Abstract
Differentiation of erythroid cells requires precise control over the cell cycle to regulate the balance between cell proliferation and differentiation. The zinc finger transcription factor, erythroid Krüppel-like factor (EKLF/KLF1), is essential for proper erythroid cell differentiation and regulates many erythroid genes. Here we show that loss of EKLF leads to aberrant entry into S-phase of the cell cycle during both primitive and definitive erythropoiesis. This cell cycle defect was associated with a significant reduction in the expression levels of E2f2 and E2f4, key factors necessary for the induction of S-phase gene expression and erythropoiesis. We found and validated novel intronic enhancers in both the E2f2 and E2f4 genes, which contain conserved CACC, GATA, and E-BOX elements. The E2f2 enhancer was occupied by EKLF in vivo. Furthermore, we were able to partially restore cell cycle dynamics in EKLF−/− fetal liver upon additional genetic depletion of Rb, establishing a genetic causal link between reduced E2f2 and the EKLF cell cycle defect. Finally, we propose direct regulation of the E2f2 enhancer is a generic mechanism by which many KLFs regulate proliferation and differentiation.
Erythroid Kruppel-like factor (EKLF/KLF1),5 the founding member of the Kruppel-like factor (KLF) family of C2H2 zinc finger transcription factors, is essential for erythropoiesis (1–3). EKLF activates a diverse set of erythroid genes that include components of the erythroid membrane and cytoskeleton such as dematin (band 4.9) (4–6), heme synthesis enzymes, the erythroid chaperone α-hemoglobin stabilizing protein (7, 8), and other transcription factors such as basic Kruppel-like factor (BKLF/KLF3) and TIEG/KLF10 (9).
Erythropoiesis is a complex process that requires a coordinated balance between proliferation/self renewal and differentiation, a process tightly linked to cell cycle control (10). In particular, regulation of the G1/S checkpoint is essential for both terminal differentiation (G0 entry) and proliferation (S-phase entry) (10). Gene targeting of specific regulators of this checkpoint has shown some of these to be critical for erythropoiesis in mice. In particular, components of the Rb-E2F complex that controls S-phase entry are essential for proper erythropoiesis. Loss of a functional Retinoblastoma (Rb) gene in mice leads to defective erythropoiesis and death in utero before E16 (11–13). Furthermore, loss of both E2f2 and E2f4, which are expressed at high levels during erythroid differentiation, leads to the development of anemia in mice and a failure of proper erythroid expansion and maturation (14–16).
Transcriptional regulation of the E2f2 gene is not well understood, particularly in erythroid cells. There are E-BOX and E2f binding sites in the proximal promoter which can bind c-Myc and E2Fs in response to growth signals, but there are no conserved CACC box elements or SP1 binding sites (17). During the preparation of this manuscript, a study by Pilon et al. (18) suggested that the E2f2 promoter is bound by EKLF, resulting in gene activation. The erythroid-specific transcription factor GATA-1 has been shown to regulate the cell cycle by activation and repression of critical cell cycle control genes, including G1 cyclins and c-Myc (19, 20). Indeed, extensive circumstantial evidence exists for cooperation between GATA-1 and EKLF in erythroid gene regulation, suggesting the two factors could work together to control aspects of the cell cycle (21), in particular the expression of E2f2.
We previously identified E2fs as potential EKLF target genes in a global profiling study (5). The focus of this study was to examine how loss of EKLF altered the cell cycle and to interrogate possible direct links between EKLF and the E2fs. Like Pilon et al. (18), we show the major cell cycle defect of EKLF−/− cells is defective S-phase entry and identify abnormal expression of E2f2 and E2f4 as the likely cause. However, in contrast to Pilon et al. (18), we suggest that EKLF binding to a previously undescribed intronic enhancer is critical for E2f2 gene regulation. We suggest that our newly discovered enhancer region is likely to act in cooperation with the promoter to drive appropriate E2f2 expression. We also show partial rescue of the cell cycle phenotype in EKLF−/− mice is achieved by depletion of the E2F-binding protein Rb, providing further evidence for a genetic link between EKLF and the E2F-Rb G1/S checkpoint.
EXPERIMENTAL PROCEDURES
Mouse Studies
Rb+/− EKLF+/− mice were obtained by mating EKLF+/− mice (3) and Rb +/− mice (12). Rb+/− EKLF +/− mice were intercrossed at 8–12 weeks of age. Genotyping of mouse lines and embryos was performed by genomic PCR using the primers indicated in supplemental Table 1.
Draq5 Cell Cycle Profiling
Peripheral blood collected at E10.5 (primitive red cells) or cells from homogenized E14.5 fetal livers (definitive red cells) were washed twice in FACS buffer (phosphate-buffered saline containing 2% fetal calf serum) and filtered through a 70-μm cell strainer. Cells were resuspended in FACS buffer at a concentration of 1 × 106 cells/ml and stained by the addition of the cell-permeant DNA dye Draq5 (Biostatus) to a concentration of 2.5 μm and incubated at room temperature in the dark for 10 min. Co-staining with 7-AAD provided an assessment of DNA content. FACS analysis was performed using an LSR II flow cytometer (BD Biosciences) and BD FACSDiva (BD Biosciences) or FlowJo (Treestar) software.
In Vivo BrdUrd Incorporation Assays
Intraperitoneal injection of 200 μl of BrdUrd solution (10 mg/ml in phosphate-buffered saline) was performed on pregnant female mice 1 h before sacrifice. Peripheral blood at E10.5 or fetal livers at E13.5 and E14.5 were collected from embryos and stained using a fluorescein isothiocyanate BrdUrd flow kit (BD Pharmingen) according to the manufacturer's recommendations. FACS analysis was performed using an LSR II flow cytometer (BD Biosciences) and BD FACSDiva (BD Biosciences) or FlowJo (Treestar) software.
Gene Expression Analyses
Total RNA was obtained using Trizol reagent (Invitrogen) according to the manufacturer's instructions from E10.5 peripheral blood cells, homogenized E14.5 fetal liver, or K1-ER cells induced by the addition of 4-hydroxytamoxifen (4-OHT, Sigma) as previously described (7). cDNA was prepared from total RNA by reverse transcription using Superscript III reverse transcriptase (Invitrogen) and oligo(dT) primers. Quantitative real-time reverse transcription-PCR reactions were performed as previously described (5) using primers designed to cross an intron-exon boundary. Primer sequences are described in supplemental Table 1.
Bioinformatics
The UCSC Genome Browser (22) and the Evolutionary Conserved Region (ECR) Browser (23) were used to align the genic and intergenic sequences of E2f2 and E2f4 between the mouse, human, and dog genomes and identify ECRs of greater than 100 bp in length and 70% similarity. Using the rVISTA (24) user-defined motif search tool we identified EKLF consensus binding sites (5′-CCNCNCCCN-3′) within these regions. Evolutionary conserved regions from the first intron of E2f2 and fifth intron of E2f4 were identified as potential erythroid specific enhancer regions and aligned using AlignX software (Invitrogen). The seven species regulatory potential track available on the UCSC Genome Browser was also used to find putative erythroid specific enhancers (25).
Luciferase Reporter Assays
The XhoI restriction site of the pGL2-Promoter vector (Promega) was used to clone a 502-bp fragment of murine E2f2 intron 1 (shown in Fig. 3) or a 222-bp fragment including murine E2f4 intron 5 (shown in supplemental Fig. 1) in either the forward (5′–3′) or reverse (3′–5′) orientation upstream of the ubiquitous minimal SV40 promoter driving expression of luciferase. Constructs were transfected into murine erythroleukemia (MEL) cells using Lipofectamine LTX (Invitrogen) according to the manufacturer's recommendations or Drosophila SL2 cells together with pPAC-EKLF or empty pPAC vector as previously reported (26). Lysates were prepared and assayed for luciferase activity using the Luciferase Assay System (Promega) and a VICTOR Light Luminescence Counter (PerkinElmer Life Sciences).
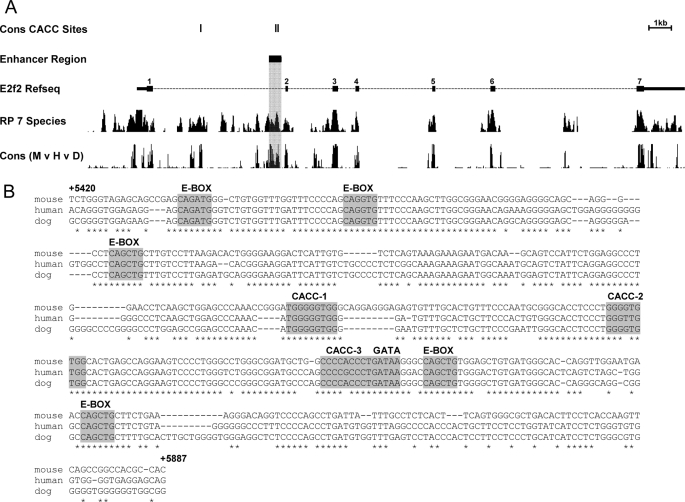
FIGURE 3.
Identification of a potential intronic enhancer in E2f2. A, the murine E2f2 gene from the UCSC Genome Browser. Gene structure is indicated by the Refseq track with exons shown by numbered black boxes and narrow portions indicating the untranslated region. Conserved CACC (EKLF binding) sites and enhancer regions identified using the Evolutionary Conserved Region browser are represented on separate tracks. Tracks utilizing conservation data (RP 7 Species (25) and Cons M v H v D) are also shown to highlight the specific intronic enhancer region. The scale bar is shown. Three-way species alignment generated (mouse, human, dog) for the shaded region of E2f2 (B) provides a more specific picture of the binding motifs present for EKLF (CACC, 5′-CCNCNCCCN-3′), GATA-1 (GATA, 5′-(A/T)GATA(A/G)-3′), and SCL/TAL1 (E-BOX, 5′-CANNTG-3′). Nucleotide positions relative to the canonical start of transcription are indicated.
Chromatin Immunoprecipitation (ChIP)
The erythroid cell line K1zf-ER was created by immortalization of EKLF−/− erythroid progenitors with J2 retrovirus as previously described (27) followed by infection with murine stem cell virus expressing the zinc finger DNA binding region (amino acids 273–376) of murine EKLF as an ERTM fusion. These cells or E14.5 fetal liver erythroid cells were used to determine in vivo chromatin occupancy of EKLF. K1zf-ER cells were induced for DNA binding activity by treating with 4-OHT (Sigma). ChIP was performed as previously described (26) using a specific ERα antibody (Ab-10; Neomarkers) together with an irrelevant mouse IgG1 control antibody or a specific EKLF antibody (3) together with preimmune rabbit serum as a control. ChIP assays to address GATA-1 binding were performed in a similar manner using a specific monoclonal GATA-1 antibody (sc-265, Santa Cruz Biotechnology) or an irrelevant rat IgG control. Enrichment of transcription factor binding was determined by real-time PCR using specific primers designed to amplify putative enhancer regions. Primer sequences are provided in supplemental Table 1.
Electrophoretic Mobility Shift Assays (EMSAs)
EMSAs were performed as previously described (28). Nuclear extracts were derived from COS7 cells that had been transfected with an EKLF expression plasmid (pSG5-EKLF) (1) or from MEL cells. Supershifts were performed using a rabbit polyclonal antibody specific for EKLF (3) or a rat monoclonal antibody specific for GATA-1 (sc-265, Santa Cruz Biotechnology). Oligonucleotide sequences used to generate radiolabeled probes are provided in supplemental Table 1 with only the forward strand shown. Reverse oligonucleotides were annealed in excess after labeling of the forward strand with T4 polynucleotide kinase (Promega) and [γ-32P]ATP (PerkinElmer Life Sciences).
RESULTS
Loss of EKLF Impairs S-phase Entry during Primitive and Definitive Erythropoiesis
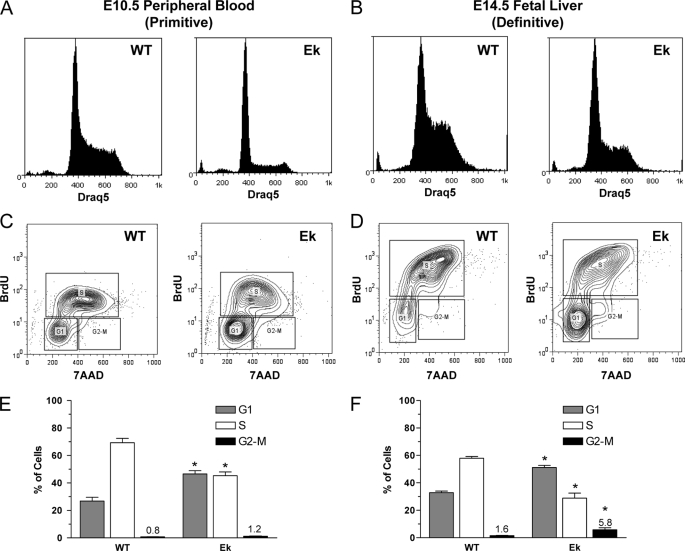
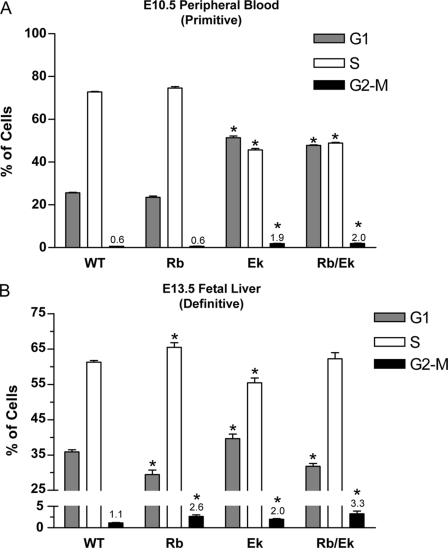
Forced overexpression studies in cell lines (27), expression profiling experiments (4, 5), and the morphology of EKLF−/− erythroid cells (3–5) suggested a cell cycle defect caused by a loss of EKLF. To examine cell cycle phasing of erythroid cells in vivo, we used the cell-permeable DNA dye Draq5. There was a significant reduction in cells with S-phase DNA content during primitive (E10.5 peripheral blood, Fig. 1A) and definitive (E14.5 fetal liver, Fig. 1B) erythropoiesis in EKLF−/− (Ek) embryos when compared with wild-type littermates (WT). To determine whether this was because of a shortened S-phase or an inability of cells to enter S-phase, we injected pregnant females with BrdUrd solution 1 h before sacrifice and collected primitive blood or fetal livers to determine the amount of BrdUrd incorporation by FACS. There was a marked reduction in BrdUrd incorporation in EKLF−/− embryonic (Figs. 1, C and E) and definitive red cells (Figs. 1, D and F) compared with litter mates, which is consistent with the Draq5 DNA content data. In addition, there was a significant increase in cells in G2-M-phase in EKLF−/− fetal liver cells, suggesting loss of EKLF in the erythroid compartment impairs progression through G2-M directly or indirectly by eliciting a G2/M checkpoint (compare Ek and WT in Figs. 1, E and F).
FIGURE 1.
Disruptions to the cell cycle during primitive and definitive erythropoiesis in EKLF−/− mice. Cell cycle profiles were obtained by Draq5 DNA staining and FACS analysis of WT and Ek embryos. A, representative DNA histogram of E10.5 peripheral blood. B, representative DNA histogram of E14.5 fetal liver. Representative FACS contour plots of E10.5 peripheral blood (C) and E14.5 fetal liver (D) from the in vivo BrdUrd incorporation assay show a reduction in cells containing BrdUrd. Quantification of BrdUrd incorporation assay data using gates are shown in C and D for E10.5 peripheral blood (E) and E14.5 fetal liver (F). Data are the mean ± S.E., n ≥ 3 for each genotype; *, p < 0.05 compared with WT by Student's t test. Percentages of cells in G2-M-phase are indicated. 7-AAD, 7-aminoactinomycin D.
Expression of S-phase Genes E2f2 and E2f4 Is Reduced in EKLF−/− Mice
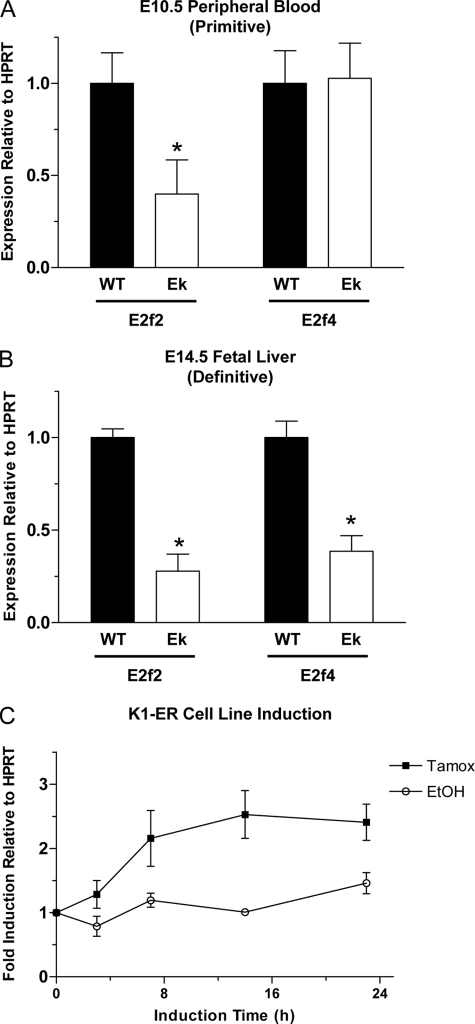
To explain the defect in S-phase entry for EKLF−/− erythroid cells, we focused our studies on the E2F family of transcription factors as potential EKLF target genes because of their critical role in S-phase progression. E2f5 and E2f6 showed no difference in expression at the mRNA level between EKLF−/− and wild-type E14.5 fetal livers (0.98 and 1.098, respectively, Ek versus WT, data not shown), whereas E2f1 and E2f3 showed only a modest decrease in mRNA levels in EKLF−/− (0.708 and 0.717, respectively, Ek versus WT, data not shown). In contrast, E2f2 and E2f4 both showed a significant reduction in mRNA expression in EKLF−/− fetal livers at E14.5 when compared with wild-type (0.28 and 0.38, respectively, Ek versus WT, Fig. 2B). To confirm that E2f2 and E2f4 were likely to be critical EKLF target genes during erythropoiesis, we investigated the mRNA expression levels during primitive erythropoiesis at E10.5 when the phenotype is subtle. E2f2 expression was also significantly reduced at E10.5 in the EKLF−/− (0.4, Ek versus WT, Fig. 2A). However, E2f4 expression was unaffected at this time (1.03, Ek versus WT, Fig. 2A). We also sought to confirm that the change in E2f2 expression levels was a direct effect of EKLF activity by utilizing the K1-ER cell line system, which contains a 4-OHT (Tamoxifen)-inducible form of EKLF (27). E2f2 is rapidly induced by 4-OHT, even faster than β-globin and α-hemoglobin-stabilizing protein in these cells (Fig. 2C) (7).
FIGURE 2.
E2f transcript levels are reduced in EKLF−/− erythrocytes. Levels of E2f2 and E2f4 mRNA were determined by quantitative real-time reverse transcription-PCR in E10.5 peripheral blood (A) and E14.5 fetal liver (B). Data are the mean ± S.E. relative to housekeeping gene hypoxanthine phosphoribosyltransferase (HPRT) and WT expression levels, n ≥ 3 for each genotype, *, p < 0.05 versus WT levels by Student's t test. C, induction of E2f2 gene expression by 4-OHT. K1-ER erythroid cells were used to show a rapid induction of E2f2 gene expression by reverse transcription-PCR upon the addition of 4-OHT (Tamox) but not upon the addition of vehicle control (EtOH). E2f2 mRNA expression levels were normalized to pretreatment controls and the housekeeping gene hypoxanthine phosphoribosyltransferase. Each point is represented as the mean ± S.E., n = 4 at each time point.
Intronic Regions of High Conservation within E2f2 and E2f4 Contain EKLF and Other Erythroid Transcription Factor Binding Sites
To investigate the possibility of direct regulation of E2f2 and E2f4 by EKLF, we looked for EKLF consensus binding sites (5′-CCNCNCCCN-3′ (29)) that were conserved between the mouse, human, and dog genomes. We identified a highly conserved region within the first intron of E2f2, with a high regulatory potential score (>0.3) (25) that contained EKLF consensus sites as well as consensus sites for the erythroid specific transcription factors GATA-1 and SCL/TAL1 (Fig. 3A). This region represents a putative erythroid-specific enhancer of E2f2 expression (hereafter referred to as E2f2-i1en). A multiple sequence alignment of this region shows that it contains three independent conserved EKLF consensus sites (designated CACC-1, CACC-2, and CACC-3, Fig. 3B) as well as a conserved GATA-1 consensus site (GATA, Fig. 3B) and five separate E-BOX binding sites for SCL/TAL1 and related proteins (E-BOX, Fig. 3B). Investigation of the E2f4 gene locus also showed a region containing conserved EKLF and GATA-1 consensus sites within intron 5 (hereafter referred to as E2f4-i5en), which has also been assigned a high regulatory potential score (>0.3, supplemental Fig. 1A). However, this region is not as highly conserved across the three genomes (supplemental Fig. 1B).
Intronic Regions of E2f2 and E2f4 Act as Enhancers in Erythroid Cells and Show EKLF Dependence
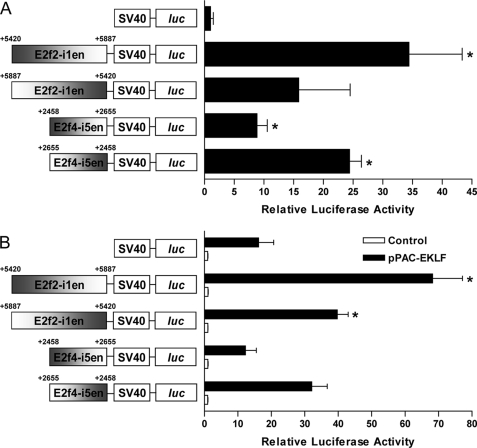
We next sought to confirm that the regions E2f2-i1en and E2f4-i5en were functional enhancers of gene expression using reporter assays. Enhancer constructs containing either E2f2-i1en or E2f4-i5en in forward and reverse orientations were tested for activity in the murine erythroleukemia cell line, MEL. Both E2f2-i1en and E2f4-i5en were found to enhance transcription of the luciferase gene in MEL cells 10–30-fold relative to a basal vector containing the SV40 promoter only (Fig. 4A). Interestingly, both E2f2-i1en and E2f4-i5en seem to display a degree of directional preference, forward for E2f2-i1en and reverse for E2f4-i5en.
FIGURE 4.
Intronic regions of E2f2 and E2f4 enhance expression in erythroid cells. The regions of E2f2 and E2f4 identified to be potential intronic enhancers (E2f2-i1en and E2f4-i5en, respectively) cloned into the pGL2-promoter vector (positions relative to transcriptional start are indicated) were transfected into MEL cells to show erythroid activity (A). Data are the mean ± S.E., n = 3; *, p < 0.05 versus empty vector by Student's t test. The same E2f2-i1en and E2f4-i5en constructs were transfected into Drosophila SL2 cells with or without an EKLF expression vector (pPAC-EKLF and control, respectively (B)). Data are the mean ± S.E., n = 3; *, p < 0.05 versus empty vector by Student's t test.
To address whether these enhancers were directly responsive to EKLF, we performed further reporter assays in Drosophila melanogaster SL2 cells, which lack any SP/KLF proteins. E2f2-i1en shows a significant response (2–3-fold induction) upon co-transfection with an EKLF expression plasmid in either the forward or reverse orientation when compared with empty vector (Fig. 4B). However, the E2f4-i5en enhancer does not display a significant response in either orientation when compared with empty vector containing the basal SV40 promoter alone (Fig. 4B), suggesting it may not be directly EKLF-responsive.
EKLF Binds to CACC Sites within Intron 1 of E2f2 in Vivo and in Vitro
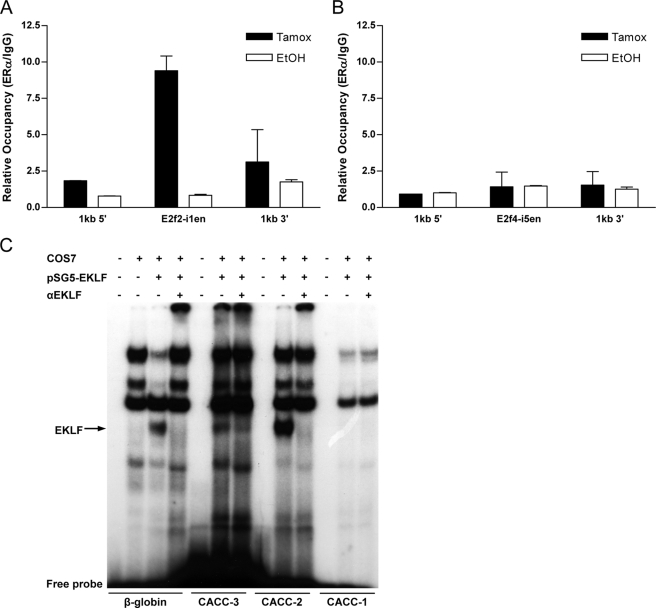
To determine whether the identified putative enhancers (E2f2-i1en and E2f4-i5en) were occupied by EKLF in vivo, we performed a ChIP assay (Fig. 5). Using a cell line containing an inducible form of the EKLF DNA binding domain (K1zf-ER, inducible by addition of 4-OHT), we found there is specific in vivo occupancy of E2f2-i1en but not regions 1 kilobase upstream or downstream and that this occupancy is dependent on 4-OHT (EKLF induced, Tamox (black bars), Fig. 5A). In contrast, there is no occupancy of E2f4-i5en by K1zf-ER in these cells (Fig. 5B). This is consistent with our observations made by reporter assay in SL2 cells (Fig. 4B). As a positive control, a previously identified site of EKLF binding in the α-hemoglobin-stabilizing protein gene promoter (7) was also occupied by K1zf-ER in these cells (data not shown).
FIGURE 5.
EKLF occupies the E2f2 intronic enhancer in vivo and in vitro. ChIP assays in an inducible EKLF cell line (K1zf-ER) show specific EKLF binding to the CACC enhancer region (E2f2-i1en) of E2f2 and not to regions upstream or downstream in the presence of 4-OHT only (Tamox (black bars) (A)). In this same system no binding was observed across the E2f4 enhancer region (E2f4-i5en (B)). The mean relative occupancy for positive (ERα) versus negative (IgG1) antibodies for two independent experiments is shown. Error bars represent the S.D. between experiments. C, EMSA using probes specific for the three predicted EKLF binding sites (CACC-1, CACC-2, CACC-3) in the E2f2-i1en region as well as a control probe from the β-globin promoter (β-globin). Nuclear extracts from COS7 cells alone or COS7 cells transfected with an EKLF expression vector (pSG5-EKLF) were used. EKLF binding was confirmed by supershift with specific EKLF antisera (αEKLF). kb, kilobases.
We were also able to show occupancy by endogenous EKLF at E2f2-i1en in primary fetal liver cells (supplemental Fig. 2). Pilon et al. (18) recently showed EKLF occupancy broadly at the E2f2 proximal promoter. Even though the CACC sites within the E2f2 proximal promoter are not conserved, we also found EKLF was enriched close to the transcriptional start site (supplemental Fig. 2).
ChIP assays have limited resolution, which is dependent on efficiencies of DNA shearing; ChIP cannot reliably distinguish occupancy of sites within ∼50–100 bp. Thus, to determine EKLF binding at E2f2-i1en at higher resolutions, we used EMSA and specific probes designed for each of the bioinformatically identified EKLF binding sites (CACC-1, CACC-2, CACC-3, Fig. 3B). EKLF is only able to bind to the CACC-3 and CACC-2 probes in vitro (Fig. 5C) as confirmed by supershift with a specific EKLF antibody. EMSAs using a probe for the CACC site of E2f4-i5en confirmed what was observed in vivo by ChIP, as EKLF also failed to bind to this site in vitro (data not shown). Taken together, these data suggest direct regulation of E2f2 by EKLF is likely to occur via binding to the enhancer region, E2f2-i1en, which contains two EKLF binding sites (CACC-2 and CACC-3). Direct regulation via binding to the promoter could also occur as suggested by Pilon et al. (18). However, we suggest that transcriptional regulation of E2f2 probably occurs via co-operating contributions of both the promoter and the E2f2-i1en element (supplemental Fig. 2).
Additional Genetic Depletion of Rb Rescues Impaired Cell Cycle Entry in EKLF−/− Erythroid Cells
We hypothesized that the defects in S-phase entry observed in EKLF−/− embryos at E10.5 in the peripheral blood and E14.5 in the fetal liver were primarily caused by a loss of EKLF-dependent E2f2 gene expression. We postulated much of the abnormal erythroid differentiation phenotype in EKLF−/− embryos could be the result of aborted entry into S-phase. To test this, we generated Rb/EKLF double heterozygous mice (3, 30) and crossed these mice for in vivo BrdUrd incorporation assays. Rb normally binds E2F2 and prevents its inappropriate activity until Rb phosphorylation by cyclin-dependent kinase 2 (CDK2), CDK4, or CDK6 allows the release of E2F2 and subsequent activation of S-phase. Thus, loss of Rb in EKLF−/− embryos should permit the activity of residual E2F2 and hence S-phase entry. Previous studies have employed this genetic strategy to partially rescue the erythropoietic defects present in E2f2−/− mice (31).
At E10.5 (embryonic erythropoiesis) there was no significant improvement in the number of cells entering S-phase in the combined Rb/EKLF−/− compared with the EKLF−/− peripheral blood (Fig. 6A, compare Ek and Rb/Ek). This result was not unexpected as BrdUrd incorporation into Rb−/− embryos was no different to that of WT embryos in embryonic red cells (Fig. 6A, compare WT and Rb). EKLF−/− and Rb/EKLF−/− embryos were significantly different to wild type in all three cell cycle phases (Fig. 6A). Thus, it is likely that Rb does not play a significant role during embryonic erythropoiesis as Rb−/− embryos were found to be phenotypically normal at this stage, as previously reported (13).
FIGURE 6.
Combined loss of EKLF and Rb rescues S-phase entry during definitive but not primitive erythropoiesis. BrdUrd incorporation assays were performed on embryonic peripheral blood at E10.5 (A) and fetal liver at E13.5 (B). Genotypes are as indicated (WT, Rb−/−, Ek). BrdUrd incorporation assay data were gated as shown in Fig. 1 and plotted as the mean ± S.E., n ≥ 3 for each genotype; *, p < 0.05 compared with WT by Student's t test. Percentages of cells in G2-M-phase are indicated for clarity.
In contrast, additional loss of Rb in EKLF−/− definitive fetal liver cells resulted in partial rescue of the cell cycle at E13.5 (Fig. 6B). Although EKLF−/− embryos showed a reduction of cells entering S-phase compared with wild-type at E13.5 as expected (55 and 61% for Ek and WT, respectively, Fig. 6A), the combined loss of Rb and EKLF provided a rescue such that there was no significant difference in S-phase entry compared with WT (62% for Rb/Ek, Fig. 6B). These observations demonstrated that a lack of EKLF activation of E2f2 was the primary cause of the cell cycle defect during definitive erythropoiesis. This result prompted us to look for signs of a phenotypic rescue to erythropoiesis. However, we found no indications of a phenotypic rescue to erythropoiesis by FACS using antibodies to Ter119 and CD71 or by morphology in May-Grunwald Giemsa-stained peripheral blood cytospins (data not shown). It was interesting to note that the proportion of cells in G1-phase between the Rb/Ek and WT embryos was significantly different (Fig. 6B). We attribute this difference due to the increased proportion of cells trapped in G2/M-phase in the Rb/ Ek embryos, independent of the restored G1/S transition. We conclude that a combined loss of EKLF and Rb does provide a partial rescue to cell cycle dynamics during definitive erythropoiesis; however, many other aspects of erythroid development remain perturbed.
DISCUSSION
EKLF Is a Master Regulator of Erythropoiesis
The essential role of EKLF in erythropoiesis was initially thought to be primarily via direct regulation of the β-globin gene (2, 3). However, it is becoming increasingly clear that EKLF is essential for other aspects of erythroid differentiation such as cytoskeletal integrity (4–6), transmembrane blood group and other non-globin protein expression (4, 5), and erythroid versus megakaryocyte lineage specification (32–34). In our study we have established a role for EKLF in erythroid differentiation via direct regulation of components of the cell cycle machinery.
We previously demonstrated a role for EKLF in cell cycle control by direct regulation of the cyclin-dependent kinase inhibitor, p18INK4c (26), which is also a target of GATA-1 (19). In this study we have shown that one of the major defects of EKLF−/− red cells is an inability to enter into S-phase of the cell cycle during definitive erythropoiesis (confirmation of the results of Pilon et al. (18)) and also during primitive erythropoiesis (Fig. 1). This was at odds with loss of p18INK4c, which would be predicted to result in increased S-phase entry, and suggested additional EKLF target genes were responsible for the cell cycle phenotype.
Direct Regulation of E2f2 Is a Critical Function of EKLF in Erythropoiesis
Herein, we have identified E2f2 as a direct target gene of EKLF via an intronic enhancer region we have called E2f2-i1en. Recently published work suggests that regions of the E2f2 gene promoter are also required for EKLF regulation (18). However, we hypothesize that the E2f2-i1en region may also be responsive to other erythroid transcription factors such as GATA-1 and SCL/TAL1 because of the presence of conserved motifs and occupancy by GATA-1 in vivo and in vitro by ChIP assay and EMSA, respectively (supplemental Fig. 3). As such, E2f2-i1en represents an erythroid-specific enhancer similar to that shown for intron 8 of the Alas2 gene (35).
E2f2 and the closely related transcription factor E2f4 are the primary members of the E2F transcription factor family expressed during erythropoiesis (31). A loss of either transcription factor in mice leads to anemia, which is associated with defects in erythroid maturation and an inadequate expansion of the erythroid compartment (14–16, 36). However, our studies suggest E2f2 to be the only member of the E2F family to be a direct target gene of EKLF. The reduced levels of E2f4 mRNA observed in EKLF−/− mice may be in part because of the failure of proper erythroid differentiation rather than as a consequence of impaired EKLF activity.
Based on the organization of E2f2-i1en, it is likely that GATA-1 and perhaps SCL/TAL1 co-operate with EKLF to regulate E2f2 gene expression, although we are not aware of ChIP experiments which confirm in vivo occupancy of this enhancer by SCL. To add to the complexity of erythroid cell cycle circuitry, GATA-1 and E2Fs themselves serve as transcriptional regulators of p18INK4c gene expression (37). GATA-1 also regulates EKLF gene expression (38), suggesting a complex regulatory network impinges upon the G1/S checkpoint in erythroid cells. Nevertheless, the consequence of loss of EKLF is a dramatic reduction in S-phase entry.
Although the regulation of E2f2 during erythropoiesis depends upon EKLF acting at the transcriptional level, regulation of E2F2 protein function occurs by association with the Rb protein to sequester its activity (39). It is interesting to note that E2F4 does not associate with Rb but, rather, with the other two members of the pocket protein family, p107 and p130 (40). We attempted to restore the cell cycle balance of EKLF−/− red cells by genetically engineering additional loss of Rb. A similar experiment was recently used to show that the E2F2-Rb interaction during erythropoiesis is critical for control of S-phase entry and erythroid maturation (31). We hypothesized that a similar result might be obtained by restoring the ability of cells to enter S-phase in the EKLF−/− mice. Indeed, the combined loss of EKLF and Rb during erythropoiesis provided a rescue to S-phase entry during definitive erythropoiesis, suggesting reduced levels of E2F2 are responsible (Fig. 6B). The failure to rescue erythroid differentiation during definitive erythropoiesis was not surprising, as EKLF regulates many other critical target genes that are not dependent on the function of Rb. We also failed to improve survival of Rb−/− mice with additional loss of EKLF. This is also not surprising, as a critical role for Rb in the erythropoietic niche and the placenta has been demonstrated (41, 42). Thus, reduction of E2F2 levels in erythroid cells (indirectly via loss of EKLF) would not be expected to rescue these aspects of the Rb−/− phenotype.
The KLF Family as Cell Cycle Regulators
Our study has identified a novel mechanism whereby EKLF, the founding member of the KLF family, regulates the cell cycle during erythropoiesis via direct binding to a novel E2f2 enhancer. There are 17 members of the KLF family and 8 members of the related SP1 family (43). Some act primarily as transcriptional activators, and others act as repressors via differential recruitment of co-activators and co-repressors such as CBP/p300 (CREB binding protein) and CtBP (C-terminal-binding protein). Because all KLFs and SP1-like proteins bind very similar GC-rich or CACC-box motifs, we suggest they might perform a similar function in cell cycle regulation via E2f2 in other tissue types. Consequently, we interrogated recent ChIP sequencing data for KLF4 occupancy in embryonic stem cells and found occupancy directly over the same E2f2-i1en (44), which coincides with specific trimethylation of H3 lysine 4 (45) (supplemental Fig. 4). In these studies no such occupancy of KLF4 was observed in the promoter of E2f2. We suggest that a critical role played by KLF2 and KLF4 in embryonic stem cell self-renewal and induction of iPS cells from differentiated adult cells (46) might be primarily enacted via regulation of E2f2 and that this regulation is likely to occur via the E2f2-i1en region alone (44, 47, 48). As further KLF ChIP sequencing and ChIP-chip data become available, we predict other members of the family will be found to directly bind E2f2-i1en in vivo. We hypothesize this will be a generic mechanism by which the KLF family of proteins control the balance between self- renewal and differentiation.
Supplementary Material
Acknowledgments
We thank Natalie Eriksson for help with maintenance and genotyping of the EKLF and Rb mouse colonies and Tyler Jacks (Massachusetts Institute of Technology) for the kind gift of the Rb mutant mice. We are also grateful to Tom Whitington for assistance with bioinformatics and use of the UCSC Genome Browser and to Simon Wilkins for critical review of the manuscript.
This work was supported by Cancer Council Queensland Grant 519718 (to A. C. P.) and Australian Research Council Discovery Grant DP0770471 (to A. C. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4 and Table 1.
- EKLF
- erythroid Kruppel-like factor
- ChIP
- chromatin immunoprecipitation
- COS7
- African green monkey kidney fibroblast cell line
- ER
- estrogen receptor
- MEL
- murine erythroleukemia cell line
- Rb
- retinoblastoma gene/protein
- SCL/TAL1
- stem cell leukemia hematopoietic transcription factor
- SL2
- Schneider's Drosophila line 2
- FACS
- fluorescence-activated cell sorter
- BrdUrd
- bromodeoxyuridine
- 4-OHT
- 4-hydroxytamoxifen
- WT
- wild type
- Ek
- EKLF−/−
- EMSA
- electrophoretic mobility shift assay.
REFERENCES
- 1.Miller I. J., Bieker J. J. (1993) Mol. Cell. Biol. 13, 2776–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nuez B., Michalovich D., Bygrave A., Ploemacher R., Grosveld F. (1995) Nature 375, 316–318 [DOI] [PubMed] [Google Scholar]
- 3.Perkins A. C., Sharpe A. H., Orkin S. H. (1995) Nature 375, 318–322 [DOI] [PubMed] [Google Scholar]
- 4.Drissen R., von Lindern M., Kolbus A., Driegen S., Steinlein P., Beug H., Grosveld F., Philipsen S. (2005) Mol. Cell. Biol. 25, 5205–5214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodge D., Coghill E., Keys J., Maguire T., Hartmann B., McDowall A., Weiss M., Grimmond S., Perkins A. (2006) Blood 107, 3359–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilson D. G., Sabatino D. E., Bodine D. M., Gallagher P. G. (2006) Exp. Hematol. 34, 705–712 [DOI] [PubMed] [Google Scholar]
- 7.Keys J. R., Tallack M. R., Hodge D. J., Cridland S. O., David R., Perkins A. C. (2007) Br. J. Haematol. 136, 150–157 [DOI] [PubMed] [Google Scholar]
- 8.Pilon A. M., Nilson D. G., Zhou D., Sangerman J., Townes T. M., Bodine D. M., Gallagher P. G. (2006) Mol. Cell. Biol. 26, 4368–4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funnell A. P., Maloney C. A., Thompson L. J., Keys J., Tallack M., Perkins A. C., Crossley M. (2007) Mol. Cell. Biol. 27, 2777–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koury M. J., Sawyer S. T., Brandt S. J. (2002) Curr. Opin. Hematol. 9, 93–100 [DOI] [PubMed] [Google Scholar]
- 11.Clarke A. R., Maandag E. R., van Roon M., van der Lugt N. M., van der Valk M., Hooper M. L., Berns A., te Riele H. (1992) Nature 359, 328–330 [DOI] [PubMed] [Google Scholar]
- 12.Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. (1992) Nature 359, 295–300 [DOI] [PubMed] [Google Scholar]
- 13.Lee E. Y., Chang C. Y., Hu N., Wang Y. C., Lai C. C., Herrup K., Lee W. H., Bradley A. (1992) Nature 359, 288–294 [DOI] [PubMed] [Google Scholar]
- 14.Humbert P. O., Rogers C., Ganiatsas S., Landsberg R. L., Trimarchi J. M., Dandapani S., Brugnara C., Erdman S., Schrenzel M., Bronson R. T., Lees J. A. (2000) Mol. Cell 6, 281–291 [DOI] [PubMed] [Google Scholar]
- 15.Li F. X., Zhu J. W., Hogan C. J., DeGregori J. (2003) Mol. Cell. Biol. 23, 3607–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rempel R. E., Saenz-Robles M. T., Storms R., Morham S., Ishida S., Engel A., Jakoi L., Melhem M. F., Pipas J. M., Smith C., Nevins J. R. (2000) Mol. Cell 6, 293–306 [DOI] [PubMed] [Google Scholar]
- 17.Sears R., Ohtani K., Nevins J. R. (1997) Mol. Cell. Biol. 17, 5227–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilon A. M., Arcasoy M. O., Dressman H. K., Vayda S. E., Maksimova Y. D., Sangerman J. I., Gallagher P. G., Bodine D. M. (2008) Mol. Cell. Biol. 28, 7394–7401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rylski M., Welch J. J., Chen Y. Y., Letting D. L., Diehl J. A., Chodosh L. A., Blobel G. A., Weiss M. J. (2003) Mol. Cell. Biol. 23, 5031–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welch J. J., Watts J. A., Vakoc C. R., Yao Y., Wang H., Hardison R. C., Blobel G. A., Chodosh L. A., Weiss M. J. (2004) Blood 104, 3136–3147 [DOI] [PubMed] [Google Scholar]
- 21.Gregory R. C., Taxman D. J., Seshasayee D., Kensinger M. H., Bieker J. J., Wojchowski D. M. (1996) Blood 87, 1793–1801 [PubMed] [Google Scholar]
- 22.Karolchik D., Kuhn R. M., Baertsch R., Barber G. P., Clawson H., Diekhans M., Giardine B., Harte R. A., Hinrichs A. S., Hsu F., Kober K. M., Miller W., Pedersen J. S., Pohl A., Raney B. J., Rhead B., Rosenbloom K. R., Smith K. E., Stanke M., Thakkapallayil A., Trumbower H., Wang T., Zweig A. S., Haussler D., Kent W. J. (2008) Nucleic Acids Res. 36, D773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ovcharenko I., Nobrega M. A., Loots G. G., Stubbs L. (2004) Nucleic Acids Res. 32, W280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loots G. G., Ovcharenko I. (2004) Nucleic Acids Res. 32, W217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H., Zhang Y., Cheng Y., Zhou Y., King D. C., Taylor J., Chiaromonte F., Kasturi J., Petrykowska H., Gibb B., Dorman C., Miller W., Dore L. C., Welch J., Weiss M. J., Hardison R. C. (2006) Genome Res. 16, 1480–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tallack M. R., Keys J. R., Perkins A. C. (2007) J. Mol. Biol. 369, 313–321 [DOI] [PubMed] [Google Scholar]
- 27.Coghill E., Eccleston S., Fox V., Cerruti L., Brown C., Cunningham J., Jane S., Perkins A. (2001) Blood 97, 1861–1868 [DOI] [PubMed] [Google Scholar]
- 28.Gordon C. T., Fox V. J., Najdovska S., Perkins A. C. (2005) Biochim. Biophys. Acta 1729, 74–80 [DOI] [PubMed] [Google Scholar]
- 29.Feng W. C., Southwood C. M., Bieker J. J. (1994) J. Biol. Chem. 269, 1493–1500 [PubMed] [Google Scholar]
- 30.Clark A. J., Doyle K. M., Humbert P. O. (2004) Blood 104, 1324–1326 [DOI] [PubMed] [Google Scholar]
- 31.Dirlam A., Spike B. T., Macleod K. F. (2007) Mol. Cell. Biol. 27, 8713–8728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frontelo P., Manwani D., Galdass M., Karsunky H., Lohmann F., Gallagher P. G., Bieker J. J. (2007) Blood 110, 3871–3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouilloux F., Juban G., Cohet N., Buet D., Guyot B., Vainchenker W., Louache F., Morlé F. (2008) Blood 112, 576–584 [DOI] [PubMed] [Google Scholar]
- 34.Siatecka M., Xue L., Bieker J. J. (2007) Mol. Cell. Biol. 27, 8547–8560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surinya K. H., Cox T. C., May B. K. (1998) J. Biol. Chem. 273, 16798–16809 [DOI] [PubMed] [Google Scholar]
- 36.Kinross K. M., Clark A. J., Iazzolino R. M., Humbert P. O. (2006) Blood 108, 886–895 [DOI] [PubMed] [Google Scholar]
- 37.Blais A., Monté D., Pouliot F., Labrie C. (2002) J. Biol. Chem. 277, 31679–31693 [DOI] [PubMed] [Google Scholar]
- 38.Crossley M., Tsang A. P., Bieker J. J., Orkin S. H. (1994) J. Biol. Chem. 269, 15440–15444 [PubMed] [Google Scholar]
- 39.Lee C., Chang J. H., Lee H. S., Cho Y. (2002) Genes Dev. 16, 3199–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavia P., Jansen-Dürr P. (1999) BioEssays 21, 221–230 [DOI] [PubMed] [Google Scholar]
- 41.Walkley C. R., Shea J. M., Sims N. A., Purton L. E., Orkin S. H. (2007) Cell 129, 1081–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu L., de Bruin A., Saavedra H. I., Starovic M., Trimboli A., Yang Y., Opavska J., Wilson P., Thompson J. C., Ostrowski M. C., Rosol T. J., Woollett L. A., Weinstein M., Cross J. C., Robinson M. L., Leone G. (2003) Nature 421, 942–947 [DOI] [PubMed] [Google Scholar]
- 43.van Vliet J., Crofts L. A., Quinlan K. G., Czolij R., Perkins A. C., Crossley M. (2006) Genomics 87, 474–482 [DOI] [PubMed] [Google Scholar]
- 44.Chen X., Xu H., Yuan P., Fang F., Huss M., Vega V. B., Wong E., Orlov Y. L., Zhang W., Jiang J., Loh Y. H., Yeo H. C., Yeo Z. X., Narang V., Govindarajan K. R., Leong B., Shahab A., Ruan Y., Bourque G., Sung W. K., Clarke N. D., Wei C. L., Ng H. H. (2008) Cell 133, 1106–1117 [DOI] [PubMed] [Google Scholar]
- 45.Mikkelsen T. S., Ku M., Jaffe D. B., Issac B., Lieberman E., Giannoukos G., Alvarez P., Brockman W., Kim T. K., Koche R. P., Lee W., Mendenhall E., O'Donovan A., Presser A., Russ C., Xie X., Meissner A., Wernig M., Jaenisch R., Nusbaum C., Lander E. S., Bernstein B. E. (2007) Nature 448, 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. (2007) Cell 131, 861–872 [DOI] [PubMed] [Google Scholar]
- 47.Bruce S. J., Gardiner B. B., Burke L. J., Gongora M. M., Grimmond S. M., Perkins A. C. (2007) BMC Genomics 8, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang J., Chan Y. S., Loh Y. H., Cai J., Tong G. Q., Lim C. A., Robson P., Zhong S., Ng H. H. (2008) Nat. Cell Biol. 10, 353–360 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.