Abstract
Promotion of remyelination is an important therapeutic strategy for the treatment of the demyelinating neurological disorders. Adult oligodendrocyte precursor cells (OPCs), which normally reside quiescently in the adult central nervous system (CNS), become activated and proliferative after demyelinating lesions. However, the extent of endogenous remyelination is limited because of the failure of adult OPCs to mature into myelinating oligodendrocytes (OLs) in the demyelinated CNS. Understanding the molecular mechanisms that regulate the differentiation of adult OPCs could lead to new therapeutic strategies to treat these disorders. In this study, we established a stable culture of adult spinal cord OPCs and developed a reliable in vitro protocol to induce their sequential differentiation. Adult OPCs expressed bone morphogenetic protein (BMP) type Ia, Ib, and II receptor subunits, which are required for BMP signal transduction. BMP2 and 4 promoted dose-dependent astrocyte differentiation of adult OPCs with concurrent suppression of OL differentiation. Treatment of OPCs with BMP2 and 4 increased ID4 expression and decreased the expression of olig1 and olig2. Overexpression of olig1 or olig2 blocked the astrocyte differentiation of adult OPCs induced by BMP2 and 4. Furthermore, overexpression of both olig1 and olig2, but not olig1 or olig2 alone, rescued OL differentiation from inhibition by BMP2 and 4. Our results demonstrated that downregulation of olig1 and olig2 is an important mechanism by which BMP2 and 4 inhibit OL differentiation of adult OPCs. These data suggest that blocking BMP signaling combined with olig1/2 overexpression could be a useful therapeutic strategy to enhance endogenous remyelination and facilitate functional recovery in CNS demyelinated disorders.
Keywords: Adult oligodendrocyte precursor cell, Spinal cord, Bone morphogenetic protein, Transcription factor, Differentiation Remyelination
Introduction
In spite of the ubiquitous distribution in the mature central nervous system (CNS), the majority of oligodendrocytes (OLs) originate from relatively discrete ventral regions along the neural axis during early embryogenesis. They subsequently migrate laterally and dorsally to populate all parts of the developing CNS before differentiating into myelin-forming OLs [1, 2]. The secreted glycoprotein Sonic Hedgehog (Shh), released from the notochord and floor plate, is primarily responsible for the initial specification of oligodendrocyte precursor cells (OPCs) in the ventral spinal cord [3–5]. More recent studies show that a small number of OLs are generated from the dorsal spinal cord or forebrain during later developmental stages in a Shh-independent manner [6 – 8]. Irrespective of their ventral or dorsal origin, however, two basic-helix-loop-helix (bHLH) transcription factors, olig1 and olig2, are ultimately required for OL development, since OLs completely fail to develop in olig1 and olig2 double-null mice [9, 10]. During CNS development, bone morphogenetic proteins (BMPs), members of the transforming growth factor-β protein family of extracellular ligands, repress oligodendrogenesis while enhancing the development of astrocytes [11, 12]. Implantation of noggin-producing cells into the early developing chicken spinal cord or anti-BMP antibody coated beads into developing Xenopus promotes the subsequent appearance of OL progenitors in dorsal neural tube [13, 14], suggesting that endogenous dorsally expressed BMPs inhibit oligodendrogenesis. Conversely, elevated BMP expression inhibits the appearance of ventral OL progenitors [13, 14], and overexpression of BMP4 enhances astrocytic lineage commitment in vivo and significantly inhibits the generation of OLs [15]. In vitro, BMP signaling promotes astrocytic differentiation at the expense of OLs from embryonic or postnatal multipotential NSCs [16–20] or OPCs [21, 22]. However, the mechanism(s) by which BMP signaling inhibits OL development remain to be elucidated.
OPCs reside in the white matter of the adult CNS [23, 24]. Although these cells remain relatively quiescent in the normal CNS, they become activated and proliferate in response to CNS injury, including demyelination [25–28]. More importantly, these cells have the potential to differentiate into mature OLs and myelinate axons both in vitro and in vivo [29–31]. However, endogenous remyelination by adult OPCs is very limited in a number of demyelinated disorders, including multiple sclerosis [32, 33] and spinal cord injury (SCI) [34, 35]. The failure of endogenous remyelination may be due to the inhibition of adult OPC differentiation and maturation into myelinating OLs. Although OPCs are present in the demyelinated adult CNS and even contact demyelinated axons, they fail to differentiate into mature OLs that remyelinate these axons [28, 36–38]. Understanding the molecular mechanism(s) that regulate the differentiation of adult OPCs may lead to new therapeutic strategies for demyelinated disorders. Most previous studies investigating the response of OPCs to environmental factors present within demyelinating lesions have used embryonic and neonatal progenitor cells. However, the proliferation and differentiation of adult and perinatal OPCs in response to growth factors are different [39–41]. The molecular mechanism(s) that control their differentiation may also be different. For example, olig1 is not required for OL generation and myelination during CNS development but is essential for remyelination following demyelinating lesions in adult mice [42].
In the present study, we established an in vitro model to directly study the proliferation and differentiation of adult spinal cord OPCs. We provide evidence that the interaction between BMP signaling and olig1 and olig2 regulates the differentiation of adult OPCs.
Materials and Methods
All animal care and surgical interventions were undertaken in strict accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and with the approval of the University of Louisville Institutional Animal Care and Use Committee and the Institutional Biosafety Committee.
Isolation of OPCs from Adult Spinal Cord
OPCs were immunopanned from adult spinal cords of Fischer rats (3–4 months old) with an O4 antibody using a protocol modified from a previous study [39]. Briefly, the dissected spinal cords were minced into 1-mm3 pieces and incubated in Hanks' balanced salt solution containing 0.1% papain, 0.1% neutral protease, and 0.01% DNase for 30 minutes at 37°C. The digestion was stopped by the addition of an equal volume of Dulbecco's modified Eagle's medium (DMEM) containing 20% fetal bovine serum (FBS). Tissues were dissociated by repeated trituration with fire-polished Pasteur pipettes and were filtered through 70-μm nylon mesh. The cells were incubated at 37°C on an anti-RAN-2 antibody-coated dish for 30 minutes to deplete type 1 astrocytes and meningeal cells and then transferred to an O4-coated dish for 45 minutes to select OPCs. The purified OPCs were removed with trypsin and cultured in poly-l-lysine/laminin (P/L)-coated dishes with DMEM/Ham's F-12 medium containing 1 × N2 and 1 × B27 supplements, 20 ng/ml fibroblast growth factor 2 (FGF2), 10 ng/ml platelet-derived growth factor aa (PDGFaa), 5 μg/ml insulin, and 0.1% bovine serum albumin. Cells were fed with fresh growth medium every other day. In all cases, an aliquot of cells was analyzed immunohistochemically or by fluorescence-activated cell sorting the next day to determine the efficiency of the immunopanning. Only those cell preparations in which >95% of the bound cells expressed O4 were used in the subsequent experiments.
OL Differentiation and Maturation of Adult OPCs
Passage 4–7 adult OPCs were plated on P/L-coated 60-mm culture dishes for Western blot or 24-well plates for immunohistochemical analyses. The following day, adult OPCs were induced to differentiate by withdrawal of FGF2 and PDGFaa from the growth medium for 3 days with or without BMP2 and 4 (R&D Systems Inc., Minneapolis, http://www.rndsystems.com). To examine the effects of BMP2 and 4 on OL maturation of adult OPCs, cells were allowed to differentiate into O1+ OLs by withdrawal of FGF2 and PDGFaa for 2 days and then cultured for 3 more days with or without BMPs. The concentration of BMP2 and 4 was 10 ng/ml except where otherwise indicated.
Olig1 and Olig2 Overexpression
Olig1, olig2, and enhanced green fluorescence protein (EGFP) cDNAs were cloned into the LZRS retroviral vector [43]. To generate high titer virus, ΦNX cells (provided by Dr. Gary Nolan, Stanford University) were transfected using GenePorter II (Gene Therapy Systems, Inc., San Diego, http://www.genlantis.com), which routinely gave transfection efficiencies of 50%–65%. Selection with 2 μg/ml puromycin began 48 hours later. To harvest viral supernatant, medium was changed to the appropriate serum-free medium overnight and harvested the next day. We routinely obtained titers of 5 × 105 to 5 × 106 transforming units/ml. Cells were treated with 1 μg/ml polybrene for 1 hour, followed by a wash and subsequent replacement of half of the medium with viral stock. After incubation at 37°C for 6 hours, the medium was changed to the appropriate growth medium. With this protocol, approximately 50% of the cells were labeled. Two days after infection, adult OPCs were induced to differentiate and mature with or without BMP2 and 4, as described above.
olig1 and olig2 Small Interference RNA
A retrovirus vector carrying small interference RNAs (siRNAs) targeting the mouse olig1 or olig2 gene was prepared using the BD Knockout RNAi System (Clontech, Palo Alto, CA, http://www.clontech.com). The polyacrylamide gel electrophoresis-purified complementary oligonucleotide pair for the hairpin siRNA was synthesized to target the coding region of mouse olig1 or olig2 mRNA as follows: 5′-gatccGCCACGAGTACAAACATCAATT-CAAGAGATTGATGTTTGTACTCGTGGTTTTTTACGCGTg-3′, 5′-aattcACGCGTAAAAAACCACGAGTACAAACATCAATC-TCTTGAATTGATGTTTGTACTCGTGGCg-3′, and 5′-gatccGT-ACAAAGATTGACTCCTTTTCAAGAGAAAGGAGTCAATCTT-TGTACTTTTTTACGCGTg-3′, 5′ -aattcACGCGTAAAAAAGTA-CAAAGATTGACTCCTTTCTCTTGAAAAGGAGTCAATCTTT-GTACg-3′, respectively. A complementary pair of oligonucleotides for a sense-only control RNA was also designed. These oligonucleotide pairs containing BamHI and EcoRI overhangs were annealed and ligated to a linearized RNAi-Ready pSIREN-RetroQ-ZsGreen vector digested with BamHI and EcoRI (Clontech). The RNAi-Ready pSIREN-RetroQ-ZsGreen vector is a self-inactivating retroviral expression vector designed to express a small hairpin RNA using the human U6 promoter. The resultant constructs were amplified, purified, and sequenced. High-titer retrovirus vectors expressing olig1 or olig2 siRNAs were produced in ΦNX cells as described above. The ZsGreen fluorescent marker yields a bright green fluorescence, permitting direct monitoring of the infection efficiency in adult OPCs.
Immunofluorescence In Vitro
To detect surface membrane antigens, cells cultured on 24-well plates were incubated with the primary antibodies A2B5, O4, and O1 (hybridoma supernatant, undiluted; American Type Culture Collection, Manassas, VA, http://www.atcc.org) at 4°C for 45 minutes. After fixation with 4% paraformaldehyde (PFA), cells were incubated in fluorescein isothiocyanate- or Texas Red-conjugated donkey anti-mouse IgM for 1 hour at room temperature (RT). For recognition of other antigens, cells cultured on 24 well plates were fixed with 4% paraformaldehyde (PFA). Mouse monoclonal antibodies against 2′,3′-cyclic nucleotide phosphodiesterase (CNPase; 1:800; Sternberger Monoclonal, Covance, Princeton, NJ, http://www.covance.com), myelin basic protein (MBP; 1:50; Chemicon, Temecula, CA, http://www.chemicon.com), glial fibrillary acidic protein (GFAP; 1:400; Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com), or proteolipid protein (PLP; 1:200; Serotec, Serotec, Raleigh, NC, http://www.serotec.com) or rabbit polyclonal antibodies against olig1 (1:100; Chemicon), Olig2 (1:2,000; a generous gift from Dr. Charles Stiles, Harvard University), or chicken polyclonal antibody anti-EGFP (1:500; Chemicon) were applied overnight at 4°C. Then, the appropriate fluorophore-conjugated secondary antibodies (1:200; Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com) were applied, and the nuclei were counterstained with 4,6-diamidino-2-phenylindole. Negative control experiments performed with the appropriate species-specific IgG or with inappropriate secondary antibodies showed negligible background. Total cellular counts for each experimental well were obtained in 10 fields with a ×20 objective from three independent culture wells. The result for each experimental condition was verified a minimum of three times.
Western Blot Experiments
Cells were harvested in ice-cold lysis buffer. Equivalent amounts of total protein extract from each sample were mixed with sample buffer, boiled, and loaded onto SDS polyacrylamide gels. Electrophoretic separation of the extracts was typically performed on 7.5%–15% (depending on the molecular weight of the protein of interest) discontinuous acrylamide gels under denaturing conditions. Proteins were then transferred to nitrocellulose membranes and probed with monoclonal-specific antibodies raised against GFAP (Sigma-Aldrich), MBP, PLP, or olig1 or with polyclonal antibodies raised against olig2, and phosphated Smad 1/5/8. In addition, an antibody against β-actin (Sigma-Aldrich) was used as a loading control. Appropriate secondary horseradish peroxidase-conjugated antibodies were used for detection with chemiluminescence ECL reagents (Amersham Biosciences, Piscataway, NJ, http://www.amersham.com).
Spinal Cord Injury and Immunohistochemistry for Tissue
After anesthetization with Nembutal (50 mg/kg, i.p.), adult female Fischer 344 rats received a dorsal laminectomy at the ninth thoracic vertebral level (T9) to expose the spinal cord, and then a 150 kdyn contusive SCI using the Infinite Horizons (Lexington, KY) impactor as described previously [44, 45]. After surviving 1 week or 1 month, animals were perfused transcardially with 0.01 M phosphate-buffered saline, pH 7.4, followed by 4% PFA in 0.1 M phosphate buffer. The injured spinal cords were removed, cryoprotected in 30% sucrose buffer overnight at 4°C, and then transversely sectioned at 20 μm on a cryostat. After blocking with 10% donkey serum in Tris-buffered saline containing 0.3% Triton X-100 (TBST) for 1 hour at RT, the sections were incubated in TBST containing 5% donkey serum, polyclonal rabbit anti-GFAP (1:500; Dako, Carpinteria, CA, http://www.dako.com)/monoclonal mouse anti-BMP2 or anti-BMP4 (1:200; Chemicon), monoclonal mouse anti-olig1 (1: 100; a generous gift from Dr. Charles Stiles, Harward University)/polyclonal anti-NG2 (1:200; Chemicon), or monoclonal mouse anti-platelet-derived growth factor receptor α (1:25; BD Pharmingen, San Diego, http://www.bdbiosciences.com/index_us.shtml)/polyclonal rabbit anti-olig2 (1:2,000) overnight at 4°C. Then, the appropriate fluorophore-conjugated secondary antibodies (1:200; Jackson Immunoresearch Laboratories) were applied. A Nikon C1 confocal microscope (Nikon, Tokyo, http://www.nikon.com) was used to capture representative images. Photomicrographs were assembled using Adobe Photoshop and Adobe Illustrator software (Adobe Systems Inc., San Jose, CA, http://www.adobe.com).
Results
Characterization of Adult OPC
In the presence of FGF2 and PDGFaa, O4+ OPCs proliferated and could be passaged many times. Proliferating OPCs displayed a characteristic adult OPC morphology, with several small short processes emanating from a small round cell body (Fig. 1A), as shown in the intact adult spinal cord by NG2 immunostaining [23, 46]. More than 95% of these cells were stained by antibodies directed against O4 (Fig. 1B, 1F), A2B5 (Fig. 1C, 1F), or NG2 (Fig. 1D, 1F). Fewer than 5% of the adult OPCs expressed the more mature OL-specific proteins O1 or CNPase and they did not express MBP, PLP, GFAP, or vimentin (data not shown). To examine their proliferative capacity, the adult OPCs were cultured in serum-free medium containing FGF2 and PDGFaa for 5 days, and then 10 μM 5-bromo-2′-deoxyuridine (BrdU) was added overnight. More than 50% of adult OPCs were BrdU+ (Fig. 1B, 1C), indicating that they were mitotically active. After proliferating for 2–8 passages, the percentage of adult OPCs expressing A2B5, NG2, or O4 did not significantly change (Fig. 1F), indicating that adult OPCs can proliferate extensively in vitro without phenotypic drift. When cultured in serum-free medium containing insulin and ciliary neurotrophic factor but lacking FGF2 and PDGFaa for 3 days, more than 85% of cells differentiated to express O1 and developed the typical process-bearing morphology of OLs (Fig. 1G). Moreover, after 6 days of differentiation, more than 80% of the cells developed into CNPase+ (Fig. 1H) or MBP+ (Fig. 1I) mature OLs with obvious membrane sheet. No GFAP+ astrocytes were observed (data not shown). In contrast, when cultured in the same differentiation medium but containing 10% FBS, more than 60% of the cells differentiated into GFAP+ astrocytes that coexpressed A2B5 with typical stellate morphology of type 2 astrocytes (Fig. 1J). These results showed that adult spinal cord OPCs were bipotential and differentiated into OLs in the absence of serum and into type 2 astrocytes in its presence.
Figure 1.
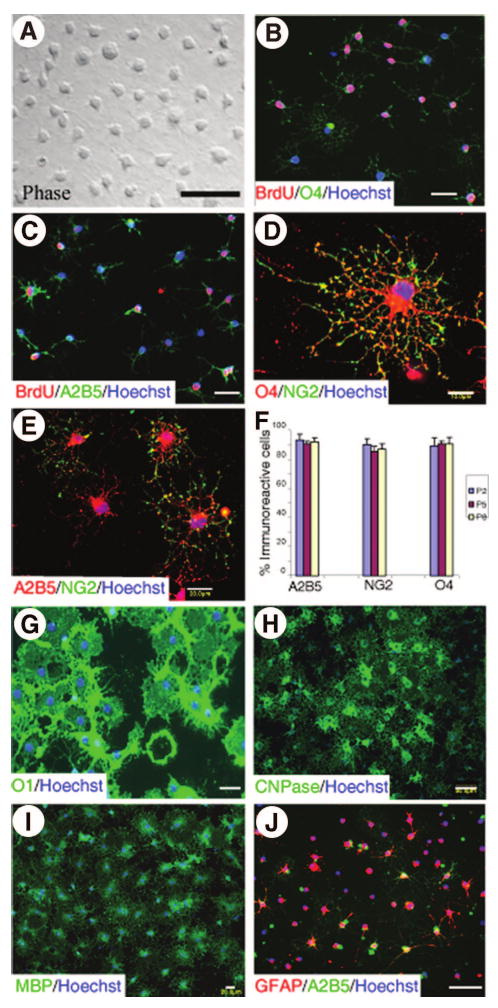
Isolation and differentiation of adult oligodendrocyte precursor cells (OPCs) in vitro. Adult OPCs were purified from the spinal cord of adult rats by immunopanning with the O4 antibody. The proliferating precursors displayed a characteristic adult OPC morphology with several small short processes emanating from a small round cell body (A). All cells expressed O4 (B), A2B5 (B), and NG2 (D, E). In the presence of fibroblast growth factor 2 (FGF2) and platelet-derived growth factor aa (PDGFaa), adult OPCs divided and are readily labeled by BrdU (B, C). These OPCs proliferated for multiple passages without changing phenotypes (F). Data in (F) were the mean ± SD of four independent experiments. Three days after withdrawal of FGF2 and PDGFaa, OPCs constitutively differentiated into OLs expressing O1 (G). Five days after differentiation, the majority of adult OPCs differentiated into mature OLs expressing CNPase (H) and MBP (I). When differentiated in the presence of 10% fetal bovine serum, more than 60% of the cells differentiated into GFAP+ astrocytes (J) that also expressed A2B5 and had a typical stellate morphology of type 2 astrocytes (J). Scale bars = 25 μm (A-C, G, J), 10 μm (D), 20 μm (E, I), and 50 μm (H). Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; CNPase, 2′,3′-cyclic nucleotide phosphodiesterase; GFAP, glial fibrillary acidic protein; MBP, myelin basic protein.
Expression of BMP Receptors by OPCs and OLs
OL differentiation in cell culture can be roughly divided into three stages: A2B5+/O4+ OPCs, O1+ immature OLs, and MBP+ mature OLs. As described above, adult OPCs readily differentiated into O1+ and MBP+ OLs after withdrawal of FGF2 and PDGFaa for 3 and 5 days, respectively. To determine whether cells in the three stages of OL development can respond to BMP, we examined their expression of BMP receptors Ia, Ib, and II using immunohistochemistry and Western blot. Adult OPCs expressed BMP receptors BMPR Ia (Fig. 2Aa), BMPR Ib (Fig. 2Ab), and BMPR II (Fig. 2Ac). With slight downregulation, immature O1+ and mature MBP+ OLs still persistently expressed these receptors (Fig. 2Ad). These findings suggest that OPCs in the different stages of OL differentiation all have the potential to respond to BMP.
Figure 2.

Inhibition of oligodendrocyte (OL) differentiation and promotion of astrocyte differentiation of adult oligodendrocyte precursor cells (OPCs) by BMP2 and 4. Adult OPCs expressed BMPR Ia (Aa), Ib (Ab), and II (Ac). Although they were slightly downregulated, all three BMPRs were persistently expressed in immature O1+ and more mature MBP+ OLs after Dif3d and Dif5d, respectively (Ad). Treatment with BMP2, 4, or both decreased the number of O1+ OLs, with concurrent increases in the number of GFAP+ astrocytes (Ba–Bd). The number of MBP+ OLs was also decreased (Be–Bh). Quantification of cells immunostained for GFAP, O1, or MBP showed a dose-dependent increase in the number of GFAP+ astrocytes and decrease of O1+ or MBP+ OLs differentiated from adult OPCs by BMP2, 4, or both, respectively (C). Data represent the mean ± SD (n = 4; one and two stars represent p < .05 and 0.01, respectively). Western blot experiments confirmed the dose-dependent activation of pSmad1/5/8, ID4, the intracellular downstream target of BMP signaling with the increasing and decreasing expression of GFAP and MBP, respectively, by BMP2, 4, or both (D). Scale bar = 20 μm (Aa–Ac) and 25 μm (Ba–Bh). Abbreviations: BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; DAPI, 4,6-diamidino-2-phenylindole; Dif3d, differentiation for 3 days; Dif5d, differentiation for 5 days; GFAP, glial fibrillary acidic protein; MBP, myelin basic protein; Undif, undifferentiated.
BMP2 and BMP4 Suppress OL Differentiation and Promote Astrocyte Differentiation by Adult OPCs
Previous studies showed that either BMP2 or BMP4 increased astrocyte differentiation and inhibited OL differentiation of neural stem cells or OPCs isolating from embryonic or postnatal developing CNS [17–19, 21, 22]. Whether BMPs have similar effects on adult OPCs has not been determined. When adult OPCs were differentiated without BMP2 or BMP4 for 3 days, almost all of the cells differentiated into O1+ OLs. No astrocyte differentiation was observed (Fig. 2Ba). After addition of BMP2, BMP4, or both in the differentiation medium, the number of OPCs that differentiated into O1+ OLs dramatically decreased, with concurrent increases of GFAP+ astrocytes (Fig. 2Bb–2Bd). The number of OPCs that differentiated into mature MBP+ OLs also decreased (Fig. 2Be–2Bh). To determine dose-dependent effects on the differentiation of adult OPCs, increasing concentrations of BMP2, 4, or both 2 and 4 were added to the medium. As shown in Figure 2C, when differentiated for 3 days in the presence of BMP2 at concentrations of 1, 2, 5, or 10 ng/ml, the percentage of adult OPCs that differentiated into GFAP+ astrocytes significantly increased from 0.4% at control to 14%, 18%, 25%, and 46%, respectively (p < .001 at all four concentrations). The percentage of O1+ OLs decreased from 77% to 60%, 45%, 34%, and 19% (p < .001), respectively, and MBP+ OLs also were suppressed from 58% to 60%, 45%, 34%, and 19% (p < .01), respectively. BMP4 was more potent than BMP2 in the inhibition of OL differentiation and promotion of astrocyte differentiation. In the presence of BMP4 at 1, 2, 5, and 10 ng/ml, the number of OPCs that differentiated into GFAP+ astrocytes were 35%, 44%, 66%, and 75%, respectively, all being significantly increased compared with 0.4% in control cultures (all p < .001). The number of O1+ and MBP+ OLs were 20%, 13%, 6%, 1% and 21%, 9%, 2%, 1%, respectively, all significantly decreased compared with 77% and 58% (all p < .001). In the presence of BMP2 and 4 at the same concentrations, further increases in the number of GFAP+ astrocytes and decreases of O1+ or MBP+ OLs were observed (Fig. 2C). These findings were confirmed by Western blot experiments (Fig. 2D). Moreover, we found that the expression of pSmad1/5/8 and ID4, two intracellular downstream targets of BMP signaling, was also increased in proportion with the dosage of BMP2, 4, or 2 and 4, suggesting that BMP signaling inhibits OL differentiation and promotes astrocyte differentiation of adult OPCs through Smad-dependent pathways.
BMP2 and BMP4 Downregulate the Expression of olig1 and olig2 During Adult OPC Differentiation
To determine the potential mechanism through which BMPs affect the differentiation of adult OPCs, we examined the expression of olig1 and olig2, two bHLH transcription factors important for OL development. When differentiating for 3 days in the absence of BMP2 and 4, the majority of cells developed into OLs (Fig. 3Aa) that expressed olig1 (Fig. 3Ab) and olig2 (Fig. 3Ac). Addition of BMP2 and 4, however, significantly decreased the number of olig1-expressing cells from 79% to 15% (p < .001) and olig2-expressing cells from 76% to 15% (p < .001) in addition to dramatically increasing the number of GFAP+ astrocytes and decreasing O1+, CNPase+, or MBP+ OLs (Fig. 3Ad–3Af, 3B). Western blot experiments confirmed that addition of BMP2 and 4 in the differentiating medium activated Smad1/5/8 to inhibit the expression of olig1, olig2, and MBP and to promote the expression of GFAP (Fig. 3C).
Figure 3.

BMP2 and BMP4 inhibit adult oligodendrocyte precursor cell (OPC) differentiation into oligodendrocytes (OLs) in vitro by downregulating olig1 and olig2. Three days after differentiation in vitro, almost all adult OPCs differentiated into OLs expressing O1 (Aa) or CNPase (Ab). A few cells expressed MBP (Ac). Addition of 10 ng/ml BMP2 and 4 dramatically decreased the number of O1-, CNPase-, or MBP-expressing OLs while significantly increasing the number of GFAP+ astrocytes (Ad–Af). BMP2 and 4 significantly decreased the number of cells immunostained for olig1 and olig2 (B). Data represent the mean ± SD (n = 4; stars indicate p < .001). Western blot experiments confirmed the immunohistochemical results and further showed that BMPs activate pSmad1/5/8 in adult OPCs (C). Similar results were obtained in four independent experiments. Scale bar = 100 μm (Aa–Af). Abbreviations: BMP, bone morphogenetic protein; CNPase, 2′,3′-cyclic nucleotide phosphodiesterase; DAPI, 4,6-diamidino-2-phenylindole; Dif3d, differentiation for 3 days; GFAP, glial fibrillary acidic protein; MBP, myelin basic protein; Undif, undifferentiated.
Previous studies have shown that adult OPCs change their responses to growth factors after culturing for a long time in the presence of FGF2 and PDGFaa [41]. To address this possibility, we tested the effects of BMP2 and 4 on differentiation of freshly isolated OPCs from adult spinal cord. As shown in supplemental online Figure 1, all OPCs expressed A2B5 (supplemental online Fig. 1A) and O4 (supplemental online Fig. 1B). After differentiation without FGF2 and PDGFaa for 3 days, most OPCs differentiated into OLs expressing O1 (supplemental online Fig. 2A), CNPase (supplemental online Fig. 2C), and MBP (supplemental online Fig. 2E). No GFAP+ astrocytes were observed (supplemental online Fig. 2A). Addition of BMP2 and 4 dramatically increased the number of GFAP+ astrocytes (supplemental online Fig. 2B) and simultaneously decreased the number of O1+ (supplemental online Fig. 2B), CNPase+ (supplemental online Fig. 2D), or MBP+ (supplemental online Fig. 2E) OLs. The number of cells expressing Olig2 (supplemental online Fig. 2C, 2D) or Olig1 (supplemental online Fig. 2E, 2F) was also significantly decreased by the treatment of BMP2 and 4. These data indicate that the effects of BMP2 and 4 on the differentiation of freshly isolated adult OPCs are identical to their effects on OPCs of passages 4–7, which we used in most of our present study. Thus, exposure to FGF2 and PDGFaa in vitro does not change the responses of adult OPCs to BMP2 and 4.
BMP2 and 4 Inhibit OL Maturation
The findings that immature O1+ OLs expressed BMP receptors (Fig. 2) and that the number of MBP+ OL decreased after the treatment with BMP2 and 4 suggested that BMP might also inhibit the maturation of OL from immature O1+ to more mature MBP+ stages. To determine the effects of BMP2 and 4 on OL maturation, adult OPCs were allowed to differentiate into O1+ OLs by withdrawing FGF2 and PDGFaa for 2 days and then continued to mature for 3 more days in the absence or presence of BMP2 and 4. As shown previously, when differentiating for 2 days after withdrawal of FGF2 and PDGFaa, more than 75% of adult OPCs developed into O1+ OLs; after 3 more days, more than 95% of cells became O1+ OLs (Fig. 4Aa), and 90% of OPCs matured into CNPase+ (Fig. 4Ab) or MBP+ (Fig. 4Ac) OLs. When 10 ng/ml BMP2 and 4 were added for the last 3 days of differentiation, a significant decrease in the number of OLs expressing either MBP+ (from 95% to 64%) or CNPase (from 93% to 58%; p < .001) was observed (Fig. 4Ad–4Af, 4B). However, the number of O1+ OLs (from 98–89%; p > .05) or GFAP+ astrocytes (from 1.1% to 5.6%; p > .05) did not significantly change (Fig. 4Aa, 4Ad, 4B). These results showed that BMP2 and 4 inhibited the maturation of O1+ into MBP+ or CNPase+ OLs.
Figure 4.

BMP2 and BMP4 inhibit oligodendrocyte (OL) maturation in vitro. Differentiated for 5 days, almost all cells matured into CNPase or MBP OLs expressing olig1 and olig2 (Aa–Ac, B). If they were differentiated for 2 days without BMPs to allow adult oligodendrocyte precursor cell to become O1+ OLs and then differentiated for 3 more days with BMPs, the number of cells expressing O1 did not change (Ad, B). The number of cells expressing CNPase, MBP, olig1, and olig2, however, significantly decreased (Ae–Af, B). No obvious GFAP+ astrocyte differentiation was observed with or without BMPs (Aa, Ad, B). Data represent the mean ± SD (n = 4; stars indicate p < .01). Western blot experiments confirmed the decreasing expression of CNPase, MBP, olig1, and olig2 with increasing pSMAD1/5/8 after BMP treatment (C). Scale bar = 25 μm (Aa–Af). Abbreviations: BMP, bone morphogenetic protein; CNPase, 2′,3′-cyclic nucleotide phosphodiesterase; DAPI, 4,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; MBP, myelin basic protein.
We further examined the expression of olig1 and olig2 during the maturation of adult OPCs. When differentiating in the presence of BMP2 and 4, the number of olig1+ cells significantly decreased from 79% at 5 days after differentiation without BMP2 and 4 to 49% (p < .01) (Fig. 4Ab, 4Ae, 4B, 4C). The number of olig2+ cells was also decreased from 80% at control to 45% (p < .05) (Fig. 4Ac, 4Af, 4B, 4C). BMP2 and 4 also inhibited the development of both CNPase- or MBP-expressing mature OLs, as shown by both immunohistochemistry (Fig. 4A, 4B) and Western blot (Fig. 4C). Our results showed that BMP2 and 4 specifically decreased the expression of both olig1 and olig2 and subsequently inhibited OL differentiation and maturation of adult OPCs.
Overexpression of olig1 and olig2 Blocks the BMP-Mediated Inhibition of Adult OPC Differentiation
When differentiating in the presence of BMP2 and 4 for 3 days, 70% of EGFP-expressing OPCs became GFAP+ astrocytes (Fig. 5A, 5D). Astrocyte differentiation in olig1- or olig2-expressing OPCs was dramatically decreased to 25% (p < .001) and 32% (p < .001), respectively (Fig. 5B–5D). However, the percentage of O1+ OLs derived from olig1- or olig2-expressing OPCs (22% and 26%, respectively) after 3 days of differentiation in the presence of BMP2 and 4 was slightly but not significantly increased compared with 17% of EGFP-OPCs (both p > .05) (Fig. 5E–5I). These results indicate that overexpression of olig1 or olig2 is sufficient to block the astrocyte differentiation of adult OPCs induced by BMP2 and 4. However, overexpression of either olig1 or olig2 failed to block the inhibition of BMP2 and 4 on OL differentiation of adult OPCs. Since BMP2 and 4 downregulate the expression of both olig1 and olig2 in the adult OPCs, overexpression of both olig1 and olig2, not olig1 or olig2 alone, may be necessary to overcome the inhibition of BMP2 and 4 on OL differentiation of adult OPCs. To test this hypothesis, we infected adult OPCs with retroviruses encoding both olig1 and olig2 and then differentiated the cells for 3 days in the presence of BMP2 and 4. As shown in Figure 5H and 5I, the percentage of O1+ OLs in olig1/olig2-expressing adult OPCs was 63%, which was significantly increased compared with 17%, 22% or 26% in EGFP-, olig1-, or olig2-expressing adult OPCs, respectively (all p < .001).
Figure 5.

Overexpression of olig1 and olig2 inhibits astrocyte differentiation and promotes OL differentiation of adult oligodendrocyte precursor cells (OPCs). Adult OPCs were infected with retroviruses encoding EGFP, Olig1, Olig2, or Olig1 and 2 and then differentiated for 3 days in the presence of 10 ng/ml BMP2 and 4. Many adult OPCs, including EGFP-infected ones (A, D) differentiated into GFAP+ astrocytes. Overexpressing Olig1 (B, D), or Olig2 (C, D) significantly decreased the number of adult OPCs differentiating into astrocytes (D). Data represent the mean ± SD (n = 4; stars indicate p < .001). The number of adult OPCs differentiating into O1+ OLs was repressed in the presence of BMP2 and 4 (E, I). Overexpression of olig1 (F, I) or olig2 (G, I) failed to result in a significant increase of O1+ OLs. Overexpression of olig1 and olig2, however, overcame the inhibition of BMP signaling to significantly increase OL differentiation from adult OPCs (H, I). Data represent the mean ± SD (n = 4; star indicates p < .01). Scale bars = 25 μm. Abbreviations: DAPI, 4,6-diamidino-2-phenylindole; EGFP, enhanced green fluorescence protein; GFAP, glial fibrillary acidic protein; OL, oligodendrocyte.
Expression of Both olig1 and olig2 Is Necessary for OL Differentiation of Adult OPCs
To further elucidate the role(s) of olig1 and olig2 in the differentiation of adult OPCs, we tested whether downregulation of olig1 or olig2 by specific siRNA would inhibit OL differentiation of adult OPCs. We first tested whether siRNAs were able to efficiently downregulate the expression of olig1 or olig2 in the adult OPCs. Expression of either olig2 (Fig. 6A–6C) or olig1 (Fig. 6D–6F) in adult OPCs infected with retroviruses encoding control scrambled siRNA did not change. However, the expression of olig2 was dramatically decreased in these adult OPCs infected with retroviruses encoding olig2 siRNA (Fig. 6A′–6C′). Expression of olig1, however, did not change in these olig2 siRNA OPCs (data not shown). Similarly, adult OPCs expressing olig1 siRNA significantly downregulated its expression of olig1 (Fig. 6D′–6F′) but not olig2 (data not shown). These results demonstrated that olig1 or olig2 siRNA specifically knocked down the expression of olig1 and olig2 in adult OPCs, respectively. We then examined whether OL differentiation of adult OPCs was inhibited following the downregulation of olig1 or olig2 with specific siRNA. Adult OPCs infected with retroviruses encoding either control scrambled, olig1, or olig2 siRNA were differentiated for 3 days. In the control group, more than 90% of infected adult OPCs differentiated into O1+ OLs (Fig. 6G, 6J). O1+ OLs, however, significantly decreased in olig1 siRNA OPCs (35%; p < .001) (Fig. 6I, 6J) or in olig2 siRNA OPCs (59%; p < .05) (Fig. 6H, 6J). These results showed that both olig1 and olig2 were necessary for OL differentiation of adult OPCs. No GFAP+ astrocyte differentiation was observed in either EGFP, olig1, or olig2 siRNA OPCs after 3 days differentiation without BMP2 and 4 (data not shown).
Figure 6.
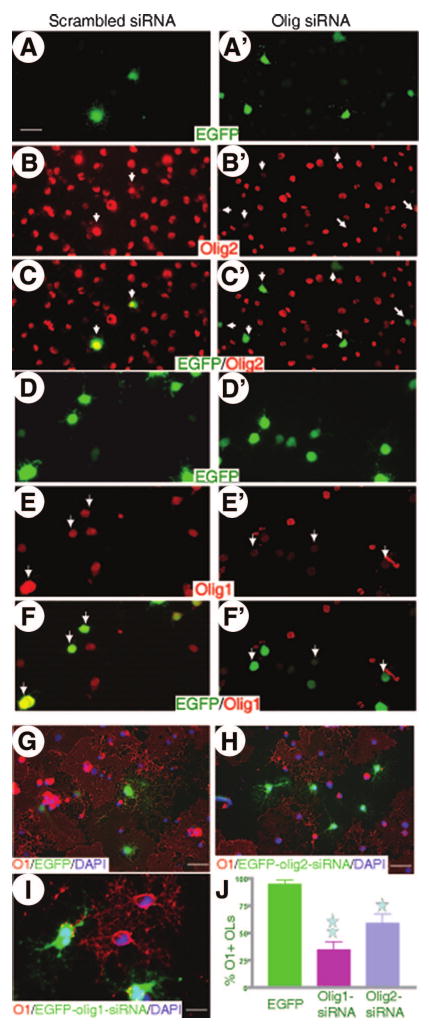
Inhibition of adult oligodendrocyte precursor cell (OPC) differentiation into oligodendrocytes (OLs) following downregulation of Olig2 and Olig1. Olig2 and Olig1 siRNA downregulated the expression of Olig2 and Olig1 in adult OPCs. Adult OPCs were infected with retroviruses that express either scrambled negative control (A–F), Olig2 (A′–C′), or Olig1 (D′–F′) siRNA. Two days later, control adult OPCs did not alter their expression of Olig2 ([A–C], arrows) or Olig1 ([D–G], arrows). However, adult OPCs infected with retroviruses expressing either Olig2 or Olig1 siRNA downregulated their expression of Olig2 ([A′–C′], arrows) or Olig1 ([D′–G′], arrows), respectively. Adult OPCs were differentiated for 3 days following infection with retroviruses encoding negative control, Olig2, or Olig1 siRNA. Uninfected OPCs ([G–I], not green cells) and OPCs infected with scrambled control siRNA ([G], green) differentiated into OLs expressing O1 ([G–I], red). Adult OPCs expressing Olig2 siRNA ([H], green cells) or Olig1 siRNA ([I], green cells) did not differentiate into O1+ OLs. Data represent the mean ± SD (n = 4; one and two stars indicate p < .05 and 0.01, respectively). Scale bar = 25 μm (A–F, A′–F′), 20 μm (G, H), and 10 μm (I). Abbreviations: DAPI, 4,6-diamidino-2-phenylindole; EGFP, enhanced green fluorescence protein; siRNA, small interference RNA.
Expression of BMP2 and 4 and olig1 and 2 in the Injured Spinal Cord
Obvious expression of BMP2 or BMP4 was not observed in the normal adult spinal cord (supplemental online Fig. 3A, 3D). At 7 days after contusion, expression of BMP2 and BMP4 was significantly increased in the reactive hypotrophic astrocytes in the injured spinal cord (supplemental online Fig. 3B, 3E). Their expression in the astrocytes continued to increase in the spinal cord at 1 month after contusion (supplemental online Fig. 3C, 3F). These results showed that expression of BMP2 and BMP4 was significantly increased after SCI.
Olig1 was localized in the nuclei of NG2+ OPCs in the normal spinal cord, and almost all NG2+ OPCs expressed olig1 (supplemental online Fig. 4A, inset, arrows). At 1 week (supplemental online Fig. 4B) and 1 month (supplemental online Fig. 4C) after the contusion, the number of NG+ OPCs significantly increased in the injured spinal cord, but the number of olig1+ cells decreased. Most of NG2+ OPCs did not express olig1 (supplemental online Fig. 4B, 4C, inset, arrowheads), whereas some OPCs remained olig1-positive (supplemental online Fig. 4B, 4C, inset, arrows). OPCs in the adult spinal cord also expressed olig2 in their nuclei (supplemental online Fig. 4D, inset, arrow). At 1 week postinjury, the number of olig2+ cells increased in the injured spinal cord, and its expression in some cells was upregulated (supplemental online Fig. 4E). However, many adult OPCs did not express olig2 (supplemental online Fig. 4E, inset, arrowheads). The number of olig2+ cells and its expression level decreased in the injured spinal cord at 1 month postinjury compared with 1 week postinjury (supplemental online Fig. 4F). More OPCs did not express olig2 (supplemental online Fig. 4F, inset, arrowheads).
Discussion
OPCs in the Adult Spinal Cord
We obtained highly purified OPCs from the adult rat spinal cord that share many fundamental properties with OPCs from perinatal and adult optic nerve or subcortical white matter. They express similar phenotype-specific antigens, O4, A2B5, and NG2. OPCs cultured from adult rat optical nerve [47] or human cortical white matter [48, 49] express O4. Approximately 80% of acutely isolated cycling progenitor cells in the adult mammalian white matter are O4+ [50]. OPCs in the adult spinal cord also express A2B5 and NG2, which is in accord with previous results from adult optic nerve [39, 47]. In the intact adult spinal cord, more than 70% of BrdU+ cycling precursor cells express NG2 [23]. Therefore, the purified A2B5+/NG2+/O4+ OPCs in this study are the most actively proliferative precursor cells in the normal adult spinal cord.
Consistent with present data with adult spinal cord OPCs, OPCs from postnatal seven days rat optic nerve can proliferate for many passages without differentiation in the presence of PDGFaa and in the absence of thyroid hormone [51, 52]. FGF2 can inhibit the differentiation of OPCs and promote their proliferation [49, 53] and can convert slowly dividing adult OPCs to rapidly dividing cells with characteristics of perinatal OPCs [41]. Moreover, the adult spinal cord OPCs differentiate into OLs in the serum-free medium and into type 2 astrocytes in medium containing serum, a property of OPCs from other CNS regions [54, 55]. The fact that adult spinal cord OPCs can be induced to differentiate into different developmental stages of OLs by withdrawing FGF2 and PDGFaa for certain times (Fig. 1) establishes a stable adult spinal cord OPC model to investigate potential molecular mechanism(s) by which adult OPCs proliferate and differentiate in vitro.
BMP Signaling Inhibits Oligodendrogenesis and Promotes Astrogliogenesis from Adult OPCs
In vitro, BMP signaling enhances astrocyte differentiation and inhibits OL differentiation from cultured neural stem cells or OPCs isolated from embryonic or perinatal CNS [16, 17, 21, 22, 56]. In vivo, BMP signaling also represses OL development [11, 13, 57]. Furthermore, overexpression of BMP4 in transgenic mice under the control of the neuron-specific enolase promoter results in a significant decrease in OLs, with a concurrent increase in astrocytes [15]. The present study extends these previous findings to show that BMP signaling also inhibits the differentiation and maturation of adult OPCs, suggesting that BMP signaling may play an important role in the regulation of remyelination of the adult CNS following traumatic or demyelinating injuries.
Importantly, present data document the molecular mechanism(s) by which BMP signaling inhibits OPC differentiation into mature OLs. One possible mechanism is the inhibition of olig1/2 activity by ID4 (Fig. 2), as suggested by a previous study [20]. Although ID4 and ID2 are expressed in proliferating OPCs, their expression decreases progressively as OPCs differentiate and mature [20, 58, 59]. Overexpression of ID2 or ID4 in OPCs inhibits OL differentiation, whereas downregulation of their expression induces premature differentiation in vitro [20, 58, 59]. ID2 and ID4 bind olig1 and olig2 in the cytoplasm of OPCs to prevent their translocation to nucleus, thereby preventing olig1 and olig2 from binding target DNA [20]. The present study further suggests that an additional mechanism by which BMP signaling inhibits OL differentiation from adult OPC is the downregulation of olig1 and olig2 (Figs. 2–5). That overexpression of olig1 and olig2 reverses the inhibitory effects of BMP signaling on OL differentiation of OPCs further confirms the suggestion that BMP signaling inhibits oligodendrogenesis from OPCs by downregulating olig1 and olig2. Therefore, downregulation of olig1 and olig2 expression and inhibition of olig1/2 activity by increased ID4 may work synergistically to inhibit OL differentiation and to enhance astrogliogenesis (Fig. 7).
Figure 7.

A schematic illustration of the regulation of adult oligodendrocyte precursor cell (OPC) differentiation by the interaction of BMP signaling and olig1/2. BMPs upregulate or downregulate the expression of ID4 and olig1/2, respectively, by Smad-dependent or -independent pathways. Increasing the expression of ID4, which blocks the translocation of olig1 and 2 to the nucleus, works synergistically with the downregulation of olig1 and 2 to inhibit oligodendrocyte (OL) differentiation and permit and/or potentiate astrocyte differentiation following BMP signaling. Olig1 and 2 are necessary to promote OL differentiation and sufficient to inhibit astrocyte differentiation by binding p300 to interrupt the formation of the Smad/p300 complex, which activates the GFAP promoter. Therefore, overexpression of olig1 and 2 overcomes the effects of BMP signaling on adult OPCs to promote OL differentiation and inhibit astrocyte differentiation. Abbreviations: BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor.
Olig1 and olig2 in the Differentiation of Adult OPCs
Transcriptional regulation orchestrates oligodendrogenesis during CNS development [1, 2, 60]. Olig1 and olig2 play important roles in generating OLs during embryogenesis [9, 10, 61, 62]. Olig2 is required for OL lineage specification during CNS development, whereas olig1 contributes more to OL differentiation and maturation [9, 10, 63, 64]. However, olig2 compensates for the function of olig1 during OL development, since OL differentiation and myelination are delayed but eventually reach normal level in olig1-null mice [9, 10]. In contrast to embryonic development, adult OPCs require both olig1 and olig2 for their differentiation and maturation. Downregulation of either olig1 or olig2 by siRNA inhibits adult OPCs differentiation into OLs (Fig. 6). Overexpression of olig1 or olig2 alone fails to overcome the inhibition of BMP to promote OL differentiation since BMP signaling decreases the expression of both olig1 and olig2. However, overexpression of both olig1 and olig2 enhances OL differentiation even in the presence of BMP2 and 4 (Fig. 5). These results are consistent with data from Arnett et al. [42], which show that olig1 is required not for the development of OLs but for remyelination in adult mice following demyelination. Taken together, these data indicate that olig1 is required for the differentiation and maturation of adult OPCs and endogenous remyelination after demyelination in adult CNS. Whether olig2 plays an important role in the differentiation and maturation and myelination of OLs during development is not known, since olig2-null mice die during the perinatal period. Interestingly, olig2 expression is retained during OL maturation, suggesting its possible role in OL differentiation. Consistent with this idea, our in vitro analyses show that olig2 is also required for OL differentiation and maturation from adult OPCs. These data also suggest that it may have a potential role during remyelination. Conditional olig2 knockout mice will be very useful to study its effects on remyelination in vivo.
In addition to OL development, olig1 and olig2 also play an important role in astrogliogenesis. Overexpression of olig1 or olig2 significantly inhibits astrocyte differentiation of adult OPCs induced by BMP signaling, suggesting that either olig1 or olig2 is sufficient to repress astrogliogenesis. These results are in good accord with the previous observation of Lu et al. [10], which show that the null mutation of either olig1 or olig2 alone is not sufficient to increase astrogliogenesis in the spinal cord. The double-null mutation of olig1 and olig2, however, results in an apparent increase in astrogliogenesis, with a complete failure of OL development [9]. Thus, downregulation of both olig1 and olig2 is required for OPCs to differentiate into astrocytes since olig1 and olig2 could complement the functions of one another sufficient to inhibit astrocyte differentiation. Consistent with this notion, we observe that both olig1 and olig2 are dramatically downregulated when astrocyte differentiation from adult OPCs is induced by BMP signaling. Our results further show that downregulation of olig1 or olig2 alone by siRNA fails to result in enhanced astrocyte differentiation even though OL differentiation is inhibited, suggesting that repression of OL differentiation alone is not sufficient to cause the astrocyte differentiation from adult OPCs if BMP signaling is not present. BMP signaling activates Smad proteins, which translocate into the nucleus to form a complex with p300 to directly activate the GFAP promoter [65, 66]. Olig2 interacts with p300 to interrupt the complex of p300 and Smad1 and thereby represses the astrocyte-specific GFAP promoter [67]. Because of their structural similarity, it is possible that olig1 may use the same mechanism to inhibit astrocyte differentiation. This would suggest that BMP signaling is able to induce adult OPCs to differentiate into astrocytes through the activation of GFAP promoter by the Smad-p300 complex only when olig1 and olig2 are also down-regulated (Fig. 7). Downregulation of both olig1 and olig2 is required, but not sufficient, for astrocyte differentiation from adult OPCs.
Implications for Remyelination
OPCs reside quiescently in the white matter of the adult CNS [23, 24]. They become activated and proliferate in response to CNS injury, including demyelination [25–28]. However, adult OPCs fail to differentiate and mature into OL capable of remyelinating demyelinated axons in multiple sclerosis lesions even though endogenous OPCs are present in the demyelinated areas and some even closely contact the affected axons [36, 38, 68–70]. Inhibitory signals that prevent OPCs from differentiation and maturation are present in the demyelinated lesions [71, 72]. We suggest that BMP signaling may be an important factor that inhibits OL differentiation and maturation in the injured CNS. In fact, expression of BMPs dramatically increases after CNS injury [73, 74]. Moreover, neuronal and OL differentiation from engrafted neural stem cells in the injured spinal cord is increased after blocking BMP signaling by expressing noggin [73]. Consistent with those findings, we also observed a significant increase of BMP2 and 4 in the hypertrophic astrocytes after traumatic SCI (supplemental online Fig. 3), and expression of olig1 and olig2 in the OPCs of the injured spinal cord decreased (supplemental online Fig. 4). Manipulation of BMP signaling may provide a new therapeutic strategy to enhance remyelination from endogenous or/and grafted OPCs after multiple sclerosis or SCI.
Supplementary Material
Acknowledgments
This work was supported by National Center for Research Resources Grant 5P20 RR 015576 (to Q.C. and S.R.W.), NIH R01 NS37717 (to M.Q.), the Kentucky Spinal Cord and Head Injury Research Trust (to Q.C. and S.R.W.), Norton Healthcare, the Commonwealth of Kentucky Research Challenge for Excellence Trust Fund, and The Christopher Reeve Paralysis Foundation (to S.R.W.). We thank George Harding of the Anatomical Sciences and Neurobiology, University of Louisville School of Medicine, for expert technical help.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors indicate no potential conflicts of interest.
References
- 1.Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 2.Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- 3.Orentas DM, Hayes JE, Dyer KL, et al. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development. 1999;126:2419–2429. doi: 10.1242/dev.126.11.2419. [DOI] [PubMed] [Google Scholar]
- 4.Soula C, Danesin C, Kan P, et al. Distinct sites of origin of oligodendrocytes and somatic motoneurons in the chick spinal cord: Oligodendrocytes arise from Nkx2.2-expressing progenitors by a Shh-dependent mechanism. Development. 2001;128:1369–1379. doi: 10.1242/dev.128.8.1369. [DOI] [PubMed] [Google Scholar]
- 5.Poncet C, Soula C, Trousse F, et al. Induction of oligodendrocyte progenitors in the trunk neural tube by ventralizing signals: Effects of notochord and floor plate grafts, and of sonic hedgehog. Mech Dev. 1996;60:13–32. doi: 10.1016/s0925-4773(96)00595-3. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Qi Y, Hu X, et al. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45:41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Fogarty M, Richardson WD, Kessaris N. A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development. 2005;132:1951–1959. doi: 10.1242/dev.01777. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 10.Lu QR, Sun T, Zhu Z, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 11.Hall AK, Miller RH. Emerging roles for bone morphogenetic proteins in central nervous system glial biology. J Neurosci Res. 2004;76:1–8. doi: 10.1002/jnr.20019. [DOI] [PubMed] [Google Scholar]
- 12.Agius E, Soukkarieh C, Danesin C, et al. Converse control of oligodendrocyte and astrocyte lineage development by Sonic hedgehog in the chick spinal cord. Dev Biol. 2004;270:308–321. doi: 10.1016/j.ydbio.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Mekki-Dauriac S, Agius E, Kan P, et al. Bone morphogenetic proteins negatively control oligodendrocyte precursor specification in the chick spinal cord. Development. 2002;129:5117–5130. doi: 10.1242/dev.129.22.5117. [DOI] [PubMed] [Google Scholar]
- 14.Miller RH, Dinsio K, Wang R, et al. Patterning of spinal cord oligodendrocyte development by dorsally derived BMP4. J Neurosci Res. 2004;76:9–19. doi: 10.1002/jnr.20047. [DOI] [PubMed] [Google Scholar]
- 15.Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev Biol. 2003;255:164–177. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 16.Gross RE, Mehler MF, Mabie PC, et al. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- 17.Mabie PC, Mehler MF, Kessler JA. Multiple roles of bone morphogenetic protein signaling in the regulation of cortical cell number and phenotype. J Neurosci. 1999;19:7077–7088. doi: 10.1523/JNEUROSCI.19-16-07077.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu G, Mehler MF, Zhao J, et al. Sonic hedgehog and BMP2 exert opposing actions on proliferation and differentiation of embryonic neural progenitor cells. Dev Biol. 1999;215:118–129. doi: 10.1006/dbio.1999.9431. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima K, Takizawa T, Ochiai W, et al. BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proc Natl Acad Sci U S A. 2001;98:5868–5873. doi: 10.1073/pnas.101109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samanta J, Kessler JA. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 2004;131:4131–4142. doi: 10.1242/dev.01273. [DOI] [PubMed] [Google Scholar]
- 21.Mabie PC, Mehler MF, Marmur R, et al. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J Neurosci. 1997;17:4112–4120. doi: 10.1523/JNEUROSCI.17-11-04112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grinspan JB, Edell E, Carpio DF, et al. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J Neurobiol. 2000;43:1–17. [PubMed] [Google Scholar]
- 23.Horner PJ, Power AE, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scolding N, Franklin R, Stevens S, et al. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain. 1998;121:2221–2228. doi: 10.1093/brain/121.12.2221. [DOI] [PubMed] [Google Scholar]
- 25.Levine JM, Reynolds R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp Neurol. 1999;160:333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds R, Cenci di Bello I, Dawson M, et al. The response of adult oligodendrocyte progenitors to demyelination in EAE. Prog Brain Res. 2001;132:165–174. doi: 10.1016/s0079-6123(01)32073-3. [DOI] [PubMed] [Google Scholar]
- 27.Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- 28.Talbott JF, Loy DN, Liu Y, et al. Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp Neurol. 2005;192:11–24. doi: 10.1016/j.expneurol.2004.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Windrem MS, Roy NS, Wang J, et al. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 2002;69:966–975. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- 30.Windrem MS, Nunes MC, Rashbaum WK, et al. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- 31.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 32.Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- 33.Dubois-Dalcq M, Ffrench-Constant C, Franklin RJ. Enhancing central nervous system remyelination in multiple sclerosis. Neuron. 2005;48:9–12. doi: 10.1016/j.neuron.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Bunge RP, Puckett WR, Becerra JL, et al. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- 35.Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- 36.Chang A, Nishiyama A, Peterson J, et al. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang CF, Lin SZ, Chiang YH, et al. Intravenous administration of bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke. 2003;34:558–564. doi: 10.1161/01.str.0000051507.64423.00. [DOI] [PubMed] [Google Scholar]
- 38.Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125:338–349. doi: 10.1093/brain/awf031. [DOI] [PubMed] [Google Scholar]
- 39.Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci. 1998;18:4627–4636. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolswijk G, Munro PM, Riddle PN, et al. Origin, growth factor responses, and ultrastructural characteristics of an adult-specific glial progenitor cell. Ann N Y Acad Sci. 1991;633:502–504. doi: 10.1111/j.1749-6632.1991.tb15640.x. [DOI] [PubMed] [Google Scholar]
- 41.Wolswijk G, Noble M. Cooperation between PDGF and FGF converts slowly dividing O-2Aadult progenitor cells to rapidly dividing cells with characteristics of O-2Aperinatal progenitor cells. J Cell Biol. 1992;118:889–900. doi: 10.1083/jcb.118.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnett HA, Fancy SP, Alberta JA, et al. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- 43.Kinsella TM, Nolan GP. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- 44.Cao Q, Zhang YP, Iannotti C, et al. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol. 2005;191(suppl 1):S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 45.Cao Q, Xu XM, DeVries WH, et al. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong RC, Dorn HH, Kufta CV, et al. Pre-oligodendrocytes from adult human CNS. J Neurosci. 1992;12:1538–1547. doi: 10.1523/JNEUROSCI.12-04-01538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gogate N, Verma L, Zhou JM, et al. Plasticity in the adult human oligodendrocyte lineage. J Neurosci. 1994;14:4571–4587. doi: 10.1523/JNEUROSCI.14-08-04571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gensert JM, Goldman JE. Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J Neurobiol. 2001;48:75–86. [PubMed] [Google Scholar]
- 51.Tang DG, Tokumoto YM, Raff MC. Long-term culture of purified postnatal oligodendrocyte precursor cells. Evidence for an intrinsic maturation program that plays out over months. J Cell Biol. 2000;148:971–984. doi: 10.1083/jcb.148.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang DG, Tokumoto YM, Apperly JA, et al. Lack of replicative senescence in cultured rat oligodendrocyte precursor cells. Science. 2001;291:868–871. doi: 10.1126/science.1056780. [DOI] [PubMed] [Google Scholar]
- 53.Grinspan JB, Reeves MF, Coulaloglou MJ, et al. Re-entry into the cell cycle is required for bFGF-induced oligodendroglial dedifferentiation and survival. J Neurosci Res. 1996;46:456–464. doi: 10.1002/(SICI)1097-4547(19961115)46:4<456::AID-JNR7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 54.Noble M, Wolswijk G, Wren D. The complex relationship between cell division and the control of differentiation in oligodendrocyte-type-2 astrocyte progenitor cells isolated from perinatal and adult rat optic nerves. Prog Growth Factor Res. 1989;1:179–194. doi: 10.1016/0955-2235(89)90010-0. [DOI] [PubMed] [Google Scholar]
- 55.Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 56.See J, Zhang X, Eraydin N, et al. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol Cell Neurosci. 2004;26:481–492. doi: 10.1016/j.mcn.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Wada T, Kagawa T, Ivanova A, et al. Dorsal spinal cord inhibits oligodendrocyte development. Dev Biol. 2000;227:42–55. doi: 10.1006/dbio.2000.9869. [DOI] [PubMed] [Google Scholar]
- 58.Wang S, Sdrulla A, Johnson JE, et al. A role for the helix-loop-helix protein Id2 in the control of oligodendrocyte development. Neuron. 2001;29:603–614. doi: 10.1016/s0896-6273(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 59.Kondo T, Raff M. The Id4 HLH protein and the timing of oligodendrocyte differentiation. EMBO J. 2000;19:1998–2007. doi: 10.1093/emboj/19.9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
- 62.Lu QR, Yuk D, Alberta JA, et al. Sonic hedgehog–regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 63.Lu QR, Cai L, Rowitch D, et al. Ectopic expression of Olig1 promotes oligodendrocyte formation and reduces neuronal survival in developing mouse cortex. Nat Neurosci. 2001;4:973–974. doi: 10.1038/nn718. [DOI] [PubMed] [Google Scholar]
- 64.Takebayashi H, Nabeshima Y, Yoshida S, et al. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- 65.Nakashima K, Yanagisawa M, Arakawa H, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 66.Fukuda S, Taga T. Cell fate determination regulated by a transcriptional signal network in the developing mouse brain. Anat Sci Int. 2005;80:12–18. doi: 10.1111/j.1447-073x.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- 67.Fukuda S, Kondo T, Takebayashi H, et al. Negative regulatory effect of an oligodendrocytic bHLH factor OLIG2 on the astrocytic differentiation pathway. Cell Death Differ. 2004;11:196–202. doi: 10.1038/sj.cdd.4401332. [DOI] [PubMed] [Google Scholar]
- 68.Chang A, Tourtellotte WW, Rudick R, et al. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- 69.Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson HC, Scolding NJ, Raine CS. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;176:162–173. doi: 10.1016/j.jneuroim.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 71.Back SA, Tuohy TM, Chen H, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 72.John GR, Shankar SL, Shafit-Zagardo B, et al. Multiple sclerosis: Re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- 73.Setoguchi T, Nakashima K, Takizawa T, et al. Treatment of spinal cord injury by transplantation of fetal neural precursor cells engineered to express BMP inhibitor. Exp Neurol. 2004;189:33–44. doi: 10.1016/j.expneurol.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 74.Setoguchi T, Yone K, Matsuoka E, et al. Traumatic injury-induced BMP7 expression in the adult rat spinal cord. Brain Res. 2001;921:219–225. doi: 10.1016/s0006-8993(01)03123-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


