Abstract
A diversity-oriented synthesis (DOS) strategy termed “Click, Click, Cyclize” is reported. This approach relies on functional group (FG) pairing between a vinyl sulfonamide and an array of functional groups to synthesize skeletally diverse sultams. Several FG pairing pathways on central tertiary vinyl sulfonamide linchpins have been developed including intramolecular Heck, aza-Michael, ring-closing enyne metathesis, Pauson—Khand, and chemoselective oxidation/Baylis—Hillman reactions.
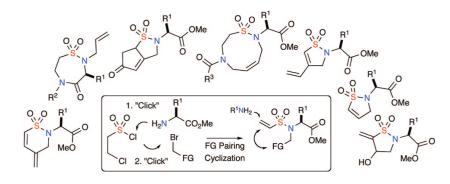
The growing demand for quick access to small molecules with novel architechures for high throughput screening has provided challenging opportunities for synthetic chemists. Despite significant advances in this field, a lack of systematic library planning strategies has been cited as a limiting factor in the process.1 Diversity-oriented synthesis (DOS)1,2 has emerged to address this deficiency as an enabling platform for the facile production of multiple scaffolds1-3 displaying skeletal diversity.2,4 Among several features that define DOS, forward-synthetic analysis1,2 and functional group (FG) pairing5,6 have surfaced as significant tools. In particular, forward-synthetic analysis focuses on overarching strategies to generate multiple scaffolds in the fewest possible steps, while FG pairing aims to selectively pair functional groups from a central functionalized core, resulting in the quick production of an array of scaffolds with minimum steps. Both concepts are at the heart of the “Build/Couple/Pair (BCP)” strategy pioneered by Schreiber and co-workers.6 This strategy involves a “Build phase” to assemble chiral building blocks containing orthogonal sets of functionality suitable for the subsequent “Coupling and FG pairing” phases en route to an array of diverse scaffolds. In this regard, interest in the generation of skeletally diverse sultams for biological screening has provided impetus for the titled FG pairing cyclization strategy termed “Click, Click, Cyclize” enroute to five-, six-, seven-, eight-, and nine-membered ring sultams.7
Sulfonamides have long been valued for their rich biological and chemical profiles and have emerged as a promising class of compounds in drug discovery.8 Sultams (cyclic sulfonamides), although not found in nature, display a wide array of potent biological activities.9,10 Traditionally, sultam syntheses have relied on classical cyclization protocols and a number of transition-metal-catalyzed processes that have recently been reported.10 In this regard, vinyl sulfonamides represent an emerging chemotype with a wide spectrum of reaction potential to generate sultams. Literature precedence has shown their reactivity in Diels—Alder,11 Heck,12 indium-initiated radical addition,13 and [3 + 2] cycloadditions.14 This chemical profile is augmented by recent work using ring-closing metathesis (RCM),10a,15 intramolecular oxa-Michael, and Baylis—Hillman cyclizations to generate sultams.16 Taken collectively, these methods represent opportunities for the implementation of DOS approaches to new sultams.
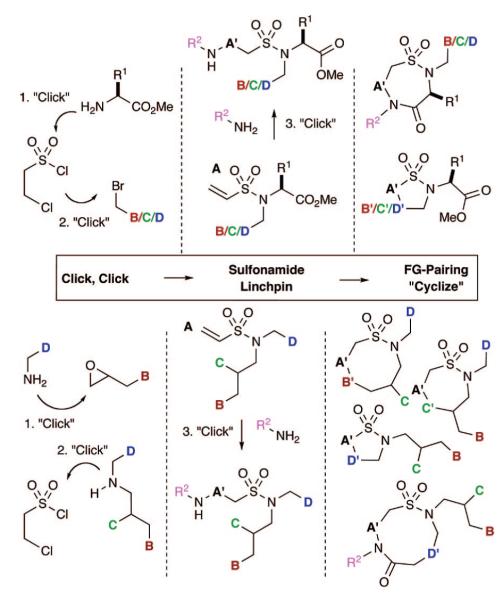
At the heart of the titled approach is the facile production of precursorary tertiary vinyl sulfonamides via use of two “Click” reactions7 (Figure 1). These two reliable reactions, include either (i) vinyl sulfonylation of amines and subsequent alkylation of the resulting secondary sulfonamides or (ii) the production of a secondary amine via an epoxide opening with an amine, followed by vinyl sulfonylation. Subsequent FG pairing in either case between the vinyl sulfonamide moiety A and FGs B, C, and D yield sultams of varying architectures.17 Alternatively, the use of a third reaction,7 an aza-Michael reaction, allows for broadening of FG pairing patterns and installation of an additional diversity element. Overall, these strategies rely on efficient and disparate FG pairing pathways on tertiary vinyl sulfonamides to establish reaction manifolds eminating from a single linchpin.
Figure 1.
“Click, Click, Cyclize” or “Click, Click, Click, Cyclize” approach to skeletally diverse sultams.
This study was initiated by investigating several FG pairing pathways between vinyl sulfonamides and disparate functional groups including intramolecular Heck, aza-Michael,18 ring-closing enyne metathesis (RCEM), Pauson-Khand (PK), and chemoselective oxidation/Baylis-Hillman reactions as outlined in Scheme 1. These methods set the foundation for the overall DOS approach outlined in Schemes 2 and 3, whereby multiplication of a single scaffold3 can be realized.
Scheme 1.
DOS via “Click, Click, Cyclize” Reaction Manifolds
Scheme 2.
“Click, Click, Cyclize” Reaction Manifolds Utilizing FG Pairing en Route to Skeletally Diverse Sultams
Scheme 3.
FG Pairing to Sultams Utilizing FGI
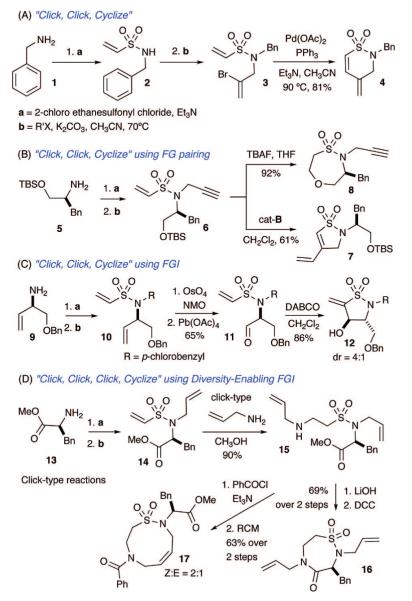
The first pathway investigated was commenced with a vinyl sulfonylation/allylation sequence yielding tertiary vinyl sulfonamide 3, which undergoes a regioselective 6-endotrig intramolecular Heck cyclization to afford the δ-sultam 4 in good yield (Scheme 1, pathway A). This pathway is amenable to variations in both the amine component and alkylation partner as shown in Scheme 2.
The use of a TBS-protected amino alcohol and propargyl bromide in the vinyl sulfonylation/alkylation, respectively, produces the tertiary sulfonamide linchpin 6 (Scheme 1, pathway B). Linchpin 6 is armed for an intramolecular enyne metathesis yielding sultam 7, whereas TBAF deprotection initiates an intramolecular oxa-Michael cyclization to afford 8 in 92% yield.
Use of FG interconversion (FGI) broadens the reaction manifold by allowing for a new FG pairing event (Scheme 1, pathway C). In this example, chemoselective oxidation of an allyl moiety in the presence of the electron-deficient vinyl sulfonamide enables FG pairing cyclization between the aldehyde and vinyl sulfonamide moieties utilizing an intramolecular Baylis—Hillman cyclization reaction. In this regard, sequential vinyl sulfonylation of amine 9 and alkylation generate vinyl sulfonamide 10 in good yield. Chemoselective oxidation with OsO4/NMO/Pb(OAc)4 followed by DABCO-initiated Baylis—Hillman cyclization affords the γ-sultam 12 in 86% yield (dr = 4:1).
A fourth pathway utilizes an aza-Michael reaction in a “Click, Click, Click, Cyclize” reaction sequence, which effects an umpulong FGI of an electrophilic vinyl sulfonamide into a nucleophilic amino sulfonamide (Scheme 1, pathway D). This latter concept represents a diversity-enabling step and offers a new pattern of FG pairing between the nucleophilic amine (NHR2) and remaining FGs.
Although use of an aza-Michael reaction on a vinyl sulfonamide has been previously reported,18 its use in sultam synthesis is limited.16 In this study, it was found that 14 undergoes facile aza-Michael reaction to afford sulfonamide 15 in excellent yield. Subsequent basic hydrolysis and intramolecular lactamization yields the seven-membered sultam 16. Alternatively, reaction with benzoyl chloride, followed by RCM, produces the nine-membered sultam 17 in 63% yield (Z/E = 2:1).
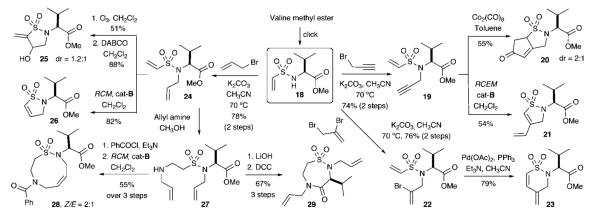
Pathways A—D outlined in Scheme 1 can be integrated into DOS strategies quite readily as outlined in Schemes 2 and 3. The first DOS reaction manifold strategy we investigated began with the readily prepared vinyl sulfonamide 18 derived from vinyl sulfonylation of valine methyl ester with 2-chloro ethanesulfonyl chloride. Subsequent alkylation with propargyl bromide, 2,3-dibromopropene, or allyl bromide arms the sulfonamide linchpin for three central reaction pathways. These include (i) propargylation to 19, followed by intramolecular Pauson—Khand cyclization to afford sultam scaffold 20 or RCEM with (IMesH2)(PCy3)-(Cl)2Ru=CHPh (Grubbs cat-B) to provide sultam 21, (ii) allylation of 18 with 2,3-dibromopropene to yield 22, followed by regioselective intramolecular Heck cyclization to produce sultam 23 in 79% yield, and (iii) allylation of 18 using allyl bromide to yield 24.
This latter allylation route opens three additional reaction pathways to broaden the overall DOS reaction manifold (Scheme 2). The first route employs selective oxidation by ozone to generate an aldehyde for subsequent intramolecular Baylis—Hillman cyclization affording the γ-sultam 25 in 88% yield with 1.2:1 diastereoselectivity. A second route utilizes RCM of 24 with cat-B to yield γ-sultam 26. A third route employs the aforementioned intermolecular aza-Michael reaction of vinyl sulfonamide 24 to afford the β-amino sulfonamide 27, which leads to yet two additional synthetic branch routes. Simple benzoylation of 27, followed by RCM reaction generates the nine-membered sultam 28 in 55% yield (Z/E = 2:1). Alternatively, hydrolysis of sulfonamide 27 and intramolecular amidation using DCC coupling affords the seven-membered sultam 29. Overall, this process yields a total of seven different sultam scaffolds in short order.
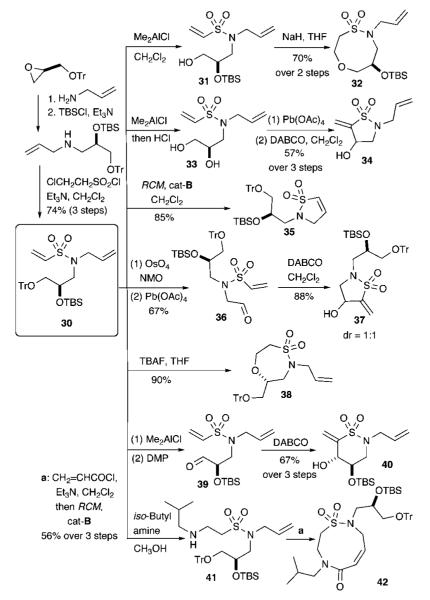
The second DOS strategy was initiated with vinyl sulfonamide 30, in which three functional groups are incorporated in the amine component for FG pairing. Seven synthetic routes have been developed. The first uses chemoselective deprotection of the trityl group of 30 using Me2AlCl, followed by NaH-promoted oxa-Michael addition to afford sultam 32 in 70% yield over two steps (Scheme 3). The second utilizes a global deprotection to produce diol 33. Subsequent oxidative cleavage with Pb(OAc)4 and Baylis—Hillman cyclization affords sultam 34 in 57% yield over three steps. The third example involves RCM of vinyl sulfonamide 30 to yield sultam scaffold 35 in 85% yield using cat-B. Alternatively, selective oxidation of the terminal olefin in vinyl sulfonamide 30 followed by intramolecular Baylis-Hillman cyclization affords sultam 37. A fifth example demonstrates facile oxa-Michael cyclization initiated by TBAF deprotection to produce sultam scaffold 38 in 90% yield. A sixth example involves selective trityl deprotection/Dess—Martin oxidation and intramolecular Baylis—Hillman to furnish sultam 40 in 67% yield over three steps with >20:1 diastereoselectivity. The seventh and final example utilizes an intermolecular aza-Michael reaction with allyl amine to generate β-amine sulfonamide 41, which was reacted with acryloyl chloride and subjected to RCM with cat-B to yield the nine-membered ring sultam-lactam 42 (Z/E = 2:1) in 56% yield over three steps. Overall, seven unique scaffolds are produced in simple reaction sequences.
In summary, we have reported several FG pairing pathways to generate sultams based on versatile vinyl sulfonamide linchpins. Incorporation of diversity-enabling FGI expands the DOS reaction manifolds and broadens the scope of the method. These results are highly amenable to library production, and ongoing efforts are in order.
Supplementary Material
Acknowledgment
This investigation was generously supported by funds provided by the NIH Center for Chemical Methodologies and Library Development at the University of Kansas (P50 GM069663) and NIGMS Pilot-Scale Libraries Program (NIH P41 GM076302). The authors also thank Daiso Co., Ltd., Fine Chemical Department for donation of trityl-protected glycidol.
Footnotes
Supporting Information Available: Experimental details and spectral charactization for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1) (a).Burke MD, Schreiber SL. Angew. Chem., Int. Ed. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]; (b) Spiegel DA, Schroeder FC, Duvall JR, Schreiber SL. J. Am. Chem. Soc. 2006;128:14766–14767. doi: 10.1021/ja065724a. [DOI] [PubMed] [Google Scholar]
- (2) (a).Schreiber SL. Science. 2000;287:1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]; (b) Pelish HE, Westwood NJ, Feng Y, Kirchhausen T, Shair MD. J. Am. Chem. Soc. 2001;123:6740–6741. doi: 10.1021/ja016093h. [DOI] [PubMed] [Google Scholar]; (c) Burke MD, Berger EM, Schreiber SL. Science. 2003;302:613–618. doi: 10.1126/science.1089946. [DOI] [PubMed] [Google Scholar]; (d) Spring DR. Org. Biomol. Chem. 2003;1:3867–3870. doi: 10.1039/b310752n. [DOI] [PubMed] [Google Scholar]; (e) Wipf P, Stephenson CRJ, Walczak MAA. Org. Lett. 2004;6:3009–3012. doi: 10.1021/ol0487783. [DOI] [PubMed] [Google Scholar]; (f) Tan DS. Nat. Chem. Biol. 2005;1:74–84. doi: 10.1038/nchembio0705-74. [DOI] [PubMed] [Google Scholar]; (g) Smith AB, Walsh PS, Frohn M, Duffey MO. Org. Lett. 2005;7:139–142. doi: 10.1021/ol047792c. [DOI] [PubMed] [Google Scholar]; (h) Sunderhaus JD, Dockendorff C, Martin SF. Org. Lett. 2007;9:4223–4226. doi: 10.1021/ol7018357. [DOI] [PubMed] [Google Scholar]; (i) Mao S, Probst D, Werner S, Chen J, Xie X, Brummond KM. J. Comb. Chem. 2008;10:235–246. doi: 10.1021/cc7001843. [DOI] [PubMed] [Google Scholar]
- (3) (a).Tempest AP, Armstrong WR. J. Am. Chem. Soc. 1997;119:7607–7608. [Google Scholar]; (b) Castellano S, Fiji HDG, Kinderman SS, Watanade M, Leon PD, Tamanoi F, Kwon O. J. Am. Chem. Soc. 2007;129:5843–5845. doi: 10.1021/ja070274n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4) (a).Arya P, Joseph R, Gan Z, Rakic B. Chem. Biol. 2005;12:163–180. doi: 10.1016/j.chembiol.2005.01.011. [DOI] [PubMed] [Google Scholar]; (b) Spiegel DA, Schroeder FC, Duvall JR, Schreiber SL. J. Am. Chem. Soc. 2006;128:14766–14767. doi: 10.1021/ja065724a. [DOI] [PubMed] [Google Scholar]; (c) Thomas GL, Spring DR, et al. Angew. Chem., Int. Ed. 2008;47:2808–2912. doi: 10.1002/anie.200705415. [DOI] [PubMed] [Google Scholar]
- (5).Comer E, Rohan E, Deng L, Porco JA. Org. Lett. 2007;9:2123–2126. doi: 10.1021/ol070606t. [DOI] [PubMed] [Google Scholar]
- (6).Nielsen TE, Schreiber SL. Angew. Chem., Int. Ed. 2008;47:48–56. doi: 10.1002/anie.200703073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).“Click” chemistry is a chemical philosophy introduced by K. Barry Sharpless in 2001 that describes chemistry tailored to generate substances quickly and reliably by joining small units together; see: Kolb H, Finn MG, Sharpless KB. Angew. Chem., Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5.
- (8) (a).Drews J. Science. 2000;287:1960–1964. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]; (b) Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Curr. Med. Chem. 2003;10:925–953. doi: 10.2174/0929867033457647. [DOI] [PubMed] [Google Scholar]
- (9) (a).Tanimukai H, Inui M, Harigushi S, Kaneko J. Biochem. Pharmacol. 1965;14:961–970. doi: 10.1016/0006-2952(65)90248-0. [DOI] [PubMed] [Google Scholar]; (b) Wroblewski T, Graul A, Castaner J. Drugs Future. 1998;23:365–369. [Google Scholar]; (d) Rabasseda X, Hopkins SJ. Drugs Today. 1994;30:557–563. [Google Scholar]; (e) Inagaki M, Tsuri T, et al. J. Med. Chem. 2000;43:2040–2048. doi: 10.1021/jm9906015. [DOI] [PubMed] [Google Scholar]; (f) Valente C, Guedes RC, Moreira R, Iley J, Gut J, Rosental PJ. Bioorg. Med. Chem. Lett. 2006;16:4115–4119. doi: 10.1016/j.bmcl.2006.04.079. [DOI] [PubMed] [Google Scholar]
- (10) (a).For an extensive list of biologically active sultams as well as classical and transition metal catalyzed cyclization strategies to sultams, see: Jiménez-Hopkins M, Hanson PR. Org. Lett. 2008;10:2223–2226. doi: 10.1021/ol800649n.Rolfe A, Young K, Hanson PR. Eur. J. Org. Chem. 2008:5254–5262. doi: 10.1002/ejoc.200800651.
- (11) (a).Brosius AD, Overman LE. J. Am. Chem. Soc. 1999;121:700–709. [Google Scholar]; (b) Greig IR, Tozer MJ, Wright PT. Org. Lett. 2001;3:369–371. doi: 10.1021/ol006863e. [DOI] [PubMed] [Google Scholar]; (c) Bernabeu MC, Chinchilla R, Falvello LR, Nájera C. Tetrahedron: Asymetry. 2001;12:1811–1815. [Google Scholar]; (d) Wanner J, Harned AM, Probst DA, Poon KWC, Klein TA, Snelgrove KA, Hanson PH. Tetrahedron Lett. 2002;43:917–721. [Google Scholar]; (e) Rogatchov VO, Bernsmann H, Schwab P, Fröhlich R, Wibbeling B, Metz P. Tetrahedron Lett. 2002;43:4753–4756. [Google Scholar]
- (12).Merten S, Fröhlich R, Kataeva O, Metz P. AdV. Synth. Catal. 2005;347:754–758. [Google Scholar]
- (13).Ueda M, Miyabe H, Nishimura A, Miyata O, Takemoto Y, Naito T. Org. Lett. 2003;5:3835–3838. doi: 10.1021/ol035357x. [DOI] [PubMed] [Google Scholar]
- (14).Chiacchio U, Corsaro A, Gumina G, Pistarà V, Rescifina A, Alessi M, Piperno A, Roeo G, Romeo R. Tetrahedron. 1997;53:13855–13866. [Google Scholar]
- (15) (a).Hanson PR, Probst DA, Robinson RE, Yau M. Tetrahedron Lett. 1999;40:4761–4764. [Google Scholar]; (b) Hanessian S, Sailes H, Therrien E. Tetrahedron. 2003;59:7047–7056. [Google Scholar]
- (16).Zhou A, Hanson RP. Org. Lett. 2008;10:2951–2954. doi: 10.1021/ol8009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Use of A’,B’,C’, and D’ denotes functional group transformation after an FG pairing event.
- (18).Makara MG, Ma Y. Tetrahedron Lett. 2001;42:4123–4125. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.