosis. This possibility has important implications for the apoptosis-related pathology, i.e. pancreatitis (49) with downstream events that include ROS production (50), calcium toxicity (51), and mitochondrial damage, all contributing to acinar cell death (52, 53). These studies suggest that apoptosis plays a protective role in the development of acute pancreatitis (13, 54–56). Manipulation of mitochondrial Ca2+ should be a much easier task for designing pro-apoptotic and anti-apoptotic drugs instead of drastic changes of the calcium store content.
Supplementary Material
This work was supported in part by Medical Research Council (MRC) Cooperative Grant G0300076 and MRC Programme Grant G8801575 and by a Wellcome Trust Prize Ph.D. studentship (to P. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- mPTP
- mitochondrial permeability transition pore
- ER
- endoplasmic reticulum
- ROS
- reactive oxygen species
- TG
- thapsigargin.
REFERENCES
- 1.Thornberry N. A., Lazebnik Y. (1998) Science 281, 1312–1316 [DOI] [PubMed] [Google Scholar]
- 2.Berridge M. J., Lipp P., Bootman M. D. (2000) Nat. Rev. Mol. Cell Biol. 1, 11–21 [DOI] [PubMed] [Google Scholar]
- 3.Huang Y., Fischer J. E., Balasubramaniam A. (1996) Peptides 17, 497–502 [DOI] [PubMed] [Google Scholar]
- 4.Gerasimenko J. V., Gerasimenko O. V., Palejwala A., Tepikin A. V., Petersen O. H., Watson A. J. M. (2002) J. Cell Science 115, 485–497 [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner H. K., Gerasimenko J. V., Thorne C., Ashurst L. H., Barrow S. L., Chvanov M. A., Gillies S., Criddle D. N., Tepikin A. V., Petersen O. H., Sutton R., Watson A. J., Gerasimenko O. V. (2007) Am. J. Physiol. Gastrointest. Liver Physiol. 293, G296–G307 [DOI] [PubMed] [Google Scholar]
- 6.Criddle D. N., Gillies S., Baumgartner-Wilson H. K., Jaffar M., Chinje E. C., Passmore S., Chvanov M., Barrow S., Gerasimenko O. V., Tepikin A. V., Sutton R., Petersen O. H. (2006) J. Biol. Chem. 281, 40485–40492 [DOI] [PubMed] [Google Scholar]
- 7.Saluja A. K., Bhagat L., Lee H. S., Bhatia M., Frossard J. L., Steer M. L. (1999) Am. J. Physiol.-Gastrointestinal Liver Physiol. 276, 835–842 [Google Scholar]
- 8.Maruyama Y., Petersen O. H. (1994) Cell Calcium 16, 419–430 [DOI] [PubMed] [Google Scholar]
- 9.Petersen O. H., Tepikin A., Park M. K. (2001) Trends Neurosci. 24, 271–276 [DOI] [PubMed] [Google Scholar]
- 10.Parekh A. B. (2000) Proc. Natl. Acad. Sci. U. S. A. 97, 12933–12934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raraty M., Ward J., Erdemli G., Vaillant C., Neoptolemos J. P., Sutton R., Petersen O. H. (2000) Proc. Natl. Acad. Sci. U. S. A. 97, 13126–13131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherwood M. W., Prior I. A., Voronina S. G., Barrow S. L., Woodsmith J. D., Gerasimenko O. V., Petersen O. H., Tepikin A. V. (2007) Proc. Natl. Acad. Sci. U. S. A. 104, 5674–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Criddle D. N., Gerasimenko J. V., Baumgartner H. K., Jaffar M., Voronina S., Sutton R., Petersen O. H., Gerasimenko O. V. (2007) Cell Death Differ. 14, 1285–1294 [DOI] [PubMed] [Google Scholar]
- 14.Hanson C. J., Bootman M. D., Roderick H. L. (2004) Curr. Biol. 14, R933–R935 [DOI] [PubMed] [Google Scholar]
- 15.Szabadkai G., Rizzuto R. (2004) FEBS Lett. 567, 111–115 [DOI] [PubMed] [Google Scholar]
- 16.Pinton P., Rizzuto R. (2006) Cell Death Diff. 13, 1409–1418 [DOI] [PubMed] [Google Scholar]
- 17.Pinton P., Ferrari D., Rapizzi E., Di Virgilio F., Pozzan T., Rizzuto R. (2001) EMBO J. 20, 2690–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scorrano L., Korsmeyer S. J. (2003) Biochem. Biophys. Res. Commun. 304, 437–444 [DOI] [PubMed] [Google Scholar]
- 19.Crompton M. (1999) Biochem. J. 341, 233–249 [PMC free article] [PubMed] [Google Scholar]
- 20.Martinou J. C., Desagher S., Antonsson B. (2000) Nat. Cell Biol. 2, E41–E43 [DOI] [PubMed] [Google Scholar]
- 21.Rizzuto R., Pozzan T. (2006) Physiol. Rev. 86, 369–408 [DOI] [PubMed] [Google Scholar]
- 22.Giacomello M., Drago I., Pizzo P., Pozzan T. (2007) Cell Death Differ. 14, 1267–1274 [DOI] [PubMed] [Google Scholar]
- 23.Hajnóczky G., Csordás G., Das S., Garcia-Perez C., Saotome M., Sinha, Roy S., Yi M. (2006) Cell Calcium 40, 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csordás G., Renken C., Várnai P., Walter L., Weaver D., Buttle K. F., Balla T., Mannella C. A., Hajnóczky G. (2006) J. Cell Biol. 174, 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jouaville L. S., Ichas F., Holmuhamedov E. L., Camacho P., Lechleiter J. D. (1995) Nature 377, 438–441 [DOI] [PubMed] [Google Scholar]
- 26.Tinel H., Cancela J. M., Mogami H., Gerasimenko J. V., Gerasimenko O. V., Tepikin A. V., Petersen O. H. (1999) EMBO J. 18, 4999–5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boitier E., Rea R., Duchen M. R. (1999) J. Cell Biol. 145, 795–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzuto R., Brini M., Murgia M., Pozzan T. (1993) Science 262, 744–747 [DOI] [PubMed] [Google Scholar]
- 29.Ichas F., Jouaville L. S., Mazat J. P. (1997) Cell 89, 1145–1153 [DOI] [PubMed] [Google Scholar]
- 30.Basso E., Fante L., Fowlkes J., Petronilli V., Forte M. A., Bernardi P. (2005) J. Biol. Chem. 280, 18558–18561 [DOI] [PubMed] [Google Scholar]
- 31.Schlattner U., Dolder M., Wallimann T., Tokarska-Schlattner M. (2001) J. Biol. Chem. 276, 48027–48030 [DOI] [PubMed] [Google Scholar]
- 32.Putney J. W., Jr., Thomas A. P. (2006) Curr. Biol. 16, R812–R815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian X., Ma X., Qiao D., Ma A., Yan F., Huang X. (2005) Mol. Cell. Biochem. 277, 33–42 [DOI] [PubMed] [Google Scholar]
- 34.Orrenius S., Gogvadze V., Zhivotovsky B. (2007) Annu. Rev. Pharmacol. Toxicol. 47, 143–183 [DOI] [PubMed] [Google Scholar]
- 35.Cao Y., Adhikari S., Clément M. V., Wallig M., Bhatia M. (2007) Am. J. Pathol. 170, 1521–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monks T. J., Hanzlik R. P., Cohen G. M., Ross D., Graham D. G. (1992) Toxicol. Appl. Pharmacol. 112, 2–16 [DOI] [PubMed] [Google Scholar]
- 37.Saxena K., Henry T. R., Solem L. E., Wallace K. B. (1995) Arch. Biochem. Biophys. 317, 79–84 [DOI] [PubMed] [Google Scholar]
- 38.Thorn P., Lawrie A. M., Smith P. M., Gallacher D. V., Petersen O. H. (1993) Cell 74, 661–668 [DOI] [PubMed] [Google Scholar]
- 39.Dolman N. J., Gerasimenko J. V., Gerasimenko O. V., Voronina S. G., Petersen O. H., Tepikin A. V. (2005) J. Biol. Chem. 280, 15794–15799 [DOI] [PubMed] [Google Scholar]
- 40.Gerasimenko O. V., Gerasimenko J. V., Rizzuto R. R., Treiman M., Tepikin A. V., Petersen O. H. (2002) Cell Calcium 32, 261–268 [DOI] [PubMed] [Google Scholar]
- 41.Gerasimenko O., Tepikin A. (2005) Cell Calcium 38, 201–211 [DOI] [PubMed] [Google Scholar]
- 42.Filippin L., Abad M. C., Gastaldello S., Magalhães P. J., Sandonà D., Pozzan T. (2005) Cell Calcium 37, 129–136 [DOI] [PubMed] [Google Scholar]
- 43.Gerasimenko J. V., Sherwood M., Tepikin A. V., Petersen O. H., Gerasimenko O. V. (2006) J. Cell Sci. 119, 226–238 [DOI] [PubMed] [Google Scholar]
- 44.Gerasimenko J. V., Flowerdew S. E., Voronina S. G., Sukhomlin T. K., Tepikin A. V., Petersen O. H., Gerasimenko O. V. (2006) J. Biol. Chem. 281, 40154–40163 [DOI] [PubMed] [Google Scholar]
- 45.Camello-Almaraz C., Pariente J. A., Salido G., Camello P. J. (2000) Biochem. Biophys. Res. Commun. 271, 311–317 [DOI] [PubMed] [Google Scholar]
- 46.Sata N., Klonowski-Stumpe H., Han B., Häussinger D., Niederau C. (1997) Free Radic. Biol. Med. 23, 844–850 [DOI] [PubMed] [Google Scholar]
- 47.Song J. Y., Lim J. W., Kim H., Morio T., Kim K. H. (2003) J. Biol. Chem. 278, 36676–36687 [DOI] [PubMed] [Google Scholar]
- 48.Szalai G., Krishnamurthy R., Hajnóczky G. (1999) EMBO J. 18, 6349–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhatia M., Brady M., Shokuhi S., Christmas S., Neoptolemos J. P., Slavin J. (2000) J. Pathol. 190, 117–125 [DOI] [PubMed] [Google Scholar]
- 50.Sweiry J. H., Mann G. E. (1996) Scand. J. Gastroenterol. Suppl. 219, 10–15 [DOI] [PubMed] [Google Scholar]
- 51.Ward J. B., Petersen O. H., Jenkins S. A., Sutton R. (1995) Lancet 346, 1016–1019 [DOI] [PubMed] [Google Scholar]
- 52.Bhatia M. (2004) Am. J. Physiol. Gastrointest. Liver Physiol. 286, G189–196 [DOI] [PubMed] [Google Scholar]
- 53.Mareninova O. A., Sung K. F., Hong P., Lugea A., Pandol S. J., Gukovsky I., Gukovskaya A. S. (2006) J. Biol. Chem. 281, 3370–3381 [DOI] [PubMed] [Google Scholar]
- 54.Hahm K. B., Kim J. H., You B. M., Kim Y. S., Cho S. W., Yim H., Ahn B. O., Kim W. B. (1998) Pancreas 17, 153–157 [DOI] [PubMed] [Google Scholar]
- 55.Bhatia M., Wallig M. A., Hofbauer B., Lee H. S., Frossard J. L., Steer M. L., Saluja A. K. (1998) Biochem. Biophys. Res. Commun. 246, 476–483 [DOI] [PubMed] [Google Scholar]
- 56.Lampel M., Kern H. F. (1977) Virchows Arch. A. Pathol. Anat. Histol. 373, 97–117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.