Abstract
To investigate the damages to the extracellular matrix in articular cartilage due to cryopreservation, the depth-dependent concentration profiles of glycosaminoglycans (GAGs) in thirty-four cartilage specimens from canine humeral heads were imaged at 13μm pixel resolution using the in vitro version of the dGEMRIC protocol in microscopic MRI (μMRI). In addition, a biochemical assay was used to determine the GAG loss from the tissue to the solution where the tissue was immersed. For specimens that had been frozen at −20 °C or −80 °C without any cryoprotectant, a significant loss of GAG (as high as 56.5%) was found in cartilage, dependent upon the structural zones of the tissue and the conditions of cryopreservation. The cryoprotective abilities of dimethyl sulfoxide (DMSO) as a function of its concentration in saline and storage temperature were also investigated. A 30% DMSO concentration was sufficient in preventing the reduction of GAG in the tissue at the −20 °C storage temperature, but a 50% concentration of DMSO was necessary for the −80 °C cryopreservation. These imaging results were verified by the biochemical analysis.
Keywords: cryopreservation, cartilage, microscopic MRI, glycosaminoglycan, dGEMRIC, biochemical assay, dimethyl sulfoxide (DMSO), DMMB
Introduction
Articular cartilage, whose degradation plays a critical role in the development of osteoarthritis (OA) and related joint diseases, consists of an extracellular matrix composed mainly of three molecules: type II collagen in the form of triple-helical fibrils, proteoglycan with heavily sulfated side chains of glycosaminoglycan (GAG), and ubiquitous water molecules [1–4].
Biomechanically, the collagen content provides a structural framework for the tissue while the GAG content is responsible for the mechanical stiffness of the tissue as a load-bearing media. Cartilage becomes mechanically weakened when its GAG content reduces, which has been regarded as one of the early signs of the tissue degradation leading to the clinical disease. Morphologically, the spatial orientation of the collagen fibrils in articular cartilage has a depth-dependent feature, which conceptually divides the tissue from the tissue surface to the tissue/bone interface into three structural zones (superficial zone, transitional zone, radial zone). This depth-dependent structure of the collagen fibrils in articular cartilage determines the tissue anisotropy as is apparent in the magic angle effect in MRI [5], birefringence in polarized light microscopy [6], and amide anisotropy in Fourier-transform infrared imaging [7].
In laboratory studies of cartilage, fresh tissue is often stored for short durations in refrigerators between the tissue harvest and the onset of any experiment. For this reason, it is important to understand the implications of this cold storage on the molecular environment in the specimens, because a prolonged storage at the refrigerator temperature can change the tissue/cell properties in many ways [8–11]. In clinical treatment of large joint defects in humans, fresh osteochondral allograft transplantation is a well-established technique [12,13]. However, fresh allograft tissue stored for more than fourteen days at 4 °C undergoes significant decrease in chondrocyte activity [9]. Cryopreservation provides an alternative means for this storage need in labs and tissue banks, often for longer durations. But cryopreservation has its own drawbacks, which in cartilage include the reduction of chondrocyte viability, the disruption of extracellular matrix due to the formation of ice crystals [14–20], and the reduction of tissue’s mechanical modulus (a consequence of the disruption of the extracellular matrix) [21,22]. Several cryopreservation methods, that include the use of cyroprotective agents, have been developed to improve the chondrocyte viability and extracellular matrix integrity [18,19,23–27]. Among the cryoprotectants, dimethyl sulfoxide ((CH3)2SO, DMSO), which is a colorless polar aprotic solvent that can penetrate the skin and other membranes easily, has been widely used in biology and medicine for the long-term storage of tissue samples at low temperatures [24,25,27], although the cellular toxicity of high DMSO concentration is still a problem to overcome [18, 24, 25, 32].
Recently, Fishbein et al [20] and Laouar et al [27] used the non-destructive MRI to investigate the influence of cryopreservation on the cartilage properties. In the work of Fishbein et al [20], T2 relaxation and magnetization transfer rate in bovine nasal cartilage were measured weekly over 4 months of time under different storage conditions. They found that water content increased while the bulk GAG content markedly decreased in all specimens. In the work of Laouar et al [27], fixed charged density and magnetization transfer rate in porcine cartilage were averaged in each of the three intensity bands in MRI. Some of these averaged MRI quantities were found to be able to detect changes in the cartilage matrix structure, and is consistent with the results from biochemical assessments after the ice formation during cryopreservation.
The goal of this project is to study the influence of several cryopreservation approaches on the integrity of the extracellular matrix in cartilage by mapping the GAG concentration at 13μm pixel resolution using microscopic MRI (μMRI) [28], which has been proved to be effective in monitoring the tissue structure and property without a prior assumption of the zonal boundaries in cartilage [29]. The in vitro version [30] of the dGEMRIC protocol [31] was used in this μMRI investigation, which quantifies the GAG concentration based on the influence of gadolinium (Gd) on T1 relaxation. Since the GAG concentration profile across the thickness (depth) of healthy cartilage is approximately a linear function of the tissue depth [30, 40], we aimed to map the changes in the GAG profiles in cartilage at high resolution and to use these changes as healthy indicators for articular cartilage. The μMRI data was substantiated with the biochemical assessments of the sulfated glycosaminoglycans through a modified 1,9-dimethylmethylene blue (DMMB) dye binding assay and were found to be in very good agreement.
Material and Methods
Cartilage Samples
Humeral heads were harvested from a group of canine specimens that were used for an unrelated cardiovascular study, and were collected immediately after sacrifice. The dogs were between one- and two-year old and showed no evidence of musculoskeletal disease throughout the course of these studies. Tissue slices of 1.75 mm thickness were cut from the humeral head using a table saw with a diamond blade. Three or four cartilage-bone plugs were cut from each slice and the plugs with a relatively flat surface were used in the experiments. Each tissue specimen is about 1.75×2×6mm in size, with a mean cartilage thickness of 600μm. For MRI experiment, a total of 34 specimens from four dogs were used in this study and divided into seven groups, as summarized in Table 1.
Table 1.
The preservation conditions of the specimens before imaging
| Imaging Group | Preservation Conditions of the Specimens | Number of the Specimen (n) |
|---|---|---|
| 1 | Imaged immediately after harvesting or after storage at 4 °C for less than 3 days | 8 |
| 2 | Immersed in saline and frozen at −20 °C for ~ 4 days | 3 |
| 3 | Immersed in saline and frozen at −80 °C for ~ 4 days | 3 |
| 4 | Snap-frozen in liquid nitrogen for 20 minutes | 2 |
| 5 | Immersed in 10% (~1.4M) DMSO/saline solution for one hour before freezing at (a) −20 °C (n=3) and (b) −80 °C (n=3) for ~ 4 days. | 6 |
| 6 | Immersed in 30% (~4.2M) DMSO/saline solution for one hour before freezing at (a) −20 °C (n=3) and (b) −80 °C (n=3) for ~ 4 days. | 6 |
| 7 | Immersed in 50% (~7.0M) DMSO/saline solution for one hour before freezing at (a) −20 °C (n=3) and (b) −80 °C (n=3) for ~ 4 days. | 6 |
Different concentrations of DMSO/saline solution were prepared by mixing 99.85% dimethyl sulfoxide (Acros, New Jersey) in normal saline [19,32]. For cartilage immersed in saline, the specimens were taken out from the refrigerator or freezer and thawed to room temperature before imaging. For cartilage frozen in the DMSO/saline solutions, the specimens were thawed and immersed in 20 ml saline solution for 10 hours with three changes of saline solution (to remove DMSO) before imaging. After this procedure, there was no distinguishable difference between the T1 profiles of DMSO processed sample and that of control sample (data not shown), indicating that most of the DMSO has diffused out of the cartilage tissue before the MRI experiment was carried out or the DMSO residue has no significant effect on the measured T1 value of cartilage.
In addition, a total of 50 specimens from 3 dogs (from the same animal source) were used for the biochemical analysis. These specimens were divided, according to the conditions in Table 1, into 5 biochemical groups. These 5 groups are: (1) fresh cartilage specimens (n=13); (2) cartilage specimens frozen at −20 °C for 24 hours in saline, thawed and GAG-analyzed immediately (n=10); (3) cartilage specimens frozen at −20 °C for 24 hours in saline, thawed and kept in saline for about 12 hours at room temperature to simulate the MRI experiment condition, and GAG-analyzed after the soaking (n=9); (4) cartilage specimens frozen at −20 °C in 10% DMSO (n=3), 30% DMSO (n=3) and 50% DMSO (n=3) for 24 hours, thawed and kept in saline for about 12 hours at room temperature to simulate the MRI experimental condition, and GAG-analyzed after the soaking; (5) cartilage specimens went through the same sequence of event as in (4), except the frozen temperature was at − 80 °C (n=9). Except for the group 1 specimens, the GAG contents in both the specimen blocks and the liquid solution where the specimens were soaked were analyzed.
Microscopic MRI (μMRI)
μMRI experiments were performed on a Bruker AVANCE II 300M NMR spectrometer equipped with a 7-Tesla/89-mm vertical-bore superconducting magnet and micro-imaging accessory (Bruker Instrument, Billerica, MA) at room temperature (~ 25°C). Quantitative T1-imaging experiments were performed using a magnetization-prepared T1-imaging sequence [28], which had two well-separated timing segments: a leading inversion-recovery pulse sequence segment and a subsequent spin echo imaging segment with an echo time of 8.6 ms in which all timings were kept constant. Since only the timing of the leading segment was altered during the experiments, the effect of T1 weighting during the leading contrast segment could be calculated unambiguously. Although T1 in cartilage has been shown to be independent of the specimen orientation in the magnetic field [28], the magic angle effect in MRI of cartilage (i.e., cartilage can have different T2 values depending upon its orientation in the magnet) can cause the laminar appearance of cartilage in MRI [5,33]. The specimens were therefore imaged at the magic angle (~55°) in the magnet, by rotating the specimen using a homebuilt in situ sample-rotating device that incorporates a 3mm solenoid coil.
To measure the GAG concentration in cartilage, T1 images of cartilage were acquired twice, before and after soaking the specimen in the 1 mM Gd-DTPA solution for about 10 hours, which is sufficiently long for the Gd-DTPA to penetrate into cartilage tissue [39]. The repetition time (TR) of the imaging experiment was 1.5 s before the tissue was soaked in 1 mM Gd-DTPA solution, and 0.5 s after soaking. The leading T1 contrast segment in imaging had five increments (0, 0.4, 1.1, 2.2, 4.0 s before Gd-DTPA immersion; 0, 0.1, 0.3, 0.5, 1.0 s in 1.0mM Gd-DTPA solution). The T1 relaxation times were calculated by fitting of the magnitude images mono-exponentially on a pixel-by-pixel basis [30]. The calculation of the GAG in cartilage from these T1 images uses a set of equations that have been well-documented in the dGEMRIC literature [30,31] The in-plane pixel resolution, which was across the depth of the cartilage tissue, was 13.0 μm, and the slice thickness was 1 mm. The spin echo readout sampling time is 20 μs, corresponding to 50 kHz spectral bandwidth. Other experimental details have been discussed earlier [28,30,34].
Biochemical Assays
A modified DMMB (1,9-dimethylmethylene blue) dye assay was used to analyze the GAG content in each specimen block. The full-thickness cartilage tissues was cut from cartilage-bone plugs and weighed before biochemical analysis. The tissue was then digested with enzyme papain (1:10 ratio at 65 °C in an oven for 48 hours). The enzyme extract and the saline aliquots were analyzed for GAG through Blyscan assay. Other experimental details for this biochemical assay can be found in our earlier work [30].
Results
μMRI Results
Fig 1 and Fig 2 compare the T1 relaxation images and profiles from a representative tissue block from Group #2 (immersed in saline and stored at − 20°C for 4 days, Table 1) with those before freezing. Because of the magic angle effect [5], the images at the 0° orientation had lower signal-to-noise ratio than the images at the magic angle of 55°. However, the T1 relaxation in cartilage is essentially isotropic, which is consistent with our previous cartilage imaging results [28]. This result shows that this storage condition (− 20 °C, 4 days) has no effect on the value and profiles of T1 relaxation time in cartilage.
Fig 1.

T1 images from a specimen that was imaged before and after a − 20°C/4day cryo-storage (without immersing the specimen in the Gd solution). The max/min T1 values for the quantitative image display are 2s/0s respectively.
Fig 2.
The T1 profiles from the central region of the 2D images shown in Fig 1. The values were averaged from 10 neighboring columns of independent data. The error bars were the standard deviations in the averaging.
The GAG concentrations in articular cartilage, however, are influenced significantly by the storage conditions employed during different preservation methods, as shown in Fig 3. For the control experiments, the shape of the T1 and GAG profiles agrees well with several of our previous reports [30,34] and literature [40], where the GAG concentration (Fig 3b) increases approximately linearly with the tissue depth. For the specimens that were ‘slowly frozen’ (i.e., Group #2 in Table 1 where the specimens were immersed in saline and frozen in refrigerator at − 20 °C), the GAG concentration stays rather constant for about the first half of the tissue (~ 300 μm) and increases nearly linearly for the second half of the tissue block (~ 300 μm). Similar results were obtained for the specimens that were snap-frozen in liquid nitrogen (Group #4, Fig 3b) and frozen at − 80°C while immersed in saline (Group #3, data not shown). Although the onset of the GAG increment among individual specimens could vary from 200 to 400μm tissue depth, a common feature for the specimens in these three groups (n=8) was the loss of the GAG as a function of tissue depth in the upper part of the tissue. The largest decline in GAG concentration as a function of tissue depth was found in specimens that were snap-frozen and kept in liquid nitrogen for 20 minutes (Group #4).
Fig 3.
The quantitative profiles of the cartilage specimens: (a) T1 relaxation time before (T1b) and after (T1a) the specimens’ immersion in the Gd solution, and (b) the GAG concentrations in the specimens. The specimen storage conditions are listed in Table 1 (Control: Group #1; − 20°C: Group #2; LN2: Group #4).
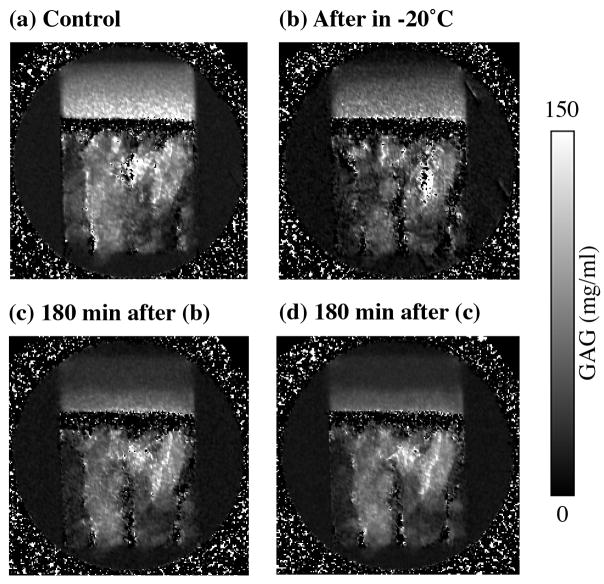
To investigate the temporal progression in the loss of the GAG concentration, one of the specimens in Group #1 was GAG-imaged when it was fresh. After freezing in the Gd solution at − 20 °C for 12 hours, the same specimen was thawed and GAG-imaged again three times, with about 180 min interval between any two experiments. On comparing with the GAG concentration in the fresh specimen (Fig 4a and Fig 5a), the GAG concentration was found reduced in the same specimen that was imaged “immediately” after thawing the specimen (Fig 4b and Fig 5b). Two further delays (the thawed specimen immersed in the Gd solution) caused more reductions in the GAG concentration, as shown clearly in Fig 4c–d and Fig 5c–d. (An experimental issue to be clarified here is that it took about 20 min to load the specimen into the probe and to setup the experiment, and about 45 min to complete the imaging experiment. Consequently, the first after-frozen experiment (Fig 4b) did not occur immediately.)
Fig 4.
2D images of GAG concentrations from one specimen from Group #1 before and after frozen in saline at − 20 °C for 12 hours.
Fig 5.
1D profiles from the GAG images shown in Fig 4.
It is of interest to note that at the same tissue depth, there is no distinguishable difference in the GAG concentration between the center and edge of the cartilage block shown in Fig 4, which suggests that the mechanism that has resulted in the loss of GAG in the thawed specimen depends only on the depth-dependent structure of the collagen matrix rather than the direct exposure of the tissue surface to the immersion solution. The averaged ‘bulk’ values of the GAG profiles shown in Fig 5 are 84.3, 51.8, 39.6, 32.3 mg/ml, for the control, ‘immediate’-imaging after freezing, 180 min-delay-imaging and 360 min-delay-imaging, respectively. These values equal to the bulk reductions of the GAG in this set of temporal imaging experiments to be 38.5%, 53.0%, 61.8% respectively.
The protective effect of DMSO in the cryopreservation is illustrated in Fig 6, as functions of DMSO concentration and preservation temperature. As the DMSO concentration increased from 10% (Group #5) to 50% (Group #7), the shape of the GAG profile increasingly assumed the shape of the control tissue (Fig 3b). At the 10% concentration of DMSO, the GAG profiles at both − 20 °C and − 80 °C lost the initial elevation in the GAG quantity, more significantly at − 80 °C. This result shows that at − 20°C, a 10% of DMSO cannot prevent the damage to the extracellular matrix in cartilage. At the 30% DMSO, the shape of the GAG profiles is temperature dependent: ‘normal’ at − 20 °C but disturbed at − 80 °C. At the 50% DMSO concentrations, the profiles at both − 20 °C and − 80 °C essentially became ‘normal’ regardless of the cryopreservation temperature, demonstrating the excellent cryopreservation ability of high concentrations of DMSO solution.
Fig 6.
The Profiles of GAG concentrations in cartilage cryopreserved at different % DMSO solutions: (a) specimens stored at − 20°C, and (b) specimens stored at − 80°C.
Biochemical Results
The quantitative results from the biochemical analysis of the cartilage specimens are summarized in Table 2. The total GAG concentrations in these cartilage specimens agree well with the results in one of our recent work [30]. It is astound to see that over 56% of GAG can be lost from the tissue by simply immersing the frozen-thawed tissue block in saline. The protective ability of DMSO is clear. These bulk biochemical results are highly consistent with the μMRI observations.
Table 2.
Bulk GAG concentrations in cartilage as determined by the biochemical methods.
| Biochemical Analysis Group Numbers (treatment conditions) | Number of Samples | GAG concentration in saline/DMSO (mg/ml, w.w.) | Total GAG Concentration in both tissue and saline/DMSO (mg/ml, w.w.) | Percentage of GAG in saline/DMSO (%) | |
|---|---|---|---|---|---|
| 1 (fresh specimens) | 13 | Not available | 59.0±7.3 | Not available | |
| 2 (thawed and analyzed immediately) | 10 | Not detectable | 61.0±10.0 | Not detectable | |
| 3 (thawed, soaked in saline, analyzed) | 9 | 34.1±8.5 | 60.4±10.5 | 56.5 | |
| 4 (frozen at −20 °C) | 10% DMSO | 3 | 27.8±4.0 | 62.5±0.8 | 44.5 |
| 30% DMSO | 3 | Not detectable | 64.2±15.9 | Not Detectable | |
| 50% DMSO | 3 | Not detectable | 76.6±5.9 | Not detectable | |
| 5 (frozen at −80 °C) | 10% DMSO | 3 | 33.3±1.1 | 68.6±4.9 | 48.5 |
| 30% DMSO | 3 | 18.5±5.8 | 64.9±5.0 | 28.5 | |
| 50% DMSO | 3 | Not detectable | 69.0±3.7 | Not detectable | |
(w.w. = wet weight)
Discussion
Since GAG is a major molecular component of the extracellular matrix of cartilage and is responsible for the stiffness of the tissue as a load-bearing material, it is critically important to monitor the concentration and distribution of GAG within cartilage. In this study, the GAG concentration in the fresh cartilage (Controls) was found to be approximately linear with the tissue depth, being lowest at the surface and highest in the deep zone (Fig 3b). This profile is consistent with the results reported in literature [30,40]. Depending upon whether the tissue was ‘slowly’ frozen to − 20 °C or snap-frozen in liquid nitrogen, the profiles of the GAG concentration in cartilage changed significantly, especially in the upper part of the tissue (the superficial zone and the transitional zone). These results confirm the general understanding that the formation of ice crystals can disrupt the extracellular matrix in articular cartilage. Since the data (Fig 4–5) in this report shows that there is a progressive reduction of the GAG concentration in cartilage within the time scale of hours, we propose the following mechanism for the GAG loss due to cryopreservation. The formation of ice crystals in cartilage causes the breakdown of the massive aggregating proteoglycans to which chondroitin sulfate and keratan sulfate glycosaminoglycan (GAG) chains are covalently attached. This damage to the tissue is physical rather than biochemical [15,25]. Once the macromolecules are broken, the smaller pieces of proteoglycans or GAG chains can easily diffuse out from the extracellular matrix of cartilage because of the strong electrostatic repulsion among the negative charges inside the tissue. This conclusion is also supported by our biochemical result, wherein we accounted for substantial amounts of sulfated glycosaminoglycans within the medium.
This gradual loss of the net GAG quantity shown in Figs 4 and 5, however, was accompanied by a curious detail: the GAG loss had an associative ‘wave-front’ that was parallel with the articular surface, despite the fact that three sides of the tissue block shown in the 2D images were in contact with the immersion solution. This detail implies that either there was no GAG loss from the left or right surface of the tissue block (unlikely) or the imaging time of 45 min was too long to map the dynamics of the GAG loss before it ‘smoothed’ itself after losing some portion of GAG from all surfaces (likely). However, this ‘smoothing’ or ‘re-distribution’ process occurs only horizontally but not vertically in the specimen, which results in the non-uniform GAG concentration observed along the depth of the tissue. Although we do not know the precise mechanism that governs this ‘smoothing’ or ‘re-distribution’ process, they must be related in some way to the histological structure of the extracellular matrix that varies significantly along the tissue depth [28]. With the gradual loss of GAG from the tissue to the surrounding saline from all surfaces, the repulsive forces reduce inside the tissue block, causing the slowdown of the GAG loss and an eventual steady state that occurs only horizontally.
The use of DMSO can help prevent the formation of ice crystals because of the vitrification process occurring inside the cartilage tissue that helps to better preserve the tissue during its storage [25]. Because of the toxicity of DMSO, however, it is critically important to decrease the concentration of cyroprotective agent thereby rendering higher viability of chondrocytes in cryopreserved tissue. Fig 6 shows clearly that the protective effect of DMSO depends upon the temperature of the cold storage and also the concentration of DMSO in saline. At − 20 °C, 30% of DMSO is sufficient to protect the extracellular matrix in cartilage; at − 80°C, in comparison, for the same protective effect also requires the use of approximate 50% DMSO in saline. Both the MRI result and biochemical result agree with each other and is also consistent with the reports in literature [24–26].
When comparing our results with the reports in literature [8,14,18,23,25,27,36], one of our experimental details should be mentioned here. To measure the GAG concentration in this project, each cartilage specimen went through the following experimental steps: (1) any cool/cryo-thaw procedure, (2) immersed in saline for the T1before imaging (~ 8 hours), (3) immersed in Gd-DTPA solution for 10 hours for a full equilibration, and (4) immersed in Gd-DTPA for the T1after imaging (~ 3 hours). So it takes about 21 hours to get the final GAG result at microscopic resolution. In a previous work where biochemical assays were used to analyze the GAG content in cartilage [30] and in the biochemical result from this report, we did not observe any significant loss of GAG from (1) the un-frozen cartilage specimens after immersion in saline and Gd-DTPA solution, and (2) the frozen/thawed cartilage specimens biochemically analyzed immediately after thawing without further immersion in saline and Gd-DTPA solution. This means that (1) the tissue with an undisrupted extracellular matrix would not lose much GAG via simple soaking for a finite time; and (2) the tissue with a disrupted extracellular matrix would not lose much GAG if little time was given for the smaller GAG pieces to diffuse out the extracellular matrix.
Proteoglycans are known to be responsible for the mechanical stiffness of articular cartilage as a load-bearing material, since the negatively charged GAG side-chains repel each other, which create an osmotic pressure that stiffens the extracellular space in cartilage. Our results in this project are consistent with the understanding of the biomechanical role of GAG in cartilage and the observations that cartilage after the freeze/thaw protocols becomes weaker biomechanically, although the conclusions from earlier reports in literature were not consistent with this work [21,22, 37,38]. For example, Kennedy et al. [21] found that a freeze/thaw cycle caused cartilage stiffness to decrease by about 31–37% and the compressive stress to be lowered by 31%. Similarly, a − 20 °C freezing protocol can cause significant reduction of the aggregate modulus and stress relaxation in porcine osteochondral specimens [22]. These authors attributed the reduction of tissue’s mechanical property to the micro-structural damage in the extracellular matrix in cartilage during the freeze/thaw process. With the GAG chains broken due to the freeze/thaw cycle, if given sufficient time of immersion, the smaller GAG pieces can diffuse out of the extracellular matrix of cartilage because of the strong electrostatic repulsion, resulting in a mechanically weaker tissue.
Finally, there is a notable feature of the T1 relaxation in articular cartilage if one compares the profiles from Fig 2, Fig 3 and Fig 5. The GAG data in Fig 3 and Fig 5 show that significant amounts of GAG were lost from fresh cartilage specimens that had been frozen in saline at − 20°C; in particular, a 38.5% reduction of GAG was found in the frozen specimen that was imaged ‘immediately’ after the thaw (Fig 5b). However, the T1 relaxation in the cartilage after a − 20°C storage changed very little (Fig 2). In contrast, the T1 relaxation in the gadolinium-doped cartilage after a − 20°C storage changed significantly (Fig 3b). These features imply that T1 relaxation is not sensitive to the amount of GAG in the native cartilage that has not been gadolinium-doped. However, since the distribution of the gadolinium ions is very sensitive to the GAG content in cartilage and T1 relaxation is very sensitive to the presence of gadolinium ions in the tissue, T1 relaxation is a sensitive indicator to the GAG concentration in the gadolinium-doped cartilage, and consequently the biomechanical properties of cartilage.
Conclusion
In summary, this combined μMRI and biochemistry project investigated the influence of the cryopreservation conditions on the depth-dependent GAG concentration profiles in articular cartilage at 13μm in-plane resolution. The biochemistry assay shows that about 50% of the bulk GAG in cartilage can be found in the solution after the frozen-thawed specimen was soaked in the solution for about 12 hours. The reductions in the GAG concentration from the superficial zone to the radial zone in cartilage were gradual and had a depth-dependent feature, illustrating the damage to the extracellular matrix in articular cartilage due to the cryopreservation procedures. The cryopreservation ability of DMSO in saline depends upon its concentration, with a 10% DMSO providing no protection to the extracellular matrix even at − 20°C and a 50% DMSO providing a good protection to the extracellular matrix at − 80 °C. The μMRI approach used in this study can be a sensitive technique to quantify the modifications to the extracellular matrix in articular cartilage due to cryopreservation and other storage processes.
Acknowledgments
Y Xia is grateful to the National Institutes of Health for the R01 grants (AR45172, AR52353). The authors are indebted to Drs. C Les and H Sabbah (Henry Ford Hospital, Detroit) for providing the canine specimens.
Grant Support: NIH R01 grants (AR 45172, AR 52353)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 1975;12:233–248. doi: 10.3233/bir-1975-123-416. [DOI] [PubMed] [Google Scholar]
- 2.Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthritic femoral head cartilage. Ann Rheum Dis. 1977;36:121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muir H. Proteoglycans as organizers of the intercellular matrix. Biochem Soc Trans. 1983;11:613–622. doi: 10.1042/bst0110613. [DOI] [PubMed] [Google Scholar]
- 4.Brandt KD, Slowman SD. Composition and glycosaminoglycan metabolism of articular cartilage from habitually loaded and habitually unloaded sites. Arthritis Rheum. 1986;29:88–93. doi: 10.1002/art.1780290112. [DOI] [PubMed] [Google Scholar]
- 5.Xia Y. Magic Angle Effect in MRI of Articular Cartilage - A Review. Invest Radiol. 2000;35:602–621. doi: 10.1097/00004424-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Xia Y, Moody J, Burton-Wurster N, Lust G. Quantitative In Situ Correlation Between Microscopic MRI and Polarized Light Microscopy Studies of Articular Cartilage. Osteoarthritis Cartilage. 2001;9:393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]
- 7.Xia Y, Ramakrishnan N, Bidthanapally A. The depth-dependent anisotropy of articular cartilage by Fourier-transform infrared imaging (FTIRI) Osteoarthritis Cartilage. 2007;15:780–788. doi: 10.1016/j.joca.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothwell AG, Bentley G. Chondrocyte multiplication in osteoarthritic articular cartilage. J Bone Joint Surg [Br] 1973;55:588–594. [PubMed] [Google Scholar]
- 9.Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, Sah RL, Bugbee WD. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg [Am] 2003;85:2111–2120. doi: 10.2106/00004623-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Williams JM, Virdi AS, Pylawka TK, Edwards RB, 3rd, Markel MD, Cole BJ. Prolonged-fresh preservation of intact whole canine femoral condyles for the potential use as osteochondral allografts. J Orthop Res. 2005;23:831–837. doi: 10.1016/j.orthres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Pylawka TK, Virdi AS, Cole BJ, Williams JM. Reversal of suppressed metabolism in prolonged cold preserved cartilage. J Orthop Res. 2008;26:247–254. doi: 10.1002/jor.20487. [DOI] [PubMed] [Google Scholar]
- 12.Gross AE, McKee NH, Pritzker KP, Langer F. Reconstruction of skeletal deficits at the knee. A comprehensive osteochondral transplant program. Clin Orthop Relat Res. 1983;174:96–106. [PubMed] [Google Scholar]
- 13.McDermott AG, Langer F, Pritzker KP, Gross AE. Fresh small-fragment osteochondral allografts. Long-term follow-up study on first 100 cases. Clin Orthop Relat Res. 1985;197:96–102. [PubMed] [Google Scholar]
- 14.Tavakol K, Miller RG, Bazett-Jones DP, Hwang WS, McGann LE, Schachar NS. Ultrastructural changes of articular cartilage chondrocytes associated with freeze-thawing. J Orthop Res. 1993;11:1–9. doi: 10.1002/jor.1100110102. [DOI] [PubMed] [Google Scholar]
- 15.Muldrew K, Novak K, Yang H, Zernicke R, Schachar NS, McGann LE. Cryobiology of articular cartilage: ice morphology and recovery of chondrocytes. Cryobiology. 2000;40:102–109. doi: 10.1006/cryo.2000.2236. [DOI] [PubMed] [Google Scholar]
- 16.Muldrew K, Novak K, Studholme C, Wohl G, Zernicke R, Schachar NS, McGann LE. Transplantation of articular cartilage following a step-cooling cryopreservation protocol. Cryobiology. 2001;43:260–267. doi: 10.1006/cryo.2001.2349. [DOI] [PubMed] [Google Scholar]
- 17.Muldrew K, Chung M, Novak K, Schachar NS, Zernicke RF, McGann LE, Rattner JB, Matyas JR. Evidence of chondrocyte repopulation in adult ovine articular cartilage following cryoinjury and long-term transplantation. Osteoarthritis Cartilage. 2001;9:432–439. doi: 10.1053/joca.2000.0409. [DOI] [PubMed] [Google Scholar]
- 18.Guan J, Urban JP, Li ZH, Ferguson DJ, Gong CY, Cui ZF. Effects of rapid cooling on articular cartilage. Cryobiology. 2006;52:430–439. doi: 10.1016/j.cryobiol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Pegg DE, Wang L, Vaughan D, Hunt CJ. Cryopreservation of articular cartilage. Part 2: mechanisms of cryoinjury Cryobiology. 2006;52:347–359. doi: 10.1016/j.cryobiol.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Fishbein KW, Canuto HC, Bajaj P, Camacho NP, Spencer RG. Optimal methods for the preservation of cartilage samples in MRI and correlative biochemical studies. Magn Reson Med. 2007;57:866–873. doi: 10.1002/mrm.21189. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy EA, Tordonado DS, Duma SM. Effects of freezing on the mechanical properties of articular cartilage. Biomed Sci Instrum. 2007;43:342–347. [PubMed] [Google Scholar]
- 22.Willett TL, Whiteside R, Wild PM, Wyss UP, Anastassiades T. Artefacts in the mechanical characterization of porcine articular cartilage due to freezing. Proc Inst Mech Eng [H] 2005;219:23–29. doi: 10.1243/095441105X9200. [DOI] [PubMed] [Google Scholar]
- 23.Marco F, Leon C, Lopez-Oliva F, Perez AJ, Sanchez-Barba A, Lopez-Duran Stern L. Intact articular cartilage cryopreservation. In vivo evaluation. Clin Orthop Relat Res. 1992;283:11–20. [PubMed] [Google Scholar]
- 24.Jomha NM, Lavoie G, Muldrew K, Schachar NS, McGann LE. Cryopreservation of intact human articular cartilage. J Orthop Res. 2002;20:1253–1255. doi: 10.1016/S0736-0266(02)00061-X. [DOI] [PubMed] [Google Scholar]
- 25.Jomha NM, Anoop PC, McGann LE. Intramatrix events during cryopreservation of porcine articular cartilage using rapid cooling. J Orthop Res. 2004;22:152–157. doi: 10.1016/S0736-0266(03)00158-X. [DOI] [PubMed] [Google Scholar]
- 26.Jomha NM, Law GK, McGann LE. Storage of porcine articular cartilage at high subzero temperatures. Cell Tissue Bank. 2006;7:55–60. doi: 10.1007/s10561-005-4521-x. [DOI] [PubMed] [Google Scholar]
- 27.Laouar L, Fishbein K, McGann LE, Horton WE, Spencer RG, Jomha NM. Cryopreservation of porcine articular cartilage: MRI and biochemical results after different freezing protocols. Cryobiology. 2007;54:36–43. doi: 10.1016/j.cryobiol.2006.10.193. [DOI] [PubMed] [Google Scholar]
- 28.Xia Y. Relaxation Anisotropy in Cartilage by NMR Microscopy (μMRI) at 14 μm Resolution. Magn Reson Med. 1998;39:941–949. doi: 10.1002/mrm.1910390612. [DOI] [PubMed] [Google Scholar]
- 29.Xia Y, Moody J, Alhadlaq H. Orientational dependence of T2 relaxation in articular cartilage: A microscopic MRI (μMRI) study. Magn Reson Med. 2002;48:460–469. doi: 10.1002/mrm.10216. [DOI] [PubMed] [Google Scholar]
- 30.Xia Y, Zheng SK, Bidthanapally A. Depth-dependent Profiles of Glycosaminoglycans in Articular Cartilage by μMRI and Histochemistry. J Magn Reson Imaging. 2008;28:151–157. doi: 10.1002/jmri.21392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation [published erratum appears in Magn Reson Med 1996 Dec;36(6):964] Magn Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 32.Sharma R, Law GK, Rekieh K, Abazari A, Elliott JA, McGann LE, Jomha NM. A novel method to measure cryoprotectant permeation into intact articular cartilage. Cryobiology. 2007;54:196–203. doi: 10.1016/j.cryobiol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Xia Y, Farquhar T, Burton-Wurster N, Lust G. Origin of cartilage laminae in MRI. J Magn Reson Imaging. 1997;7:887–894. doi: 10.1002/jmri.1880070518. [DOI] [PubMed] [Google Scholar]
- 34.Xia Y, Zheng SK, Bidthanapally A. Imaging the Molecular Concentrations in Articular Cartilage 2007. the Ninth International Conference on Magnetic Resonance Microscopy; Aachen, Germany. 203. [Google Scholar]
- 35.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41:857–865. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson S, Dannucci GA, Sharkey NA, Pool RR. The fate of articular cartilage after transplantation of fresh and cryopreserved tissue-antigen-matched and mismatched osteochondral allografts in dogs. J Bone Joint Surg [Am] 1989;71:1297–1307. [PubMed] [Google Scholar]
- 37.Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res. 1991;9:330–340. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- 38.Kerin AJ, Wisnom MR, Adams MA. The compressive strength of articular cartilage. Proc Inst Mech Eng [H] 1998;212:273–280. doi: 10.1243/0954411981534051. [DOI] [PubMed] [Google Scholar]
- 39.Nieminen MT, Rieppo J, Silvennoinen J, Toyras J, Hakumaki JM, Hyttinen MM, Helmineen HJ, Jurvelin JS. Spatial assessment of articular cartilage proteoglycans with Gd-DTPA-enhanced T1 imaging. Magn Reson Med. 2002;48:640–648. doi: 10.1002/mrm.10273. [DOI] [PubMed] [Google Scholar]
- 40.Buschmann MD, Maurer AM, Berger E, Perumbuli P, Hunziker Ruthenium hexaammine trichloride chemography for aggrecan mapping in cartilage is a sensitive indicator of matrix degradation. J Histochem Cytochem. 2000;48:81–88. doi: 10.1177/002215540004800108. [DOI] [PubMed] [Google Scholar]