Abstract
Autosomal dominant polycystic kidney disease is caused by mutations in the genes encoding polycystin-1 (PC-1) and polycystin-2 (PC-2). PC-1 cleavage releases its cytoplasmic C-terminal tail (CTT), which enters the nucleus. To determine whether PC-1 CTT cleavage is influenced by PC-2, a quantitative cleavage assay was utilized, in which the DNA binding and activation domains of Gal4 and VP16, respectively, were appended to PC-1 downstream of its CTT domain (PKDgalvp). Cells cotransfected with the resultant PKDgalvp fusion protein and PC-2 showed an increase in luciferase activity and in CTT expression, indicating that the C-terminal tail of PC-1 is cleaved and enters the nucleus. To assess whether CTT cleavage depends upon Ca2+ signaling, cells transfected with PKDgalvp alone or together with PC-2 were incubated with several agents that alter intracellular Ca2+ concentrations. PC-2 enhancement of luciferase activity was not altered by any of these treatments. Using a series of PC-2 C-terminal truncated mutations, we identified a portion of the PC-2 protein that is required to stimulate PC-1 CTT accumulation. These data demonstrate that release of the CTT from PC-1 is influenced and stabilized by PC-2. This effect is independent of Ca2+ but is regulated by sequences contained within the PC-2 C-terminal tail, suggesting a mechanism through which PC-1 and PC-2 may modulate a novel signaling pathway.
Autosomal dominant polycystic kidney disease (ADPKD)2 is one of the most common genetic disorders, affecting 1 of every 1000 people (1). It is characterized by the slow development of multiple bilateral cysts in the kidneys, resulting in progressive renal failure in 50% of patients by their 6th decade of life. ADPKD is a systemic disease with a number of extrarenal manifestations, including hepatic cysts, cardiac valvular anomalies, intracranial aneurysms, and colonic diverticulae (1). More than 95% of cases of ADPKD are because of mutations in two recently identified genes, PKD1 and PKD2, which encode the polycystin-1 (PC-1) and polycystin-2 (PC-2) proteins, respectively (2, 3).
PC-1 is composed of a very large N-terminal extracellular region that incorporates a combination of functional motifs, followed by 11 transmembrane segments, and an ∼240-amino acid intracellular C terminus (4). PC-1 is believed to play a role in cell-cell or cell-matrix interactions, renal tubulogenesis, and intracellular signaling pathways (5–8). Embedded within the N-terminal extracellular domain immediately proximal to the first transmembrane domain is a G protein-coupled receptor proteolytic site (GPS) (9). Qian et al. (5) have demonstrated that PC-1 undergoes cleavage at the GPS domain after its synthesis in vivo, and a large proportion of the population of the ∼350-kDa N-terminal fragment generated through this cleavage remains tethered at the cell surface. The second fragment generated by this cleavage is a polypeptide of ∼150 kDa that is comprised of all of the transmembrane domains and the cytoplasmic tail (5). Chauvet et al. (10) have recently reported that the PC-1 protein is subject to an additional proteolytic cleavage. This proteolytic cleavage releases the polycystin-1 cytoplasmic C-terminal tail (CTT), which can enter the nucleus where it is able to regulate the expression of genes involved in cellular proliferation and differentiation. This cleavage appears to be stimulated, at least in part, by the absence of mechanical stimuli detected by the primary cilium (10). Moreover, PC-1 is subjected to an additional cleavage that leads to the release of the C-terminal half of the cytoplasmic tail. This C-terminal half of the PC-1 tail interacts with the transcription factor STAT6 and the coactivator P100, and it stimulates STAT6-dependent gene expression (11). In summary, PC-1 undergoes several different cleavages, each of which generates fragments that may participate in specific signaling processes.
Polycystin-2 is predicted to possess six membrane-spanning segments, with both the N and C termini exposed at the cytosolic surface of the membrane (3, 12). The C-terminal tail of PC-2 contains a calcium-binding EF hand (3, 13) and several potential phosphorylation sites (3). Polycystin-2 is predicted to form homomultimers as well as heteromultimers, particularly with PC-1 via coiled-coil domains that are predicted to reside in each of their cytoplasmic tails (14, 15). These two proteins are thought to interact and to participate in common signaling pathways. Coexpression of PC-1 with PC-2 has been shown to promote the translocation of PC-2 to the plasma membrane, where PC-2 can function as a nonselective Ca2+-permeable cation channel (16). It remains unclear, however, whether PC-1 is an obligate component of the channel complex or merely acts as a chaperone or anchoring partner. The mechanosensitive channel activity located on the primary cilia has been hypothesized to include the PC-1-PC-2 complex. It has been suggested that PC-1 senses flow and activates its binding partner PC-2, which then mediates Ca2+ entry into the cell (17). Koulen et al. (18) have demonstrated that PC-2 can also act as a Ca2+-activated Ca2+-release channel in the ER that is dependent upon the activation of the inositol trisphosphate receptor. This mechanism does not require coassembly with PC-1 (18).
We wondered whether PC-2 and the formation of the PC-1-PC-2 complex may regulate the PC-1 C-terminal tail cleavages. Toward this end, we took advantage of an assay that allows us to detect and quantify the cleavage and translocation of the PC-1 CTT to the nucleus. We demonstrate that the cleavage of polycystin-1 C-terminal tail is increased in the presence of polycystin-2. This mechanism, however, does not appear to require the coiled-coil domain interaction, and it is not modulated by Ca2+-signaling pathways.
EXPERIMENTAL PROCEDURES
Generation of DNA Constructs
Full-length cDNA encoding mouse PC-1 was modified to include the N-terminal FLAG and C-terminal HA epitopes (19) and was subcloned into pcDNA3.1 (Invitrogen). The DNA sequence encoding the Gal4VP16 tag was PCR-amplified using CMV-Gal4VP16 (CMV-GV) as template, and BsiWI restriction enzyme sites were added at each end. Gal4VP16 was inserted at the BsiWI site located in the middle of one HA tag in the C terminus of PC-1, generating a PC-1-Gal4VP16 fusion protein (Fig. 1A, PKDgalvp). GL4.31[luc2P/GAL4UAS/Hygro] (F) (Promega, Madison, WI) was used as a Gal4 promoter-driven firefly luciferase reporter construct. This reporter vector contains five consensus binding sequences for the Gal4 DNA-binding protein upstream of an adenoviral promoter, followed by the firefly luciferase gene. In transient transfection experiments, a vector constitutively expressing Renilla luciferase (R), pRL-TK, was included as an internal control to normalize for transfection differences. pRL-TK and CMV-GV vectors were generous gifts from Dr. Yung-Feng Liao (Institute of Zoology, Academia Sinica, Taiwan). The p200galvp construct, corresponding to the last 200 amino acids of mouse PC-1 protein, was generated by PCR from PKDgalvp and subcloned into pcDNA 3.1. The generation of the human PC-2 construct has already been described (20). All PCR primer details are available upon request.
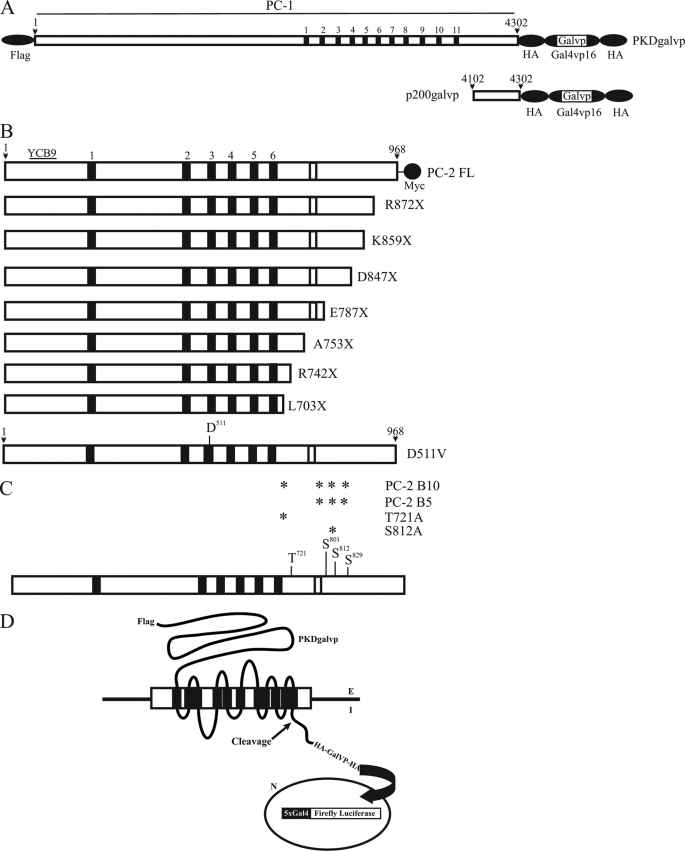
FIGURE 1.
Schematic representations of PC-1 and PC-2 constructs. A, schematic representation of the full-length polycystin-1 construct tagged at its CTT with 2× HA-epitope/galvp and at its N terminus with a FLAG epitope (PKDgalvp). The predicted CTT includes residues 4048–4302. The p200galvp construct contains the final 200 amino acids of PC-1 plus the 2× HA-epitope/galvp of the PKDgalvp and extends from residues 4102 to 4302. The predicted transmembrane domains are indicated by the black bars and are numbered 1–11. B, schematic representations of the full-length polycystin-2 protein (PC-2 FL) with a Myc tag in its C-terminal tail, C-terminal truncated PC-2 constructs (R872X, K859X, D847X, E787X, A753X, R742X, and L703X), and the point mutation in amino acid 511 (D511V) of PC-2. The relative location of the YCB9 immunogenic sequence is shown. The predicted transmembrane domains are indicated by the black bars and are numbered 1–6. The EF-hand in the C terminus is represented as a white bar. C, schematic diagram showing the predicted phosphorylation sites in the C terminus of PC-2. The asterisks indicate the residue(s) substituted by alanine in each of the four constructs. The predicted transmembrane domains are indicated by the black bars. The EF-hand in the C terminus is represented as a white bar. D, schematic representation of the luciferase reporter assay. Proteolytic cleavage of the PC-1 fusion protein releases Gal4-VP16, which translocates to the nucleus and activates a Gal4-responsive luciferase reporter gene. Therefore, luciferase expression reflects the PC-1 CTT cleavage.
The D511V mutant cDNA construct was made by introducing GTT (Val) to replace GAT (Asp) at codon 511 of PC-2 by site-directed mutagenesis in pcDNA3.1 (18) (Fig. 1B) using the QuickChange kit (Stratagene, La Jolla, CA).
In addition, we produced a PC-2 clone truncated after leucine 703 (L703X) by introduction of an influenza virus hemagglutinin protein epitope tag and an in-frame stop codon as has been described previously (20) (Fig. 1B). We also introduced two naturally occurring human PC-2 mutations, R742X and R872X (3), into PC-2 by site-directed mutagenesis using the QuickChange kit. Additional truncation constructs were produced by introducing stop codons at lysine 859 (K859X), aspartate 847 (D847X), glutamate 787 (E787X), and alanine 753 (A753X) (Fig. 1B). These mutagenized clones were constructed in pcDNA3.1 and were confirmed by direct sequencing.
Amino acid substitution point mutations in predicted phosphorylation sites of the C terminus of PC-2 were introduced by site-directed mutagenesis using the QuickChange kit (21). Briefly, we substituted alanine at Thr721 (T721A) or Ser812 (S812A) as single point substitutions and in combinations of three (Ser801, Ser812, and Ser829, clone PC-2 B5; Fig. 1C) or four sites (Thr721, Ser801, Ser812, and Ser829, clone PC-2-B10; Fig. 1C). PC-2 clones were generated using PC-2 subcloned into the pcDNA3.1 vector.
Transient Transfection and Luciferase Assay
COS-7 cells were cultured in a 5% CO2, 95% air humidified incubator at 37 °C in α-MEM (Invitrogen) supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. COS-7 cells were transfected using Lipofectamine 2000 (Invitrogen). Briefly, COS-7 cells were plated onto 6-well tissue culture plates and grown to about 60–80% confluency prior to transfection. GL4.31[luc2P/GAL4UAS/Hygro] (1 μg), pRL-TK (0.2 μg), PKDgalvp (1 μg), and PC2 (6 μg) were mixed with 4 μl of Lipofectamine 2000. Transfection mixtures were added dropwise into cell culture medium and incubated at 37 °C for 40 h. The amount of DNA in each well was equalized by the addition of a control plasmid, pcDNA3.1, which was also used for the mock transfection. Transfected cells were harvested with PBS and lysed with 250 μl of 1× passive lysis buffer (Promega, Madison, WI). Cell lysates were subjected to luciferase assay using the dual luciferase assay reagent kit (Promega, Madison, WI). Luciferase signals were determined in a GloMaxTM 20/20 luminometer (Promega, Madison, WI).
All the experiments were compared with parallel experiments in which we cotransfected the CMV-GV vector, firefly and Renilla luciferases in the absence of PKDgalvp. Luciferase values in these experiments did not change in the presence of PC-2, its mutants, or when we added any of the chemical compounds tested (data not shown).
Several series of experiments were performed to assess the effects of a variety of bioactive compounds on the PC-1 cleavage assay. Clasto-lactacystin β-lactone (15 μm) was added to the media 16 h before luciferase assay. To assess the potential role of extracellular calcium, cells were incubated in calcium-free medium containing dialyzed calcium-free serum for 6 h prior to the luciferase assay. In addition, cells in calcium-containing medium were subjected to incubation with EGTA, gadolinium (Gd3+), A23187, ionomycin, [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetate-acetoxymethyl ester] (BAPTA-AM), or thapsigargin at the indicated concentrations (Fig. 4) for 6 h before the luciferase assay. Extracellular ATP disodium salt, at the indicated concentration (Fig. 4), was added for 9 h prior to the luciferase assay.
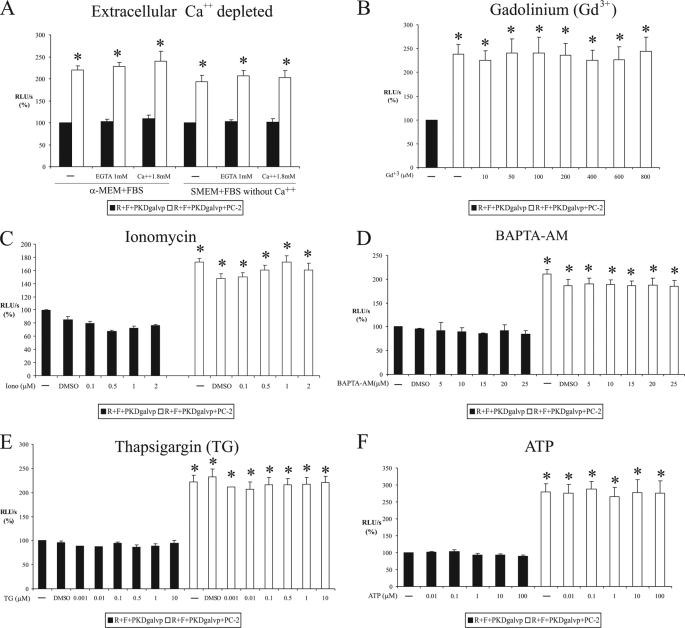
FIGURE 4.
Stimulation of C-terminal tail cleavage of PC-1 by PC-2 is independent of Ca2+. COS-7 cells were transiently transfected with PKDgalvp, PC-2, firefly luciferase reporter construct, and Renilla luciferase (F + R, respectively) and exposed to extracellular Ca2+ depletion (A), increasing concentrations of Gd3+ (B), ionomycin (C), BAPTA-AM (D), thapsigargin (E), or ATP (F). Changes in extracellular or intracellular Ca2+ concentrations did not modify the increase of CTT PC-1 cleavage induced by coexpression of PC-2. Luciferase activity was expressed as RLU/s after normalization for Renilla activity (R). Results of six (B, D, and E) or four (A, C, and F) experiments performed in duplicate are expressed as means ± S.E. (*, p < 0.01, compared with R + F + PKDgalvp without PC-2).
Immunoblot Analysis
COS-7 cells from a 10-cm dish were lysed in 400 μl of cold lysis buffer containing non-ionic detergent (1% Nonidet P-40) and a mixture of protease inhibitors (Complete, Roche Applied Science). Cell lysates were exposed to brief probe sonication to thoroughly disrupt nuclei. The protein concentration of each sample was determined via a colorimetric protein concentration assay (Bio-Rad), and equal amounts of each sample were loaded and separated on an 8% SDS-polyacrylamide gel. The gel was then electrophoretically transferred to a nitrocellulose membrane (Bio-Rad), incubated in blocking buffer (150 mm NaCl, 20 mm Tris, 5% (w/v) powdered milk, 0.1% Tween) for 60 min, and then incubated with one of the following primary antibodies at 4 °C overnight: monoclonal anti-HA antibody (1:1000, Invitrogen), monoclonal anti-FLAG (1:3000, Sigma), or anti-PC-2 (YCB9) polyclonal antibody (20). Subsequently, primary antibody binding was detected with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (1:10,000; Jackson ImmunoResearch Laboratories, West Grove, PA), and proteins were visualized with an enhanced chemiluminescence detection kit (ECL, Amersham Biosciences).
Nuclear Preparations
The nuclear preparation protocol was modified from Lin et al. (22). COS-7 cells grown in 10-cm dishes were harvested in cold PBS, centrifuged for 5 min at 500 × g, and resuspended in hypotonic buffer (10 mm HEPES, 1.5 mm MgCl2, 10 mm KCl, and a standard mixture of protease inhibitors). Cells were homogenized in a tight-fitting Dounce homogenizer, chilled on ice for 10 min, and then shaken for 15 min with an Eppendorf mixer 5432. Nonidet P-40 was added (1% final concentration), and the preparations were shaken for an additional 15 min. The lysates were centrifuged at 1500 × g for 5 min. The resulting supernatant formed the non-nuclear fractions. The pellets (nuclear fractions) were washed in hypotonic buffer, resuspended in high salt buffer (20 mm HEPES, 420 mm NaCl, 1.5 mm MgCl2, 0.2 mm EDTA, 25% glycerol, and protease inhibitors), shaken for 20 min, and finally centrifuged at 14,000 × g for 30 min. The pellets were sonicated and prepared for immunoblot analysis. All steps were performed at 4 °C. The protein concentration of each sample was determined using a Bio-Rad colorimetric protein concentration assay.
Medium Concentration
COS-7 cells grown in 10-cm dishes were transfected as was described above. At 8 h after transfection, cell monolayers were washed twice with serum-free medium, after which the cultures were maintained in serum-free medium for 34 h. The culture medium was then collected, subjected to centrifugation to remove residual cells, and concentrated 200-fold by using a 100K Amicon membrane filter (Amicon-Ultra, Millipore, Tullagreen, Ireland). Each sample was stored at −80 °C.
Immunofluorescent Cell Staining
COS-7 cells were grown on glass coverslips, fixed in 2% paraformaldehyde dissolved in PBS, permeabilized in PBS, 0.3% Triton, 0.1% BSA blocked in GSDB (16% goat serum, 120 mm sodium phosphate, 0.3% Triton X-100, and 450 mm NaCl), and incubated with primary antibody at room temperature. In clasto-lactacystin β-lactone experiments, full-length PKDgalvp construct was detected with a polyclonal antibody against HA (1:200 dilution; Invitrogen) and a monoclonal antibody against FLAG (1:200 dilution; Sigma). Fluorescein isothiocyanate-conjugated anti-rabbit IgG (1:100 dilution; Sigma) and rhodamine-conjugated anti-mouse IgG (1:100 dilution; Chemicon International, Billerica, MA) were used as secondary antibodies. In experiments involving full-length PC-2 or its mutants, HA and FLAG epitope tags in PKDgalvp were detected with monoclonal antibodies against HA and FLAG, respectively (1:200; Sigma), and fluorescein isothiocyanate-conjugated anti-mouse IgG (1:100, Sigma) was used as the secondary antibody. PC-2 or PC-2 mutants were detected with rabbit polyclonal YCB9 (N terminus) anti-PC-2 antibody (1:10,000) (20) and detected with rhodamine-conjugated anti-rabbit IgG (1:100; Chemicon International, Billerica, MA). Nuclei were stained with Hoechst 33342 (Molecular Probes, Invitrogen). Coverslips were mounted in Vectashield (Vector Laboratories, Burlingame, CA) and were examined using either an Olympus BX51 epifluorescent microscope equipped with a ×40 objective and a Magnafire digital camera or a Leica confocal microscope, using a ×40 oil-immersion objective. To quantify the nuclear localization of PC-1, a line was traced following the major axis of each nucleus, and the mean intensity along this line was determined for either PKDgalvp-HA or PKDgalvp-FLAG and for Hoechst staining. No differences were observed in nuclear Hoechst staining in each of the different treatments. Thus, Hoechst staining intensity was used to normalize nuclear CTT signals measured in different studies.
Statistical Analysis
Results are expressed as means ± S.E. Differences between means were evaluated using Student's t test or analysis of variance as appropriate. Values of p < 0.05 were considered to be significant.
Reagents
The following reagents were obtained from commercial sources: clasto-lactacystin β-lactone (Calbiochem), BAPTA-AM (Calbiochem), thapsigargin (Calbiochem), ATP disodium salt (Sigma), ionomycin calcium salt (Sigma), calcimycin A23187 (Sigma), and SMEM medium (Invitrogen). All the other reagents were at least molecular biology grade and obtained from standard suppliers.
RESULTS
C-terminal Tail of PC-1 Is Released and Enters the Nucleus
The goal of this study was to define the nature of the mechanisms responsible for PC-1 CTT cleavage. Our strategy made use of a sensitive and quantitative reporter gene assay. We generated a fusion protein in which a chimera of Gal4 and VP16 was inserted into full-length PC-1 downstream of the intracellular CTT domain (PKDgalvp). Gal4 is a yeast transcription factor, and VP16 is the transcriptional activating domain of the viral VP16 protein. The PKDgalvp construct also has a FLAG epitope tag at its N terminus and two HA epitope tags at its C terminus, one on either side of Gal4VP16 (Fig. 1A). The resultant cDNA encoding PKDgalvp was cotransfected with a Gal4-driven firefly luciferase reporter gene in COS-7 cells. Proteolytic cleavage of the PKDgalvp fusion protein will release the CTT, which may then translocate to the nucleus and activate expression of a firefly luciferase reporter gene under the control of the Gal4 promoter. Therefore, luciferase expression is a function of PC-1 CTT cleavage, and luciferase activity could be measured using a quantitative assay. When the PKDgalvp protein was expressed, we observed the maximum luciferase activity after 36–42 h post-transfection. All data in Fig. 2 and in subsequent figures are normalized to the values obtained with triple transfection of Renilla luciferase + Gal4 promoter-driven firefly luciferase reporter construct + PKDgalvp, which is set to 100%. No increases in luciferase activity were observed in COS-7 cells transfected with R alone or with R + PKDgalvp or R + F (1.54 ± 0.45, 2.18 ± 0.72, and 2.56 ± 0.62% of the RLU/s value obtained with R + F + PKDgalvp, respectively; Fig. 2A, p, NS). Transfection with R + F + PKDgalvp produced a dramatic (∼100-fold) increase in luciferase activity (Fig. 2A). It should be noted that a very similar assay was developed and successfully employed to study the C-terminal tail cleavage of fibrocystin, the product of the gene mutated in autosomal recessive polycystic kidney disease. These investigators performed a control for nonspecific cleavage that made use of the human low density lipoprotein receptor, a single pass membrane protein, with Gal4-VP16 appended at its C terminus. They observed that this construct is not subjected to proteolysis, demonstrating the specificity of the assay (23).
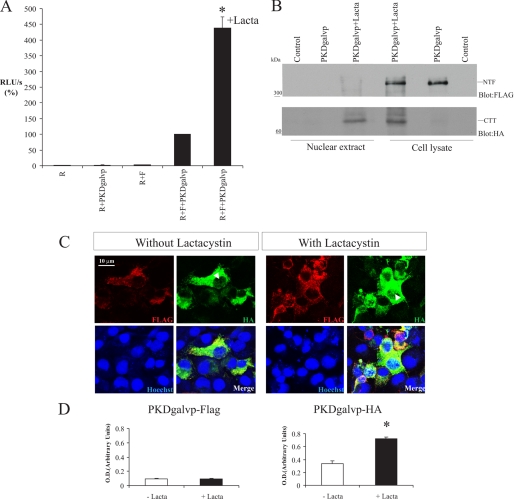
FIGURE 2.
C-terminal tail of PC-1 undergoes proteasomal degradation. A, COS-7 cells were transiently transfected with vectors expressing PKDgalvp as well as the firefly luciferase reporter plasmid and a Renilla luciferase plasmid (F + R, respectively). Luciferase activity stimulated by PKDgalvp increased significantly after treatment for 16 h in the presence of 15 μm of clasto-lactacystin β-lactone (Lacta) (*, p < 0.001, compared with R + F + PKDgalvp; n = 6 determined in duplicate each time). Luciferase activity was expressed as RLU/s after normalization for Renilla (R) activity. B, Western blot of total cell lysates and nuclear extracts from COS-7 cells transfected with the PKDgalvp construct, in the absence or presence of clasto-lactacystin β-lactone. A band of high molecular weight (∼350 kDa) was detected only in total cell lysates, either with or without clasto-lactacystin β-lactone, with an anti-FLAG antibody, which recognizes the N terminus of PC-1. A band of ∼65 kDa, corresponding to the predicted weight of the CTT of PC-1 coupled to galvp, was observed in the total lysate and in nuclear extracts in the presence of clasto-lactacystin β-lactone with anti-HA antibody, which recognizes the C terminus of the PC-1 construct. Each transfection contained the same ratios of DNA used for the luciferase assays. C, in COS-7 cells transfected with PKDgalvp, the N-terminal FLAG epitope was detected by immunofluorescence microscopy in association with intracellular membranes (red) with or without the addition of clasto-lactacystin β-lactone. In contrast, the C-terminal HA epitope (green) was detected in the nucleus, and nuclear staining was increased in the presence of clasto-lactacystin β-lactone. Arrowheads indicate nuclear localization of the PC-1 C terminus with the HA antibody. Nuclei were stained with Hoechst 33342 (blue). The scale bar indicates 10 μm. D, relative intensity of PKDgal-HA and PKDgalvp-FLAG standardized against nuclear Hoechst staining shows nuclear translocation of PKDgal-HA (CTT) after treatment with clasto-lactacystin β-lactone (*, p < 0.01, compared with the condition without clasto-lactacystin β-lactone; total cells analyzed = 25 for each condition).
Chauvet et al. (10) have demonstrated that PC-1 is subject to a proteolytic cleavage in response to alterations in mechanical stimuli, which releases the cytoplasmic CTT of the protein. The CTT can enter the nucleus, where it regulates the expression of genes involved in cellular proliferation and differentiation. It has also been shown that Siah-1, a protein that functions to mediate the ubiquitin-dependent degradation of target proteins, regulates the ubiquitination and degradation of PC-1 C-terminal fragments by the proteasome pathway (24). To test whether the signaling capacity of the PC-1 CTT is decreased by proteasome degradation, we used COS-7 cells transiently transfected with PKDgalvp with or without the addition of clasto-lactacystin β-lactone, an irreversible inhibitor of the proteasome. In the presence of clasto-lactacystin β-lactone, luciferase activity increased significantly (∼4-fold) compared with R + F + PKDgalvp (438.22 ± 36.20% of the RLU/s value obtained with R + F + PKDgalvp, p < 0.001; Fig. 2A). Furthermore, Western blotting with an anti-HA epitope tag antibody that recognizes the C terminus of PKDgalvp revealed a band corresponding to the molecular weight of the CTT of PC-1 plus gal4/vp16 in total lysates when clasto-lactacystin β-lactone was added (Fig. 2B) but not in its absence. This band was also present when we performed Western blotting with the anti-HA antibody on nuclear fractions prepared from COS-7 cells transiently transfected with PKDgalvp and treated with clasto-lactacystin β-lactone (Fig. 2B). When an antibody against FLAG, which recognizes the tag located at the N terminus of the PKDgalvp protein, was used, a band corresponding to the size of the PC-1 N-terminal fragment (NTF) produced by autocatalytic cleavage at the GPS site was observed in cell lysates with or without clasto-lactacystin β-lactone. This band was not detected in the nuclear extract preparations (Fig. 2B).
To confirm that this increase in luciferase activity was because of an increase in nuclear translocation of the CTT, COS-7 cells transiently transfected with PKDgalvp and incubated in the presence or absence of the proteosome inhibitor were examined by immunocytochemistry using an antibody directed against the HA epitope tag. An increase in nuclear staining was observed in the cells that had been treated with the proteasome inhibitor (Fig. 2, C and D). This nuclear signal was not seen by immunofluorescence when an antibody against the FLAG tag was used. Together, these data demonstrate that the released CTT of PC-1 appears to undergo rapid proteasomal degradation and that its nuclear accumulation is enhanced by proteasome inhibition.
Polycystin-2 Expression Stimulates the C-terminal Tail Cleavage
It has been shown that PC-1 and PC-2 interact directly via their C-terminal domains (14, 15). To test the possibility that the interaction of PC-2 with PC-1 could modulate CTT cleavage and its nuclear translocation, we transiently coexpressed full-length PC-2 and the PKDgalvp construct in COS-7 cells. In the presence of PC-2, luciferase activity was significantly increased almost 3-fold compared with R + F + PKDgalvp (294.53 ± 18.96% of the RLU/s value obtained with R + F + PKDgalvp alone, p < 0.01; Fig. 3A). Moreover, this increase in luciferase activity proved to be PC-2 concentration-dependent (Fig. 3B).
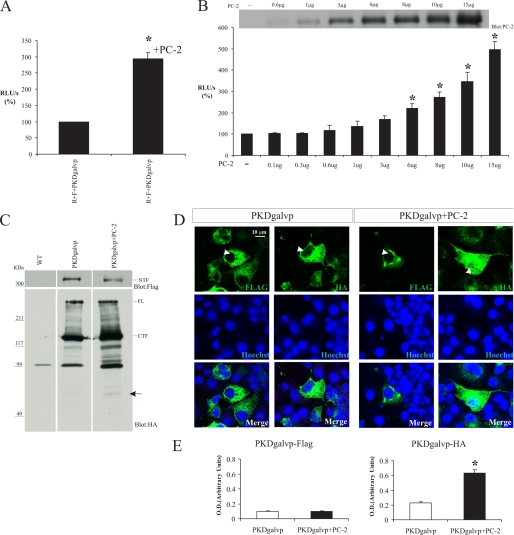
FIGURE 3.
PC-1 C-terminal tail cleavage is increased in the presence of wild type PC-2. A, COS-7 cells were transiently transfected with PKDgalvp, PC-2, firefly luciferase reporter construct, and Renilla luciferase (F + R, respectively). Luciferase activity significantly increased in the presence of PC-2 (p < 0.05, compared with no PC-2, n = 5 determined by duplicate each time). B, COS-7 cells were transiently transfected with PKDgalvp and with different concentrations of PC-2, firefly luciferase reporter construct, and Renilla luciferase (F + R, respectively). The increase in luciferase activity was PC-2 concentration-dependent (*, p < 0.01, compared with no PC-2; n = 3 experiments performed in duplicate). Top, representative Western blot of total cell lysates from COS-7 cells transfected with different concentrations of PC-2 and probed with YCB9 antibody. Luciferase activity was expressed as RLU/s after normalization for Renilla activity (R). C, representative Western blot of total cell lysates from COS-7 cells transfected with both the PKDgalvp and the PC-2 constructs. A band of high molecular weight (∼350 kDa, NTF) was detected in the presence or absence of PC-2 with the anti-FLAG antibody, which recognizes the N terminus of PC-1 (upper blot). Full-length PC-1 (FL) and a band of ∼150 kDa (CTF) were observed in total lysates with the anti-HA antibody, which recognizes the C terminus of PC-1 (lower blot). Furthermore, a band of ∼65 kDa, corresponding to the CTT of PC-1 plus galvp, was detected by anti-HA antibody only in the presence of PC-2 (arrow). Each transfection contained the same ratios of DNA used for the luciferase assays. All three lanes shown are from a single immunoblot. WT, wild type. D, COS-7 cells were transiently transfected with PKDgalvp alone or together with PC-2 and were immunostained with the anti-FLAG or anti-HA antibodies that recognize the N and C terminus of PC-1, respectively. The N-terminal FLAG epitope was detected in association with intracellular membranous structures in the presence or absence of PC-2. In contrast, the C-terminal HA epitope was detected in the nucleus, and nuclear staining was increased in the presence of PC-2. Arrowheads indicate nuclear localization. Nuclei were stained with Hoechst (blue). The scale bar indicates 10 μm. E, relative intensity of PKDgal-HA and PKDgalvp-FLAG standardized against nuclear Hoechst staining shows nuclear translocation of PKDgal-HA (CTT) in the presence of PC-2 (*, p < 0.01, compared with no PC-2; total cells analyzed = 30 for each condition).
Total cellular lysates of COS-7 cells transfected with PKDgalvp alone or PKDgalvp and PC-2 were analyzed by Western blotting. When the anti-HA antibody was used, a band corresponding to the molecular weight of the PC-1 full-length protein was detected. It has been shown recently that PC-1 undergoes an autocatalytic cleavage at a site that is homologous to a GPS (5). This cleavage essentially removes the entire extracellular N terminus of the protein (∼350-kDa fragment, NTF) and generates an ∼150-kDa C-terminal fragment that is composed of all of the transmembrane domains and the CTT of PC-1 (CTF). The CTF band was also observed when we used the anti-HA antibody (Fig. 3C, lower blot). When we used the anti-FLAG antibody against the tag located at the N terminus of PC-1 full length, a band corresponding to the expected size of the NTF of PC-1 was observed, but the density of this band decreased in the presence of PC-2 (Figs. 3C and 5D). Using the anti-HA antibody, we were able to detect an increase in the level of the CTT cleavage fragment of PC-1 in the presence of PC-2, even in absence of clasto-lactacystin β-lactone (arrow in Fig. 3C and Fig. 5B). Moreover, by immunofluorescence we were able to detect that the subpopulations of cells that were simultaneously cotransfected with PC-2 and PKDgalvp constructs (PC-2 staining not shown) showed readily detectable nuclear staining with the anti-HA tag antibody. No nuclear signal was observed when we used anti-FLAG antibody (Fig. 3, D and E). Taken together, these data suggest that the presence of PC-2 enhances the CTT cleavage of PC-1 and increases the size of the population of the cleaved CTT fragment.
FIGURE 5.
D511V mutant of PC-2 increases CTT production without inducing Gal4 reporter activation. A, COS-7 cells were transiently transfected with PKDgalvp, PC-2, or two PC-2 mutants, D511V or L703X, firefly luciferase reporter construct, and Renilla luciferase (F + R, respectively). Although the full-length PC-2 protein stimulates luciferase activity, this increase does not occur with either the D511V or the L703X mutants. Luciferase activity was expressed as RLU/s after normalization for Renilla activity (R). *, p < 0.01, compared with R + F + PKDgalvp; n = 6 assays performed in duplicate. B, representative Western blots of total cell lysates from COS-7 cells transfected with PKDgalvp alone or with wild type PC-2, D511V, or L703X constructs. Full-length PC-1 was observed in total lysates with anti-HA antibody, which recognizes the C terminus of PC-1. Furthermore, two bands of ∼150 and ∼65 kDa, corresponding to the PC-1 CTF and CTT plus galvp, respectively, were detected with anti-HA. CTF expression was decreased in the presence of the PC-2 mutants. CTT expression was increased in the presence of PC-2 and D511V. Immunoblotting with antibodies to PC-2 and β-actin are also shown. The variation in migration of wild type PC-2 versus D511V is because of the presence of a Myc tag in the C-terminal tail of the wild type PC-2 construct. Each transfection contained the same ratios of DNA used for the luciferase assays. C, densitometric analysis of PKDgalvp-FL, PKDgalvp-CTF, and PKDgalvp-CTT bands detected with the anti-HA antibody shows the mean ± S.E. of optical density in arbitrary units (*, p < 0.01, compared with PKDgalvp; n = 4 experiments). D, representative Western blots of total cell lysates and tissue culture medium from COS-7 cells transfected with PKDgalvp alone or with wild type PC-2, D511V, or L703X constructs. A band of 350 kDa (NTF) was detected in the presence or absence of PC-2, D511V, or L703X by anti-FLAG antibody, which recognizes the N terminus of PC-1. In total cell lysates and tissue culture medium, the NTF expression was decreased in the presence of both PC-2 mutants.
Increase of CTT Cleavage by PC-2 Is Independent of Extracellular Ca2+
Polycystin-2 is a transmembrane protein that has been shown to function as a Ca2+ channel in vivo (18, 25). To determine whether the Ca2+ channel function of PC-2 is important for CTT cleavage regulation, we performed several manipulations designed to change extracellular and intracellular calcium levels. In our initial experiments, we transfected COS-7 cells with PKDgalvp alone or with PKDgalvp and PC-2 and maintained the cells for 6 h in media lacking extracellular Ca2+. When we used medium with Ca2+ (α-MEM), medium without Ca2+ (SMEM), or Ca2+-free medium supplemented with EGTA to chelate extracellular Ca2+, we were not able to detect any changes in the capacity of PC-2 to increase the luciferase activity (in α-MEM control, 219.71 ± 10.25% versus EGTA 228.45 ± 8.83%; in SMEM control, 192.67 ± 15.76% versus EGTA 206.51 ± 12.77% of the RLU/s value obtained with R + F + PKDgalvp alone, p, NS; Fig. 4A). The re-addition of extracellular Ca2+ to Ca2+-free medium also did not produce any change in PC-2-stimulated luciferase activity (in α-MEM control, 219.71 ± 10.25% versus Ca2+ 1.8 mm, 240.03 ± 22.82%; in SMEM control, 192.67 ± 15.76% versus Ca2+ 1.8 mm, 203.13 ± 14.93% of the RLU/s value obtained with R + F + PKDgalvp alone, p, NS; Fig. 4A). Cantiello and co-workers (25) have demonstrated that PC-2 channel activity is sensitive to Gd3+ blockage. When we blocked cell surface Ca2+ channel activity with different concentrations of Gd3+, luciferase activity did not change in the presence of PC-2 (control, 238.13 ± 19.86% versus 800 μm Gd3+, 44.08 ± 30.13% of the RLU/s value obtained with R + F + PKDgalvp alone, p, NS; Fig. 4B). These data suggest that neither extracellular calcium nor cell surface calcium channel activity is required for PC-2 to exert its stimulatory effect on PC-1 CTT cleavage.
Stimulation of CTT Cleavage by PC-2 Is Independent of Intracellular Ca2+
It has been reported that the PC-2 Ca2+ channel activity is positively regulated by intracellular Ca2+ (18, 26). To determine whether intracellular Ca2+ is necessary for the increase of CTT cleavage induced by PC-2, COS-7 cells transfected with PKDgalvp alone or with PKDgalvp and PC-2 were incubated with different concentrations of two different calcium ionophores (A23187 and ionomycin) or with the permeable intracellular calcium chelator BAPTA-AM. We found that the increase of CTT cleavage induced by PC-2 was unaffected by the treatment with ionomycin (control, 172.70 ± 5.91%; DMSO, 147.93 ± 7.07%; 2 μm ionomycin, 160.92 ± 10.20% of the RLU/s value obtained with R + F + PKDgalvp alone, p, NS; Fig. 4C) or A23187 (control, 179.06 ± 3.28%; DMSO, 169.51 ± 2.85%; 2 μm A23187, 166.17 ± 0.45% of the RLU/s value obtained with R + F + PKDgalvp alone, p, NS) or BAPTA-AM (control, 210.87 ± 9.17%; DMSO, 186.50 ± 13.05%; 25 μm BAPTA-AM, 184.77 ± 13.10% of the RLU/s value obtained with R + F + PKDgalvp alone, p, NS; Fig. 4D).
To determine whether calcium derived from intracellular stores could be involved in the increase of CTT cleavage induced by PC-2, the intracellular stores were depleted by preincubating COS-7 cells with thapsigargin (TG) in the absence (data not shown) or presence of extracellular Ca2+ (Fig. 4E). The increase of CTT cleavage induced by PC-2 was not altered by the addition of TG (control, 221.84 ± 14.50%; DMSO, 232.53 ± 15.77%; 10 μm TG, 220.86 ± 13.18% of the RLU/s value obtained with R + F + PKDgalvp alone, p, NS; Fig. 4E).
In nonexcitable cells, it has been reported that extracellular ATP acts via the plasma membrane P2 purinergic receptor to generate the release of intracellular Ca2+ from the ER in response to a stimulus-induced rise in inositol 1,4,5-trisphosphate and causes an increase in intracellular Ca2+ concentrations (27, 28). When COS-7 cells cotransfected with PKDgalvp and PC-2 were treated with different ATP concentrations, the PC-2-induced PC-1 CTT cleavage was unaffected (control, 279.65 ± 23.59%; 100 mm ATP, 275.01 ± 36.28% of the RLU/s value obtained with R + F + PKDgalvp alone, p, NS; Fig. 4F). Taken together, all of these data suggest that the CTT cleavage of PC-1 induced by PC-2 is independent of any alterations in intracellular Ca2+ concentrations.
D511V-PC-2 Mutant Induces CTT Cleavage but Prevents Its Nuclear Localization
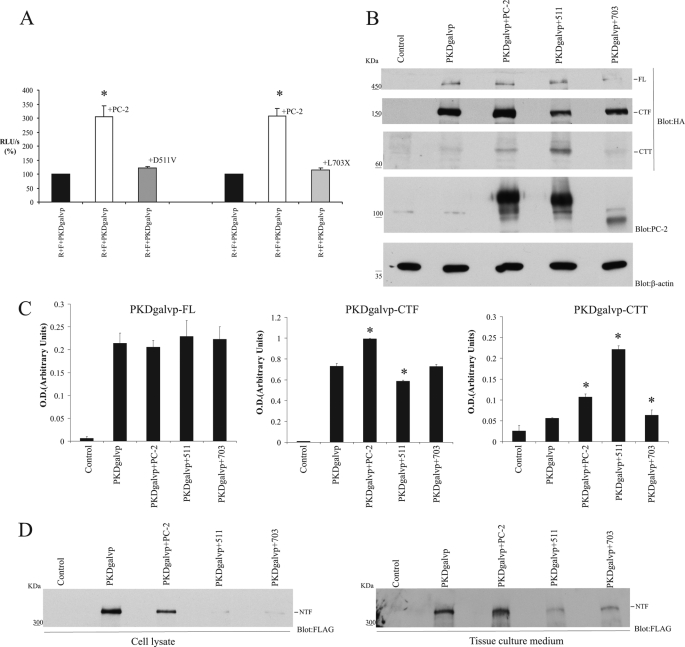
To determine which features of the PC-2 protein are important for stimulating the CTT cleavage, we coexpressed PKDgalvp with the D511V or the 703X PC-2 mutants (29). The D511V mutation abrogates the channel activity of PC-2. The D511V protein has an intact C-terminal tail. L703X lacks the C-terminal tail of PC-2 and as a result lacks several putative protein interaction domains, including the ER retention signal (3, 14, 20) and the coiled-coil domain (14). We observed that although the wild type full-length PC-2 protein stimulated luciferase activity (Fig. 3A and Fig. 5A), both D511V and L703X mutant forms failed to increase luciferase activity (PC-2, 305.7 ± 39.2% versus D511V, 122.10 ± 5.81% and PC-2, 306.9 ± 27.8% versus L703X, 114.49 ± 7.27% of the RLU/s value obtained with R + F + PKDgalvp alone, p, NS; Fig. 5A).
When lysates from the cells used in the preceding experiments were analyzed by Western blot using an anti-HA antibody against the HA tag located at the C terminus of the PKDgalvp protein, we observed bands corresponding to molecular weight of full-length PC-1, as well as the CTF and the CTT bands (Fig. 5B). We were surprised to find that although the quantity of CTT detected in these blots was not altered substantially in cells expressing the L703X mutant, it was increased in cells expressing the D511V mutant (Fig. 5, B and C). These data suggest that the D511V mutant may stimulate cleavage but prevents the cleaved fragment from entering the nucleus and activating luciferase expression.
When we used the anti-FLAG antibody directed against the tag located at the N terminus of the full-length PKDgalvp protein, we were able to detect a band corresponding to the expected size of the NTF of PC-1 in cell lysates, but the density of this band decreased in presence of PC-2 (Fig. 3C and Fig. 5D) and decreased even more in presence of both the D511V or L703X mutants (Fig. 5D). The CTF expression detected with the anti-HA antibody also showed a decrease in cells expressing the D511V mutant (Fig. 5, B and C). Qian et al. (5) have demonstrated that most of the N-terminal fragment remains tethered at the cell surface in a noncovalent fashion, although a small amount is released and secreted. To assess whether the effects of the PC-2 mutants on NTF levels might be due, at least in part, to NTF shedding into the cell culture medium, we analyzed the NTF protein levels in collected and concentrated culture medium by Western blotting. Surprisingly, the density of this band was also decreased in the presence of both D511V and L703X mutants (Fig. 5D). These data suggest that total NTF expression as well as that of the CTT of PC-1 is modulated by wild type PC-2 and its mutants.
C Terminus of Polycystin-2 Is Necessary for PC-2 Stimulation of PC-1 CTT Cleavage
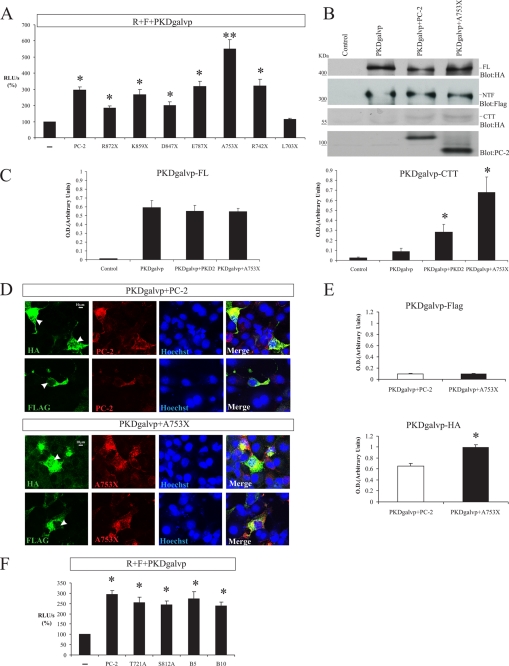
Although the full-length PC-2 protein stimulates PC-1 cleavage-dependent luciferase activity (Fig. 3A and Fig. 5A), the L703X mutant that lacks the C-terminal tail of PC-2 did not increase CTT cleavage (Fig. 5A). To find out which amino acids of the C-terminal tail of PC-2 are involved in stimulating PC-1 CTT cleavage, we used a series of truncation mutant constructs of PC-2 that were generated by introducing premature termination codons into the C terminus of the cDNA encoding PC-2 (Fig. 1B). Two of these constructs, R742X and R872X, are naturally occurring disease-causing human PC-2 mutations (3). All of the PC-2 truncation products studied here are incapable of homomultimerization or association with PC-1 (14). We found that although the PC-2 L703X truncation does not stimulate cleavage, PC-2 constructs that terminate at residues Arg872, Lys859, Asp847, Glu787, or Arg742 stimulated PC-1 CTT cleavage to roughly the same extent as full-length PC-2 (PC-2, 294.5 ± 18.9%; R872X, 185.7 ± 11.7%; K859X, 266.2 ± 31.8%; D847X, 199.6 ± 22.3%; E787X, 317.3 ± 33.4%; and R742X, 319.8 ± 41.4% of the RLU/s value obtained with R + F + PKDgalvp alone; Fig. 6A). Surprisingly, the A753X truncated construct appeared to increase PC-1 CTT cleavage beyond the levels obtained with full-length PC-2 (PC-2, 294.5 ± 18.9% versus A753X, 549.8 ± 46.6% of the RLU/s value obtained with R + F + PKDgalvp alone, p < 0.05 compared with R + F + PKDgalvp + PC-2; Fig. 6A). Total cellular lysates from COS-7 cells transiently transfected with PKDgalvp alone or with full-length PC-2 or A753X were analyzed by Western blot. Immunoblotting with the anti-HA antibody that recognizes the C terminus of PC-1 showed increased levels of the PC-1 CTT cleavage product in the presence of A753X as compared with those detected with full-length PC-2 (Fig. 6, B and C). Immunofluorescence analysis of transiently transfected COS-7 cells also showed that expression of the mutant A753X PC-2 protein increased nuclear localization of the PC-1 CTT cleavage product (Fig. 6, D and E), as detected with an anti-HA antibody that recognizes the C terminus of PKDgalvp.
FIGURE 6.
PC-2 C-terminal tail truncation mutants increase PC-1 CTT cleavage. A, COS-7 cells were transiently transfected with PKDgalvp alone or with different C-terminally truncated PC-2 constructs (R872X, K859X, D847X, E787X, A753X, and R742X), together with the firefly luciferase reporter construct and Renilla luciferase (F + R, respectively). R872X, K859X, D847X, E787X, and R742X stimulated PC-1 CTT cleavage to the same extent as full-length PC-2 (*, p < 0.01, compared with R + F + PKDgalvp). The A753X truncated construct increased PC-1 CTT cleavage beyond the levels obtained with wild type PC-2 (**, p < 0.05, compared with R + F + PKDgalvp + PC-2), n = 4 assays performed in duplicate. B, representative Western blot of total cell lysates from COS-7 cells transfected with both PKDgalvp and the full-length (FL) PC-2 or A753X constructs, using the anti-HA antibody that recognizes the C terminus of PC-1. The immunoblot shows a larger increase in CTT cleavage of PC-1 in the presence of A753X than was observed with wild type PC-2. Immunoblotting with antibody to PC-2 and the FLAG epitope is also shown. C, densitometric analysis of PKDgalvp-FL and PKDgalvp-CTT bands detected using the anti-HA antibody show mean ± S.E. of optical density in arbitrary units (*, p < 0.01, compared with PKDgalvp; n = 3 experiments). D, COS-7 cells were transiently transfected with PKDgalvp and with the PC-2 wild type or A753X constructs and were immunostained with anti-FLAG or anti-HA antibodies, which recognize the N and C terminus of PC-1, respectively. The N-terminal FLAG epitope was detected in association with intracellular membranous structures in the presence or absence of either PC-2 or A753X. In contrast, the C-terminal HA epitope was detected in the nucleus in the presence of both PC-2 and A753X, but A753X increased the nuclear localization of PC-1 CTT. YCB9 antibody was used to detect the N terminus of PC-2 and A753X (red). Arrowheads indicate nuclear localization. Nuclei were stained with Hoechst (blue). The scale bar indicates 10 μm. Each transfection contained the same ratios of DNA used for the luciferase assays. E, relative intensity of PKDgal-HA and PKDgalvp-FLAG standardized against nuclear Hoechst staining shows increased nuclear translocation of PKDgal-HA (CTT) in the presence of A753X (*, p < 0.01, compared with PC-2; total cells analyzed = 30 for each condition). F, COS-7 cells were transiently transfected with a vector expressing PKDgalvp alone or with different C-terminal phosphorylation site mutants of PC-2 (S812A, T721A, PC-2 B10, and PC-2 B5), as well as the firefly luciferase reporter plasmid and Renilla luciferase plasmid (F + R, respectively). Phosphorylation site mutants of the PC-2 C terminus stimulated PC-1 CTT cleavage to the same extent as wild type PC-2. Luciferase activity was expressed as RLU/s after normalization for Renilla activity (R). *, p < 0.01, compared with R + F + PKDgalvp, n = 5 experiments performed in duplicate.
PC-2 is predicted to have at least four serine/threonine phosphorylation sites in its cytoplasmic C terminus (3). To determine whether phosphorylation sites in PC-2 are involved in PC-1 CTT cleavage, we substituted Thr721 (T721A) or Ser812 (S812A) with alanine to create single point substitution mutant proteins or proteins carrying these mutations in various combinations of three (Ser801, Ser812, and Ser829, clone PC-2 B5; Fig. 1C) (21) or four (Thr721, Ser801, Ser812, and Ser829, clone PC-2 B10; Fig. 1C) (21). Phosphorylation site mutants stimulated PC-1 CTT cleavage to the same extent as wild type PC-2 (PC-2, 294.5 ± 18.9%; T721A, 253.3 ± 27.9%; S812A, 243.8 ± 18.7%; B5, 272.8 ± 34.8%; and B10, 239.7 ± 18.6% of the RLU/s value obtained with R + F + PKDgalvp alone; Fig. 6F). These data demonstrate that the release of the CTT of PC-1 is influenced by the C terminus of PC-2, and this mechanism seems to be regulated by two sequences contained within the regions of the PC-2 C-terminal tail defined by A753X and the sequence between R742X and L703X.
PC-2 and the D511V-PC2 Mutant Increase Nuclear Accumulation of the Last 200 Amino Acids of PC-1
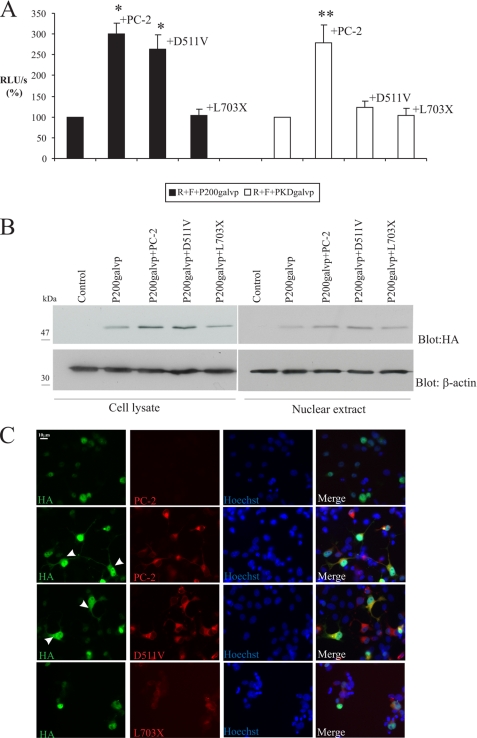
The cytoplasmic tail of PC-1 is thought to encompass approximately the last 226 residues of the C terminus of the protein. To generate a cytosolic fragment of PC-1 that is not covalently linked to a transmembrane segment, we created a soluble protein containing the last 200 amino acids of PC-1, which is designated as p200. This construct was engineered to carry the galvp sequence (additional ∼27 kDa) and two intact HA tags at its C terminus (Fig. 1A). The resultant p200galvp construct migrates in SDS-PAGE as a protein with a molecular mass of ∼52 kDa. Chauvet et al. (10) demonstrated that p200 accumulates in the nucleus of COS-7 cells. To determine whether nuclear accumulation of the last 200 amino acids of PC-1 is altered in the presence of PC-2 or its mutants, p200galvp alone or with either wild type PC-2 or the D511V and L703X PC-2 mutant was transfected in COS-7 cells. In the presence of PC-2 wild type or D511V, luciferase activity increased 3-fold (300.7 ± 25.8% and 263.6 ± 33.6% of the RLU/s value obtained with R + F + p200galvp, respectively, p < 0.01; Fig. 7A) compared with R + F + p200galvp. On the other hand, the L703X construct did not increase luciferase activity (103.3 ± 15.2% of the RLU/s value obtained with R + F + p200galvp; Fig. 7A). Thus, although the D511V form of PC2 does not stimulate luciferase production when it is expressed in association with full-length PKDgalvp, it appears to increase the transcriptional activity of p200galvp, perhaps by binding to this protein and preventing its proteosome-mediated degradation.
FIGURE 7.
PC-2 and the D511V-PC2 mutant increase functional nuclear accumulation of the last 200 amino acids of PC-1. A, COS-7 cells were transfected with either p200galvp or PKDgalvp alone and with wild type PC-2, D511V, or L703X, as well as the firefly luciferase reporter construct and Renilla luciferase plasmid (F + R, respectively). In COS-7 cells transfected with p200galvp, luciferase activity increased in the presence of wild type PC-2 and with D511V, whereas in COS-7 cells transfected with PKDgalvp, functional nuclear accumulation of the CTT increased only in the presence of the wild type PC-2 protein. Luciferase activity was expressed as RLU/s after normalization for Renilla activity (R). *, p < 0.01, compared with R + F + p200galvp; **, p < 0.05, compared with PKDgalvp alone; n = 3 experiments performed in duplicate. B, expression levels of the p200galvp construct were determined by Western blotting using anti-HA antibody. Total cell lysates prepared from the same COS-7 cells transiently transfected with p200galvp as in A showed that p200galvp expression increased in the presence of wild type PC-2 and with the D511V mutant. In nuclear fractions, p200galvp expression also increased with wild type PC-2 and D511V. The p200 protein migrated with an apparent molecular mass of ∼52 kDa. Immunoblotting with antibody to β-actin is also shown. C, COS-7 cells were transiently transfected with p200galvp alone or with wild type PC-2 or the D511V or L703X mutants and were immunostained with the anti-HA antibody that recognizes the C terminus of p200 (green) or YCB9 antibody that recognizes the N termini of PC-2, D511V, and L703X (red). The C-terminal HA epitope was detected in the nucleus in the presence of p200galvp alone or with L703X, but nuclear and cytoplasmic staining were detected when p200galvp was expressed with wild type PC-2 and the D511V mutant. Arrowheads indicate cytoplasmic localization. Nuclei were stained with Hoechst (blue). The scale bar indicates 10 μm.
To test this possibility, total cellular lysates and nuclear fractions of COS-7 cells transfected with p200galvp alone or together with PC-2, D511V or L703X were analyzed by Western blot. When we probed these blots with an anti-HA antibody against the tag located at the C terminus of the p200, we were able to see that p200 expression was increased in the presence of wild type PC-2 or D511V in cell lysates and nuclear preparations, but this expression was not enhanced when p200galvp and L703X were coexpressed (Fig. 7B). Immunocytochemistry showed that in COS-7 cells expressing PC-2 or the D511V mutant, the p200galvp protein has a cytoplasmic and nuclear localization, whereas it has only a nuclear localization in COS-7 cells expressing p200galvp with the L703X PC-2 mutant (Fig. 7C). C-terminal truncated constructs and phosphorylation site mutants of PC-2 enhanced p200galvp accumulation to the same extent as full-length PC-2 (data not shown). These results suggest that PC-2 and the D511V mutant stabilize the expression of the p200 construct as well as that of the cleaved native PC-1 CTT.
DISCUSSION
Chauvet et al. (10) have demonstrated that in renal epithelial cells overexpressing full-length PC-1, the CTT PC-1 was cleaved from the membrane and targeted to the nucleus. Nuclear CTT may be able to regulate genes involved in cellular proliferation and differentiation. In this study, we explore further the mechanisms involved in PC-1 CTT cleavage. To monitor the cleavage of PC-1 protein with a sensitive and quantitative method, we took advantage of a unique reporter gene assay. We generated a fusion protein in which a chimera of the Gal4 transcription factor and VP16 transcriptional activator was inserted downstream of the PC-1 intracellular CTT domain. We expected that if the CTT of PC-1 is cleaved, we should see an increase in luciferase activity in cells cotransfected with a cDNA encoding luciferase under the control of the Gal4 promoter. We find that the cleavage of the PC-1 C-terminal tail is increased in the presence of polycystin-2 (PC-2) and that this effect is independent of any alterations in Ca2+ concentrations. Furthermore, this PC-2 stimulation does not appear to require the coiled-coil domain interaction between PC-1 and PC-2.
We found that the cleaved PC-1 CTT could be detected by Western blotting and immunocytochemical analysis in the presence but not in absence of clasto-lactacystin β-lactone, an irreversible inhibitor of the proteasome (Fig. 2B) when using an anti-HA antibody that specifically recognizes an HA tag inserted at the C terminus of PC-1. In addition, PC-1 CTT nuclear accumulation was enhanced by proteasome inhibition. Rechsteiner and Rogers (30) have observed that many short lived eukaryotic proteins contain a PEST sequence (30), which targets proteins for rapid degradation in the proteasome. The PEST motif seems to be important for regulatory molecules that require a fast turnover or proteins that are subjected to rapid degradation. Tsiokas et al. (15) have observed that cells transfected with the C-terminal 226 amino acids of PC-1 express very low levels of this protein but express much higher levels of a construct comprising the last C-terminal 113 amino acids of PC-1. These investigators suggested that the presence of a potential PEST sequence spanning amino acids 93–109 may account for this effect. Our data suggest that the cleaved C-terminal tail of PC-1 may be similarly susceptible to PEST-mediated destruction, because lactacystin inhibition of proteasome activity dramatically increases the accumulation of the cleaved C-terminal tail. Interestingly, Kim et al. (24) demonstrated that the human homologue of Drosophila Seven in Absentia (Siah-1) interacts with the C-terminal fragment of PC-1 in vivo. This interaction induced the ubiquitination and degradation of PC-1 via the ubiquitin-dependent proteasome pathway, shortening its half-life.
Mutations in either mammalian PC-1 or PC-2 result in almost identical phenotypes, suggesting that the polycystins participate in a common and evolutionarily conserved signaling pathway (31). Both polycystins have been shown to interact physically with one another (14, 15), and these proteins have been implicated in a wide array of diverse cellular signaling pathways. Delmas et al. (33) have found that full-length PC-1 functioned as a nontraditional G protein-coupled receptor that activates Gαi/o-type trimeric G proteins, releases Gβγ subunits, and is antagonized by PC-2. Parnell et al. (34) have demonstrated that the Gβγ subunits of heterotrimeric G proteins and protein kinase Cα mediate PC-1-induced JNK/AP-1 activation, whereas PC-2 expression up-regulated JNK/AP-1 upon activation of protein kinase Cϵ (35). Bhunia et al. (36) have shown that PC-1 inhibits cyclin-dependent kinase-2 by up-regulating its inhibitor, p21CIP1/WAF1. In addition, PC-1 activates the JAK-STAT signaling pathway in a process that requires PC-2 and that leads to activation of p21CIP1/WAF1 (36). In the primary cilia the activation of PC-1 by an as yet unidentified stimulus appears to lead to an activation of PC-2 that releases Ca2+ from the ER (17). The PC-1-PC-2 complex may also be anchored to the cytoskeleton through a number of actin-binding adapter proteins (37). These studies suggest that PC-1 and PC-2 function in a cooperative manner to regulate processes involved in the control of cell proliferation (38), cell adhesion (39), and mechanotransduction (17). Thus, PC-1 and PC-2 appear to work in conjunction with each other. It remains unclear, however, whether the interaction of PC-2 with PC-1 may modulate cleavage and nuclear translocation of the PC-1 CTT. Our data demonstrate that the presence of the wild type PC-2 protein enhances the CTT cleavage of wild type PC-1 and increases the soluble pool of the cleaved CTT fragment. In the presence of PC-2, Gal4-driven luciferase activity increased almost 3-fold (Fig. 3A) compared with that in the absence of PC-2 in cells expressing a PC-1 construct in which Gal4VP16 is appended to the CTT. Moreover, by immunofluorescence analysis we were able to observe nuclear staining with the anti-HA tag antibody, which recognizes the C terminus of PC-1, and we were able to detect an increase in CTT cleavage by Western blot, even in the absence of clasto-lactacystin β-lactone, when PC-1 is coexpressed with PC-2 (Fig. 3B). This effect could be due either to a stimulatory effect of PC-2 on PC-1 cleavage or to a stabilization of the cleaved CTT. Because PC-2 increases cellular levels of the soluble CTT construct p200, we hypothesize that PC-2 stabilizes the quantity of PC-1 CTT, perhaps by inhibiting its proteasomal degradation. It remains to be determined whether and how the presence of full-length PC-2 inhibits the proteasomal degradation of CTT. It is possible that at least some fraction of the PC-2-induced increase in the quantity of the PC-1 CTT could be attributable to stabilization of PC-1 levels by PC-2 expression, leading to an increase in the total cellular population of PC-1 and thus perhaps an increase, by simple mass action, of PC-1 cleavage and CTT generation. The data presented in Figs. 3C and 5B, however, indicate that any PC-2-induced increases in PC1 levels are relatively small and could not account directly for the 3-fold increase in luciferase activity that PC-2 expression produces in the luciferase reporter assay.
The physiological role of PC-2 appears to depend upon its subcellular localization. PC-2 has been proposed to function as a Ca2+-activated Ca2+-release channel in the ER requiring activation of the inositol trisphosphate receptor (18), as a nonselective Ca2+ channel in the presence or absence of PC-1 on the plasma membrane (16, 25), or as a Ca2+ channel that activates intracellular ryanodine receptors through Ca2+-induced Ca2+ release from the primary cilium in association with PC-1 acting as a mechanoreceptor (17). To determine whether the Ca2+ channel function of PC-2 is important for CTT cleavage regulation or stabilization, we used different reagents to alter extracellular and intracellular calcium levels. The data from these experiments suggest that the increase of CTT cleavage by PC-2 may be independent of Ca2+ permeation mediated by the PC-2 channel activity. Lan et al. (40) have reported the discovery of another proteolytic cleavage of full-length PC-1, which occurs near the 6th transmembrane domain. This cleavage is independent of PC-2 channel activity but requires intact PC-2. Our results are similar, because the nuclear translocation of the cleaved PC-1 CTT requires the intact transmembrane domains of PC-2 but is independent of PC-2 channel activity.
A pathogenic missense mutation of PC-2 (D511V), in which a single amino acid residue in the third membrane-spanning domain is altered, results in the loss of PC-2 channel activity (18). This missense mutant retains its proper subcellular localization and its capacity for C-terminally mediated protein interactions (18). Because we observed that full-length PC-2 increases PC-1 CTT cleavage in a Ca2+-independent manner, we expected that in presence of D511V luciferase activity would increase similarly. Surprisingly, our data demonstrated that luciferase activity did not increase in the presence of the D511V-PC-2 mutant (Fig. 5A), whereas the quantity of cleaved PC-1 CTT detected by Western blotting was increased in the presence of the D511V mutant as compared with that detected in the presence of wild type PC-2 (Fig. 5B). A previous study has identified an alternatively spliced form of PC-2 that lacks exon 7 (PC-2Δ7) and consequently lacks completely the third transmembrane domain of this protein. These authors demonstrated that the detection of the PC-1 CTT was enhanced in the presence of wild type PC-2 and even more so with PC-2Δ7 (41). These data are consistent with our observed increase in the expression of the PC-1 CTT in the presence of D511V, and they suggest that the D511V mutant may stimulate PC-1 CTT cleavage but then retain the resultant CTT cleavage product in the cytoplasm, thus preventing this enhanced cleavage from being detected in the Gal4-driven luciferase reporter assay.
We generated a p200galvp construct that consists of the last 200 amino acids of the cytosolic fragment of PC-1 with galvp appended at its C terminus. This construct is not covalently linked to a transmembrane segment and accumulates in the nucleus of COS-7 cells (10). The cytoplasmic C-terminal portion of the PC-1 polypeptide regulates several important cell signaling pathways, and its deletion is sufficient to cause autosomal dominant polycystic kidney disease (42). Chauvet et al. (10) have demonstrated that the coexpression of PC-2 with p200 construct led to a reduction in AP-1 activation, suggesting that PC-2 may modulate the downstream effects of PC-1. To determine whether the nuclear accumulation of the last 200 amino acids of PC-1 is differentially regulated by wild type PC-2 or its mutants, p200galvp was coexpressed with wild type PC-2 or the D511V or L703X PC-2 mutants in COS-7 cells. Although the L703X mutant did not alter the nuclear localization of p200galvp or the luciferase activity it induced, wild type PC-2 and the D511V mutant increased luciferase activity significantly (Fig. 7A). Western blots of cell lysates and nuclear fractions showed that the level of detectable p200 increased in the presence of PC-2 and D511V (Fig. 7B) suggesting that PC-2 and the D511V mutant may stabilize the expression of p200 as they do the expression of the PC-1 CTT.
It is not clear whether the N-terminal cleavage precedes and is a prerequisite for the C-terminal cleavage in the PC-1. Although the PC-2Δ7 construct increased PC-1 CTT cleavage, it decreased NTF and CTF expression (41). These data are consistent with the increase in the expression of PC-1 CTT and the decrease in PC-1 NTF and CTF expression (Figs. 5B and D) that we observe in the presence of D511V. These data suggest that stimulation of PC-1 CTT cleavage by PC-2 may be related to effects on GPS cleavage.
The normal function of the PC-2 protein is thought to require the presence of its intact C-terminal domain (18). L703X, R742X, A753X, and E787X are different C-terminal truncations lacking the EF-hand, the ER retention domain, and the coiled-coil domain, respectively. Previous studies demonstrated that in cells overexpressing L703X, R742X, or E787X, these PC-2 mutant proteins predominantly localized at the plasma membrane, whereas the wild type PC-2 is largely retained in the ER (16, 20). Although L703X possesses channel activity with altered voltage dependence and a Ca2+-independent open probability, R742X does not exhibit any channel activity. R872X is predominantly localized to the ER and is unable to associate with PC-1 (14). To study whether the C terminus of PC-2 is also important in the PC-1 CTT cleavage, we cotransfected COS-7 cells with PKDgalvp and one of these C-terminal truncation mutants of PC-2. We found that although the PC-2 L703X truncation does not stimulate cleavage, PC-2 constructs that end at residues Arg742, Glu787, Asp847, Lys859, or Arg872 stimulated PC-1 CTT cleavage to the same extent as full-length PC-2. Surprisingly, the A753X truncated construct appears to increase PC-1 CTT cleavage beyond the levels obtained with full-length PC-2 (Fig. 6A). This finding suggests the following: 1) the PC-2-induced increase in cleavage of PC-1 and the subsequent translocation of the CTT to the nucleus do not require coassembly with the PC-2 cytoplasmic tail; 2) the release of the CTT of PC-1 is influenced by the C terminus of PC-2, and this mechanism seems to be regulated by two PC-2 C-terminal tail sequences defined by A753X and the sequence between R742X and L703X.
Several studies have suggested functional involvement of protein phosphorylation in the pathogenesis of ADPKD (32, 34). PC-2 contains six predicted phosphorylation sites, of which five are in the C terminus (3). Four of these five are conserved in mouse PC-2. Ser801, a predicted protein kinase C phosphorylation site, and Ser812, a casein kinase II site, are located within the ER retention domain in the cytosolic C terminus of PC-2 (20). A pair of predicted protein kinase A/G sites are located upstream of the EF-hand domain and downstream of the ER retention domain at Thr721 and Ser829, respectively. Cai et al. (21) demonstrated that PC-2-B10 (substituted alanine at Thr721, Ser801, Ser812, and Ser829) and S812A mutants interact with PC-1 in a manner similar to wild type PC-2. The B10, B5 (substituted alanine at Ser801, Ser812, and Ser829), and T721A proteins did not have channel activity. PC-2 is constitutively phosphorylated at Ser812, and phosphorylation at this site does not affect the subcellular localization of the PC-2 protein or the interaction of PC-2 with PC-1. Moreover, single channel studies showed that loss of phosphorylation at Ser812 results in a significant decrease in the sensitivity of the PC-2 channel to calcium stimulation. In this study, we sought to determine whether these C-terminal phosphorylation sites of PC-2 are involved in PC-1 CTT cleavage. Our data demonstrate that PC-1 CTT cleavage was stimulated to the same extent as that obtained with wild type PC-2 when we used B5, B10, Thr721, or Ser812 mutants (Fig. 6F). These data suggest that PC-2 stimulation of PC-1 CTT cleavage does not depend on the C-terminal phosphorylation of PC-2.
Taken together, our work demonstrates that PC-2 plays an integral role in the translocation of the CTT of PC-1 to the nucleus. Insight into precisely how PC-2 acts to regulate the cleavage-dependent functions of PC-1 will require additional studies, including identification of the responsible protease. Elucidation of these new PC-2-specific functions may reveal novel molecular and cellular mechanisms involved in the pathogenesis of polycystic kidney disease.
Acknowledgments
We thank Dr. Yung-Feng Liao (Institute of Zoology, Academia Sinica, Taiwan) for the generous gifts of the pRL-TK and CMV-GV vectors and all of the members of the Caplan laboratory for helpful discussion.
This work was supported, in whole or in part, by National Institutes of Health Grant DK57328 (to S. S. and M. J. C.). This work was also supported by fellowships from the National Kidney Foundation (to C. A. B.) and the National Science Foundation (to H. C. C.).
- ADPKD
- autosomal dominant polycystic kidney disease
- PC-1
- polycystin-1
- PC-2
- polycystin-2
- CTT
- cytoplasmic C-terminal tail
- PKDgalvp
- PC-1-Gal4VP16 fusion protein
- F
- Firefly luciferase
- R
- Renilla luciferase
- Gd3+
- gadolinium
- BAPTA-AM
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetate-acetoxymethyl ester
- TG
- thapsigargin
- YCB9
- anti-PC-2 polyclonal antibody
- GPS
- G protein-coupled receptor proteolytic site
- HA
- hemagglutinin
- RLU
- relative light unit
- α-MEM
- α-minimum Eagle's medium
- ER
- endoplasmic reticulum
- JNK
- c-Jun N-terminal kinase
- CTF
- C-terminal fragment
- PBS
- phosphate-buffered saline
- NTF
- N-terminal fragment
- NS
- not significant.
REFERENCES
- 1.Gabow P. A. (1993) N. Engl. J. Med. 329, 332–342 [DOI] [PubMed] [Google Scholar]
- 2.Burn T. C., Connors T. D., Dackowski W. R., Petry L. R., Van Raay T. J., Millholland J. M., Venet M., Miller G., Hakim R. M., Landes G. M., KIinger K. W., Qian F., Onuchic L. F., Watnick T., Germino G. G., Doggett N. A. (1995) Hum. Mol. Genet. 4, 575–582 [DOI] [PubMed] [Google Scholar]
- 3.Mochizuki T., Wu G., Hayashi T., Xenophontos S. L., Veldhuisen B., Saris J. J., Reynolds D. M., Cai Y., Gabow P. A., Pierides A., Kimberling W. J., Breuning M. H., Deltas C. C., Peters D. J., Somlo S. (1996) Science 272, 1339–1342 [DOI] [PubMed] [Google Scholar]
- 4.Hughes J., Ward C. J., Peral B., Aspinwall R., Clark K., San Millán J. L., Gamble V., Harris P. C. (1995) Nat. Genet. 10, 151–160 [DOI] [PubMed] [Google Scholar]
- 5.Qian F., Boletta A., Bhunia A. K., Xu H., Liu L., Ahrabi A. K., Watnick T. J., Zhou F., Germino G. G. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 16981–16986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boletta A., Germino G. G. (2003) Trends Cell Biol. 13, 484–492 [DOI] [PubMed] [Google Scholar]
- 7.Arnould T., Kim E., Tsiokas L., Jochimsen F., Grüning W., Chang J. D., Walz G. (1998) J. Biol. Chem. 273, 6013–6018 [DOI] [PubMed] [Google Scholar]
- 8.Kim E., Arnould T., Sellin L. K., Benzing T., Fan M. J., Grüning W., Sokol S. Y., Drummond I., Walz G. (1999) J. Biol. Chem. 274, 4947–4953 [DOI] [PubMed] [Google Scholar]
- 9.Moy G. W., Mendoza L. M., Schulz J. R., Swanson W. J., Glabe C. G., Vacquier V. D. (1996) J. Cell Biol. 133, 809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauvet V., Tian X., Husson H., Grimm D. H., Wang T., Hiesberger T., Igarashi P., Bennett A. M., Ibraghimov-Beskrovnaya O., Somlo S., Caplan M. J. (2004) J. Clin. Invest. 114, 1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low S. H., Vasanth S., Larson C. H., Mukherjee S., Sharma N., Kinter M. T., Kane M. E., Obara T., Weimbs T. (2006) Dev. Cell 10, 57–69 [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T., Mochizuki T., Reynolds D. M., Wu G., Cai Y., Somlo S. (1997) Genomics 44, 131–136 [DOI] [PubMed] [Google Scholar]
- 13.Li Q., Liu Y., Zhao W., Chen X. Z. (2002) FEBS Lett. 516, 270–278 [DOI] [PubMed] [Google Scholar]
- 14.Qian F., Germino F. J., Cai Y., Zhang X., Somlo S., Germino G. G. (1997) Nat. Genet. 16, 179–183 [DOI] [PubMed] [Google Scholar]
- 15.Tsiokas L., Kim E., Arnould T., Sukhatme V. P., Walz G. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanaoka K., Qian F., Boletta A., Bhunia A. K., Piontek K., Tsiokas L., Sukhatme V. P., Guggino W. B., Germino G. G. (2000) Nature 408, 990–994 [DOI] [PubMed] [Google Scholar]
- 17.Nauli S. M., Alenghat F. J., Luo Y., Williams E., Vassilev P., Li X., Elia A. E., Lu W., Brown E. M., Quinn S. J., Ingber D. E., Zhou J. (2003) Nat. Genet. 33, 129–137 [DOI] [PubMed] [Google Scholar]
- 18.Koulen P., Cai Y., Geng L., Maeda Y., Nishimura S., Witzgall R., Ehrlich B. E., Somlo S. (2002) Nat. Cell Biol. 4, 191–197 [DOI] [PubMed] [Google Scholar]
- 19.Grimm D. H., Cai Y., Chauvet V., Rajendran V., Zeltner R., Geng L., Avner E. D., Sweeney W., Somlo S., Caplan M. J. (2003) J. Biol. Chem. 278, 36786–36793 [DOI] [PubMed] [Google Scholar]
- 20.Cai Y., Maeda Y., Cedzich A., Torres V. E., Wu G., Hayashi T., Mochizuki T., Park J. H., Witzgall R., Somlo S. (1999) J. Biol. Chem. 274, 28557–28565 [DOI] [PubMed] [Google Scholar]
- 21.Cai Y., Anyatonwu G., Okuhara D., Lee K. B., Yu Z., Onoe T., Mei C. L., Qian Q., Geng L., Wiztgall R., Ehrlich B. E., Somlo S. (2004) J. Biol. Chem. 279, 19987–19995 [DOI] [PubMed] [Google Scholar]
- 22.Lin S. Y., Makino K., Xia W., Matin A., Wen Y., Kwong K. Y., Bourguignon L., Hung M. C. (2001) Nat. Cell Biol. 3, 802–808 [DOI] [PubMed] [Google Scholar]
- 23.Hiesberger T., Gourley E., Erickson A., Koulen P., Ward C. J., Masyuk T. V., Larusso N. F., Harris P. C., Igarashi P. (2006) J. Biol. Chem. 281, 34357–34364 [DOI] [PubMed] [Google Scholar]
- 24.Kim H., Jeong W., Ahn K., Ahn C., Kang S. (2004) J. Am. Soc. Nephrol. 15, 2042–2049 [DOI] [PubMed] [Google Scholar]
- 25.González-Perrett S., Kim K., Ibarra C., Damiano A. E., Zotta E., Batelli M., Harris P. C., Reisin I. L., Arnaout M. A., Cantiello H. F. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anyatonwu G. I., Estrada M., Tian X., Somlo S., Ehrlich B. E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6454–6459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandyopadhyay A., Shin D. W., Kim D. H. (2000) Biochem. J. 348, 173–181 [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon J. L. (1986) Biochem. J. 233, 309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds D. M., Hayashi T., Cai Y., Veldhuisen B., Watnick T. J., Lens X. M., Mochizuki T., Qian F., Maeda Y., Li L., Fossdal R., Coto E., Wu G., Breuning M. H., Germino G. G., Peters D. J., Somlo S. (1999) J. Am. Soc. Nephrol. 10, 2342–2351 [DOI] [PubMed] [Google Scholar]
- 30.Rechsteiner M., Rogers S. W. (1996) Trends Biochem. Sci. 21, 267–271 [PubMed] [Google Scholar]
- 31.Barr M. M., DeModena J., Braun D., Nguyen C. Q., Hall D. H., Sternberg P. W. (2001) Curr. Biol. 11, 1341–1346 [DOI] [PubMed] [Google Scholar]
- 32.Li X., Li H. P., Amsler K., Hyink D., Wilson P. D., Burrow C. R. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 9260–9265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delmas P., Nomura H., Li X., Lakkis M., Luo Y., Segal Y., Fernández-Fernández J. M., Harris P., Frischauf A. M., Brown D. A., Zhou J. (2002) J. Biol. Chem. 277, 11276–11283 [DOI] [PubMed] [Google Scholar]
- 34.Parnell S. C., Magenheimer B. S., Maser R. L., Rankin C. A., Smine A., Okamoto T., Calvet J. P. (1998) Biochem. Biophys. Res. Commun. 251, 625–631 [DOI] [PubMed] [Google Scholar]
- 35.Arnould T., Sellin L., Benzing T., Tsiokas L., Cohen H. T., Kim E., Walz G. (1999) Mol. Cell. Biol. 19, 3423–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhunia A. K., Piontek K., Boletta A., Liu L., Qian F., Xu P. N., Germino F. J., Germino G. G. (2002) Cell 109, 157–168 [DOI] [PubMed] [Google Scholar]
- 37.Ong A. C., Harris P. C. (2005) Kidney Int. 67, 1234–1247 [DOI] [PubMed] [Google Scholar]
- 38.Boletta A., Qian F., Onuchic L. F., Bhunia A. K., Phakdeekitcharoen B., Hanaoka K., Guggino W., Monaco L., Germino G. G. (2000) Mol. Cell 6, 1267–1273 [DOI] [PubMed] [Google Scholar]
- 39.Charron A. J., Nakamura S., Bacallao R., Wandinger-Ness A. (2000) J. Cell Biol. 149, 111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lan Z., Xu H., He X., Zhou Q., Germino G. G., Watnick T., Qian F. (2004) J. Am. Soc. Nephrol. 15, 218A [Google Scholar]
- 41.Hackmann K., Markoff A., Qian F., Bogdanova N., Germino G. G., Pennekamp P., Dworniczak B., Horst J., Gerke V. (2005) Hum. Mol. Genet. 14, 3249–3262 [DOI] [PubMed] [Google Scholar]
- 42.Vandorpe D. H., Chernova M. N., Jiang L., Sellin L. K., Wilhelm S., Stuart-Tilley A. K., Walz G, Alper S. L. (2001) J. Biol. Chem. 276, 4093–4101 [DOI] [PubMed] [Google Scholar]