Abstract
Adipose tissue depots originate from distinct precursor cells, are functionally diverse, and modulate disease processes in a depot-specific manner. However, the functional properties of perivascular adipocytes, and their influence on disease of the blood vessel wall, remain to be determined. We show that human coronary perivascular adipocytes exhibit a reduced state of adipocytic differentiation as compared with adipocytes derived from subcutaneous and visceral (perirenal) adipose depots. Secretion of anti-inflammatory adiponectin is markedly reduced, whereas that of pro-inflammatory cytokines IL-6, IL-8, and MCP-1, is markedly increased in perivascular adipocytes. These depot-specific differences in adipocyte function are demonstrable in both freshly isolated adipose tissues and in vitro differentiated adipocytes. Murine aortic arch perivascular adipose tissues likewise express lower levels of adipocyte-associated genes as compared with subcutaneous and visceral adipose tissues. Moreover, two weeks of high fat feeding caused further reductions in adipocyte-associated gene expression, while up-regulating pro-inflammatory gene expression, in perivascular adipose tissues. These changes were observed in the absence of macrophage recruitment to the perivascular adipose depot. We conclude that perivascular adipocytes exhibit reduced differentiation and a heightened pro-inflammatory state, properties that are intrinsic to the adipocytes residing in this depot. Dysfunction of perivascular adipose tissue induced by fat feeding suggests that this unique adipose depot is capable of linking metabolic signals to inflammation in the blood vessel wall.
Keywords: Perivascular adipose tissue, adipocytes, adventitia, adipokines, cytokines
Introduction
Atherosclerosis is traditionally viewed as a disease of the vascular intima, following a paradigm of endothelial cell dysfunction, inflammatory cell recruitment, and foam cell formation1–4. More recently, dysfunction of medial smooth muscle cells and adventitial cells has also been demonstrated to play a role in the pathogenesis of atherosclerosis5,6. While reactive fibroblasts and inflammatory cells in the adventitia have been the focus of extensive investigations6,7, very little is known about perivascular adipocytes that reside at the adventitial border of atherosclerosis-prone blood vessels. Adipocytes secrete numerous factors that could potentially modulate the development of vascular disease, including pro-inflammatory cytokines and adipokines, angiogenic molecules, and stem cell homing factors8–10. Recent evidence indicates that the periadventitial adipose depot is a functional component of the vasculature, exerting paracrine influences on blood vessel contractility11,12.
Inflammatory cell infiltration is markedly increased in perivascular adipose tissue surrounding atherosclerotic human aorta as compared with non-diseased aorta13. Moreover, inflammatory gene expression is upregulated14,15, and expression of adiponectin, an anti-inflammatory adipokine, is downregulated16, in perivascular adipose tissues surrounding human coronary arteries. The mechanisms underlying these observations are unknown. It is possible that perivascular adipose inflammation stems from disease of the blood vessel wall that passively extends into the surrounding adipose tissue. Conversely, depending on their functional properties, perivascular adipocytes could potentially initiate vascular wall inflammation. In this regard, emerging evidence suggests that anatomically separated adipose tissue depots are functionally diverse, originate from distinct precursor cells17,18, and exert both systemic and local (paracrine) effects on tissue and organ function. Insulin sensitivity, and the balance of pro- and anti-inflammatory adipokine and cytokine expression, varies widely among regional visceral and subcutaneous adipose depots19. Moreover, high fat feeding induces inflammation of visceral adipose tissues20, which may contribute to insulin resistance and dyslipidemia. However, responses of perivascular adipose tissues to high fat feeding have not been examined.
Accordingly, we characterized the molecular and functional properties of human perivascular adipose tissues and adipocytes and determined the effects of high fat feeding on perivascular adipose tissues in a mouse model. Studies were conducted in adipose tissues of humans and mice devoid of atherosclerotic disease in order to eliminate confounding influences of pre-existing vascular wall inflammation. Our results suggest that human perivascular adipocytes exhibit a reduced state of adipocytic differentiation, and a remarkably higher level of pro-inflammatory cytokine expression and release, as compared with adipocytes from other regional depots. Moreover, we observed that mouse perivascular adipose tissue is strikingly responsive to the effects of short-term high fat feeding. Thus, by virtue of their unique functional and biochemical properties, we propose that perivascular adipocytes may play a primary role in establishing adventitial inflammation in atherosclerosis.
Material and Methods
Animals
Eight-week old male C57BL/6J mice, maintained on chow diet after weaning, were continued on chow diet or placed on a high-fat western diet (Harlan Teklad, 42% calories from fat) ad libitum for 2 weeks before sacrifice. Subcutaneous, visceral (epididymal and perirenal), and perivascular (aortic arch) adipose tissues were dissected, rinsed, snap-frozen in liquid nitrogen, and stored at −80°C before use. All animal procedures were approved by the Institutional Animal Care and Use Committeeof the University of Cincinnati.
Human adipose tissue collection and processing
Human adipose tissue samples were collected from candidates for organ donation (see Online Table I for donor demographic information). Institutional review boards at the University of Cincinnati and the University of Iowa approved our protocols. Adipose tissues were obtained from subcutaneous, visceral (perirenal), perivascular (coronary artery), omental, and epicardial (right ventricle) sites. Tissues were harvested, rinsed, placed in DMEM/F12, and rapidly transported to the laboratory. Tissues were separated for cell culture (see below) or fixed with 10% buffered formalin, paraffin embedded, and stained with hematoxylin and eosin. Stained sections were examined microscopically, and cell diameters were determined using Leica image processing system.
Isolation and differentiation of human preadipocytes
Tissues were minced and digested with collagenase type I (Worthington), after which isolation and culture of preadipocytes was performed as previously described18,21. Cells were maintained in DMEM/F12/10% FBS and passaged or subjected to the differentiation protocol when confluent. Adipocytic differentiation was accomplished by switching to a commercially available differentiation medium (Cell Applications), which was replaced every 4 days. Differentiated adipocytes were examined by DIC microscopy to visualize cytoplasmic lipid droplet accumulation. All experiments were performed on cells at passage 3 or less.
Oil Red O staining and spectrophotometric quantification of lipids
Differentiating adipocytes were washed with PBS, fixed in 10% formalin, stained with Oil Red O (Sigma) in 2-propanol, air dried, and photographed. In some experiments, Oil Red O-stained cells were extracted with 2-propanol in 4% Nonidet P-40 (Sigma), and the color intensity was measured spectrophotometrically at 510 nm. Cellular protein was determined by Micro BCA Protein Assay Kit (Pierce), and data were expressed as OD units per mg protein.
RNA Extraction and Quantitative RT-PCR
Total RNA from tissues and cells was extracted using RNasey Lipid Mini Kits (Qiagen). Quantitative RT-PCR was performed using Brilliant II QRT-PCR Kits (Stratagene). The levels of acidic ribosomal phosphoprotein P0 RNA were used as endogenous controls for normalization of human and mouse RNA. The relative gene expressions were calculated using cycle threshold (Ct) values in accordance with the ΔΔCt method as described previously 22. Primer sequences used are available upon request.
ELISA determination of adiponectin, IL-8, IL-6, MCP-1, and leptin
Preadipocytes were placed in differentiation medium, as described above. At the designated time, 100 μl of medium was removed from each culture and stored at −30°C until assayed. Adiponectin, IL-8, IL-6, MCP-1, and leptin in the culture medium were quantified using ELISA Kits (R&D Systems). Values were normalized to cellular protein.
Statistics
Data are presented as mean ± SEM. Differences between mean values were evaluatedby ANOVA, followed by Student-Newman-Keuls testing. Statistical significance was defined as P<0.05.
Results
Morphological features of perivascular adipocytes
Figure 1 shows representative images of coronary perivascular (PV), perirenal (PR) and subcutaneous (SQ) adipose tissues. Perivascular adipose tissue is an integral part of the blood vessel wall, as noted by invasion of adipocytes into the adventitia (red arrows). Perivascular adipocytes are more irregularly shaped and smaller in size than perirenal or subcutaneous adipocytes. Comparative analysis of adipocyte size in situ is shown in Table 1.
Figure 1.
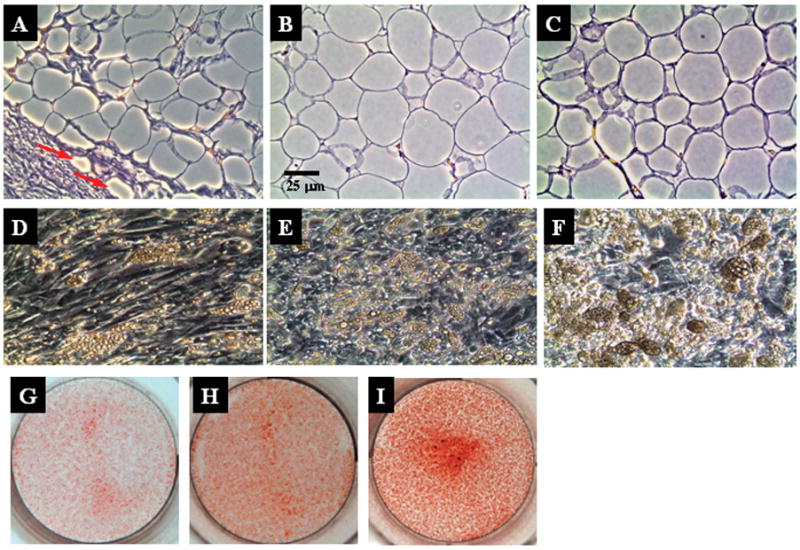
Top panel, Light microscopic appearance of human perivascular (A), perirenal (B), and subcutaneous (C) adipose tissues in situ. Tissues were harvested from the same patient, stained with hematoxylin and eosin, sectioned and photographed (X 20 magnification). Red arrows indicate adventitial infiltration of adipocytes. Middle panel, Lipid droplet accumulation within in vitro differentiated adipocytes from perivascular (D), perirenal (E), and subcutaneous (F) adipose depots. Preadipocytes were isolated from each depot and differentiated for 28 days as described in Methods. Cells were fixed and imaged using phase contrast microscopy (X 20 magnification). Bottom panel, Images of oil red O stained, perivascular (G), perirenal (H), and subcutaneous (I) differentiated adipocytes in vitro.
Table 1.
Depot-specific differences in tissue adipocyte diameter in situ, and cellular lipid accumulation within in vitro differentiated adipocytes
| Tissue |
Adipocyte diameter (μM)a |
|---|---|
| SQ | 42.32 ± 2.74 |
| PR | 60.68 ± 2.63b |
| PV | 22.53 ± 1.53b,c |
| Cells |
Oil Red O-positive materials (OD units/mg protein)d |
| SQ | 8053 ± 180 |
| PR | 4975 ± 39b |
| PV | 3032 ± 116b,c |
, data represent mean ± SEM.
, p < 0.05 vs. SQ;
, p < 0.05 vs. PR.
, values are expressed as mean OD at 510 nm/mg cellular protein ± SEM.
Preadipocytes from perivascular adipose tissue exhibit reduced capacity for adipocytic differentiation
Having observed that perivascular adipocytes are smaller than subcutaneous and perirenal adipocytes in situ, we next investigated the capacity of preadipocytes derived from these adipocyte depots to differentiate into mature adipocytes in vitro. All cell cultures were derived from patients without a preexisting history of cardiovascular disease, and the preadipocytes were isolated from each depot using identical techniques and culture conditions. For these and subsequent studies, perivascular adipocytes were cultured from adipose tissues overlying coronary arteries. The cultured, undifferentiated preadipocytes from each depot displayed similar morphologic features when grown in standard medium (not shown). When exposed to differentiating medium, subcutaneous and perirenal preadipocytes exhibited robust accumulation of cytoplasmic lipid droplets (an index of adipocyte differentiation) within 14 days. In contrast, relatively few perivascular adipocytes displayed cytoplasmic lipid droplets by 14 days after initiation of differentiation protocol, although approximately 50–70% of the cells formed lipid droplets by 28 days. A representative DIC microscopic picture of the live cells after 28 days of adipocytic differentiation is shown in Figure 1 (D–F). Oil Red O staining confirmed that lipid accumulation was dramatically lower in differentiated perivascular adipocytes as compared with their subcutaneous and perirenal counterparts, as shown in Figure 1 (G–I) and quantified in Table 1.
Reduced expression of the adipocyte-associated genes PPARγ, C/EBPα, and FABP4 in perivascular adipocytes
We next compared the levels of expression of adipocyte-associated genes PPARγ, C/EBPα, and FABP4 in perivascular, subcutaneous and perirenal adipose tissues, and in differentiated adipocytes derived from these depots. In intact adipose tissues (Figure 2A) and in vitro differentiated adipocytes (Figure 2B), the relative order of expression of all three adipocyte-associated genes was subcutaneous>perirenal>perivascular. Consistent with reduced lipid droplet formation in perivascular adipocytes, lower levels of expression of these genes, which are considered to be master regulators of adipogenesis, suggest that perivascular adipocytes exhibit a reduced state of differentiation as compared with subcutaneous and perirenal adipocytes. Moreover, the strong concordance in adipocyte-associated gene expression between in vitro differentiated adipocytes and intact adipose tissues suggests that our cell culture system provides a valid model for investigating the molecular and biochemical properties of perivascular adipocytes.
Figure 2.
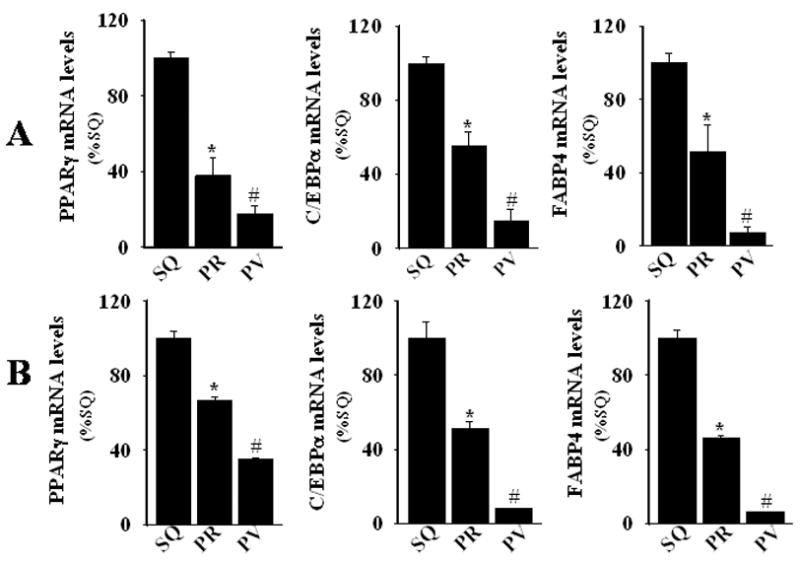
Real-time PCR determination of mRNA levels of PPARγ, C/EBPα, and FABP4 in (A) human subcutaneous (SQ), perirenal (PR), and perivascular (PV) adipose tissues and in (B) 28-day in vitro differentiated adipocytes from these depots. RNA was subjected to quantitative PCR, normalized, and expressed relative to levels observed in corresponding subcutaneous adipose tissues or cells. Values represent the mean ± SEM of three different donors. *, p < 0.05 versus SQ; #, p < 0.05 versus SQ and PR.
Expression of adipocytic differentiation markers is reduced in perivascular adipocytes
To provide further support for the hypothesis that adipocytic differentiation is reduced in perivascular adipocytes, we examined transcript levels of fatty acid synthase (FAS), glycerol 3-phosphodehydrogenase 1 (GPDH), lipoprotein lipase (LPL), hormone sensitive lipoprotein lipase (HSL), adipose triglyceride lipase (ATGL), and perilipin (a lipid droplet-associated protein) in differentiated adipocytes isolated from subcutaneous, perirenal, and perivascular adipose tissues. As shown in Figure 3, these adipocyte differentiation/maturation-related genes tended to be expressed at lower levels in perivascular adipocytes. Likewise, adiponectin transcript expression was lower in perivascular as compared with subcutaneous and perirenal adipose tissues (Figure 4A), and in in vitro differentiated perivascular adipocytes as compared with subcutaneous and perirenal adipocytes (Figure 4B).
Figure 3.
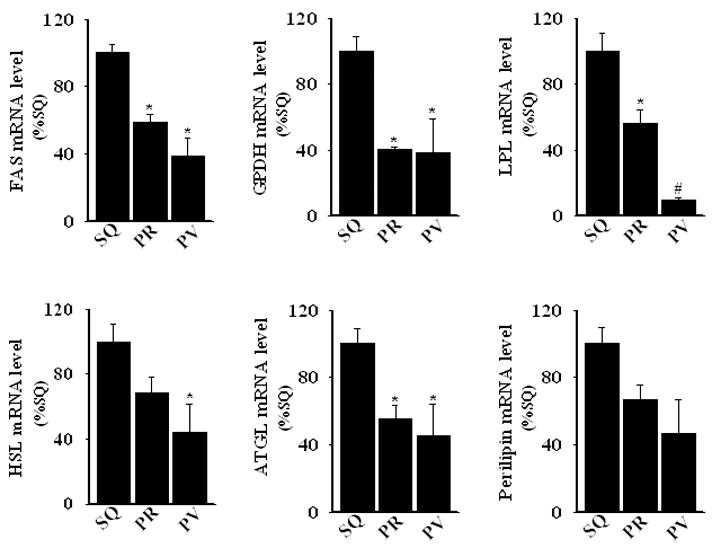
Real-time PCR determination of mRNA levels of FAS, GPDH, LPL, HSL, ATGL, and perilipin in human SQ, PR, and PV 28-day in vitro differentiated adipocytes. Methods are as described for Figure 3; results represent the mean ± SEM of 3–4 different donors. *, p < 0.05 versus SQ; #, p < 0.05 versus SQ and PR.
Figure 4.
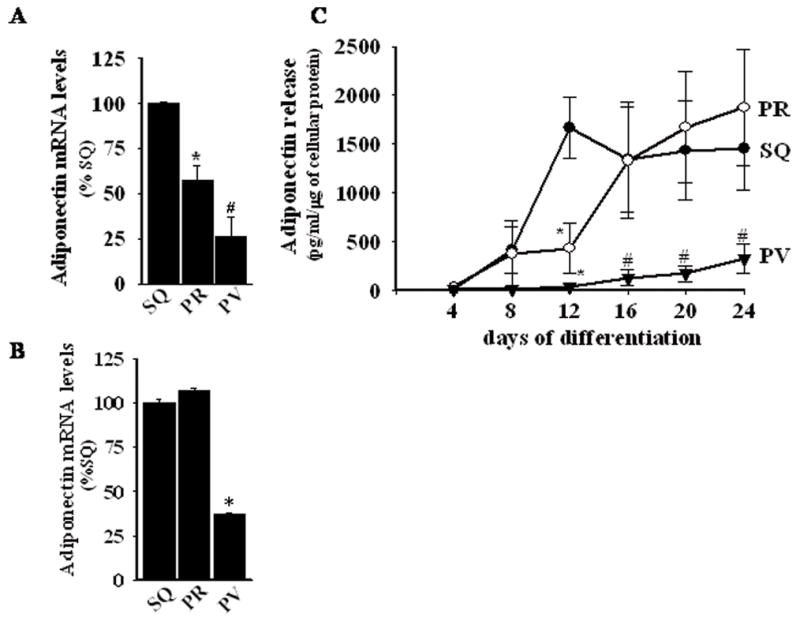
Panels A and B, Real-time PCR determination of mRNA levels of adiponectin in (A) adipose tissues and in (B) in vitro differentiated adipocytes from human SQ, PR, and PV adipose depots. Methods are as described for Figure 3. Panel C, Adiponectin release by in vitro differentiating adipocytes as a function of time in differentiation medium (ELISA). Values represent the mean ± SEM of 3–4 different donors. *, p < 0.05 versus SQ; #, p < 0.05 versus SQ and PR for panels A–C.
The secretion of adiponectin protein by differentiated adipocytes from each depot was also compared. Baseline adiponectin release by subcutaneous, perirenal, and perivascular preadipocytes was very low (not shown) and remained so early during the course of adipocytic differentiation. By day 8 after initiation of differentiation protocol, increased adiponectin release was detected from subcutaneous and perirenal cells, which peaked at day 12 and 16, respectively (Figure 4C). In contrast, the level of adiponectin released from perivascular cells remained very low throughout the course of the differentiation protocol. Taken together, these findings confirm that human perivascular adipocytes display reduced maturation/differentiation as compared with subcutaneous and perirenal adipocytes.
Expression of inflammatory cytokines is increased in perivascular adipocytes
Adiponectin possesses anti-inflammatory effects, suggesting that perivascular adipocytes, which secrete very little adiponectin, could exhibit a heightened inflammatory state as compared with subcutaneous and perirenal adipocytes. Moreover, inflammation is an integral component of metabolic syndrome and insulin resistance23 and can both cause and result from adipocyte dysfunction. Therefore, we examined the expression of several pro-inflammatory cytokines associated with adipocyte dysfunction. IL-8 transcript expression was higher in perivascular as compared with perirenal and subcutaneous adipose tissues (Figure 5A), and in differentiated perivascular adipocytes as compared with their subcutaneous and perirenal counterparts (Figure 5B). Moreover, differentiated perivascular adipocytes released considerably more IL-8 (Figure 5C) and IL-6 (Figure 5D) into the medium as compared with subcutaneous and perirenal adipocytes.
Figure 5.
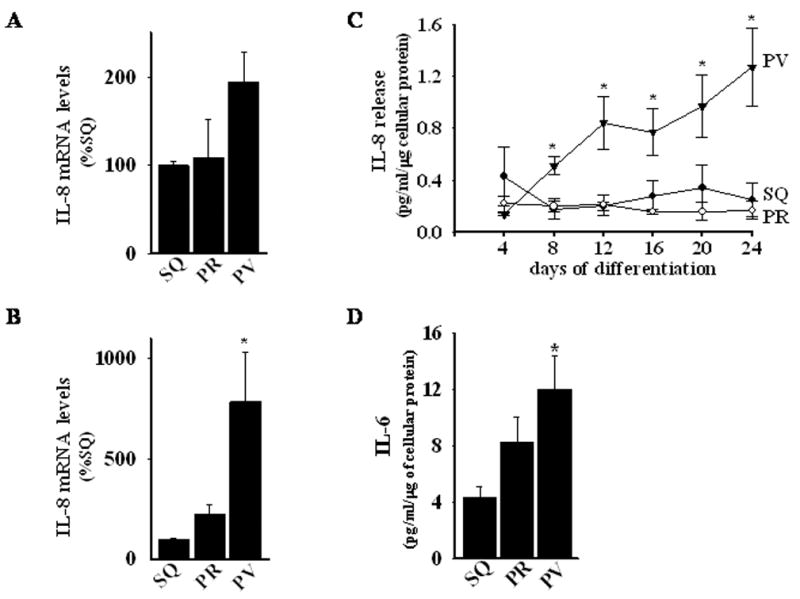
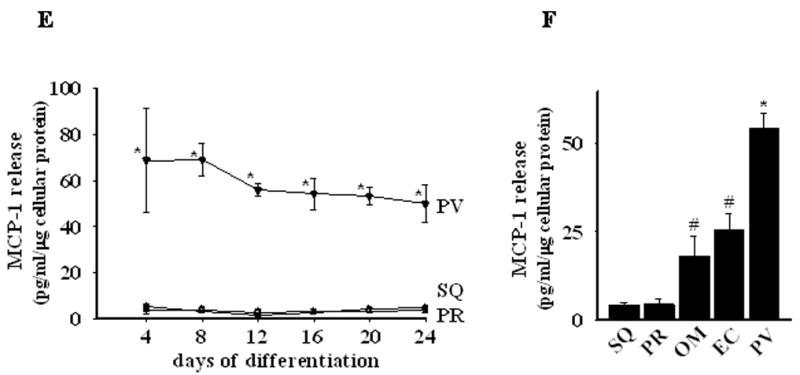
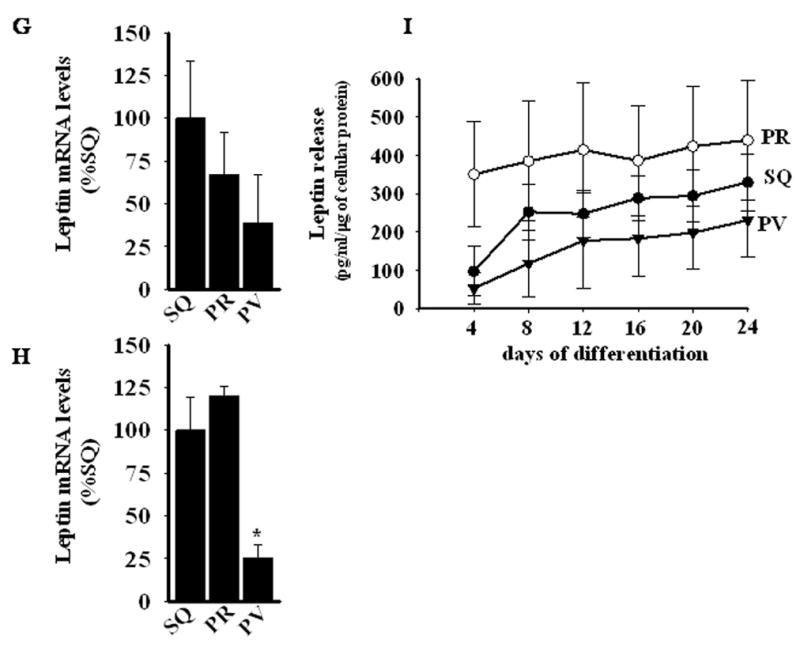
Panels A and B, Real-time PCR determination of mRNA levels of IL-8 in (A) adipose tissues and in (B) in vitro differentiated adipocytes from human SQ, PR, and PV adipose depots. *, p< 0.05 versus SQ and PR. Panel C, IL-8 release by in vitro differentiating adipocytes as a function of time in differentiation medium (ELISA). *, p < 0.05 versus SQ and PR. Panel D, IL-6 release by 24-day in vitro differentiated adipocytes from SQ, PR, and PV adipose tissues. *, p < 0.05 versus SQ. Panel E, MCP-1 release by in vitro differentiating adipocytes as a function of time in differentiation medium (ELISA). *, p < 0.05 versus SQ and PR. Panel F, Comparison of MCP-1 release by 24-day in vitro differentiated adipocytes from SQ, PR, omental (OM), epicardial (EC), and PV adipose tissues. (p values) Panel G and H, Real-time PCR determination of mRNA levels of leptin in (A) adipose tissue and in (B) in vitro differentiated adipocytes from human SQ, PR, and PV adipose depots. *, p < 0.05 versus SQ and PR.
All values are expressed as mean ± SEM of 3–5 different donors and are expressed relative to levels observed in SQ adipose tissues or cells.
MCP-1 is critically involved in the recruitment of macrophages to adipose tissues and in the subsequent development of insulin resistance24,25. We therefore investigated MCP-1 release from differentiated perivascular, perirenal, and subcutaneous adipocytes. The level of MCP-1 protein released by subcutaneous and perirenal cells was low and remained stable throughout the three-week course of the differentiation protocol (Figure 5E). In contrast, perivascular cells released substantial amounts of MCP-1 at baseline and throughout the course of the study (approximately 10–40 fold higher than perirenal and subcutaneous adipocytes).
Epicardial fat may share a common embryonic origin with omental fat, which is known for its powerful pro-inflammatory properties and its important role in insulin resistance26. We therefore examined MCP-1 release by differentiated omental adipocytes and epicardial (EC) adipocytes isolated from the surface of the right ventricle, away from the coronary arteries. As expected, differentiated omental and EC adipocytes released significant amounts of MCP-1, at levels exceeding those released by subcutaneous and perirenal adipocytes isolated from the same patients (Figure 5F). Nevertheless, differentiated perivascular adipocytes released approximately two- to three-fold more MCP-1 as compared to their omental and EC counterparts.
Leptin is an adipocyte-derived hormone whose secretion is stimulated by insulin. Hyperleptinemia is common in obesity and is independently associated with insulin resistance and cardiovascular disease27. Considering that leptin stimulates MCP-1 production28, and that plasma leptin levels correlate with inflammatory markers29, we examined leptin expression in perivascular, subcutaneous and perirenal adipose tissues and in differentiated adipocytes derived from these depots. Leptin mRNA levels were lower in perivascular adipocytes as compared to subcutaneous and perirenal adipocytes, respectively (Figure 5G and H). Likewise, perivascular adipocytes tended to release less leptin into the cell culture medium as compared to subcutaneous and perirenal adipocytes, although statistical significance was not met (Figure 5I). These data suggest that the pro-inflammatory phenotype of perivascular adipocytes is not related to increased leptin expression.
Expression of brown adipocyte-related genes and development/pattern-forming genes
Perivascular adipocytes have been suggested to be brown adipocytes30, which exhibit distinct patterns of adipocyte-related gene expression and enhanced cytokine production. We therefore examined expression of PRDM16, PGC1α/β, UCP-1, CPT1b, genes that are highly expressed in brown adipocytes,31,32 in freshly isolated subcutaneous, perirenal, and perivascular adipose tissues (Figure 6A) and in vitro differentiated adipocytes (Figure 6B). As expected, subcutaneous adipose tissue and adipocytes exhibited low levels of expression of these genes. As compared with subcutaneous adipocytes, perirenal and perivascular adipocytes exhibited higher levels of expression of some of the brown adipocyte-related genes; however, gene expression patterns were inconsistent, and levels of expression were several orders of magnitude lower than has been reported for brown adipocytes. For example, UCP-1, a prototypical marker of brown adipocytes, is expressed at ~1000 fold higher levels in brown versus white adipose tissues32. Our results therefore suggest that human perivascular adipocytes are white rather than brown adipocytes.
Figure 6.
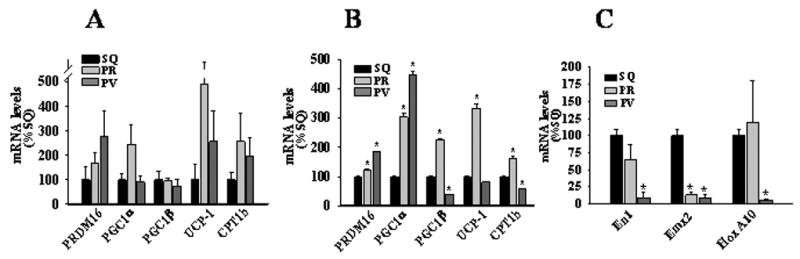
Panel A and B, Real-time PCR determination of PRDM16, PGC1α/β, UCP-1, and CPT1b mRNA expression in human adipose tissues (A) and in vitro differentiated adipocytes (B). Panel C, Real-time PCR determination of En1, Emx2, and HoxA10 mRNA expression in in vitro differentiated subcutaneous, perirenal, and perivascular adipocytes. Values represent mean ± SEM from 3–4 different donors. *, p < 0.05 compared to SQ.
Phenotypic differences in adipocytes could stem from developmental divergence of the precursor cells from which the mature adipocytes are derived33,34. We therefore compared expression of developmental and pattern-forming genes En-1, Emx-2, and Hox-A10, whose expression levels differ dramatically between human subcutaneous versus omental preadipocytes34. We found that expression of all three genes was markedly reduced in perivascular as compared with subcutaneous adipocytes (Figure 6C); interestingly, perirenal adipocytes displayed an intermediate pattern of gene expression (Figure 6C). These findings are consistent with the notion that adipocytes residing in these depots are derived from distinct precursor cells, which may underlie the phenotypic differences observed in our study.
Mouse perivascular adipose tissue gene expression: response to high fat feeding
Having established that human coronary perivascular adipocytes exhibit reduced levels of adipocytic differentiation and a heightened pro-inflammatory state, we next investigated gene expression in mouse perivascular (aortic arch), subcutaneous, and visceral (perirenal and epididymal) adipose tissues. At 8 weeks of age, mice were either maintained on a normal chow diet or switched to a high fat diet for two weeks. There was no significant difference in body weight between the two groups of mice at the completion of the study (chow 25.6±1.1 vs. high fat 27.8±0.4 g; n=6, p=0.09). In chow-fed mice, the transcript levels of adiponectin, PPARγ, FABP4, and leptin tended to be lower in perivascular adipose tissues as compared with the other adipose depots, while expression of the pro-inflammatory MIP1α gene was similar amongst all depots (Figure 7A). In mice fed a high fat diet for two weeks, expression of adiponectin, PPARγ, and FABP4 was further reduced in perivascular adipose tissues as compared with chow-fed mice, while expression of the pro-inflammatory leptin and MIP1α genes was markedly up-regulated (Figure 7B). The responses were much more robust than was observed in the other adipocyte depots.
Figure 7.
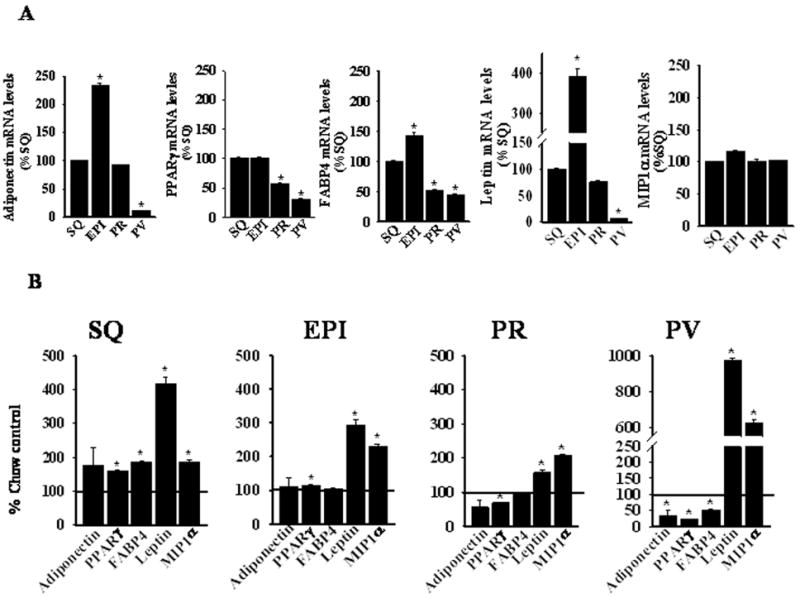
Real-time PCR determination of adiponectin, PPARγ, FABP4, leptin, and MIP1α mRNA expression in SQ, epididymal (EPI), PR, and PV adipose tissues of C57BL/6 mice fed (A) a chow or (B) a high fat diet for two weeks. mRNA levels in panel A are expressed relative to subcutaneous adipose tissue data, while in panel B, levels are expressed relative to the corresponding depot in chow-fed mice. Values represent the mean ± SEM of 3–5 mice per group. *, p < 0.05 versus SQ data (Panel A) or versus corresponding chow-fed data (Panel B).
Since inflammatory cells can modulate adipocyte function35, we examined macrophage and T cell infiltration into the adipose depots by quantifying expression of CD68 (macrophage marker) and CD3 (T lymphocyte marker) mRNA. In chow-fed mice, CD3 expression was lower in perivascular as compared with subcutaneous adipose tissues (see Online Table II, while CD68 expression was similar amongst the various depots (data not shown). Thus, the reduced state of differentiation of perivascular adipocytes in chow-fed mice is not associated with increased inflammatory cell infiltration. Following two weeks of high fat feeding, CD3 expression increased only in perivascular adipose tissues (see Online Table II), to levels comparable to subcutaneous adipose tissues, while CD68 levels remained unchanged in all depots, consistent with an earlier report20 (data not shown). Thus, increased inflammatory cell infiltration is not likely sufficient to account for the marked changes in perivascular adipocyte gene expression observed after two weeks of high fat feeding.
Discussion
Perivascular adipose tissue inflammation has recently been observed in conjunction with atherosclerotic lesions14,15. Here, we show for the first time that human perivascular adipocytes exhibit a heightened pro-inflammatory state and reduced adipocytic differentiation under basal conditions. We also report that murine perivascular adipose tissue is highly sensitive to the effects of high fat feeding, which causes further reductions in adipocyte-associated gene expression while up-regulating pro-inflammatory gene expression. Importantly, these characteristics were observed in perivascular adipose tissues from humans and mice without atherosclerotic disease. Taken together, these findings suggest that perivascular adipocytes are poised to play a primary role in development of adventitial inflammation, which in turn may contribute to atherosclerotic lesion development.
Obesity is increasing at alarming rates in industrialized societies36, and abundant evidence indicates that it is an important risk factor for atherosclerosis37. Obesity is associated with insulin resistance and dyslipidemia, as well as increases in circulating inflammatory factors, all of which may contribute to atherosclerosis. In this regard, the distribution of adipose tissue is thought to be important, with visceral adipose tissue generally viewed to be a stronger predictor of atherogenic risk than subcutaneous adipose tissue38. Perivascular adipose tissue surrounding the great vessels (the focus of the present investigation) is not traditionally considered a visceral depot based upon its anatomic location39. However, epicardial fat thickness was shown to correlate with abdominal visceral fat and fasting insulin levels in humans, suggesting that it behaves like visceral fat40. Nevertheless, the functional properties of the adipocytes that comprise epicardial and perivascular fat depots surrounding the great vessels have not been defined.
Here, we undertook a detailed comparison of human coronary perivascular, subcutaneous and visceral (perirenal) adipocytes, performing morphologic studies and examining gene expression profiles in intact adipose tissues and in in vitro differentiated adipocytes. The in vitro differentiated adipocytes isolated from the various tissue depots displayed a similar profile of adipocyte-associated gene expression as compared with their in situ counterparts. Moreover, the smaller size of perivascular adipocytes as compared with subcutaneous and perirenal adipocytes in situ was paralleled by reduced lipid droplet accumulation in differentiated perivascular adipocytes in vitro. Cumulatively, our observations suggest that adipocytes differentiated in vitro under our defined culture conditions retain their depot-specific characteristics and provide a valid model to examine the characteristics of perivascular adipocytes.
We investigated the mechanisms responsible for decreased adipocyte-associated gene expression and reduced differentiation characteristic of perivascular adipocytes. Our data suggest that coronary perivascular adipocytes are white adipocytes that are derived from distinct precursor cells, and that they inherently display a pro-inflammatory phenotype. Inflammation of adipose tissues occurs in diet-induced obesity, and cytokines such as TNFα and MCP-1 present in inflamed adipose tissues are thought to trigger insulin resistance in the adipocytes41. We observed that preadipocytes and differentiated adipocytes from all depots expressed very little TNFα mRNA, and TNFα release was below the detection limit of our ELISA (data not shown). Likewise, subcutaneous and perirenal preadipocytes produced very little IL-8 or MCP-1 during adipocytic differentiation, while secreting significant amounts of anti-inflammatory adiponectin. In contrast, perivascular adipocytes produced very little adiponectin but released substantial amounts of pro-inflammatory IL-6, IL-8, and MCP-1, while exhibiting reduced adipocytic differentiation. These novel results suggest that inflammatory cytokine release by perivascular adipocytes could modulate insulin sensitivity and cellular function in an autocrine or paracrine manner while attracting macrophages to the depot, further exacerbating inflammation and adipocyte dysfunction.
In addition, in response to two weeks of high fat feeding in mice, expression of adiponectin, PPARγ, and FABP4 in perivascular adipose tissue fell dramatically, while expression of the pro-inflammatory MIP1α and leptin genes were markedly up-regulated. Interestingly, we were able to detect up-regulation of CD3, but not CD68, expression in perivascular adipose tissues after two weeks of high fat feeding, suggesting infiltration of T cells, but not macrophages, in this short time period. However, the level of CD3 expression in perivascular adipose tissues did not exceed that detected in subcutaneous adipose tissues, suggesting that inflammatory cell infiltration per se is not sufficient to account for the dramatic changes in gene expression observed in perivascular adipose tissues. Together, these data suggest that perivascular adipose tissue is highly sensitive to the harmful influences of excess dietary fat. This may serve as a driving force for inflammatory cell recruitment to the vascular wall, which potentially could contribute to atherosclerosis.
Collectively, our study provides novel insight into the characteristics of perivascular adipocytes. Our data suggest that human perivascular adipocytes originate from distinct progenitor cells and exhibit reduced differentiation and a heightened pro-inflammatory state. We also report that high fat feeding in mice causes further reductions in adipocyte-associated gene expression while up-regulating pro-inflammatory gene expression. These results suggest that perivascular adipocytes may play a primary role in transducing adventitial inflammation in atherosclerosis.
Acknowledgments
Sources of Funding: This study was funded by HL076684 and HL62984 from the National Institutes of Health, and by an AARG award from Pfizer Corporation.
Footnotes
Disclosures: None.
References
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. The pathogenesis of atherosclerosis: a prospective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 5.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autocrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovasc Res. 2007;75:679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res. 2007;101:27–39. doi: 10.1161/CIRCRESAHA.107.151621. [DOI] [PubMed] [Google Scholar]
- 9.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 10.Rajala MW, Scherer PE. The adipocyte—at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 11.Löhn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 12.Verlohren S, Dubrovska G, Tsang S-Y, Essin K, Luft FC, Huang Y, Gollasch M. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- 13.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 14.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 15.Baker AR, da Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1–7. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, Gallo P, Rosaria C, di Gioia T. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse A, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 19.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endoc Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, DePonte M, Stevenson M, Guo W, Han J, Waloga G, Lash TL, Jensen MD, Kirkland JL. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Ateroscler Thromb Vasc Biol. 2007;27:2276–2283. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- 24.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 25.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fain JN, Sacks HS, Buehrer B, Bahouth SW, Garrett E, Wolf RY, Carter RA, Tichansky DS, Madan AK. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes (Lond) 2008;32:810–815. doi: 10.1038/sj.ijo.0803790. [DOI] [PubMed] [Google Scholar]
- 27.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzmán M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–25100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 29.Qasim A, Mehta NN, Tadesse MG, Wolfe ML, Rhodes T, Girman C, Reilly MP. Adipokines, insulin resistance, and coronary artery calcification. J Am Coll Cardiol. 2008;52:231–236. doi: 10.1016/j.jacc.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gálvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, Ruiz-Gayo M, Huber M, Wehland M, Kreutz R, Fernandez-Alfonso MS. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol. 2008;197:55–64. doi: 10.1677/JOE-07-0284. [DOI] [PubMed] [Google Scholar]
- 31.Farmer SR. Molecular determinants of brown adipocyte formation and function. Genes & Dev. 2008;22:1269–1275. doi: 10.1101/gad.1681308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse RA, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 35.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 37.Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: Exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27:996–1003. doi: 10.1161/ATVBAHA.106.131755. [DOI] [PubMed] [Google Scholar]
- 38.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, Sr, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 39.Mårin P, Andersson B, Ottosson M, Olbe L, Chowdhury B, Kvist H, Holm G, Sjöström L, Björntorp P. The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism. 1992;41:1242–1248. doi: 10.1016/0026-0495(92)90016-4. [DOI] [PubMed] [Google Scholar]
- 40.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 41.Poulain-Godefroy O, Lecoeur C, Pattou F, Fruhbeck G, Froguel P. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1–R7. doi: 10.1152/ajpregu.00926.2007. [DOI] [PubMed] [Google Scholar]


