Abstract
AIM: To determine whether Lactobacillus casei strain Shirota (Yakult®) can alter small intestinal bacterial overgrowth (SIBO), as tested by the lactulose breath test, and whether this is associated with changes in symptoms in irritable bowel syndrome (IBS).
METHODS: 18 patients with IBS (Rome II criteria), who showed an early rise in breath hydrogen with lactulose (ERBHAL), consumed 65 mL of Yakult® daily for 6 wk. Lactulose breath test was repeated at the end of the treatment period. Symptoms were recorded daily using a 10 cm visual analogue scale.
RESULTS: 14 patients completed the study, 9 (64%) had reversal of ERBHAL, with the median time of first rise in breath hydrogen increasing from 45 to 75 min (P = 0.03). There was no significant improvement in the symptom score with probiotic therapy, except for wind (P = 0.04). Patients commencing with at least moderate symptoms and who no longer had ERBHAL at the end of treatment, showed improvement in the overall symptoms scores [median final score 5.3 (IQR 3.9-5.9), 55% reduction; n = 6] to a greater extent than those who had had persisting ERBHAL [final score 6.9 (5.0-7.0), 12% reduction; n = 5; P = 0.18].
CONCLUSION: Yakult® is effective in altering fermentation patterns in the small bowel, consistent with reducing SIBO. The loss of ERBHAL was associated with reduced symptoms. The true interpretation of these findings awaits a randomised, controlled trial.
Keywords: Probiotics, Small intestinal bacterial overgrowth, Breath hydrogen testing, Functional gut symptoms
INTRODUCTION
Irritable bowel syndrome (IBS) is the most common gastrointestinal disorder, thought to affect approximately 15% of the population[1]. Differences in gut microflora are evident between IBS sufferers and controls, with the former demonstrating significantly lower concentrations of bifidobacteria and lactobacilli, and higher concentrations of Streptococcus, E. coli and Clostridia[2,3]. Small intestinal bacterial overgrowth (SIBO) occurs in up to 78% patients with IBS, and may be directly related to the genesis of IBS symptoms[4,5]. The mechanism by which symptoms are generated from SIBO is most probably through fermentation, with rapid production of hydrogen, methane and carbon dioxide. This results in distention of the small intestine, leading to discomfort, sensation of bloating and secondary disturbances in motility.
SIBO is difficult to assess, but can be detected indirectly through the use of lactulose breath hydrogen or 14C-xylose breath tests. In the case of the lactulose hydrogen breath test, a rise in breath hydrogen is expected approximately 90 min after the solution is consumed. An early rise in breath hydrogen after lactulose (ERBHAL) leads to one of two conclusions: either bacterial fermentation is occurring in the small intestine due to bacterial overgrowth, or there is rapid small intestinal transit[6].
The role of SIBO in the genesis of IBS symptoms has been reviewed recently[7]. Several clinical trials have played a key role in examining this association, in which antibiotic use has not only normalized lactulose breath hydrogen tests but has also led to an improvement in the symptoms of IBS[5,8-10]. It has also been suggested that malabsorption of fructose and lactose may result from SIBO, which, when corrected with antibiotics (as demonstrated by normalisation of the lactulose breath test), is associated with improved absorption of these sugars[5,8].
Apart from antibiotics, other potential therapies have been suggested for the treatment of SIBO. Dietary restriction of fermentable carbohydrates is promising, as shown by the use of elemental diets[11]. However, an elemental diet is not a practical solution. Secondly, prokinetic agents, such as tegaserod, may play a role in promoting clearance of the lumen[12]. Finally, probiotics may influence bacterial populations in the small intestinal biofilm, manifesting as reduction in the total count or as a change in the activity, such that symptoms are not induced. It has been well documented that probiotics modify fermentation in the large intestine by increasing the absolute count of probiotic-type bacteria and reducing the Streptococcus counts[13]. It has also been shown that some probiotic strains improve symptoms of bloating, wind and pain in IBS patients[14,15]. However, the impact of probiotics specifically on SIBO in IBS patients has not been formally investigated.
The primary aim of the present study was to address the hypothesis that probiotics reverse SIBO, as defined by the breath hydrogen criteria. The secondary aim was to determine whether changes in the symptoms were associated with changes in breath test patterns. To examine this, an open label, pilot study was performed to assess the effect of L. casei strain Shirota (Yakult®) on ERBHAL in patients with IBS and ERBHAL.
MATERIALS AND METHODS
Patients
Patients referred for breath testing, who were found to have ERBHAL and fulfilled Rome II criteria for IBS, were invited to participate in the study. Patients were excluded if they were currently taking IBS medication, had previous specific dietary therapy, had taken probiotics for greater than 2 wk within the previous six months, or antibiotics in the previous 2 mo, were ingesting aspirin, non-steroidal anti-inflammatory drugs or alcohol in excess of 20 g/d, or had any clinically significant co-morbidity. Eighteen patients were recruited. The mean age (range) was 44 (20-70) years; five subjects were male. All patients fulfilled Rome II criteria for IBS, with 8 (44%) being constipation-predominant, 4 (22%) diarrhoea-predominant, and 6 (33%) having alternating bowel habits.
Experimental protocol
Patients undertook a 1 to 2 wk run in period during which they completed a daily symptom diary. Daily recordings were made regarding symptom severity and, at the end of each week, the patients rated their symptoms overall, including the following specific symptoms-abdominal pain/discomfort, bloating, satisfaction with stool consistency, passage of wind, tiredness, and nausea-using a 10 cm visual analogue scale (VAS). The symptoms were classified as “mild” if VAS was < 3 cm, “moderate” 3 to < 7 cm and “severe” ≥ 7 cm. The patients were then started on probiotic therapy, comprising of 65 mL (one bottle, 6.5 billion bacteria, 1 g lactose per dose) of L. casei strain Shirota in a solution containing sucrose, skim milk powder and dextrose (Yakult®, Yakult Ltd, Melbourne Australia), which was taken each morning prior to breakfast, for 6 wk. The same symptom diary and weekly questionnaire was completed throughout the treatment period. The patients were reviewed after 3 and 6 wk of treatment. During the last week of the study, the lactulose breath test was repeated.
Patients whose symptoms worsened significantly, terminated the study early and, if possible, the lactulose breath hydrogen test was performed prior to the cessation of the probiotic therapy. The symptom scores were considered valid if the patient had completed at least 3 wk of treatment.
Breath hydrogen test
Prior to the breath hydrogen test, dietary restriction (minimising intake of fibre and poorly absorbed short-chain carbohydrates) was advised for 24 h and the subjects fasted overnight. The patients consumed 15 g lactulose, made up to a 100 mL solution with water. Breath hydrogen samples were collected at baseline and every 15 min for at least 2 h.
ERBHAL was defined as a rise of breath hydrogen of 10 ppm or greater above the baseline breath hydrogen on two consecutive 15-min samples before 90 min, following the ingestion of lactulose. The time of the first rise in breath hydrogen of 10 ppm or more was recorded.
Statistical analysis
Data were expressed as mean and standard error of the mean (SEM), or median and interquartile range (IQR) according to the distribution of the findings. Changes in the end-points were compared using a Wilcoxon matched pairs test. Univariate regression analyses were performed and Pearson’s correlation coefficients were calculated. All statistical tests were performed using Microsoft Office® Excel 2003 and GraphPad Prism (version 3.00 for Windows, GraphPad Software, San Diego California USA). P ≤ 0.05 was considered statistically significant.
RESULTS
Patient baseline symptom characteristics
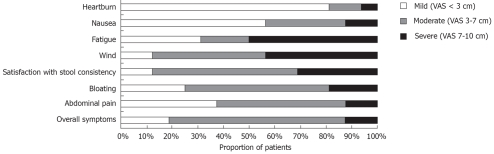
On the self-rated scales, the overall symptom severity varied across the patients, with three patients experiencing very mild symptoms, 11 moderate symptoms and two severe symptoms. However, all patients, rated at least one specific abdominal symptom as ≥ 3 cm, with the passage of wind and dissatisfaction with stool consistency being the most frequently reported symptoms. The proportion of patients with mild, moderate or severe baseline symptoms is shown in Figure 1.
Figure 1.
Individual and overall symptom scores at baseline, based on the visual analogue scale (VAS).
Adherence to protocol and treatment
Two patients terminated the study after taking Yakult® for < 2 wk due to intolerable symptoms (increasing nausea). They were excluded from the analysis as per protocol. An additional patient terminated after 3 wk, also because of increasing nausea but did not agree for a repeat hydrogen breath test. One patient commenced antibiotics for an unrelated illness after taking Yakult® for 3 wk and was withdrawn from the study due to the confounding influence of antibiotic therapy. Thus, breath test assessment was performed as per protocol in 14 patients and the effect of probiotics on symptoms was evaluable in 16 patients.
Adherence to the study treatment was > 95% in all the participants, as judged by the returned open and unused probiotic bottles.
Effect of Yakult® on end-points
Prior to treatment, the median time after ingestion of lactulose when breath hydrogen deviated by ≥ 10 ppm from the baseline was 45 min (IQR 30-60) (Figure 2). During probiotic therapy, the median time for the first breath hydrogen rise increased to 75 min (IQR 60-90) (P = 0.03) with 9 patients (64%) no longer demonstrating an ERBHAL, according to the entry criterion (Figure 2).
Figure 2.
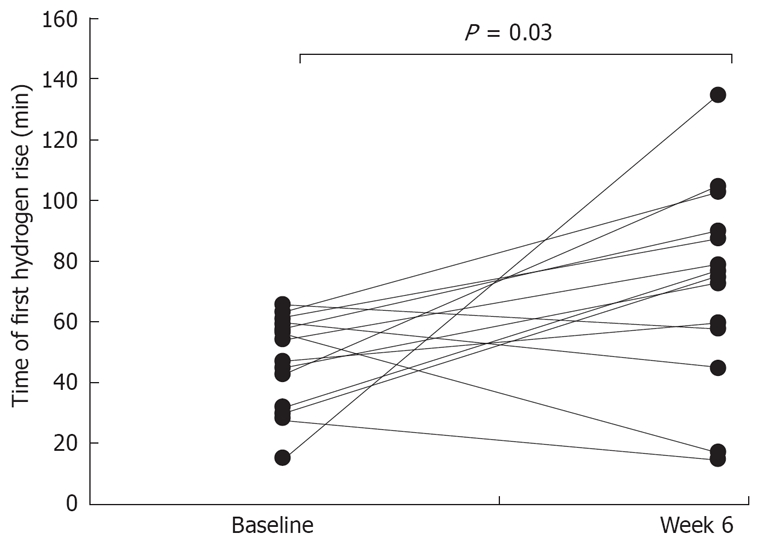
The time of the first rise at or above 10 ppm in breath hydrogen after lactulose during the baseline period, and during treatment with L. casei strain Shirota. The time during the treatment period was significantly later compared to at baseline (P = 0.03, Wilcoxon matched pairs test).
There was no significant difference in the VAS score for the overall abdominal symptoms from baseline (mean ± SEM, 4.8 ± 0.56 cm) to the final week of treatment (4.2 ± 0.68 cm) (P = 0.33). The effect of probiotic treatment was examined for each individual symptom. Only the VAS score for the passage of wind improved significantly with Yakult® (6.1 ± 0.5 cm to 4.4 ± 0.56 cm; P = 0.04), while none of the symptoms worsened significantly. There was no association of change in the symptoms with the subtype of IBS (data not shown).
Relationship between final ERBHAL status and change in symptoms
Patients were divided into two groups: those who no longer had ERBHAL at the end of 6 wk’s therapy (“no-ERBHAL” group; n = 9), and those with continuing ERBHAL (“ERBHAL” group; n = 5). The change in VAS for the overall symptoms in the two groups is shown in Figure 3. Seven of the 9 patients in the no-ERBHAL group had moderate overall symptoms prior to treatment; 5 improved by at least 2 cm on the VAS, compared with none of 5 patients in the ERBHAL group (P = 0.06; Fisher’s exact test). The change in the overall symptom score in patients with at least moderate symptoms prior to treatment was greater in those who no longer had ERBHAL [median final score 5.3 (IQR 3.9-5.9), 55% reduction; n = 6] compared to patients with continuing ERBHAL [final score 6.9 (5.0-7.0), 12% reduction; n = 5; P = 0.18; Mann Whitney t-test].
Figure 3.
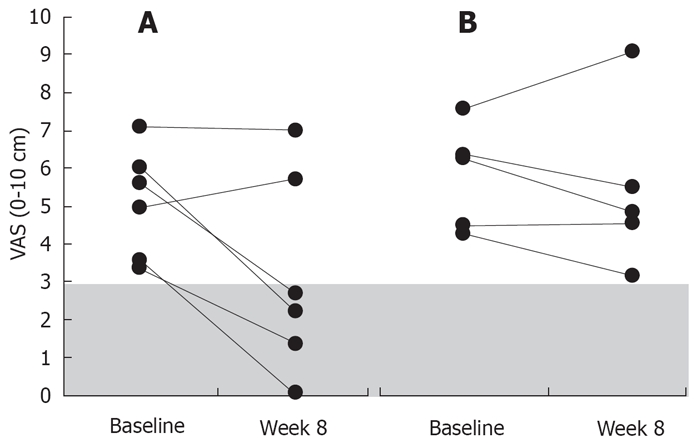
The overall abdominal symptom score at baseline in patients with more than minimal symptoms (VAS < 3 cm), compared with scores at week 6 of treatment, with and without ERBHAL at the conclusion of the treatment. A: Without ERBHAL; B: With continuing ERBHAL. Shading indicates the minimal symptom score category. These changes were statistically not significant.
DISCUSSION
This is the first study on the effect of a probiotic agent in patients with IBS and ERBHAL. The findings show that Yakult®-induced change in the fermentation pattern resulted in 64% patients no longer fulfilling the definition of ERBHAL at the end of the treatment period. While such a change may represent regression towards the mean due to the repetition of the breath test[16], it more likely reflects a physiological change. This could conceivably be due to slower oro-caecal transit, however, there are no reports of L. casei altering gastric emptying or small intestinal transit time. Alternatively, the effect may represent a more distal intestinal shift in the initial fermentation of lactulose. Formal radionuclide transit tests could address this issue in future studies, but the findings that ERBHAL represents SIBO in the majority of patients has been observed previously, along with the findings of reduction in functional gut symptoms, with the use of antibiotics and elemental diet[5,8,9,11].
The criteria used for the diagnosis of SIBO by the lactulose breath test have varied with different investigators. Much of the earlier work used bacterial cultures of the proximal small bowel as the gold standard[6,17]. However, such methodology is not helpful in detecting an increase in bacterial biofilm, if it occurs in the distal small bowel. The presence of findings such as a double peak in hydrogen production after lactulose, or high basal breath hydrogen are not well validated in patients with IBS, and are no longer considered appropriate diagnostic features. Glucose breath hydrogen test is useful for the detection of proximal bacterial overgrowth, but since glucose is readily absorbed in the proximal small intestine, SIBO occurring more distally is not detected[18]. The most consistent and accepted measure is the time of the first significant rise (usually ≥ 10 ppm) in breath hydrogen after lactulose, which therefore was applied in the present study.
No consistent effect of treatment with Yakult® was observed on abdominal symptoms or fatigue; some patients worsened whereas others improved. Three patients terminated the study early because of increasing nausea. All three complained of nausea at baseline and only one had previously been tested for lactose intolerance (that person was not lactose intolerant). However, regardless of whether these patients were lactose intolerant, it is unlikely that the small amount of lactose in 1 bottle of Yakult® (1 g) was the cause of these symptoms, given that up to 7 g of lactose in one sitting is well tolerated even in true lactose intolerant patients[19].
The loss of ERBHAL resulted in improvement in the overall abdominal symptom VAS score by at least 2 cm in 71% of the patients with moderate to severe baseline symptoms compared to 0% who maintained the early rise. However, one patient who lost ERBHAL developed more severe symptoms compared to the baseline findings. It cannot be expected that all patients will improve with the correction of ERBHAL, since only 35% improved symptomatically when ERBHAL was corrected with the use of antibiotics[9]. Previous studies on IBS have identified a particular ability of probiotics to improve bloating and wind[20-22]. While bloating did not improve with Yakult®, a significant improvement was seen in the passage of wind. A pathogenic link between the passage of wind, bacterial overgrowth and fermentation can be postulated. The present study was not designed to specifically address the effect on symptoms. However, our findings raise the possibility that symptomatic benefit can be obtained when ERBHAL is corrected with the use of Yakult®.
In conclusion, the hypothesis that treatment with Yakult® alters the fermentation pattern, exhibited by hydrogen breath testing after lactulose was supported in this uncontrolled, proof-of-concept study. A small dose of L. casei strain Shirota led to a statistically significant shift in the time of early rise of breath hydrogen after lactulose, suggesting a reduction in SIBO. Furthermore, the overall abdominal symptoms tended to improve when ERBHAL was corrected. Our findings suggest that probiotics may have a beneficial effect, but further work is required to determine the dose effects and clinical relevance with well powered, double blind, placebo-controlled trials including the measurement of transit time to support the interpretation of ERBHAL.
COMMENTS
Background
Small intestinal bacterial overgrowth (SIBO) is a common feature of irritable bowel syndrome (IBS) and may be directly related to the genesis of IBS symptoms. An early rise in breath hydrogen after lactulose (ERBHAL) may indicate SIBO. The use of antibiotics and elemental diets has been shown to be effective in treating SIBO, but the efficacy of probiotics is untested.
Research frontiers
This was a pilot study designed to determine the effect of Lactobacillus casei strain Shirota (Yakult®) on the intestinal fermentation pattern in IBS, using the lactulose breath test. The results indicate that Yakult alters the fermentation pattern suggesting a reduction in SIBO. ERBHAL may also indicate rapid transit and therefore, in order to confirm the effect of Yakult on SIBO, future studies should include monitoring of transit time in addition to a placebo control group.
Innovations and breakthroughs
The most promising treatments to date for SIBO are antibiotics and elemental diets, the side effects and practicalities of which make them undesirable options. This is the first study evaluating the effect of a probiotic on SIBO. The shift of the time of the first rise of hydrogen following lactulose indicating SIBO was no longer present at the end of 6 wk of treatment. These results offer a promising alternative treatment for SIBO in IBS. A placebo controlled trial is required to confirm these findings.
Applications
The results indicate that Yakult® may be an effective treatment for SIBO in IBS. Our findings have important implications in the treatment of IBS if these results are supported by a placebo controlled trial.
Peer review
The shift in fermentation patterns and the trend towards improvement of symptoms indicates the need for further work to examine the dose effects and clinical relevance with well powered, double blind, placebo controlled trials including the measurement of transit time to support the interpretation of ERBHAL. This is a well written paper with a nice literature review.
Acknowledgments
JSB was in receipt of the Sir Robert Menzies Memorial Research Scholarship in Allied Health Sciences; RBG was supported by Pharmatel Fresenius Kabi IBD Fellowship and the New Zealand Society of Gastroenterology-Ferring Pharmaceuticals Fellowship; PMI was supported by a Fellowship from Nycomed.
Footnotes
Supported by Yakult Ltd, Melbourne Australia
Peer reviewer: Christina Surawicz, MD, Christina Surawicz, Harborview Medical Center, 325 9th Ave, #359773, Seattle WA 98104, United States
S- Editor Li DL L- Editor Anand BS E- Editor Lin YP
References
- 1.Markowitz MA, Jhingran P, Asgharian A, Harris WA, Brod M, Wentz AL, Gordon SH, Carter E. IBS Prevalence: Results from a community based patient registry based on the Rome II criteria. Am J Gastroenterol. 2000;95:2634. [Google Scholar]
- 2.Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli G. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185–194. [PubMed] [Google Scholar]
- 3.Kassinen A, Krogius-Kurikka L, Makivuokko H, Rinttila T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–858. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 5.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–3506. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 6.Riordan SM, McIver CJ, Walker BM, Duncombe VM, Bolin TD, Thomas MC. The lactulose breath hydrogen test and small intestinal bacterial overgrowth. Am J Gastroenterol. 1996;91:1795–1803. [PubMed] [Google Scholar]
- 7.Vanner S. The small intestinal bacterial overgrowth. Irritable bowel syndrome hypothesis: implications for treatment. Gut. 2008;57:1315–1321. doi: 10.1136/gut.2007.133629. [DOI] [PubMed] [Google Scholar]
- 8.Nucera G, Gabrielli M, Lupascu A, Lauritano EC, Santoliquido A, Cremonini F, Cammarota G, Tondi P, Pola P, Gasbarrini G, et al. Abnormal breath tests to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005;21:1391–1395. doi: 10.1111/j.1365-2036.2005.02493.x. [DOI] [PubMed] [Google Scholar]
- 9.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 10.Dear KL, Elia M, Hunter JO. Do interventions which reduce colonic bacterial fermentation improve symptoms of irritable bowel syndrome? Dig Dis Sci. 2005;50:758–766. doi: 10.1007/s10620-005-2570-4. [DOI] [PubMed] [Google Scholar]
- 11.Pimentel M, Constantino T, Kong Y, Bajwa M, Rezaei A, Park S. A 14-day elemental diet is highly effective in normalizing the lactulose breath test. Dig Dis Sci. 2004;49:73–77. doi: 10.1023/b:ddas.0000011605.43979.e1. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri M. Treating irritable bowel syndrome: overview, perspective and future therapies. Br J Pharmacol. 2004;141:1237–1248. doi: 10.1038/sj.bjp.0705741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter JO, Lee AJ, King TS, Barratt MEJ, Linggood MA, Blades JA. Enterococcus faecium strain PR88: An effective probiotic. Gut. 1996;38:A62. [Google Scholar]
- 14.Gawronska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007;25:177–184. doi: 10.1111/j.1365-2036.2006.03175.x. [DOI] [PubMed] [Google Scholar]
- 15.Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, Matuchansky C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26:475–486. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 16.Davis CE. The effect of regression to the mean in epidemiologic and clinical studies. Am J Epidemiol. 1976;104:493–498. doi: 10.1093/oxfordjournals.aje.a112321. [DOI] [PubMed] [Google Scholar]
- 17.Corazza GR, Menozzi MG, Strocchi A, Rasciti L, Vaira D, Lecchini R, Avanzini P, Chezzi C, Gasbarrini G. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98:302–309. doi: 10.1016/0016-5085(90)90818-l. [DOI] [PubMed] [Google Scholar]
- 18.Simren M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vesa TH, Korpela RA, Sahi T. Tolerance to small amounts of lactose in lactose maldigesters. Am J Clin Nutr. 1996;64:197–201. doi: 10.1093/ajcn/64.2.197. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, Thomforde G, Zinsmeister AR. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 21.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, Zinsmeister AR. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]