Abstract
In Alzheimer disease (AD) and frontotemporal dementia the microtubule-associated protein Tau becomes progressively hyperphosphorylated, eventually forming aggregates. However, how Tau dysfunction is associated with functional impairment is only partly understood, especially at early stages when Tau is mislocalized but has not yet formed aggregates. Impaired axonal transport has been proposed as a potential pathomechanism, based on cellular Tau models and Tau transgenic mice. We recently reported K369I mutant Tau transgenic K3 mice with axonal transport defects that suggested a cargo-selective impairment of kinesin-driven anterograde transport by Tau. Here, we show that kinesin motor complex formation is disturbed in the K3 mice. We show that under pathological conditions hyperphosphorylated Tau interacts with c-Jun N-terminal kinase- interacting protein 1 (JIP1), which is associated with the kinesin motor protein complex. As a result, transport of JIP1 into the axon is impaired, causing JIP1 to accumulate in the cell body. Because we found trapping of JIP1 and a pathological Tau/JIP1 interaction also in AD brain, this may have pathomechanistic implications in diseases with a Tau pathology. This is supported by JIP1 sequestration in the cell body of Tau-transfected primary neuronal cultures. The pathological Tau/JIP1 interaction requires phosphorylation of Tau, and Tau competes with the physiological binding of JIP1 to kinesin light chain. Because JIP1 is involved in regulating cargo binding to kinesin motors, our findings may, at least in part, explain how hyperphosphorylated Tau mediates impaired axonal transport in AD and frontotemporal dementia.
The microtubule-associated protein Tau is predominantly found in the axonal compartment of neurons, where it binds to microtubules (1). In human brain, six isoforms of Tau are expressed, due to alternative splicing of exons 2, 3 and 10 (2). Tau consists of an amino-terminal projection domain followed by 3 or 4 microtubule binding repeats (3R or 4R), due to splicing of exon 10, and a carboxyl-terminal tail region. In the AD3 and FTD brain, Tau forms filamentous inclusions (3). They are found in nerve cell bodies and apical dendrites as neurofibrillary tangles (NFTs), in distal dendrites as neuropil threads, and in the abnormal neurites that are associated with some amyloid plaques (neuritic plaques) (3). Hyperphosphorylation of Tau is thought to be an initiating step (4), as it detaches Tau from microtubules and makes it prone to form aggregates (1, 5). Whereas in AD no mutations have been identified in the MAPT gene encoding Tau, so far 42 intronic and exonic mutations have been found in familial forms of FTD (6). Their identification assisted in the generation of transgenic mouse models that reproduce NFT formation and memory impairment (7).
The models were also instrumental in testing hypotheses that had been brought forward to link Tau pathology to functional impairment (8–10). In particular, defects in axonal transport have been implicated in neurodegenerative disorders (11, 12). Tau binding to microtubules affects axonal transport (13), and in cell culture overexpression of Tau was shown to lead to impaired transport of mitochondria and vesicles (14, 15). Axonal transport defects have also been reproduced in wild-type Tau transgenic mice (16) and in K369I mutant Tau K3 mice (17), whereas Tau expression failed to inhibit axonal transport in other systems (18, 19). This apparent discrepancy may depend on the type of cargos analyzed and, specifically, the experimental paradigm, e.g. using phosphorylated (16, 17, 20) versus non-phosphorylated Tau (18).
To dissect Tau-mediated axonal transport defects at a molecular level, we used K3 mice that overexpress human Tau carrying the pathogenic FTD K369I mutation (17). We observed a pronounced hyperphosphorylation of transgenic Tau in many brain areas. Clinically, the mice present with an early onset motor phenotype that is, at least in part, caused by impairment of axonal transport in neurons of the substantia nigra. Interestingly, only selected aspects of anterograde axonal transport were impaired, in particular those of kinesin-I motor complex-driven vesicles and mitochondria. Our data suggest a selective impairment of axonal transport rather than a generalized, non-selective blockage of microtubules that has been established in cell culture systems, which fail to phosphorylate Tau at the high levels that are found in vivo even under physiological conditions. More importantly, in AD and FTD Tau is even more phosphorylated, i.e. hyperphosphorylated at physiological sites and de novo at pathological sites, preventing it from binding to microtubules (1).
Based on our findings of an impaired kinesin-I-driven axonal transport in the K3 mice, we speculated that hyperphosphorylated Tau may impair anterograde transport by interfering directly with components of the kinesin-I motor complex rather than disrupting the binding of the kinesin heavy chain (see below) to microtubules. Axonal transport along microtubules is mediated by members of the kinesin superfamily (KIF) of motor proteins (21–23). The KIFs typically consist of an ATPase domain that interacts with microtubules and drives movement and a domain that links to cargos, either directly or indirectly, as in the case of KIF5, by assembling with the kinesin light chain (KLC) to form the kinesin-I (KIF5/KLC) motor complex (24). In addition, increasing evidence suggests that scaffolding proteins mediate and regulate the binding of cargos to KIFs (21, 25–27). These include the scaffold protein JNK-interacting protein (JIP) that is involved in the linkage of cargos to the kinesin-I motor complex via KLC (25, 28–33).
Here, by using the K3 mouse model, we identified a novel interaction of Tau and JIP in neurons that causes a trapping of JNK interacting protein 1 (JIP1) in the cell body of K3 mice, cell culture systems, and human AD brain. We found that the pathological interaction of hyperphosphorylated Tau and JIP1 competes with the physiological binding of JIP1 to KLC.
EXPERIMENTAL PROCEDURES
Mice
The generation of K3 mice expressing K369I mutant human Tau has been described (17). Animal experiments were approved by the Animal Ethics Committee of the University of Sydney, Australia.
Histology and Immunohistochemistry
Ketamine/xylazine (Troy Laboratories)-anesthetized mice were perfused with 20 ml of cold phosphate-buffered saline followed by 20 ml of 4% paraformaldehyde (Sigma), and tissue was dissected and post-fixed overnight at 4 °C. Tissue embedding was done in paraffin in a Shandon Excelsior processor (Thermo). Human AD and control tissue was obtained through the Australian Brain Bank Program as described (10). Immunohistochemistry on 3-μm sections has been described in detail before (34). Primary antibodies to Tau phosphorylated at Thr-231/Ser-235 (AT180 (Pierce)) and to JIP1 (Zymed Laboratories Inc.) were visualized using the ABC Elite Kit (Vector) or Alexa-labeled secondary antibodies (Molecular Probes). For subsequent NFT staining, coverslips were removed after taking fluorescence images followed by Gallyas silver staining using a standard protocol (35). Overlays were made using landmarks.
Western Blotting and Immunoprecipitation
Proteins were extracted from brain tissues in radioimmunoprecipitation assay buffer (50 mm Tris, pH 8.0, 150 mm sodium chloride, 1% Nonidet P-40, 5 mm EDTA, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (all Sigma)) containing Complete protease inhibitors (Roche Applied Science). Protein concentration was determined with the Dc-Protein assay (Bio-Rad) to ensure equal gel loading. For Western blotting (36) we used primary antibodies for Tau (Tau-5), KLC, kinesin heavy chain (Kif5B), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), JIP1 (all Santa Cruz), JIP1 (Zymed Laboratories Inc.), V5 and Myc (Invitrogen), Tau (DAKO), Tau phosphorylated at Ser-202/Thr-205 (AT8), at Thr-231/Ser-235 (AT180) and at Thr-181 (AT270) (all Pierce) and at Ser-396/Ser-404 (PHF-1) (Peter Davies), and non-phosphorylated Tau (TAU1) together with alkaline phosphatase-coupled secondary antibodies (Sigma), developed with the ImmunStar substrate (Bio-Rad) in a VersaDoc 4000 detection system (Bio-Rad). Quantification of bands was performed with the Quantity One software (Bio-Rad) and normalized to GAPDH expression.
Immunoprecipitation (IP) was performed as described previously (36). Briefly, tissue was lysed with a glass Dounce homogenizer in buffer containing 20 mm Tris-HCl, pH 7.5, 150 mm sodium chloride, 1% Triton X-100 (Sigma) and Complete Protease inhibitor (Roche Applied Science). After a 1-h preincubation with bovine serum albumin (Sigma)-saturated protein G-coupled beats (Pierce), extracts were incubated with antibodies overnight at 4 °C. Antibodies were subsequently precipitated with bovine serum albumin-saturated protein G-coupled beads that were then washed with buffers of increasing sodium chloride concentration (150–400 mm). The precipitate was recovered from the beads by boiling in hot sample buffer and separated by SDS-PAGE. IP of cultured cells were carried out in 50 mm HEPES, pH 7.5, 140 mm NaCl, 0.2% Nonidet P-40 substitute (Sigma) using protein G-coupled magnetic beads (Invitrogen).
Expression Vectors
JIP1, KLC, and Tau encoding full-length cDNA was amplified from a human cDNA library using Pfu Ultra polymerase (Stratagene), cloned into TOPO gateway entry vectors (Invitrogen), and sequenced. Subsequently, the coding sequence was transferred into Myc, V5, or His6 expression vectors using the LR clonase reaction according to the manual (Invitrogen).
Pulldown Experiments
Pulldown experiments were carried out with His6-tagged recombinant proteins using the TALON metal affinity resin (Clontech) following the manufacturer's instructions. Briefly, carboxyl-terminal His6-V5-tagged proteins were expressed in BL21-AI E. coli and purified after lysis by sonication in immunoprecipitation buffer. Tau was phosphorylated in vitro as described previously (37). Subsequently, the resin-bound proteins were incubated with extracts from wild-type mouse brains overnight at 4 °C. The columns were then washed with buffers of increasing sodium chloride concentration (150–400 mm). The precipitate was recovered from the resin by boiling hot sample buffer and separated by SDS-PAGE.
Cell Culture and Immunofluorescence
All cell culture media and reagents were supplied by Invitrogen unless stated otherwise. COS7 cells maintained in Dulbecco's modified Eagle's medium/F-12 containing 10% fetal bovine serum (Hyclone) and transfected as previously described (36). Cells were incubated with okadaic acid (OA) in culture medium for 10 h at 37 °C, 5%CO2.
Primary hippocampal neurons were cultured following an established protocol (38, 39). Primary hippocampal neurons were transfected by calcium/DNA precipitation as described before (40).
For immunofluorescence staining, coverslips were removed after 7 days and fixed with 4% paraformaldehyde in 80 mm PIPES, 1 mm MgCl2, and 1 mm EGTA, pH 6.8. Cells were permeabilized with 0.1% Triton in phosphate-buffered saline and stained with antibodies to V5 and JIP1.
Quantification of Immunofluorescence Staining
Immunofluorescence intensity was measured on images taken with an IX81-X microscope (Olympus, Japan). Images were background-subtracted and analyzed for fluorescence intensity using the MetaMorph 6.1 software (Molecular Devices).
Statistics
Statistical analysis was done with the Prizm 4 for Windows software (GraphPad Software) using Student's t test. All values are given as the means ± S.E.
RESULTS
Disturbed Interaction of JIP1 with Kinesin-I Motor Complexes in K3 Mice
Tau overexpression in vitro (41) and in wild-type human Tau (16, 42, 43) and K369I mutant Tau transgenic K3 mice (17) results in axonal transport defects. In cell culture and, in particular, in K3 brains, transport of distinct kinesin-driven cargos is impaired in the presence of transgenic Tau, whereas transport of other cargos remains unaffected (17, 19, 41). These observations raised the question of whether Tau would interfere with components of the axonal transport machinery directly. Several of the cargos whose transport is affected by Tau expression are driven by the kinesin-I motor complex. This complex is formed by the kinesin heavy chain (KIF5; previously termed KHC), which interacts with microtubules, and kinesin light chain (KLC), which mediates cargo linkage. In addition, scaffolding proteins are involved such as the JIP1 that binds to KLC (21). Hence, we questioned whether transgenic Tau expression in K3 mice would interfere with the integrity of the kinesin-I complex and thereby cause impaired axonal transport (17).
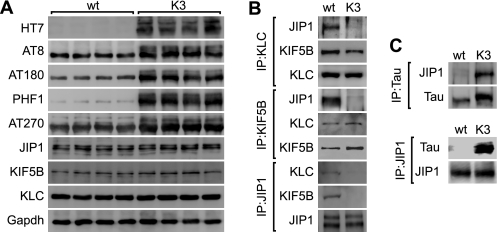
To address this, we performed a series of co-immunoprecipitation experiments from K3 compared with wild-type control brain extracts using antibodies to KIF5B, KLC, and JIP1. Transgenic expression of K369I mutant human Tau resulted in increased levels of phosphorylated Tau, as revealed by Western blot analysis of wild-type and K3 brain extracts (Fig. 1A). Protein levels of KIF5B, KLC, and JIP1, however, were comparable in wild-type and K3 mice (Fig. 1A). Intact kinesin-I complexes were present in wild-type brain extracts, as revealed by co-immunoprecipitation of KIF5B and JIP1 with an antibody to KLC, of KLC and JIP1 with an antibody to KIF5B, and of KIF5B and KLC with an antibody to JIP1 (Fig. 1B). Similarly, from K3 brain extracts, KLC co-immunoprecipitated together with KIF5B using antibodies to KLC and KIF5B, respectively. Interestingly, and different from wild-type brain, hardly any JIP1 co-immunoprecipitated from K3 brain extracts using either antibodies to KLC or KIF5B, and neither KLC nor KIF5B co-immunoprecipitated when an antibody to JIP1 was used. Taken together, KIF5B·KLC·JIP1 complexes exist in wild-type brain, whereas in K3 brain, JIP1 appears to be excluded from KIF5B·KLC complexes.
FIGURE 1.
Disturbed interaction of JIP1 with the kinesin-I motor complex in K3 mice. A, K369I mutant human Tau expressing K3 mice (HT7) phosphorylate Tau at multiple sites, including AT8, AT180, PHF-1, and AT270, as shown for 4-month-old mice compared with wild-type (wt). Despite axonal transport defects in K3 mice (17), protein levels of the motor proteins kinesin heavy chain (KIF5B), KLC, and JIP1 are not altered. GAPDH served as control for equal loading. Brain extracts from four different mice per group were analyzed. B, although an intact JIP1·KLC·KIF5B complex could be immunoprecipitated from wild-type mouse brain extracts with either an antibody against JIP1, KLC, or KIF5B, in K3 brain only the KLC/KIF5B interaction could be revealed, with no JIP1 in the complex. Representative blots from at least three independent experiments are shown. C, IP of Tau from K3 and wild-type brain extracts reveals a Tau/JIP1 interaction in K3 and not wild-type brain. Similarly, IP with a JIP1 antibody co-precipitates Tau from K3 but not wild-type mouse brain. Representative blots from three experiments are shown.
Tau/JIP1 Interaction in K3 Mice
The exclusion of JIP1 from KIF5B·KLC complexes in K3 brain raised the question of whether a reduced kinesin/JIP1 interaction would be a secondary effect of transgenic Tau expression or whether Tau would directly interfere with complex formation. To address the latter possibility, we performed additional co-immunoprecipitation experiments with antibodies to JIP1 and total Tau using wild-type and K3 brain extracts. Neither did JIP1 co-immunoprecipitate with an antibody to Tau, nor did Tau co-precipitate with an antibody to JIP1 using wild-type brain extracts (Fig. 1C). However, using the same experimental conditions, JIP1 co-immunoprecipitated from K3 brain extracts using an antibody to Tau. As a substantial amount of JIP1 was co-immunoprecipitated, this suggests a strong Tau/Jip-1 interaction. Thus, the lack of kinesin/JIP1 interaction occurs together with an aberrant Tau/JIP1 interaction in K3 brains.
Altered Sequestration of JIP1 in K3 and AD Brains
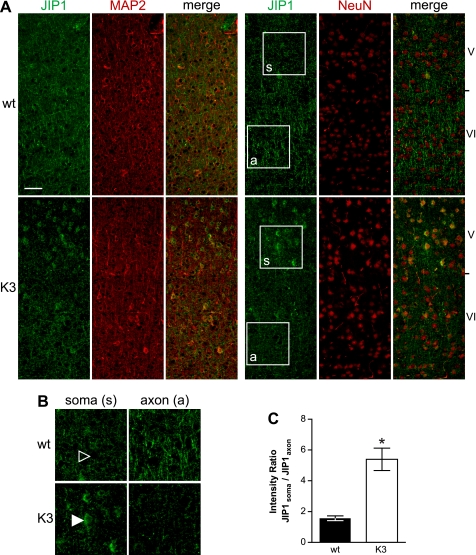
As the reduced interaction of JIP1 with kinesin motors may alter the subcellular localization of JIP1, we compared its distribution on brain sections of K3 and wild-type mice by immunohistochemistry (Fig. 2A). In wild-type cortex, JIP1 immunohistochemistry revealed a pre-dominantly axonal staining. In contrast, cortical K3 neurons showed a pronounced staining of cell bodies (soma), whereas their axons hardly stained, as revealed by co-immunofluorescence staining for JIP1 and the neuronal markers microtubule-associated protein 2 and neuronal nuclei. Quantification revealed a 3.2-fold increased soma:axon ratio for JIP1 in K3 compared with wild-type cortex (Fig. 2, B and C). Thus, JIP1 is retained in cell bodies of cortical K3 neurons.
FIGURE 2.
Tau-dependent re-distribution of JIP1 in K3 neurons. A, immunohistochemistry of sagittal brain sections reveals a predominantly axonal staining of JIP1 (green) in wild-type mice, with little staining of neuronal cell bodies. In contrast, JIP1 accumulates in the cell body of cortical neurons of K3 mice, whereas axons hardly contain JIP1. Neurons were counter-stained with microtubule-associated protein 2 (MAP2) or neuronal nuclei (NeuN) (red). Scale bar, 100 μm. B, higher magnification (boxes in A) of layer V neuronal cell bodies (soma (s)) and their axons (a) passing through neuronal layer VI. JIP1 accumulates in K3 neuronal cell bodies (arrowhead), whereas they are hardly stainable in wild-type (wt) neurons. C, thus, the ratio of somatic versus axonal JIP1 is significantly increased in K3 mice, indicating trapping of JIP1 in the cell body (*, p < 0.0001).
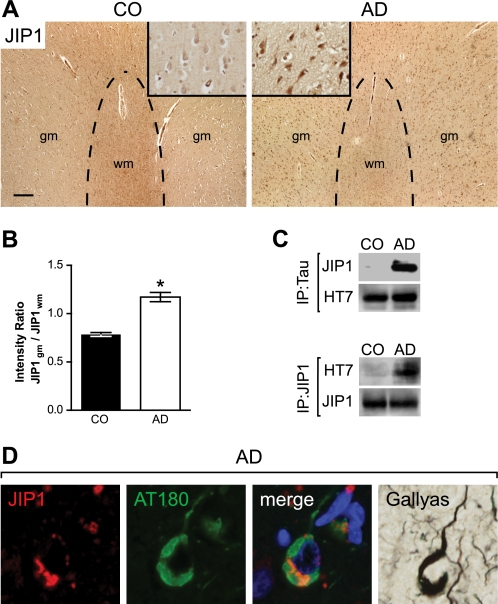
In AD, Tau is known to become hyperphosphorylated and redistributed from the axon to the somato-dendritic compartment. We questioned whether Tau redistribution in AD brain would also be associated with a somatic retention of JIP1, as has been observed by us in K3 mice. Therefore, we quantified JIP1 staining of AD and control human brain sections, as we had done for mice. We found that in temporal cortex of AD, and not control brain, neuronal cell bodies showed a pronounced JIP1 staining (Fig. 3A), whereas reactivity in the axons of the white matter within the same brain area was markedly reduced, resulting in an increased gray matter to white matter ratio of JIP1 in AD compared with controls (Fig. 3B).
FIGURE 3.
Pathological re-distribution of JIP1 in AD brain. A, low magnification images of JIP1-stained (brown) sections from the temporal cortex of control (CO) brains show a low signal in gray matter (gm) (inset at higher magnification) and intensive staining of axons in the white matter (wm). In contrast, the white matter of AD tissue sections stains less for JIP1, whereas gray matter neurons (inset) show intensive JIP1 staining. Scale bar, 250 μm. B, quantification reveals a re-distribution of JIP1 from the axon to the soma, as shown by an increased ratio of somatic versus axonal staining (*, p < 0.0001). C, JIP1 co-immunoprecipitates with Tau from human AD but not control (CO) brain extracts. Similarly, IP using a JIP1 antibody co-precipitates Tau from AD but not CO brains. Representative blots from three experiments are shown. D, JIP1 (red)/AT180 (green) co-immunofluorescence staining shows co-localization (merge) in NFTs, which stain with Gallyas silver.
Next, we addressed Tau-JIP1 complex formation in AD compared with human control brains. As done before for the mouse brains, we performed co-immunoprecipitation experiments with antibodies to JIP1 and Tau using cortical AD and control brain extracts. Both precipitations revealed the presence of Tau-JIP1 complexes in AD, but not control, brain (Fig. 3C). In addition, we found that JIP1 co-localized with phosphorylated Tau in NFTs using JIP1/AT180 co-immunofluorescence staining with subsequent visualization of NFTs by Gallyas silver impregnations (Fig. 3D). Taken together, we have shown that, first, Tau interacts with JIP1 in AD brain and, second, that JIP1 is retained in the soma of AD neurons.
Sequestration of JIP in Tau-expressing Primary Neurons
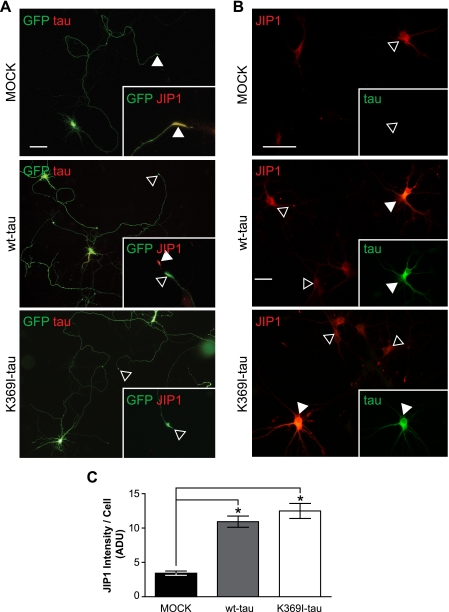
K3 mice express mutant Tau, whereas in AD Tau is not mutated. To address the role of the K369I mutation of Tau in the trapping of JIP1 in the neuronal soma, we transfected primary neuronal cultures with different expression constructs. We found that in mock-transfected hippocampal neurons, endogenous JIP1 showed an axonal distribution with an increased accumulation toward the axonal growth cones, as is typical for axon-transported proteins (Fig. 4A). We speculated that upon Tau transfection, the kinesin/JIP1 interaction would be disrupted, causing JIP1 to accumulate in the cell body and, hence, to be reduced in the growth cones. When we co-transfected primary hippocampal neurons with a green fluorescent protein marker for axonal tracing together with Tau, both in the presence and absence of the K369I mutation, JIP1 staining was decreased in the growth cone and accumulated in the cell body. Also, when we co-transfected Tau together with JIP1, this resulted in somatic retention of JIP1 (Fig. 4, B and C). Taken together, our data show that expression of Tau, irrespective of the presence of the K369I mutation, induces a redistribution and trapping of JIP1 in the cell body and, hence, a reduced localization to axons and the growth cone.
FIGURE 4.
Impaired axonal distribution of JIP1 is not caused by the K369I mutation of Tau. A, to determine whether the impaired axonal localization of JIP1 is due to the presence of K369I mutant Tau rather than elevated levels of hyperphosphorylated Tau per se, primary hippocampal neurons were transfected with MOCK, V5-wt-Tau, or V5-K369I mutant Tau together with green fluorescent protein (GFP) to visualize axonal tracing. In MOCK co-transfected neurons, JIP1 undergoes axonal transport and accumulates in growth cones (arrowheads; inset). In contrast, neurons co-transfected with V5-Tau (yellow merge) fail to accumulate JIP1 in growth cones (open arrowheads; inset). Note the intense JIP1 staining of the growth cone in untransfected neurons (arrowhead). Co-transfection of V5-K369I mutant Tau also results in a decreased JIP1 staining of growth cones. Scale bar, 50 μm. wt, wild type. B, co-transfection of primary hippocampal neurons with either V5-wt-Tau or V5-K369I mutant Tau together with Myc-JIP1 results in intensive JIP1 staining of the cell body (arrowheads), that is not found when the neurons are only transfected with the Myc-JIP1 construct (open arrowhead). Inset, staining for Tau. Scale bar, 50 μm. C, when staining is quantified, Myc-JIP1 fluorescence intensity shows increased levels of JIP1 in neurons that co-express either V5-wt-Tau or V5-K369I mutant Tau compared with co-transfection with an empty plasmid (MOCK; *, p < 0.0001). Thus, an increase of Tau in the cell body of neurons results in retention of JIP1, along with a decreased axonal distribution.
Hyperphosphorylation of Tau Is Needed for the Pathological Interaction of Tau with JIP1
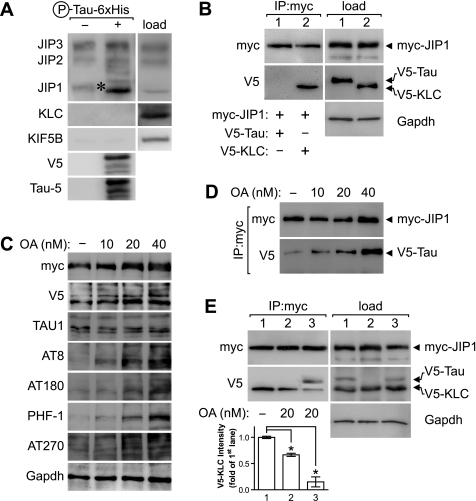
As we had identified Tau-JIP1 complexes in both K3 and AD but not wild-type murine and control human brains, we speculated that pathological changes in Tau, such as hyperphosphorylation, are needed for the pathological interaction of Tau with JIP1. This notion is supported by our finding that recombinant bacterial human Tau, whichis not phosphorylated, fails to pull down JIP1 from mouse brain extracts (data not shown). Only when recombinant Tau obtained from Escherichia coli was phosphorylated in vitro, it pulled down substantial amounts of JIP1 from brain extracts, providing for the first time experimental evidence that phosphorylation of Tau is required for its pathological interaction with JIP1 (Fig. 5A).
FIGURE 5.
Hyperphosphorylation of Tau is required for Tau to compete with KLC in the interaction with JIP1. A, recombinant hyperphosphorylated Tau carrying a carboxyl-terminal V5 and six-histidine tag (P-Tau-6×His) was used as bait to identify interaction partners of the kinesin-I-JIP complex from wild-type mouse brain extracts (load). JIP1 (asterisk) was pulled down with P-Tau-His6. Neither JIP2, JIP3, KLC, nor kinesin heavy chain (KIF5B) co-precipitated with P-Tau-His6. Recombinant Tau was visualized with V5 and Tau-5 antibodies. B, COS7 cells were co-transfected with Myc-JIP1 and V5-Tau (lane 1) or V5-KLC (lane 2). Immunoprecipitation with a Myc antibody precipitates JIP1 and co-precipitates V5-KLC but not V5-Tau. GAPDH served as loading control. C, treatment of COS7 cells that have been co-transfected with Myc-JIP1 and V5-Tau with increasing concentrations of the protein phosphatase inhibitor OA causes increased phosphorylation of Tau at multiple sites including AT8, AT180, PHF-1, and AT270. OA treatment did not affect Tau levels, as revealed by Tau-1. GAPDH served as control for equal loading. D, co-immunoprecipitation from OA-treated transfected cells (d) results in a dose-dependent increase of V5-Tau co-precipitated with Myc-JIP1. E, COS7 cells transfected with Myc-JIP1 and V5-KLC with (lanes 1 and 3) and without V5-Tau (lane 2) treated with 20 nm OA. OA impedes co-precipitation of V5-KLC with Myc-JIP1, most significantly in the presence of V5-Tau that then co-precipitates with Myc-JIP1. Quantification is shown for three independent experiments normalized for levels in the absence of OA (lane 1; *, p < 0.001).
Therefore, we performed co-immunoprecipitation experiments from transiently transfected COS7 cells. First, we expressed Myc-tagged JIP1 together with either V5-tagged Tau or KLC followed by immunoprecipitation with an anti-Myc antibody. As expected, KLC, but not Tau co-immunoprecipitated with JIP1 (Fig. 5B), as Tau in transfected COS7 cells is hardly at all phosphorylated (Fig. 5C). However, treatment of cells with the protein phosphatase inhibitor OA caused a dose-dependent hyperphosphorylation of Tau at multiple sites, including AT8, AT180, AT270, and PHF-1, without changing total Tau levels (Fig. 5C) (44). Therefore, we next performed a co-immunoprecipitation with an anti-Myc antibody from extracts of COS7 cells that had been transfected with Myc-tagged JIP1 and V5-tagged Tau and treated with increasing doses of OA. Supporting the notion that the Tau/JIP1 interaction depends on hyperphosphorylation of Tau, increasing amounts of Tau co-immunoprecipitated with JIP1 when increasing doses of OA were used (Fig. 5D). Thus, phosphorylation of Tau is needed for the interaction of Tau with JIP1.
Finally, we determined whether Tau can compete with KLC for the interaction with JIP1. Co-immunoprecipitation with an antibody to Myc from COS7 cells co-transfected with Myc-tagged JIP1 and V5-tagged KLC and Tau revealed co-precipitation of KLC·JIP1 but not Tau-JIP1 complexes (Fig. 5E, lane 1). OA treatment of cells that had been co-transfected with Myc-tagged JIP1 and V5-tagged KLC reduced the amount of co-immunoprecipitated KLC·JIP1 complexes (Fig. 5E, lane 2). However, co-expression of Tau together with Myc-tagged JIP1 and V5-tagged KLC resulted in a marked 85% reduction of co-immunoprecipitated KLC·JIP1 complexes upon OA treatment (Fig. 5E, lane 3). As expected, JIP1 co-precipitated Tau under these conditions. Taken together, our data reveal that hyperphosphorylated Tau competes with KLC for the interaction with JIP1.
DISCUSSION
In the present study, which combines the analysis of transgenic brain, human brain samples, primary cultures, and in vitro experiments, we found that hyperphosphorylated Tau disrupts the functional binding of JIP1 to the kinesin-I motor complex. We found that Tau pathologically interacts with JIP1 both in our K3 mouse model and in AD brain, causing a re-localization of JIP1 from axons to cell bodies. When Tau was expressed in primary hippocampal neurons, this also resulted in a decreased axonal localization concomitant with an accumulation of JIP1 in cell bodies. Finally, we showed that the Tau/JIP1 interaction is phosphorylation-dependent and that this pathologic interaction competes with the binding of JIP1 to KLC.
Regulation of axonal transport remains poorly understood, but it has been suggested recently that it may be regulated at the level of cargo binding to the motors (21, 26). Of the proteins involved in cargo binding, JIP1 has been found to interact specifically with KLC (29, 45). JIP is also a scaffolding protein for different components of the JNK signaling cascade, including JNK and its upstream kinases (46–48). JNK activation has been shown to regulate the kinesin/cargo binding by mediating the release of cargos once they have reached their final destination in the distal axon (26). Axonal transport defects in spinal and bulbar muscle atrophy have been suggested to result from increased JNK activity and subsequent phosphorylation of kinesin (27).
Impaired anterograde axonal transport has also been implicated in the pathogenesis of diseases such as AD (8). Many of these disorders are associated with Tau pathology. Thus, the aberrant interaction we have identified for phosphorylated Tau and the scaffold protein JIP1, whether direct or indirect, may explain, at least in part, impaired axonal transport. Mechanistically, we propose that the Tau/JIP1 interaction competes with the physiological binding of JIP1 to KLC. Hence, JIP1 fails in scaffolding regulatory kinases to the kinesin-I motor complex in axons. As a consequence, anterograde axonal transport of cargos specifically transported by the kinesin-I complex is impaired, eventually disturbing normal neuronal function. This pathomechanism may explain the clinical symptoms of Tau transgenic mice (17) and, possibly, neuronal dysfunction in AD and FTD.
Our in vivo finding that JIP1 fails to interact with KLC and is instead bound by phosphorylated Tau and retained in the cell body is consistent with an increased JIP1 staining in the soma of neurons in both K3 mice and in AD (49). Because JIP1 is also a scaffolding protein for several kinases, many of which phosphorylate Tau, it cannot be excluded that JIP1 mislocalization may also promote hyperphosphorylation of Tau in the soma, establishing a vicious cycle.
Although we cannot rule out that the Tau/JIP1 interaction may also have a physiological function in axonal transport, our data indicate that breaking up the aberrant Tau/JIP1 interaction may restore anterograde axonal transport and, hence, represent a novel approach in treating neurodegenerative diseases with Tau pathology.
Acknowledgments
We thank Dr. Stefan Kins for support and helpful comments, Dr. Thomas Fath for help with primary cultures, Dr. Nicole Schonrock for help with cloning, and Mian Bi and Fabien Delerue for technical assistance. We thank Dr. Peter Davies for antibodies.
This research was supported by grants from the University of Sydney, The Medical Foundation (University of Sydney), the National Health and Medical Research Council (NHMRC), the Judith Jane Mason and Harold Stannett Williams Memorial Foundation, the Australian Research Council (ARC), and the New South Wales Government through the Ministry for Science and Medical Research (BioFirst grant) (to J. G.) and by the NHMRC, the ARC, the University of Sydney, and the Deutsche Forschungsgemeinschaft (to L. M. I.).
- AD
- Alzheimer disease
- FTD
- frontotemporal dementia
- NFT
- neurofibrillary tangle
- KLC
- kinesin light chain
- JIP
- JNK-interacting protein
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- IP
- immunoprecipitation
- OA
- okadaic acid
- KIF
- kinesin superfamily
- JNK
- c-Jun NH2-terminal kinase
- PIPES
- 1,4-piperazinediethanesulfonic acid.
REFERENCES
- 1.Lee V. M., Goedert M., Trojanowski J. Q. (2001) Annu. Rev. Neurosci. 24, 1121–1159 [DOI] [PubMed] [Google Scholar]
- 2.Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. (1989) Neuron 3, 519–526 [DOI] [PubMed] [Google Scholar]
- 3.Goedert M., Wischik C. M., Crowther R. A., Walker J. E., Klug A. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 4051–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso A. C., Grundke-Iqbal I., Iqbal K. (1996) Nat Med 2, 783–787 [DOI] [PubMed] [Google Scholar]
- 5.Chen F., David D., Ferrari A., Götz J. (2004) Curr. Drug Targets 5, 503–515 [DOI] [PubMed] [Google Scholar]
- 6.Cruts M., Van Broeckhoven C. (2008) Trends Genet. 24, 186–194 [DOI] [PubMed] [Google Scholar]
- 7.Götz J., Ittner L. M. (2008) Nat. Rev. Neurosci. 9, 532–544 [DOI] [PubMed] [Google Scholar]
- 8.Götz J., Ittner L. M., Kins S. (2006) J. Neurochem. 98, 993–1006 [DOI] [PubMed] [Google Scholar]
- 9.David D. C., Hauptmann S., Scherping I., Schuessel K., Keil U., Rizzu P., Ravid R., Dröse S., Brandt U., Müller W. E., Eckert A., Götz J. (2005) J. Biol. Chem. 280, 23802–23814 [DOI] [PubMed] [Google Scholar]
- 10.David D. C., Ittner L. M., Gehrig P., Nergenau D., Shepherd C., Halliday G., Götz J. (2006) Proteomics 6, 6566–6577 [DOI] [PubMed] [Google Scholar]
- 11.Roy S., Zhang B., Lee V. M., Trojanowski J. Q. (2005) Acta Neuropathol. 109, 5–13 [DOI] [PubMed] [Google Scholar]
- 12.Stokin G. B., Goldstein L. S. (2006) Annu. Rev. Biochem. 75, 607–627 [DOI] [PubMed] [Google Scholar]
- 13.Dixit R., Ross J. L., Goldman Y. E., Holzbaur E. L. (2008) Science 319, 1086–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandelkow E. M., Thies E., Trinczek B., Biernat J., Mandelkow E. (2004) J. Cell Biol. 167, 99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baas P. W., Qiang L. (2005) Trends Cell Biol. 15, 183–187 [DOI] [PubMed] [Google Scholar]
- 16.Ishihara T., Hong M., Zhang B., Nakagawa Y., Lee M. K., Trojanowski J. Q., Lee V. M. (1999) Neuron 24, 751–762 [DOI] [PubMed] [Google Scholar]
- 17.Ittner L. M., Fath T., Ke Y. D., Bi M., van Eersel J., Li K. M., Gunning P., Götz J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15597–16002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morfini G., Pigino G., Mizuno N., Kikkawa M., Brady S. T. (2007) J. Neurosci. Res. 85, 2620–2630 [DOI] [PubMed] [Google Scholar]
- 19.Yuan A., Kumar A., Peterhoff C., Duff K., Nixon R. A. (2008) J. Neurosci. 28, 1682–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuchillo-Ibanez I., Seereeram A., Byers H. L., Leung K. Y., Ward M. A., Anderton B. H., Hanger D. P. (2008) FASEB J. 22, 3186–3195 [DOI] [PubMed] [Google Scholar]
- 21.Hirokawa N., Takemura R. (2005) Nat. Rev. Neurosci. 6, 201–214 [DOI] [PubMed] [Google Scholar]
- 22.Vale R. D., Reese T. S., Sheetz M. P. (1985) Cell 42, 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brady S. T. (1985) Nature 317, 73–75 [DOI] [PubMed] [Google Scholar]
- 24.Hirokawa N., Pfister K. K., Yorifuji H., Wagner M. C., Brady S. T., Bloom G. S. (1989) Cell 56, 867–878 [DOI] [PubMed] [Google Scholar]
- 25.Horiuchi D., Barkus R. V., Pilling A. D., Gassman A., Saxton W. M. (2005) Curr. Biol. 15, 2137–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horiuchi D., Collins C. A., Bhat P., Barkus R. V., Diantonio A., Saxton W. M. (2007) Curr. Biol. 17, 1313–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morfini G., Pigino G., Szebenyi G., You Y., Pollema S., Brady S. T. (2006) Nat. Neurosci. 9, 907–916 [DOI] [PubMed] [Google Scholar]
- 28.Inomata H., Nakamura Y., Hayakawa A., Takata H., Suzuki T., Miyazawa K., Kitamura N. (2003) J. Biol. Chem. 278, 22946–22955 [DOI] [PubMed] [Google Scholar]
- 29.Verhey K. J., Meyer D., Deehan R., Blenis J., Schnapp B. J., Rapoport T. A., Margolis B. (2001) J. Cell Biol. 152, 959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelkar N., Standen C. L., Davis R. J. (2005) Mol. Cell. Biol. 25, 2733–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowman A. B., Kamal A., Ritchings B. W., Philp A. V., McGrail M., Gindhart J. G., Goldstein L. S. (2000) Cell 103, 583–594 [DOI] [PubMed] [Google Scholar]
- 32.Matsuda S., Matsuda Y., D'Adamio L. (2003) J. Biol. Chem. 278, 38601–38606 [DOI] [PubMed] [Google Scholar]
- 33.Taru H., Iijima K., Hase M., Kirino Y., Yagi Y., Suzuki T. (2002) J. Biol. Chem. 277, 20070–20078 [DOI] [PubMed] [Google Scholar]
- 34.Ittner L. M., Wurdak H., Schwerdtfeger K., Kunz T., Ille F., Leveen P., Hjalt T. A., Suter U., Karlsson S., Hafezi F., Born W., Sommer L. (2005) J. Biol. 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Götz J., Chen F., van Dorpe J., Nitsch R. M. (2001) Science 293, 1491–1495 [DOI] [PubMed] [Google Scholar]
- 36.Ittner L. M., Koller D., Muff R., Fischer J. A., Born W. (2005) Biochemistry 44, 5749–5754 [DOI] [PubMed] [Google Scholar]
- 37.Goedert M., Spillantini M. G., Cairns N. J., Crowther R. A. (1992) Neuron 8, 159–168 [DOI] [PubMed] [Google Scholar]
- 38.Meberg P. J., Bamburg J. R. (2000) J. Neurosci. 20, 2459–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fath T., Ke Y. D., Gunning P., Götz J., Ittner L. M. (2009) Nat. Protoc. 4, 78–85 [DOI] [PubMed] [Google Scholar]
- 40.Köhrmann M., Haubensak W., Hemraj I., Kaether C., Lessmann V. J., Kiebler M. A. (1999) J. Neurosci. Res. 58, 831–835 [PubMed] [Google Scholar]
- 41.Ebneth A., Godemann R., Stamer K., Illenberger S., Trinczek B., Mandelkow E. (1998) J. Cell Biol. 143, 777–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Probst A., Götz J., Wiederhold K. H., Tolnay M., Mistl C., Jaton A. L., Hong M., Ishihara T., Lee V. M., Trojanowski J. Q., Jakes R., Crowther R. A., Spillantini M. G., Bürki K., Goedert M. (2000) Acta Neuropathol. 99, 469–481 [DOI] [PubMed] [Google Scholar]
- 43.Spittaels K., Van den Haute C., Van Dorpe J., Bruynseels K., Vandezande K., Laenen I., Geerts H., Mercken M., Sciot R., Van Lommel A., Loos R., Van Leuven F. (1999) Am. J. Pathol. 155, 2153–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merrick S. E., Trojanowski J. Q., Lee V. M. (1997) J. Neurosci. 17, 5726–5737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blasius T. L., Cai D., Jih G. T., Toret C. P., Verhey K. J. (2007) J. Cell Biol. 176, 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitmarsh A. J., Cavanagh J., Tournier C., Yasuda J., Davis R. J. (1998) Science 281, 1671–1674 [DOI] [PubMed] [Google Scholar]
- 47.Yasuda J., Whitmarsh A. J., Cavanagh J., Sharma M., Davis R. J. (1999) Mol. Cell. Biol. 19, 7245–7254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickens M., Rogers J. S., Cavanagh J., Raitano A., Xia Z., Halpern J. R., Greenberg M. E., Sawyers C. L., Davis R. J. (1997) Science 277, 693–696 [DOI] [PubMed] [Google Scholar]
- 49.Helbecque N., Abderrahamani A., Meylan L., Riederer B., Mooser V., Miklossy J., Delplanque J., Boutin P., Nicod P., Haefliger J. A., Cottel D., Amouyel P., Froguel P., Waeber G. (2003) Mol. Psychiatry 8, 413–422, 363 [DOI] [PubMed] [Google Scholar]