Abstract
Background
Over 80% of patients entering cardiac rehabilitation (CR) are overweight and >50% have metabolic syndrome. Current CR exercise protocols result in little weight loss and minimal changes in cardiac risk factors. We sought to design an exercise protocol that would lead to greater weight loss and risk factor change.
Methods and Results
We performed a randomized-controlled clinical trial to evaluate the effect of high-caloric expenditure exercise (3000-3500 kcal/week exercise-related energy expenditure) compared with standard CR exercise (7-800 kcal/week) on weight loss and risk factors in 74 overweight patients with coronary heart disease (CHD). Both groups were counseled for weight loss and taking evidence-based preventive medications. High-caloric exercise resulted in double the weight loss (8.2±4 vs 3.7±5 kg, P<0.001) and fat mass loss (5.9±4 vs 2.8±3 kg, P< 0.001) and a greater waist reduction (-7±5 vs. -5±5 cm, P=0.02) than standard CR exercise at 5-months. High-caloric exercise reduced insulin resistance, measured with the euglycemic hyperinsulinemic clamp, along with the Cholesterol/HDL-Cholesterol ratio and components of the metabolic syndrome more than standard CR exercise (each P<0.01). Overall, fat mass loss best predicted improved metabolic risk and the prevalence of metabolic syndrome decreased from 59% to 31%. Changes in cardiac risk factors included decreased insulin resistance, increased HDL-Cholesterol and decreased measures of insulin, triglycerides, blood pressure, plasminogen activator inhibitor-1 and the Cholesterol/HDL-Cholesterol ratio (each P<0.05). Significant weight loss was maintained at 1-year.
Conclusions
High-caloric exercise promotes greater weight loss and more favorable cardio-metabolic risk profiles than standard CR for overweight coronary patients.
Keywords: Coronary Artery Disease, Obesity, Exercise, Metabolic Syndrome, Weight Loss
Introduction
While cardiac rehabilitation (CR) has been shown to reduce cardiac and total mortality in patients after a coronary event (1,2), current CR exercise protocols were developed in the 1970's when profound deconditioning after lengthy hospitalizations was common (3,4). Profiles of contemporary CR patients have changed as the prevalence of obesity has skyrocketed (5,6) and cardiac hospitalizations have shortened (7). From 1996 to 2006 the mean body mass index (BMI) for patients entering CR increased from 28.5 to 30.1 kg/m2 (5). Presently, > 80% of CR patients are overweight (BMI>25), the prevalence of obesity (BMI>30) is >40% and >50% have insulin resistance manifest as metabolic syndrome (5,8-10). Additionally, a focus on secondary prevention and risk reduction in CR has emerged (11,12). Yet, CR exercise protocols have remained largely unchanged.
Obesity and metabolic syndrome predict an increased risk of death and recurrent events after myocardial infarction (13-17) due, in part, to their association with an adverse risk factor profile even with patients taking evidence-based preventive therapies (18). While some epidemiologic studies have questioned the link between BMI and coronary events, often termed “the obesity paradox” (19), recent data has shown an association between obesity and an earlier presentation of acute myocardial infarction with increasing BMI (20). Mortality from CHD has decreased 50% from 1980-2000 (21). However, further declines were offset by increases in rates of obesity and type II diabetes (21). Weight loss in CR has been linked to diminished cardiovascular events over 6-years follow-up (22). Unfortunately, current CR protocols, result in little weight loss or risk factor change (23,24) due, in part, to the low CR-related energy expenditure of 7-800 kcal/week (25-28). Accordingly, we performed a randomized-controlled trial in patients with CHD that compared higher caloric-expenditure CR exercise (longer distance, almost daily walking) to standard CR exercise (shorter distance, 3 times weekly on multiple exercise modalities). Both groups received behavioral weight loss counseling. Primary endpoints were measures of body composition, insulin sensitivity and metabolic predictors of cardiovascular risk.
Methods
Subject Selection
Patients with CHD, BMI of >27 and waist circumference of >102 cm (men), >88 cm (women) were eligible for participation. We chose these criteria vs. a BMI >25 to avoid individuals with borderline excess adiposity with only a minimal need for weight reduction. Patients were enrolled from 2002-2006. A flow diagram of the recruiting process is detailed in Figure 1. We evaluated 114 subjects for eligibility. Subjects with diabetes mellitus were excluded although 25 patients with a fasting glucose of 100-126 mg/dL were enrolled. Patients with severe deconditioning (peak aerobic capacity of <14 mlO2 × kg-1 × min-1) were excluded because in a preliminary non-randomized trial such subjects were unable to markedly increase their exercise-related caloric expenditure and less successful at accomplishing weight loss (28). All subjects were ≥3 months since any hospitalizations. The study population ultimately consisted of 74 individuals; 60 males and 14 females. Cardiac diagnoses included past myocardial infarction (N= 34), coronary bypass surgery (N=34), percutaneous coronary intervention (N=28) or chronic stable angina (N= 6). This protocol was approved by the University of Vermont Committee on Human Research and registered as a clinical trial (NCT00628277). After informed consent, each patient underwent a clinical review to ascertain that evidence-based pharmacologic therapies were being taken (29). Baseline measures were taken at the University of Vermont General Clinical Research Center. Exercise was restricted for a minimum of 36 hours prior to all metabolic and exercise testing procedures.
Figure 1.
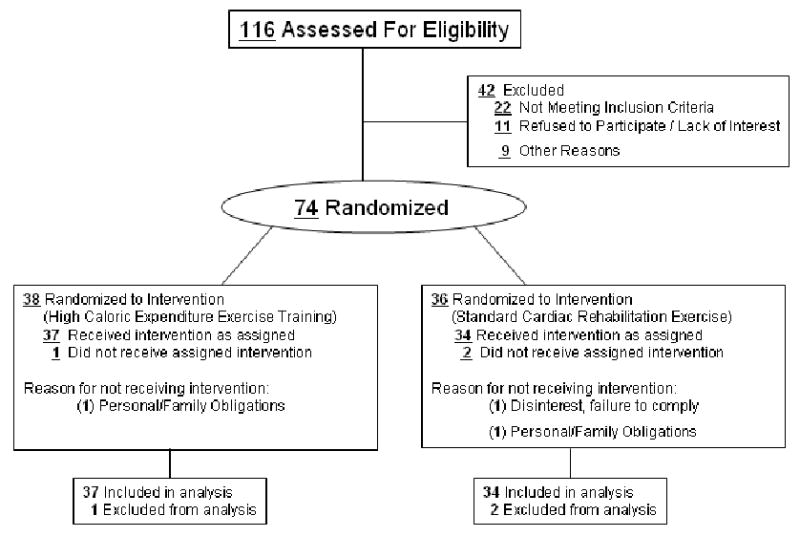
Flow diagram of the recruiting process.
Body Composition and Physical Activity
Body composition measures included body weight, BMI, waist circumference, fat mass, and fat free mass by dual energy x-ray absorptiometry (General Electric Lunar Prodigy, Madison, WI). Abdominal visceral and subcutaneous fat area was measured by CT scanning (General Electric Medical Systems, Milwaukee, WI, Philips Electronics N.V., Eindhoven, the Netherlands) (30). Physical activity energy expenditure was measured over 7-days using the doubly labeled water technique providing a non-invasive assessment of free-living activity energy expenditure after subtracting resting metabolic rate and thermic effect of food (31).
Coronary risk factor assessments included insulin-stimulated glucose disposal, insulin, glucose, lipid profiles, resting blood pressure (BP), high sensitivity C-reactive protein and plasminogen activator inhibitor-1 (PAI-1). Medications were withheld the morning of testing. BP was measured after 5 minutes in the seated position, using a Dynamap automated BP cuff. A mean of 3 measurements constituted the resting BP. Components of the metabolic syndrome were assessed including; waist circumference > 102 cm (men), >88 cm (women), systolic blood pressure ≥ 135 mm Hg or diastolic blood pressure ≥ 85 mm Hg, fasting triglycerides > 150 mg/dL, HDL cholesterol < 40 mg/dL (men), <50 mg/dL (women), and fasting blood glucose of > 100 mg/dL (32). The presence of ≥ 3 components defined the metabolic syndrome.
Insulin-stimulated glucose disposal was determined after an overnight fast using the euglycemic hyperinsulinemic clamp technique (30,33). Testing was preceded by 3 days of standardized meals consisting of 200-250 grams carbohydrate and 12 grams fiber per 1000 kcalories/day. Insulin-stimulated glucose-uptake, an index of insulin sensitivity, was the average dextrose infusion rate during the final 30 min of the 3-hour clamp plus residual endogenous glucose production (30) expressed relative to fat-free mass.
The concentration of PAI-1 was determined by ELISA (Tintelize, Biopool, Umea, Sweden)(34). High-sensitivity C-reactive protein was measured using a colorimetric ELISA (35). Isolated values of ≥ 10 mg/dL (3/74 at baseline) were eliminated from analysis unless it was elevated both before and after the 5 month intervention (1/71).
Peak aerobic capacity (peak VO2) was measured during a symptom-limited treadmill test using a graded modified-Balke protocol until volitional fatigue, cardiovascular symptoms, or ≥ 2 mm electrocardiographic ST-segment depression. Expired gas was analyzed using a SensorMedics Vmax 29c (Yorba Linda, CA) with measurement of Peak VO2 in mlO2 × kg-1 × min-1. Dietary macronutrient intake was estimated at baseline, week 15 and week 51 using 3-day dietary diaries (Food Intake Analysis System, Houston,TX).
Study Framework
Subjects were randomized to high-caloric exercise or standard CR using a distance function method in sets of four, balancing gender and BMI. After the 4-month intervention, subjects entered a 1-month weight stabilization phase, continuing to exercise but maintaining weight at < 1kg variation from the 4-month weight. We present data measured at 5-months as the primary outcome time-point. Subjects underwent identical testing as at baseline. Subsequently, subjects entered a maintenance phase performing most, but not all of their recommended exercise off-site, keeping exercise records. Each group was instructed to adhere to the exercise protocol established during the 5-month exercise intervention. There were monthly on-site reviews of dietary and exercise records with the dietician and exercise physiologist and biweekly calls from the dietician. Data are also presented at one year. Preventive medications were kept steady throughout the 12-month protocol.
Behavioral Weight Loss
The behavioral weight loss program included 16 hourly group-counseling sessions led by a dietician (36) emphasizing dietary records, itemization of food and caloric content. The daily caloric goal was 500 kcal less than predicted maintenance calories (36) independent of the exercise program to which they were assigned. There were no specific recommendations regarding macronutrient intake. Meal replacement plans were not used. Features of behavior modification included self-monitoring, stimulus control, problem solving, social assertion, goal setting, feedback, relapse prevention, and family involvement (36).
Exercise Protocols
The exercise prescription for the high-caloric CR group emphasized longer duration (45-60 minutes vs. 25-40 minutes per session), lower intensity (50-60% Peak V02 vs 65-70%) and more frequent (5-7 vs. 3 times/week) exercise than the standard CR group. Walking was the preferred exercise modality to maximize caloric expenditure vs. weight-supported exercises (cycling or rowing) which burns fewer calories (37). Simplistically, the high-caloric exercise training protocol was to “walk often and walk far”. The high-caloric exercise group had a weekly exercise expenditure goal of ≥ 3000-3500 kcal/week, attained after 2-4 weeks of gradually lengthening exercise bouts. After performing all sessions on-site for 2-weeks, high-caloric exercise subjects performed 2-4 sessions/week in the home environment using a heart rate monitor, subsequently downloaded at the CR center to ascertain duration of exercise. Both groups eventually performed 1-3 sessions/week on-site with home exercise logs. Exercise logs were reviewed weekly with the exercise physiologist to estimate caloric expenditure and ascertain compliance. The Standard CR group protocol included 25 minutes of treadmill walking and 8 minutes on 2 of 3 ergometers; cycle, rowing or arm. After 5-months, subjects transitioned to a primarily home-based exercise program continuing the same exercise prescription. Subjects were allowed to perform up to one session/week at the CR facility. The authors had full access to data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Statistical Analyses
Results are presented as the mean ± standard deviation. Repeated measure analysis of variance was used to determine overall treatment efficacy and to examine secondary outcomes between groups. When results indicated a differential treatment effect, the analysis was followed by an F-Test for Simple Effects to examine changes over time in each group separately. A two-sided P value of < 0.05 indicated statistical significance. Analyses were based upon all subjects who remained in the study at 5-months and 12-months, without imputation of missing values. Regression analysis was used to explore factors associated with fat mass loss and metabolic syndrome components during the intervention period, controlling for group assignment.
Multivariate analysis of variance was conducted on the change from baseline for a panel of cardio-metabolic risk factors associated with obesity. The majority of the variables were approximately normally distributed. Log transformations for several variables were considered but did not change the conclusions. Thus, all results presented are based upon untransformed data. The presence of the metabolic syndrome and the number of components present was computed both at baseline and at the end of treatment, with a logistic regression used to examine whether the presence of this syndrome changed differentially in each group following treatment. Differences in changes in the number of components of the metabolic syndrome were examined using Friedman's Test while Generalized Estimating Equations were used to examine changes across time in the prevalence of components of the metabolic syndrome. We estimated that a sample size of 34 subjects/group was required to detect a differential group fat mass loss of 1.9 kg, assuming a between-subjects standard deviation for the weight change of 2.5 kg with an alpha of 0.05 and power of 80% based upon a preliminary study of high-caloric expenditure exercise (28)
Results
Baseline Characteristics
The mean age of study participants was 64 ± 9 years (range 44-84), mean BMI was 32 ± 4, (range 27-45) and mean waist circumference was 110 ± 10 cm. Study groups were similar at baseline by age, gender, body weight and body fat distribution (Table 1). Groups exhibited a similar distribution of cardiac risk factor measures including BP, glucose disposal, glucose, insulin and cardiorespiratory fitness however, total cholesterol, triglycerides and total cholesterol/HDL cholesterol ratios were higher in the high-caloric exercise group (Table 2). All subjects were taking preventive cardiovascular medications in accordance with evidence-based guidelines (27). These included aspirin (99%), statins (84%), B-blockers (72%), angiotensin-inhibitors/blockers (36%) and clopidogrel (28%) with no differences in use between study groups. Study subjects had a low level of aerobic fitness comparable to patients entering clinical CR programs (Table 2) (9) thus, were not a physically elite subset of CHD patients. A total of 3 subjects dropped out of the study, all during the first month, 1 in the high-caloric group and 2 in the standard CR group with no dropouts from month 5 to month 12.
Table 1.
Body Composition and Fat Distribution Response by Group
| Total Population | High Caloric Exercise | Standard Cardiac Rehabilitation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 5-Months | P- Value Within Group | Baseline | 5-Months | P- Value Within Group | Baseline | 5-Months | P- Value Within Group | P Value Between Group | |
| Age (years) | 64 ± 9 | 64 ± 9 | 63 ± 9 | |||||||
|
Males Females |
60 14 |
58 13 |
30 8 |
29 8 |
30 6 |
29 5 |
||||
| Body Weight (kg) | 94.7 ± 14.9 | 88.5 ±14.7 | <0.001 | 93.5 ±16.2 | 85.3 ±14.6 | <0.001 | 95.4 ± 13.9 | 91.7±14.3 | <0.001 | <0.001 |
| Body Mass Index (kg/meters2) | 32.2 ± 4.1 | 30.1 ± 4.2 | <0.001 | 32.2 ± 3.7 | 29.4 ± 3.7 | <0.001 | 32.0 ± 4.5 | 30.7 ± 4.5 | <0.001 | <0.001 |
| Fat Mass (kg) | 33.8 ± 7.8 | 29.2 ± 8.1 | <0.001 | 33.4 ± 7.6 | 27.5 ± 8.1 | <0.001 | 33.7 ± 4.0 | 30.9 ± 8.1 | 0.003 | <0.001 |
| Fat Free Mass (kg) | 57.9 ± 10.6 | 56.1 ± 9.6 | <0.001 | 57.1 ± 11.8 | 54.9±10.2 | <0.001 | 58.7 ± 9.4 | 57.4 ± 8.8 | <0.001 | 0.13 |
| Waist Circumference (cm) | 110 ± 10 | 105 ± 10 | <0.001 | 110 ± 10 | 103 ± 10 | <0.001 | 111 ± 11 | 106 ± 10 | <0.001 | 0.018 |
| Total Abdominal Fat (cm2) | 579 ± 133 | 493 ± 138 | <0.001 | 593 ± 136 | 473 ± 137 | <0.001 | 565 ± 131 | 513 ± 139 | 0.004 | <0.001 |
| Intra-Abdominal Fat (cm2) | 238 ± 82 | 196 ± 75 | <0.001 | 248 ± 84 | 185 ± 74 | <0.001 | 231 ± 79 | 208 ± 76 | 0.003 | <0.001 |
Results presented as mean ± standard deviation
Table 2.
Risk Factor Responses by Group
| Total Population | High Caloric Exercise | Standard Cardiac Rehabilitation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 5-Months | P- Value Within Group | Baseline | 5-Months | P- Value Within Group | Baseline | 5-Months | P Value within group | P Value Between Group | |
|
Males Females |
60 14 |
58 13 |
30 8 |
29 8 |
30 6 |
29 5 |
||||
| Systolic Blood Pressure [BP] (mm Hg) | 133 ± 18 | 125 ± 21 | 0.0001 | 133 ± 18 | 125 ± 23 | <0.007 | 134 ± 19 | 124 ± 19 | 0.0003 | 0.73 |
| Diastolic BP (mm Hg) | 74 ± 9 | 69 ± 9 | 0.0001 | 74 ± 11 | 67 ± 8 | <0.001 | 75 ± 8 | 71 ± 10 | 0.051 | 0.12 |
| Total Cholesterol (mg/dL) | 155 ± 33 | 153 ± 32 | 0.299 | 162 ± 36 ‡ | 155 ± 33 | 0.059 | 148 ± 27 | 149 ± 30 | 0.72 | 0.11 |
| Triglycerides (mg/dL) | 132 ± 80 | 114 ± 62 | 0.007 | 149 ± 80 ‡ | 118 ± 67 | 0.001 | 114 ± 28 | 116 ± 76 | 0.11 | 0.07 |
| High Density Lipoprotein [HDL] Cholesterol (mg/dL) | 41 ± 10 | 44 ± 11 | 0.001 | 39 ± 9 | 42 ± 9 | 0.001 | 42 ± 12 | 44 ± 12 | 0.11 | 0.24 |
| Low Density Lipoprotein Cholesterol (mg/dL) | 89 ± 24 | 87 ± 23 | 0.183 | 94 ± 27 | 89 ± 24 | 0.08 | 84 ± 20 | 84 ± 21 | 0.86 | 0.26 |
| Total Cholesterol/HDL Ratio | 4.0 ±1.1 | 3.6 ± 1.0 | 0.001 | 4.3 ± 1.2 † | 3.8 ± 1.0 | <0.001 | 3.6 ± 0.9 | 3.5 ± 1.0 | 0.34 | 0.01 |
| High Sensitivity C-Reactive Protein (ng ml-1) | 3.2 ± 3.6 | 2.7 ± 3.1 | 0.04 | 3.3 ± 4.1 | 3.1 ± 4.7 | 0.14 | 3.2 ± 3.0 | 2.8 ± 2.3 | 0.11 | 0.43 |
| Glucose (mg/dL) | 96 ± 12 | 92 ± 12 | 0.007 | 96 ± 14 | 89 ± 9 | 0.003 | 96 ± 10.0 | 95 ± 14 | 0.34 | 0.14 |
| Insulin (μIU ml-1) | 19 ± 7 | 14 ± 6 | 0.001 | 19 ± 7 | 13 ± 5 | <0.001 | 19 ± 8 | 16 ± 6 | <0.001 | 0.17 |
| Glucose Disposal (mg*FFM-1*minute-1) | 7.1 ± 2.7 | 8.5 ± 3.2 | 0.0001 | 6.9 ±3.1 | 8.7 ± 3.5 | 0.0001 | 7.2 ± 2.3 | 8.2 ± 2.4 | 0.001 | 0.008 |
| Plasminogen Activator Inhibitor [PAI-1] (ng ml-1) | 22 ± 9 | 19 ± 16 | 0.06 | 24 ± 11 | 19 ± 21 | 0.05 | 20 ± 8 | 19 ± 11 | 0.49 | 0.37 |
| Peak Oxygen Uptake (mLO2*kg-1*minute-1) | 22 ± 5 | 24 ± 7 | 0.0002 | 22 ± 6 | 24 ± 8 | 0.004 | 22 ± 5 | 24 ± 6 | 0.01 | 0.85 |
Results presented as mean ± standard deviation
‡ = P = 0.05, † = P < 0.005, Baseline comparison between groups, otherwise P = NS
For conversion to International Units (mmol/L):
1) Multiply Total Cholesterol, LDL-Cholesterol, and HDL-Cholesterol by 0.02586
2) Multiply Triglycerides by 0.01129
3) Multiply Glucose by 0.05555
Combined Group Responses
To evaluate the overall effects of exercise and weight loss we combined study groups (N=71). Mean weight loss was 6.2 ± 5.1 kg, fat mass loss was 4.6 ± 3.9 kg and peak aerobic capacity increased by 8% from 22 ± 5 to 24 ± 7 mlO2 × kg-1 × min-1 (P=0.0002)(Tables 1,2). These changes were associated with pronounced favorable effects on cardiac risk factors including reductions in insulin resistance and fasting concentrations of insulin, glucose, serum triglycerides, Chol/HDL ratio, high-sensitivity C-reactive-protein, PAI-1 and an increased HDL-cholesterol (all P<0.05) (Table 2). The prevalence of metabolic syndrome was reduced from 59 % to 31 % (P<0.0001). The average number of metabolic syndrome components decreased from 2.76 ± 1.02 to 1.96 ± 1.05 (P<0.001). High-sensitivity C-reactive protein decreased after exercise and weight loss only in patients not taking a statin (N= 9) (4.3 ± 3.7 to 2.8 ± 3.5, P=0.002) with no effect in patients taking a statin (3.0 ± 3.5 to 2.7 ± 3.0 P=0.25) (N= 57), (P<0.05 between groups). Fat mass change was the only independent predictor of change in metabolic score (R= 0.26, P<0.02, adjusting for group) when compared with changes in other anthropometric variables, fitness, physical activity and dietary intake.
Body Composition by Group Assignment
High-caloric exercise training yielded more than double the weight loss (8.2±4 vs 3.7±5 kg, P<0.001), and fat mass loss (5.9±4 vs 2.8±3 kg, P<0.001) than standard CR exercise, and a greater reduction in waist circumference (7±5 vs 5±5 cm, P<0.02). (Table 1) (Figure 2). Additionally, high-caloric exercise yielded a greater loss of total and intra-abdominal fat, measured by CT scan (Table 1). Our goal of increasing exercise energy expenditure was fulfilled, with physical activity energy expenditure increasing 615 ± 427 kcal/day (P<0.001) in the high-caloric exercise group vs. 169 ± 318 kcal/day (P<0.001) in the standard CR group (P<0.001 between groups) (Table 3). The deficit in dietary caloric intake was similar between groups (-312 ± 532 kcal/d in the high-caloric group vs. -254 ± 424 cal/d, in the standard CR group; P < 0.01 within groups, P= 0.69 between groups). Success with weight reduction did not differ by gender (results not shown). Using multiple regression analysis, controlling for group assignment, exercise compliance (R=0.54, P<0.01) and physical activity energy-expenditure (R=0.29, P=0.02) were the only independent predictors of fat mass change (cumulative R2=0.54). Adherence with the study interventions was good with exercise attendance in the high-caloric exercise and standard CR groups at 85% and 84% respectively, and attendance at weight-loss sessions 77% and 72% respectively (both P=NS between groups). A total of 5 subjects in the high-caloric group and 3 in standard CR missed ≥ 1 exercise session (baseline to month-5) due to an exercise-related medical problem (p=0.76). These were mostly aggravations of previously existing musculo-skeletal conditions in addition to musculo-skeletal overuse injuries rather than acute injuries. There were no exercise-related cardiac events though 1 individual in the high-caloric group and 2 subjects in standard CR missed sessions for cardiac hospitalizations. Subjects did not experience greater difficulty accomplishing the high-caloric training protocol. This was assessed using a study-specific physical-activity satisfaction questionnaire (7-point Likert scale: 1=high level of satisfaction with physical activity, 7=low level of satisfaction with physical activity). Study groups had identical mean baseline scores of 2.7±1.2 with an overall increase in physical activity satisfaction at 5 months (combined group score of 2.3 +1.7, P<0.01) with no difference in 5-month scores between groups (2.1±1.1 in the high-caloric group vs. 2.5±1.3 in the standard CR group, P=NS).
Figure 2.
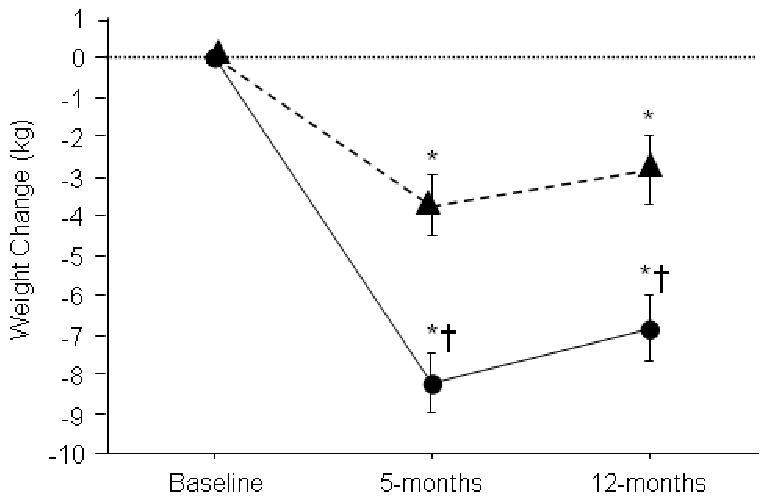
Weight Change by Group Assignment. Diamonds represent patients in standard CR exercise program. Circles represent patients in high-caloric exercise group. * = P<0.05 compared with preceding time point, within group. † = P<0.05 vs. standard CR group at same time point.
Table 3.
Physical Activity Energy Expenditure and Daily Dietary Intake
| High Caloric Exercise | Standard Cardiac Rehabilitation | P Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Time Effect | P Value Between Group | |||||||
| Baseline | 5-Months | P Value | Baseline | 5-Months | P Value | |||
| Physical Activity Energy Expenditure (kcals/day) | 717 ± 496 | 1332 ± 650 | <0.001 | 916 ± 624 | 1185 ± 537 | <0.001 | <0.001 | <0.001 |
| Caloric Intake (kcals/day) | 2080 ± 525 | 1768 ± 479 | <0.001 | 1881 ± 601 | 1626 ± 399 | <0.001 | <0.001 | 0.61 |
Results presented as mean ± standard deviation
Insulin Sensitivity and Cardiac Risk Factors by Group Status
The high-caloric exercise group experienced a significantly greater increase in insulin-mediated glucose disposal reflecting a decrease in insulin resistance, the hallmark of the insulin resistance/metabolic syndrome (Table 2). The high-caloric exercise group also experienced a greater reduction in the total cholesterol/HDL-cholesterol atherogenic ratio (Table 2) of -13% vs -3% (P<0.01). When effects of study interventions on a panel of cardiac risk factors (insulin sensitivity, waist circumference, systolic and diastolic blood pressure, plasma glucose, triglycerides, HDL-cholesterol, LDL-cholesterol, cardiorespiratory fitness, PAI-1, and high-sensitivity C-reactive protein) were entered into a model using a multivariate analysis of variance, the high-caloric group experienced a significantly greater improvement in overall cardiometabolic risk profile than the standard CR group (p=0.03). The high-caloric exercise group also experienced a greater reduction in the number of components of the metabolic syndrome compared with patients in standard CR: 3.1 ± 1.2 to 1.9 ± 1.1 components vs 2.4 ± 0.7 to 2.0 ± 1.0 components (P=0.01 between groups) (Figure 3). These differences in weight reduction and cardiac risk factors were related to the differential exercise protocol as group subjects restricted calories similarly. Cardiorespiratory fitness improved similarly by group (each P<0.01 within groups, P=NS between groups) (Table 2) from an identical baseline of 22 mlO2*kg-1min-1 to 24 mlO2*kg-1min-1.
Figure 3.
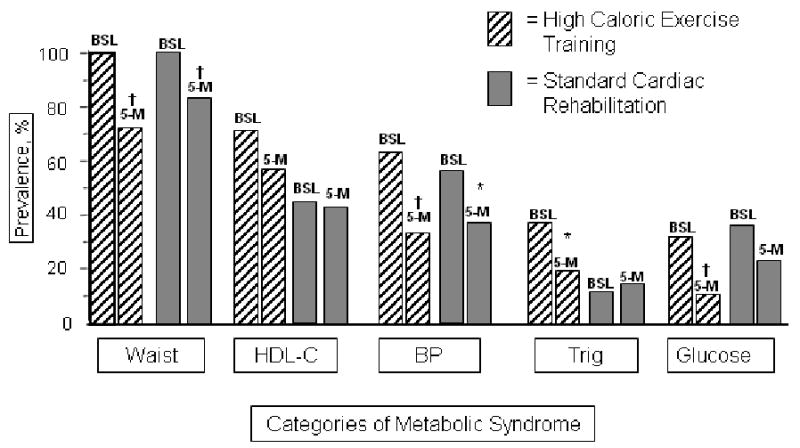
For each paired component of metabolic syndrome, the left bar represents the prevalence of the component at baseline, the right bar is the prevalence at 5-months. * = P<0.05, †= P<0.01. Changes between groups were not statistically significant.
One Year Results
Results at one year show that both groups exhibited a weight regain of 1 kg (1.3 kg for high-caloric group vs. 0.9 kg for standard CR group (each P<0.05, P=N.S. between groups) compared with the 5-month measures but body weight and body fat remained significantly lower than at baseline (each P<0.001). Favorable alterations in Chol/HDL, HDL, peak aerobic capacity and PAI-1, evident at 5 months in the high-caloric exercise group, were maintained at one year (results not shown).
Discussion
The primary finding of this study is that high-caloric expenditure exercise is superior to standard CR exercise in accomplishing weight loss and favorably altering cardiometabolic risk factors, in particular insulin resistance, in overweight patients with CHD. It was not described as being more unpleasant to accomplish and was not associated with an increased rate of exercise-related overuse injuries. Thus, high-caloric expenditure exercise should be considered the preferred exercise protocol for almost 80% of patients in the U.S. referred for CR.
While this is the first randomized study to assess the therapeutic value of high-caloric exercise and weight loss on cardio-metabolic predictors of risk in coronary heart patients, metabolic benefits of exercise and weight loss in healthy overweight adults have been amply demonstrated. Exercise and weight loss prevents type II diabetes (38), improves insulin sensitivity (39), clotting parameters (40), lipid values (41,42), fasting glucose levels (41), blood pressure (41) and systemic inflammation (43). In patients with established CHD, lipid abnormalities (44), blood pressure measures (45), PAI-1 levels (46) C-Reactive Protein (47), the presence of diabetes (45,48) and insulin resistance syndrome (17) are powerful predictors of prognosis. Thus, it is highly likely, but remains to be demonstrated, that the multiple risk factor benefits of high-caloric exercise training and behavioral weight loss would favorably affect long-term clinical outcomes.
Exercise compliance and change in physical activity energy expenditure were the only independent predictors of fat mass loss, which, in turn, was best predicted by improvements in cardiac/metabolic risk. This strongly supports the concept that exercise programs for risk reduction in overweight patients with CHD should focus on enhancing caloric expenditure. Furthermore, due to its lower exercise intensity, the high-caloric exercise program may be safer to perform in community-based programs. Finally, the high-caloric exercise regimen elicited comparable increases in peak aerobic capacity compared with standard CR exercise, suggesting that this paradigm does not compromise cardiorespiratory benefits of CR.
We demonstrated lifestyle-induced improvements of cardiometabolic risk factors beyond effects of preventive medications and these most likely can be attributed to improvements in insulin sensitivity, which in the clinical setting is usually left untreated. Clinicians should not ignore that insulin resistance is thus, a treatable risk factor. Indeed, we have previously shown that insulin sensitivity was the best predictor of the majority of risk factors prior to induction of the exercise and weight loss program (18). While our exercise and weight loss interventions yielded a more modest effect on high sensitivity C-Reactive Protein than has been previously demonstrated (49) this may have been due to higher statin use in the present study. Our 1-year results document that significant weight loss was maintained over a longer-term follow-up period although some regain occurred. We speculate that if our 5-month to 12-month intervention was more intensive than the monthly on site dietary meetings, incorporating more frequent on-site dietary and exercise sessions, this slight weight regain might have been prevented.
Strengths of our study include the state of the art techniques utilized, including the hyperinsulinemic euglycemic clamp to measure insulin sensitivity, the doubly-labeled water technique to measure free-living physical activity and abdominal CT scanning and dual x-ray absorptiometry to measure abdominal fat and body composition. The randomized-controlled format negates the criticism that only highly motivated and selected overweight patients would be able to accomplish the study intervention. Additionally, the high-caloric energy expenditure exercise program, a novel approach to CR exercise, was remarkably well-accepted by patients. The successful maintenance of weight loss at one year provides optimism that weight loss can be maintained if patients are supported to continue their efforts at favorably altering the balance between energy intake and exercise–related caloric expenditure. Finally, the demonstrated benefits of high-caloric exercise occurred in the setting of ongoing intensive pharmacologic prevention are quite relevant to the care of patients in the clinical setting. We note that comparisons of exercise programs were made in the context of a hypocaloric diet, however, we have previously shown in a less well-controlled study that high-caloric expenditure exercise in the context of an isocaloric diet also leads to significant weight loss and lower insulin levels (28).
Because the goal of the study was to compare the effectiveness of high-caloric exercise vs. standard CR exercise, there was no control group that neither exercised nor participated in behavioral weight loss. However, patients with CHD rarely lose weight with usual care. For example in the GISSI-Prevenzione Trial, only 3.6% of 7,027 patients after a recent (< 3months) MI and a BMI of >25 accomplished significant weight loss (>10% body weight) over a 6-month period (16). A second limitation of the present study was that we did not include patients with diabetes mellitus. This limitation was imposed because of our goal to measure insulin resistance as a primary outcome. The measurement of insulin resistance would be inaccurate with patients taking hypoglycemic medications, particularly if, as expected, medication dose would need to be adjusted after exercise and weight loss. A third limitation is that weight regain follow-up was limited to just one year. We also note that the benefits of exercise and weight loss on risk factors might have been amplified if the subjects were not taking medications which influence lipid levels, blood pressure and C-reactive protein. This, however, would have rendered the results inapplicable to the clinical care of contemporary CHD patients and would not have acknowledged the clear benefits of these medications. Finally, the study population (n=74) was not of a size that was statistically powered to study coronary event rates.
In summary, high-caloric exercise is substantially more effective than standard CR exercise at inducing weight loss and risk factor change in overweight patients with CHD. These findings do not obviate the established benefits of standard CR (including non-exercise components such as counseling) on mortality and clinical outcomes (1,2,11) but rather, optimizes the exercise intervention to maximize risk factor benefits. Risk-reduction benefits beyond those afforded by evidence-based pharmacologic treatments alone were accomplished. Considering the negative consequences and increasing prevalence of obesity and metabolic syndrome, high caloric exercise training combined with a hypocaloric diet, should be considered the exercise approach of choice for overweight patients with CHD. We suspect that the general applicability of the high-caloric expenditure exercise program in CR programs will be broad although CR staff and patients alike will need to be comfortable with performing much of a 5-6 day per week exercise program away from the highly monitored CR facility. Some individuals with no exercise experience whatsoever may initially benefit from a standard CR exercise protocol and then gradually evolve to a 4-6 session/week as they improve their fitness. Further research on the value of exercise and weight loss in overweight coronary patients could include refinements of the exercise protocol such as to add resistance training to minimize loss of muscle mass. Additionally, the effect of exercise and weight loss on long-term coronary events would be important to determine.
Acknowledgments
Supported by NIH grants RO1-HL72851 (P. Ades P.I.) and the General Clinical Research Center, University of Vermont (UVM) College of Medicine (RR-109).
Footnotes
Clinical Summary: Over 80% of patients entering cardiac rehabilitation (CR) are overweight and greater than 50% have metabolic syndrome. Current CR protocols, however, result in little weight loss and minimal changes in cardiac risk factors. We designed a CR exercise protocol aimed at maximizing exercise-related caloric expenditure and compared its efficacy with standard CR exercise to induce weight loss and favorably affect cardiac risk factors. Both study groups received similar nutritional counseling to reduce caloric intake. The high-caloric exercise protocol consisting of almost daily, longer distance walking and resulted in double the weight loss and a greater fat mass loss and waist reduction than standard CR exercise. The high-caloric expenditure exercise protocol resulted in reduced insulin resistance, a reduction of the cholesterol/HDL cholesterol ratio and of components of the metabolic syndrome to a greater degree vs. standard CR exercise. After combining study groups, the overall prevalence of metabolic syndrome was reduced from 59% to 31% and improvements in insulin resistance, lipid parameters, blood pressure, C-Reactive Protein and plasminogen activator inhibitor-1 were noted highlighting the multi-risk benefits of exercise and weight loss. These results demonstrate that high-caloric expenditure exercise should be the exercise modality of choice to maximize weight loss and reduce cardiac risk predictors for overweight patients in CR programs. Implementation of this approach to CR exercise will require substantial modifications to currently employed exercise programs as a large component of the exercise program needs to be accomplished beyond the confines of the usual 3-days per week, on-site exercise prescription.
Disclosures: No conflicts of interest to disclose.
References
- 1.Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260:945–50. [PubMed] [Google Scholar]
- 2.Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skidmore B, Stone JA, Thompson DR, Oldridge N. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–92. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Dorn J, Naughton J, Imamura D, Trevisan M. Results of a multicenter randomized clinical trial of exercise and long-term survival in myocardial infarction patients: the National Exercise and Heart Disease Project (NEHDP) Circulation. 1999;100:1764–9. doi: 10.1161/01.cir.100.17.1764. [DOI] [PubMed] [Google Scholar]
- 4.Pashkow FJ. Issues in contemporary cardiac rehabilitation: a historical perspective. J Am Coll Cardiol. 1993;21:822–34. doi: 10.1016/0735-1097(93)90116-i. [DOI] [PubMed] [Google Scholar]
- 5.Audelin MC, Savage PD, Ades PA. Changing Clinical Profile of Patients Entering Cardiac Rehabilitation/Secondary Prevention Programs: 1996 to 2006. J Cardiopulm Rehabil Prev. 2008;28:299–306. doi: 10.1097/01.HCR.0000336139.48698.26. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obesity. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 7.Newby LK, Eisenstein EL, Califf RM, Thompson TD, Nelson CL, Peterson ED, Armstrong PW, Van de Werf F, White HD, Topol EJ, Mark DB. Cost effectiveness of early discharge after uncomplicated acute myocardial infarction. N Engl J Med. 2000;342:749–55. doi: 10.1056/NEJM200003163421101. [DOI] [PubMed] [Google Scholar]
- 8.Ades PA, Savage PD, Brawner CA, Lyon CE, Ehrman JK, Bunn JY, Keteyian SJ. Aerobic capacity in patients entering cardiac rehabilitation. Circulation. 2006;113:2706–12. doi: 10.1161/CIRCULATIONAHA.105.606624. [DOI] [PubMed] [Google Scholar]
- 9.Bader DS, Maguire TE, Spahn CM, O'Malley CJ, Balady GJ. Clinical profile and outcomes of obese patients in cardiac rehabilitation stratified according to National Heart, Lung, and Blood Institute criteria. J Cardiopulm Rehabil. 2001;21:210–7. doi: 10.1097/00008483-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Savage PD, Banzer JA, Balady GJ, Ades PA. Prevalence of metabolic syndrome in cardiac rehabilitation/secondary prevention programs. Am Heart J. 2005;149:627–31. doi: 10.1016/j.ahj.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 11.Ades PA. Cardiac rehabilitation and secondary prevention of coronary heart disease. N Engl J Med. 2001;345:892–902. doi: 10.1056/NEJMra001529. [DOI] [PubMed] [Google Scholar]
- 12.Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, Sanderson B, Southard D. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association. Circulation. 2007;115:2675–82. doi: 10.1161/CIRCULATIONAHA.106.180945. [DOI] [PubMed] [Google Scholar]
- 13.Wolk R, Berger P, Lennon RJ, Brilakis ES, Somers VK. Body mass index: a risk factor for unstable angina and myocardial infarction in patients with angiographically confirmed coronary artery disease. Circulation. 2003;108:2206–11. doi: 10.1161/01.CIR.0000095270.85646.E8. [DOI] [PubMed] [Google Scholar]
- 14.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GG, Olsson AG, Szarek M, Sasiela WJ. Relation of characteristics of metabolic syndrome to short-term prognosis and effects of intensive statin therapy after acute coronary syndrome: an analysis of the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) trial. Diabetes Care. 2005;28:2508–13. doi: 10.2337/diacare.28.10.2508. [DOI] [PubMed] [Google Scholar]
- 16.Levantesi G, Macchia A, Marfisi R, Franzosi MG, Maggioni AP, Nicolosi GL, Schweiger C, Tavazzi L, Tognoni G, Valagussa F, Marchioli R, GISSI-Prevenzione Investigators Metabolic syndrome and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2005;46:277–83. doi: 10.1016/j.jacc.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 17.Daly CA, Hildebrandt P, Bertrand M, Ferrari R, Remme W, Simoons M, Fox KM, EUROPA investigators Adverse prognosis associated with the metabolic syndrome in established coronary artery disease: data from the EUROPA trial. Heart. 2007;93:1406–11. doi: 10.1136/hrt.2006.113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ades PA, Savage PD, Toth MJ, Schneider DJ, Audelin MC, Bunn JY, Ludlow M. The influence of obesity and consequent insulin resistance on coronary risk factors in medically treated patients with coronary disease. Int J Obes (Lond) 2008;32:967–74. doi: 10.1038/ijo.2008.6. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–78. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 20.Madala MC, Franklin BA, Chen AY, Berman AD, Roe MT, Peterson ED, Ohman EM, Smith SC, Jr, Gibler WB, McCullough PA, CRUSADE Investigators Obesity and age of first non-ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;52:979–85. doi: 10.1016/j.jacc.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 21.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–98. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 22.Sierra-Johnson J, Romero-Corral A, Somers VK, Lopez-Jiminez F, Thomas RJ, Squires RW, Allison TG. Prognostic importance of weight loss in patients with coronary heart disease regardless of initial body mass index. Eur J Cardiovasc Prev Rehabil. 2008;15:336–340. doi: 10.1097/HJR.0b013e3282f48348. [DOI] [PubMed] [Google Scholar]
- 23.Brochu M, Poehlman ET, Savage P, Fragnoli-Munn K, Ross S, Ades PA. Modest effects of exercise training alone on coronary risk factors and body composition in coronary patients. J Cardiopulm Rehabil. 2000;20:180–8. doi: 10.1097/00008483-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Lavie CJ, Milani RV. Effects of cardiac rehabilitation, exercise training, and weight reduction on exercise capacity, coronary risk factors, behavioral characteristics, and quality of life in obese coronary patients. Am J Cardiol. 1997;7:397–401. doi: 10.1016/s0002-9149(97)89239-9. [DOI] [PubMed] [Google Scholar]
- 25.Savage PD, Brochu M, Scott P, Ades PA. Low caloric expenditure in cardiac rehabilitation. Am Heart J. 2000;140:527–33. doi: 10.1067/mhj.2000.109219. [DOI] [PubMed] [Google Scholar]
- 26.Schairer JR, Kostelnik T, Proffitt SM, Faitel KI, Windeler S, Rickman LB, Brawner CA, Keteyian SJ. Caloric expenditure during cardiac rehabilitation. J Cardiopulm Rehabil. 1998;18:290–4. doi: 10.1097/00008483-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Mertens DJ, Kavanagh T, Campbell RB, Shephard RJ. Exercise without dietary restriction as a means to long-term fat loss in the obese cardiac patient. J Sports Med Phys Fitness. 1998;38:310–6. [PubMed] [Google Scholar]
- 28.Savage PD, Brochu M, Poehlman ET, Ades PA. Reduction in obesity and coronary risk factors after high caloric exercise training in overweight coronary patients. Am Heart J. 2003;146:317–23. doi: 10.1016/S0002-8703(02)94706-X. [DOI] [PubMed] [Google Scholar]
- 29.Smith SC, Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pasternak RC, Pearson T, Pfeffer MA, Taubert KA, AHA/ACC; National Heart, Lung, and Blood Institute AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–72. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 30.Toth MJ, Sites CK, Cefalu WT, Matthews DE, Poehlman ET. Determinants of insulin-stimulated glucose disposal in middle-aged, premenopausal women. Am J Physiol. 2001;281:E113–E121. doi: 10.1152/ajpendo.2001.281.1.E113. [DOI] [PubMed] [Google Scholar]
- 31.Schoeller D, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jéquier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol. 1986;250:R823–R830. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F, American Heart Association; National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 33.DeFronzo R, Tobin J, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E233. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 34.Chmielewska J, Wiman B. Determination of plasminogen activator and its “fast” inhibitor in plasma. Clin Chem. 1986;32:482–485. [PubMed] [Google Scholar]
- 35.Macy E, Hayes T, Tracy R. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 36.Harvey-Berino J. Weight loss in the clinical setting: applications for cardiac rehabilitation. Coronary Artery Disease. 1998;9:795–798. doi: 10.1097/00019501-199809120-00003. [DOI] [PubMed] [Google Scholar]
- 37.Zeni AI, Hoffman MD, Clifford PS. Energy expenditure with indoor exercise machines. JAMA. 1996;275:1424–7. [PubMed] [Google Scholar]
- 38.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 40.Calles-Escandon J, Ballor D, Harvey-Berino J, Ades P, Tracy R, Sobel B. Amelioration of the inhibition of fibrinolysis in elderly, obese subjects by moderate energy intake restriction. Am J Clin Nutr. 1996;64:7–11. doi: 10.1093/ajcn/64.1.7. [DOI] [PubMed] [Google Scholar]
- 41.Villareal DT, Miller BV, 3rd, Banks M, Fontana L, Sinacore DR, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006;84:1317–23. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- 42.Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial. JAMA. 1995;274:1915–21. doi: 10.1001/jama.1995.03530240025035. [DOI] [PubMed] [Google Scholar]
- 43.Tchernof A, Nolan A, Sites CK, Ades PA, Poehlman ET. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation. 2002;105:564–569. doi: 10.1161/hc0502.103331. [DOI] [PubMed] [Google Scholar]
- 44.Rossouw JE, Lewis B, Rifkind BM. The value of lowering cholesterol after myocardial infarction. N Engl J Med. 1990;323:1112–9. doi: 10.1056/NEJM199010183231606. [DOI] [PubMed] [Google Scholar]
- 45.Wong ND, Cupples LA, Ostfeld AM, Levy D, Kannel WB. Risk factors for long-term coronary prognosis after initial myocardial infarction: the Framingham Study. Am J Epidemiol. 1989;130:469–80. doi: 10.1093/oxfordjournals.aje.a115360. [DOI] [PubMed] [Google Scholar]
- 46.Malmberg K, Båvenholm P, Hamsten A. Clinical and biochemical factors associated with prognosis after myocardial infarction at a young age. J Am Coll Cardiol. 1994;24:592–9. doi: 10.1016/0735-1097(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 47.Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, Hsia J, Gersh BJ, Rifai N, Ridker PM, Pfeffer MA, Braunwald E, PEACE Investigators Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–36. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 48.Norhammar A, Malmberg K, Diderholm E, Lagerqvist B, Lindahl B, Rydén L, Wallentin L. Diabetes mellitus: the major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. J Am Coll Cardiol. 2004;43:585–591. doi: 10.1016/j.jacc.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 49.Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43:1056–61. doi: 10.1016/j.jacc.2003.10.041. [DOI] [PubMed] [Google Scholar]


