Abstract
A divergent synthetic approach to new sultams utilizing intramolecular oxa-Michael and Baylis—Hillman reactions of readily prepared vinyl sulfonamides and suitably protected amino alcohols, is reported. A variety of seven- and eight-membered ring sultam scaffolds were synthesized using oxa-Michael pathways, whereas both five- and six-membered rings were synthesized using Baylis—Hillman methods. Baylis—Hillman reactions proceed with good to excellent levels of diastereoselectivity, and oxa-Michael reactions leading to eight-membered ring sultams provide empirical evidence validating 8-endo-trig cyclization pathways.
Sulfonamides and their analogs have a rich chemical and biological history and have emerged as a promising class of compounds in drug discovery.1 Sultams (cyclic sulfonamides), although not found in nature, have also shown potent biological activity, including several displaying a wide spectrum of activities.2 The more prominent include the antiepileptic agent Sulthiame3 (Figure 1), Brinzolamide4 for the treatment of glaucoma, the COX-2 inhibitors Ampiroxicam5 and S-2474,6 and selective inhibitors of cysteine proteases involved in the progression of malaria.7 In addition, a number of benzodithiazine dioxides and benzoxathiazepine 1,1-dioxides displaying anti-HIV-1 activity8 and the ability to activate glucokinase9 (type II diabetes), respectively, have been uncovered.
Figure 1.
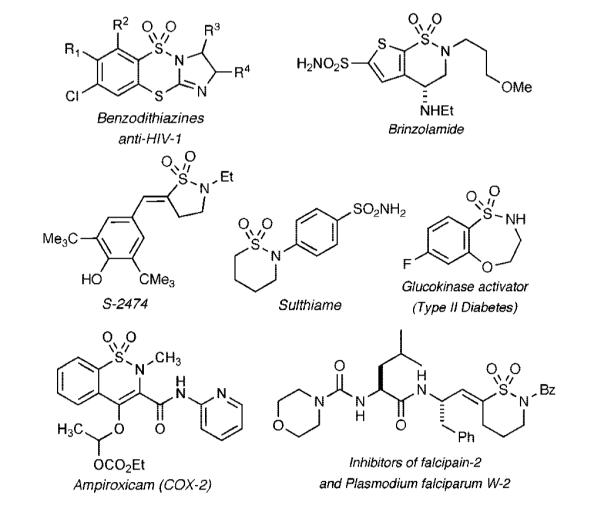
Biologically active sultams.
Traditionally, sultam syntheses have relied on classical cyclization protocols and a number of transition-metal-catalyzed processes that have recently been reported.10 Interest in the generation of new sultams for biological screening has provided impetus for exploring the Michael-accepting ability of vinyl sulfonamides. In particular, we aimed to study the titled intramolecular oxa-Michael and Baylis—Hillman approaches for the synthesis of chiral, nonracemic sultams.
The oxa-Michael11 and Baylis—Hillman12,13 reactions have emerged as simple yet powerful methods for C—O and C—C bond formation. Both processes necessitate the presence of a competent electron-deficient Michael accepting moiety. The intermolecular oxa-Michael reaction has a long history dating back to its original discovery in 1878, roughly 5 years before the well-known Michael reaction was revealed.11 The intramolecular version of the oxa-Michael reaction has seen a renaissance and has been extensively utilized in natural product synthesis for over 2 decades.14
In contrast, the Baylis—Hillman reaction was discovered in 1972.12 Intramolecular versions of the Baylis—Hillman reaction have only recently gained favor,13 where elegant work in this area has elevated the status of the intramolecular variant.15 Although the Michael accepting ability of vinyl sulfones is well-documented,16 their vinyl sulfonamide counterparts have assumed a far less prominent role as viable Michael acceptors.10,17 We herein report the first examples of intramolecular oxa-Michael and Baylis—Hillman reactions of vinyl sulfonamides to afford an array of novel five-, six-, seven-, and eight-membered ring sultam scaffolds in good to excellent yields. The Baylis—Hillman reactions proceed with good to excellent levels of diastereoselectivity, ultimately yielding interesting sultam scaffolds. Overall, this divergent route can be used to produce skeletally diverse scaffolds from a single percursor in excellent yields.
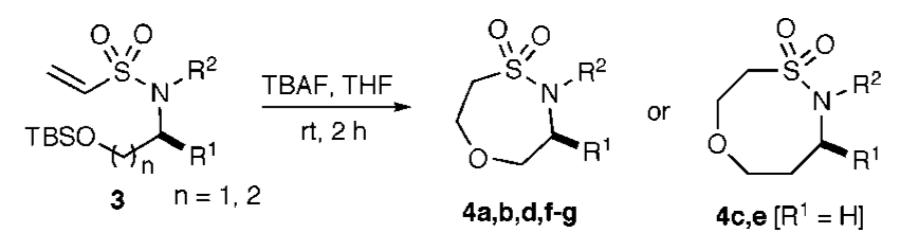
The titled route began with the assembly of TBS-protected amino alcohols, which represent cheap and versatile building blocks for sultam scaffold synthesis. These TBS-protected alcohols were reacted with 2-chloroethanesulfonyl chloride to produce vinyl sulfonamides 2 in good yields (Scheme 1). Subsequent benzylation/allylation reactions of vinyl sulfonamides 2 yielded vinyl sulfonamides 3 in good yields over three steps. The intramolecular oxa-Michael reaction was initiated by TBS-deprotection with TBAF in THF to afford the unique seven- and eight-membered ring sultams, respectively, in excellent yield under mild condition.
Scheme 1.
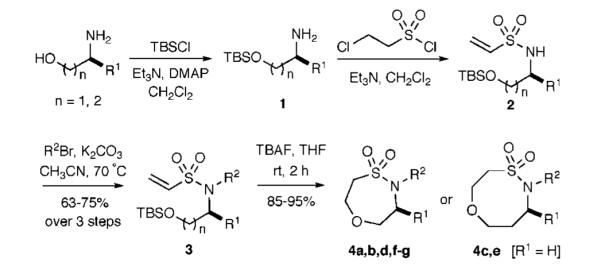
Synthesis of Seven- and Eight-Membered Ring Sultams via Intramolecular Oxa-Michael Reactions
Several examples of this straighforward procedure were examined as outlined in Table 1. In all cases the cyclization proceeded without incident. In several cases, the entire linear sequence could be run without the need for chromatography, ultimately resulting in the production of oxathiazepine- and oxathiazocine-dioxides, seven- and eight-membered ring systems, in excellent yields, respectively. To the best of our knowledge, this work represents the first report of using intramolecular oxa-Michael reactions to generate an eight-membered ring and enriches Baldwin’s rules for ring closure.18
Table 1.
Synthesis of Seven- and Eight-Membered Ring Sultams via Intramolecular Oxa-Michael Reactions
 | |||||
|---|---|---|---|---|---|
| entry | product | n | R1 | R2 | total yielda (%) |
| 1 | 4a | 1 | i-Pr | CH2CH=CH2 | 61 |
| 2 | 4b | 1 | CH2Ph | CH2Ph(o-Br) | 60 |
| 3 | 4c | 2 | H | CH2CH=CH2 | 54 |
| 4 | 4d | 1 | CH2Ph | CH2Ph(o-Me) | 59b |
| 5 | 4e | 2 | H | CH2Ph(o-F) | 53b |
| 6 | 4f | 1 | i-Bu | CH2Ph(m-Cl) | 58b |
| 7 | 4g | 1 | i-Bu | CH2Ph | 61b |
Total yield of all four steps.
Sultams 4d—g were synthesized through chromatography-free linear synthesis; see Supporting Information for reaction procedure.
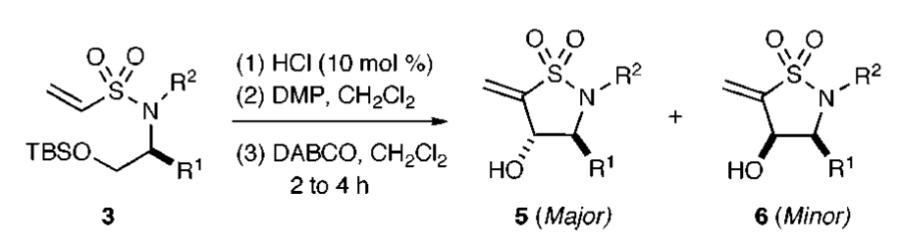
An orthogonal pathway leading to an intramolecular Baylis—Hillman reaction was next investigated. We observed that the intramolecular oxa-Michael reaction pathway was not operative under acidic conditions, and thus, a 10 mol % aqueous solution of HCl was implemented for TBS-deprotection of vinylsulfonamide 3. Subsequent Dess—Martin oxidation yielded the aldehyde vinyl sulfonamide, which underwent smooth Baylis—Hillman reaction upon the addition of DABCO (10 mol %). Overall, the intramolecular Baylis—Hillman reaction was completed within 2–4 h, affording the vinyl sultam 5 in excellent yields and with moderate to good levels of diastereoselectivity (Table 2). Several other organocatalysts such as DMAP, DBU, quinine, quinidine, and brucine were also screened in this process. Overall, DABCO proved to be the most efficient for both yield and competitive diastereoselectivity.19,20
Table 2.
Intramolecular Baylis—Hillman Reactions
 | |||||
|---|---|---|---|---|---|
| entry | product | R1 | R2 | yielda (%) | dr (5/6) |
| 1 | 5a/6a | i-Pr | CH2CH=CH2 | 69 | 63:37 |
| 2 | 5b/6b | i-Bu | CH2Ph(o-Br) | 71 | 62:38 |
| 3 | 5c/6c | i-Bu | CH2CH=CH2 | 67 | 76:24 |
| 4 | 5d/6d | CH2Ph | CH2CCH | 69 | 77:23 |
| 5 | 5e/6e | CH2Ph | CH2Ph(o-Br) | 72 | 88:12 |
| 6 | 5f/6f | CH2Ph | CH2Ph | 71 | 90:10 |
Yield of three steps.
The TBS-protected prolinol-derived vinyl sulfonamide 7 was next studied where treatment with TBAF resulted in the production of the bicyclic sultam 8 in excellent yields via an oxa-Michael pathway (Scheme 2). In contrast, treatment of 7 with the aforementioned orthogonal conditions involving acidic deprotection, oxidation, and addition of DABCO yielded the bicyclic sultam 10 with good yield and excellent diastereoselective ratio.
Scheme 2.
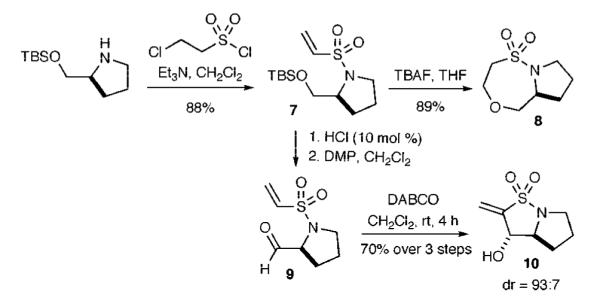
Synthesis of Bicyclic Sultam Scaffold
The method was extended to the readily prepared secondary alcohol, TBS-protected trans-2-amino-cyclohexanol (Scheme 3). Vinylsulfonylation and benzylation yielded the vinyl sulfonamide 12 in excellent yields. Treatment with TBAF yielded the bicyclic sultam 13 in excellent yield via an oxa-Michael pathway, and treatment with the orthogonal Baylis—Hillman conditions yielded the unique bicyclic vinyl sultam 15 in modest yield and extended reaction time (53%, 72 h) yet with excellent diastereoselectivity (dr > 95:5).
Scheme 3.
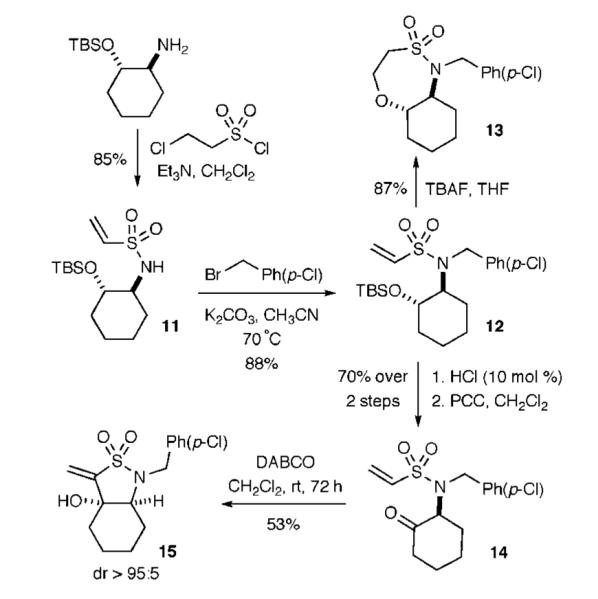
Baylis—Hillman Reaction via Keto Vinyl Sulfonamide
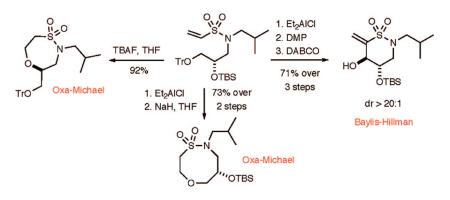
We next investigated the feasibility of extending the method to the generation of δ-sultams (Scheme 4). To address this question, chiral, nonracemic amino TBS-protected alcohol 16 was synthesized starting from (S)-glycidyl trityl ether.21 Subsequent TBS-protection and vinylsulfonylation with 2-chloroethanesulfonyl chloride afforded vinyl sulfonamide 18. TBS-deprotection with TBAF afforded the δ-sultam 19 in excellent yield via an intramolecular oxa-Michael pathway. The Baylis—Hillman pathway was initiated by use of a selective, orthogonal deprotection of the trityl ether in 18 at -10 °C using Me2AlCl (CH2Cl2 solution) to yield vinylsulfonamide 20 in good yield. The resulting alcohol was oxidized to aldehyde 21 using the Dess—Martin agent as previously described. Treatment with DABCO afforded the δ-sultam scaffold 22 in excellent yield and diastereoselectivity (dr > 95:5).22 The intramolecular oxa-Michael reaction of 20 was also pursued to afford the eight-membered-ring sultam by using NaH in THF. This reaction proceeded quickly and gave a high yield of the eight-membered-ring sultam 23.
Scheme 4.
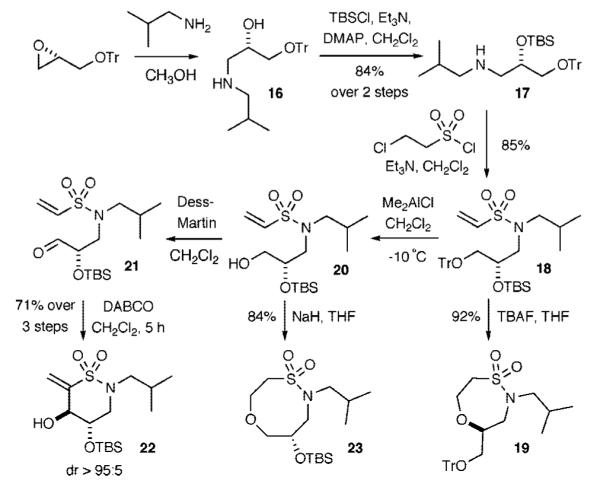
Highly Diastereoselective Intramolecular Baylis—Hillman Reaction to Generate δ-Sultam
In summary, the first examples of intramolecular oxa-Michael and Baylis—Hillman reactions to synthesize five-, six-, seven-, and eight-membered ring sultams have been attained. Moreover, empirical evidence validating 8-endo-trig cyclization pathways in oxa-Michael reactions has been presented that enriches Baldwin’s rules for ring closure. The titled oxa-Michael and Baylis—Hillman reactions are completed within short order and occur with excellent yields. In addition, good to excellent levels of diastereoselectivity were achieved throughout the Baylis—Hillman study. Overall, these two reactions can be conveniently combined into one synthetic route to produce skeletally diverse scaffolds from a single percursor in excellent yields. In addition, the method is highly amenable to library generation and current efforts are currently engaged in this endeavor.
Supplementary Material
Acknowledgment
This investigation was generously supported by funds provided by the National Institutes of General Medical Sciences Center for Chemical Methodologies and Library Development at the University of Kansas (P50 GM069663) and NIGMS Pilot-Scale Libraries Program (NIH P41 GM076302). The authors thank Dr. David VanderVelde (Director of the NMR Laboratory at The University of Kansas) for helpful discussions regarding NMR structure assignments. The authors also thank Daiso Co., Ltd., Fine Chemical Department for donation of trityl-protected glycidol. (email: akkimura@daiso.co.jp).
Footnotes
Supporting Information Available: Experimental details and spectral characterization for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1) (a).Drews J. Science. 2000;287:1960–1964. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]; (b) Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Curr. Med. Chem. 2003;10:925–953. doi: 10.2174/0929867033457647. [DOI] [PubMed] [Google Scholar]
- (2) (a).Silvestri R, Marfè G, Artico M, La Regina G, Lavecchia A, Novellino E, Morgante M, Di Stefano C, Catalano G, Filomeni G, Abruzzese E, Ciriolo MR, Russo MA, Amadori S, Cirilli R, La Torre F, Salimei PS. J. Med. Chem. 2006;49:5840–5844. doi: 10.1021/jm0602716. [DOI] [PubMed] [Google Scholar]; (b) Lebegue N, Gallet S, Flouquet N, Carato P, Pfeiffer B, Renard P, Léonce S, Pierré A, Chavatte P, Berthelot P. J. Med. Chem. 2005;48:7363–7373. doi: 10.1021/jm0503897. [DOI] [PubMed] [Google Scholar]
- (3).Tanimukai H, Inui M, Hariguchi S, Kaneko Z. Biochem. Pharmacol. 1965;14:961–970. doi: 10.1016/0006-2952(65)90248-0. [DOI] [PubMed] [Google Scholar]
- (4).Wroblewski T, Graul A, Castaner J. Drugs Future. 1998;23:365–369. [Google Scholar]
- (5).Rabasseda X, Hopkins SJ. Drugs Today. 1994;30:557–563. [Google Scholar]
- (6).Inagaki M, Tsuri T, Jyoyama H, Ono T, Yamada K, Kobayashi M, Hori Y, Arimura A, Yasui K, Ohno K, Kakudo S, Koizumi K, Suzuki R, Kato M, Kawai S, Matsumoto S. J. Med. Chem. 2000;43:2040–2048. doi: 10.1021/jm9906015. [DOI] [PubMed] [Google Scholar]
- (7).Valente C, Guedes RC, Moreira R, Iley J, Gut J, Rosental PJ. Biorg. Med. Chem. Lett. 2006;16:4115–4119. doi: 10.1016/j.bmcl.2006.04.079. [DOI] [PubMed] [Google Scholar]
- (8).Brzozowski F, Saczewski F, Neamati N. Bioorg. Med. Chem. Lett. 2006;16:5298–5302. doi: 10.1016/j.bmcl.2006.07.089. [DOI] [PubMed] [Google Scholar]
- (9).McKerrecher D, Pike KG, Waring MJ. PCT Int. Appl. 2006 2006125972. [Google Scholar]
- (10).For an extensive list of both classical and transition-metal-catalyzed cyclization reactions, see: Jiménez-Hopkins M, Hanson PR. Org. Lett. 2008;10:2223–2226. doi: 10.1021/ol800649n.
- (11) (a).Loydl F. Justus Liebigs Ann. C. 1878;192:80–89. [Google Scholar]; (b) Berkessel A. Methods of Organic Chemistry (Hueben-Weyl) 4th ed. Vol. E21e. G. Thieme; Stuttgart: 1952. pp. 4818–4856. and references therein. [Google Scholar]; (c) Enders D, Haertwig A, Raabe G, Runsink J. Angew Chem., Int. Ed. 1996;35:2388–2390. and references therein. [Google Scholar]; (d) Shi Y-L, Shi M. Org. Biomol. Chem. 2007;5:1499–1504. doi: 10.1039/b618984a. [DOI] [PubMed] [Google Scholar]
- (12).Baylis AB, Hillman MED. 2155113 German Patent. 1972; Chem. Abstr. 1972;77:34174q. [Google Scholar]
- (13).Basavaiah D, Rao AJ, Satyanarayana T. Chem. Rev. 2003;103:811–891. doi: 10.1021/cr010043d. [DOI] [PubMed] [Google Scholar]
- (14) (a).See ref 11c and references therein.; (b) Nicolaou KC, Hwang C-K, Duggan ME. J. Am. Chem. Soc. 1989;111:6682–6690. [Google Scholar]; (c) Evans DA, Gauchet-Prunet JA. J. Org. Chem. 1993;58:2446–2453. [Google Scholar]; (d) Li M, O’Doherty GA. Org. Lett. 2006;8:6087–6090. doi: 10.1021/ol062595u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ahmed MM, Mortensen MS, O’Doherty GA. J. Org. Chem. 2006;71:7741–7746. doi: 10.1021/jo061200h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chandrasekhar S, Rambabu C, Shyamsunder T. Tetrahedron Lett. 2007;48:4683–4685. [Google Scholar]
- (15) (a).Roth F, Gygax P, Frater G. Tetrahedron Lett. 1992;33:1045–1048. [Google Scholar]; (b) Wang L-C, Luis AL, Agapiou K, Jang H-Y, Krische MJ. J. Am. Chem. Soc. 2002;124:2402–2403. doi: 10.1021/ja0121686. [DOI] [PubMed] [Google Scholar]; (c) Frank SA, Mergott DJ, Roush WR. J. Am. Chem. Soc. 2002;124:2404–2405. doi: 10.1021/ja017123j. [DOI] [PubMed] [Google Scholar]; (d) Keck GE, Welch DS. Org. Lett. 2002;4:3687–3690. doi: 10.1021/ol026638s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Huddleston RR, Krische MJ. Synlett. 2003;12:21. [Google Scholar]; (f) Reddy LR, Saravanan P, Corey EJ. J. Am. Chem. Soc. 2004;126:6230–6231. doi: 10.1021/ja048613p. [DOI] [PubMed] [Google Scholar]; (g) Yeo JE, Yang X, Kim HJ, Koo S. Chem. Commun. 2004:236–237. doi: 10.1039/b311951c. [DOI] [PubMed] [Google Scholar]; (h) Aroyan CE, Vasbinder MM, Miller SJ. Org. Lett. 2005;7:3849–3851. doi: 10.1021/ol0513544. [DOI] [PubMed] [Google Scholar]; (i) Shi M, Chen LH, Li CQ. J. Am. Chem. Soc. 2005;127:3790–3800. doi: 10.1021/ja0447255. [DOI] [PubMed] [Google Scholar]; (j) Krafft ME, Haxell TFN, Seibert KA, Abboud KA. J. Am. Chem. Soc. 2006;128:4174–4175. doi: 10.1021/ja057595o. [DOI] [PubMed] [Google Scholar]; (k) Teng W-D, Huang R, Kwong CK-W, Shi M, Toy PH. J. Org. Chem. 2006;71:368–371. doi: 10.1021/jo051802l. [DOI] [PubMed] [Google Scholar]; (l) Nakano A, Takahashi K, Ishihara J, Hatakeyama S. Org. Lett. 2006;8:5357–5360. doi: 10.1021/ol0622561. [DOI] [PubMed] [Google Scholar]
- (16) (a).Truce WE, Wellisch E. J. Am. Chem. Soc. 1952;74:2881–2884. [Google Scholar]; (b) Luis A-L, Krische MJ. Synthesis. 2004:2579–2585. [Google Scholar]; (c) Meadows DC, Gervay-Hague J. Med. Res. Rev. 2006;26:793–814. doi: 10.1002/med.20074. [DOI] [PubMed] [Google Scholar]; (d) Oh K. Org. Lett. 2007;9:2973–2975. doi: 10.1021/ol0710663. [DOI] [PubMed] [Google Scholar]; (e) Esteves AP, Silva ME, Rodrigues LM, Oliveira-Campos AMF, Hrdina R. Tetrahedron Lett. 2007;48:9040–9043. [Google Scholar]; (f) Das I, Pal TK, Pathak T. J. Org. Chem. 2007;72:9181–9189. doi: 10.1021/jo701378d. [DOI] [PubMed] [Google Scholar]
- (17).Reddick JJ, Cheng J, Roush WR. Org. Lett. 2003;5:1967–1970. doi: 10.1021/ol034555l. [DOI] [PubMed] [Google Scholar]
- (18).Although Baldwin defined 7-endo-trig as favorable, 8-endo-trig pathways were not defined; see: Baldwin JE. J. Chem. Soc., Chem. Commun. 1976;73:4–736.
- (19).The stereochemistry of major product 5 was determined by 1D-NOE and NOESY experiments; see Supporting Information.
- (20).To the best of our knowledge, this work represents the first report of using an intramolecular Baylis—Hillman reaction to generate a sultam.
- (21).Regioselective ring-opening of glycidyl trityl ether with isobutylamine gave the amino alcohol 16 in excellent yield.
- (22).Confirmed by 1H measurements in different NMR solvents; see Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.