Abstract
AIM: To estimate the test characteristics of Helicobacter pylori (H pylori) serology and of C14-urea breath test (C14-UBT) in two different peptic ulcer populations and in community controls. Second, the aim was to explore the association between the level of H pylori IgG antibodies and severity of inflammation as to active peptic ulceration in the same populations.
METHODS: Vagotomized (n = 83), medically treated peptic ulcer patients (n = 73) and one reference group of community controls (n = 88) were gastroscoped. H pylori status was determined by histology, bacterial growth, C14-UBT and serology. Based on the updated Sydney System, cumulative scores from biopsies from the prepyloruos, incisura angularis, corpus and fundus were calculated.
RESULTS: The prevalence of H pylori infection varied from 70% to 79%. The C14-UBT had high accuracy compared to the serology test. The sensitivity of the serology test was good, but the specificity was low (41%-71%). The association between H pylori IgG antibodies and scores of gastric mucosal inflammation and current or previous peptic ulcer were weak.
CONCLUSION: The accuracy of C14-UBT to diagnose H pylori infection was good, and the clinical utility of a negative H pylori serology test was substantial, while the gain in clinical information of a positive test was meagre. Positive H pylori titres could not distinguish between subjects with or those without active peptic ulceration.
Keywords: C14-urea breath test, Gastric inflammation, Helicobacter pylori serology, Peptic ulcers, Test characteristics
INTRODUCTION
Serology and C14-urea breath test (C14-UBT) are the most commonly used non-invasive tests of Helicobacter pylori (H pylori) infection[1-3]. Knowledge about the diagnostic validity of particular serological tests is mandatory for inferring its test results[4]. In the diagnostics of H pylori infection, most commercially available serological tests are reported to have both a high sensitivity and a high specificity[5]. The diagnostic characteristics of the tests depend also on the prevalence of H pylori infection in the population tested[5-7]. Higher prevalence’s would imply higher sensitivity and lower specificity[5-7]. There are reports suggesting that there is an association between the level of H pylori IgG antibodies and the severity of inflammation of the gastric mucosa and also between antibody level and a current peptic ulcer[8-10]. If so, the level, not only positively or not, of H pylori IgG antibody tests might be of clinical importance.
The aim of this study was to estimate the test characteristics of H pylori serology compared to the urea breath test (C14-UBT) in two different peptic ulcer populations and in a randomly selected group of community controls without known peptic ulcer disease. Second, the aim was to explore the association between the level of H pylori IgG antibodies and severity of inflammation as to active peptic ulceration in the same populations.
MATERIALS AND METHODS
Based upon a questionnaire survey[11], three groups of subjects were invited to participate in an upper endoscopic investigation: one group of vagotomized peptic ulcer patients; one group of medically treated peptic ulcer patients and one reference group of community controls without known peptic ulcer disease.
Vagotomized peptic ulcer patients
The medical records of all patients operated with a vagotomy for peptic ulcer disease from 1967 to 1990 at Tromsø University Hospital were reviewed, totally 1 038 records. Seven hundred and twenty one were alive and received a postal questionnaire with 105 different questions on abdominal and dyspeptic complaints, medications, use of health services, health, life style, diet and social conditions. Two hundred and eighty two answered that they were interested in a gastroscopic examination if offered. By binominal distribution 106 of these 282 vagotomized patients were randomly selected and invited into the study. Sixteen patients were excluded because they had undergone gastric resections in addition to the vagotomy operation and seven due to interrupted endoscopic examination according to the patient’s wishes. Accordingly, 83 patients in these groups completed the investigation protocol. Fifty nine had been electively vagotomized, whereas 24 had been vagotomized on emergency indications.
Medically treated, non-operated, peptic ulcer patients
Two hundred and thirty one medically treated patients with radiographically (barium meal) or endoscopically verified peptic ulcer disease diagnosed in the period 1979 to 1986 received the same questionnaire as the vagoto-mized patients. One hundred and five were interested in an endoscopic examination if offered. All of these were invited. Seventy four finally accepted the invitation; one patient failed to swallow the endoscope. Accordingly, 73 patients fulfilled the investigation protocol.
Community controls
For comparison a group of community controls was included. Seven hundred and sixty two inhabitants of the local municipality were randomly selected from the National Population Registry. They were all without known peptic ulcer disease and were invited to participate in the same questionnaire survey as the peptic ulcer patients to serve as a community reference group in the comparison of abdominal and dyspeptic complaints. They were group-matched with the vagotomized patients regarding sex distribution and mean age. Two hundred and twenty five persons responded positively to the offered endoscopic examination. By binominal distribution, 105 subjects were randomly selected and invited to the endoscopic study. Ninety six finally accepted the invitation of which 7 were excluded due to interrupted endoscopic examination according to the patient’s wishes, and one because of previous gastric surgery. Accordingly, 88 community subjects completed the investigation protocol.
The Regional Ethical Committee for Medical Sciences and the Norwegian Social Science Data Services approved the study design and the data security. There was no financial gain or hints of health benefits associated with participation in the study.
After an overnight fast, all subjects were pre-medicated with a topical anaesthetic spray (lidocaine hydrochloride, 10 mg/dose, Astra, Sweden). No additional sedation was used. The same endoscopist (ROL) examined all patients, and he was unaware of the subjects’ peptic ulcer history, any previous treatment or current dyspeptic or abdominal complaints. All endoscopies were recorded by a Sony Hi-8 video-recorder using a Pentax gastroscope EG 2901.
None of the participants in the study received long term or continuous medical acid suppressive treatment prior to the endoscopic examination or any known treatment against H pylori infection.
Detection of H pylori
The culture, PCR and the serology tests were performed in the laboratories at the Department of Microbiology, Tromsø University Hospital (accredited according to the ISO 45001 standard).
Histology
The biopsy specimens for histological detection of H pylori infection was taken from the greater curvature of the duodenal bulb, from the greater curvature in the prepyloric antrum 2-3 cm from the pyloric channel, from the angulus (incisura angularis) of the lesser curvature, from the corpus of the greater curvature 10 cm from the cardia, and from the fundic top. Separate biopsy specimens were taken from all lesions. All biopsies were fixed in neutral-buffered 4% formaldehyde. Hematoxilin-eosin stains of paraffin-embedded biopsies were used for histological evaluation[12]. Histology was considered positive if H pylori like organisms were found in any of the four gastric biopsy sites, and negative if H pylori like organisms were not found in any of the biopsies. All histological specimens were evaluated by the same experienced pathologist (TJE) who was blinded for the subjects’ medical history and other H pylori test results. The histological evaluations were performed according to the updated Sydney System recommendation[13].
Gastric inflammation score
Semi-quantitative descriptions of the updated Sydney System were transformed to exact numbers from 1-4 (1 = no/none/no H pylori, 2 = slight/mild/H pylori in 1-3 pits, 3 = moderate/H pylori in more than 3 pits, but not all, 4 = severe/H pylori in all pits). The score from the 4 local biopsy sites were added up and the total scores were grouped in order to describe the histologically evaluated gastric inflammation of the total gastric mucosa. The same semi-quantitative terms were used for the chronic gastritis score. A score of 4 denotes no chronic gastritis/no H pylori, 5-8 denotes mild chronic gastritis/few H pylori, 9-12 denotes moderate chronic gastritis/some H pylori, 13-16 denotes severe chronic gastritis/much H pylori. The inflammatory activity was described as 1 = no inflammatory activity, 2 = slight/moderate inflammatory activity, 3 = severe inflammatory activity. In the scoring of the inflammatory activity 4 denotes no inflammatory activity, 5-6 slight inflammatory activity, 7-8 moderate inflammatory activity, 9-12 severe inflammatory activity.
H pylori culture growth
Two biopsies taken from the angulus of the lesser curvature were placed in a transport medium (Portagerm pylori, bioMèrieux, Lyon, France) and immediately transported to the Department of Microbiology for culture and detection of the H pylori DNA by PCR. Culture was preformed using selective (Oxoid SR 147E supplement) and nonselective columbia agar (Oxoid CM 331) with 7% lysed horse blood at 37°C. The plates were incubated under a microaerobic atmosphere (5% = 2, 7% CO2, 8% H2, 80% N2) for at least 7 d. The culture was classified as positive when oxidase-, catalase- and urease positive colonies showed typical H pylori morphology on Gram staining.
C14 urea breath test
Fifty milliliters of tap water with 2.5 μCi carbon-14 labelled urea was given orally to the overnight fasted patient. Double breath samples were taken immediately before and at 10, 20 and 30 min after the ingestion of the urea solution and analysed for 14CO2 in a Beta-counter as a measure of H pylori urease activity in the stomach. H pylori negative patients do not expire 14C labelled CO2. A 14C urea breath test was positive when the accumulated 14CO2 in the breath samples adjusted for the patients’ bodyweight and the background radiation was more than 1.5% of the ingested 14C dose[14].
Enzyme-linked immunoabsorbent assay (ELISA) detection of IgG and IgA H pylori antibodies
ELISA was used for detection of serum IgG and IgA antibodies according to the specifications of the manufacturer (Pyloriset EIA-G®, Orion Diagnostica, Finland). A titre ≥ 300 was interpreted as a positive IgG serology result.
Reference standard
As reference tests were chosen the combination of histologically detected presence of H pylori-like organisms in any of the four biopsy sites[15] augmented by H pylori culture growth. A patient was considered as H pylori positive when having a positive test for H pylori by histological examination and/or by culture. The biopsies from the gastric mucosa were taken according to recommended biopsy sites and procedures[16].
Statistical analysis
The microcomputer software Confidence Interval analysis[17] estimated 95% confidence intervals for the various proportions and Epiinfo 6[18] was used to analyze dichotomous variables by the Mantel-Haenszel χ²-test with Yates correction.
RESULTS
The prevalence of H pylori positive individuals among the vagotomized peptic ulcer patients was 79% (95% CI: 69-88), 75% (95% CI: 63-85) among the medically treated peptic ulcer patients, and 70% (95% CI: 59-80) among the community controls.
Anti H pylori IgG at a cut-off value 300 had comparable properties to C14-UBT in detecting the true positive patients in all three groups with sensitivities around 95% (Table 1). When increasing the cut-off value to 500, the sensitivities decreased 8%-10% in all three groups. The combination of anti H pylori IgG at a cut-off value 300 and anti H pylori IgA at a cut-off value 500 did not improve the sensitivity or specificity of the test in any of the groups. The specificity of anti H pylori IgG and IgA were between 32%-50% among the peptic ulcer patients and 58%-71% among the controls, and far lower than C14-UBT with a specificity ranging from 80%-90% in all three groups.
Table 1.
Sensitivity, specificity and likelihood ratio of positive test (LR+) of C14-UBT and IgG and IgA antibodies against H pylori at cut-off values 300 and 500 in vagotomized (vag) or medically (med) treated peptic ulcer patients (med) and in community controls (con)
| Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | |||||||
| vag | med | con | vag | med | con | vag | med | con | |
| Method of detection | |||||||||
| C14-UBT | 94 (85-99) | 92 (81-98) | 96 (87-100) | 85 (62-97) | 80 (56-94) | 90 (73-98) | 6.3 (2.4-31.5) | 4.6 (1.7-17.6) | 9.3 (3.0-46.6) |
| Serology IgG300 | 95 (86-99) | 98 (90-100) | 93 (83-98) | 41 (21-64) | 50 (28-72) | 68 (49-83) | 1.6 (0.9-3.2) | 2.0 (1.0-4.2) | 2.9 (1.5-6.4) |
| Serology IgG500 | 87 (76-94) | 90 (79-97) | 83 (70-91) | 55 (32-76) | 50 (28-72) | 71 (52-86) | 1.9 (1.0-4.2) | 1.8 (0.9-3.9) | 2.8 (1.4-6.6) |
| Serology IgG/IgA1 | 95 (86-99) | 98 (90-100) | 93 (83-98) | 32 (14-55) | 50 (28-72) | 58 (39-76) | 1.4 (0.8-2.7) | 2.0 (1.0-4.2) | 2.2 (1.2-4.4) |
H pylori infection detected by a positive histology and/or by a positive culture was defined as reference standard.
Serology IgG/IgA: IgG ≥ 300 and/or IgA ≥ 500.
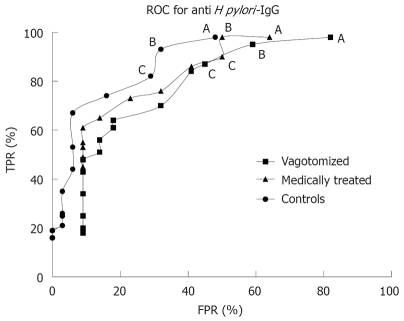
When combining the sensitivity and specificity expressed by the ROC (receiver operating characteristic) curve for anti H pylori IgG curves for each group, the area under the curve was largest in the control group (Figure 1).
Figure 1.
Receiver operating characteristic (ROC) curve for different cut-off values for H pylori IgG antibodies in vagotomized or medically treated peptic ulcer patients and in community controls. Cut-off values were 200, 300, 500, 600 and at intervals of 300 for IgG values above 600 to 4800. TRP = True positive rate (sensitivity), FPR = False positive rate (1-specificity). A: Cut-off 200; B: Cut-off 300; C: Cut-off 500.
Incomplete or missing biopsies for H pylori detection by both histology and culture growth occurred in 14 cases (n = 230, histology HP and growth, Table 2), whereas 215 biopsies were finally included to evaluate inflammation in the angulus. Two of these had missing culture growth biopsies (n = 213, anginflam and growth, Table 2).
Table 2.
H pylori status by histology and inflammatory activity in the gastric angulus (anginflam) in different combinations with culture growth, related to level of IgG antibodies against H pylori in each category
| n | Range | Mean (95% CI) | Median | |
| IgG when histologyHP neg and growth neg | 66 | 100-12800 | 700 (290-1120) | 200 |
| IgG when histologyHP pos and growth neg | 9 | 100-18000 | 4960 (530-9390) | 4000 |
| IgG when histologyHP pos and growth pos | 143 | 100-20000 | 3480 (2740-4220) | 1600 |
| IgG when histologyHP neg and growth pos | 12 | 200-7000 | 2660 (1270-4050) | 2250 |
| IgG when anginflam neg and growth neg | 60 | 100-18000 | 690 (90-1290) | 200 |
| IgG when anginflam pos and growth neg | 8 | 300-9000 | 3830 (1420-6240) | 3750 |
| IgG when anginflam pos and growth pos | 126 | 100-20000 | 3500 (2700-4300) | 1650 |
| IgG when anginflam neg and growth pos | 19 | 100-13000 | 2840 (960-4720) | 1200 |
Anti H pylori IgG had a median value of 200 when the reference standard (no H pylori like bacteria in any of the four biopsy sites and negative culture growth) was negative. Signs of H pylori either by histology or by bacterial growth, or by inflammation in the angulus were associated to elevate anti H pylori IgG levels (Table 2). This association was dichotomous and independent of severity of active inflammation or quantitative histological evaluation of H pylori (Figure 2).
Figure 2.
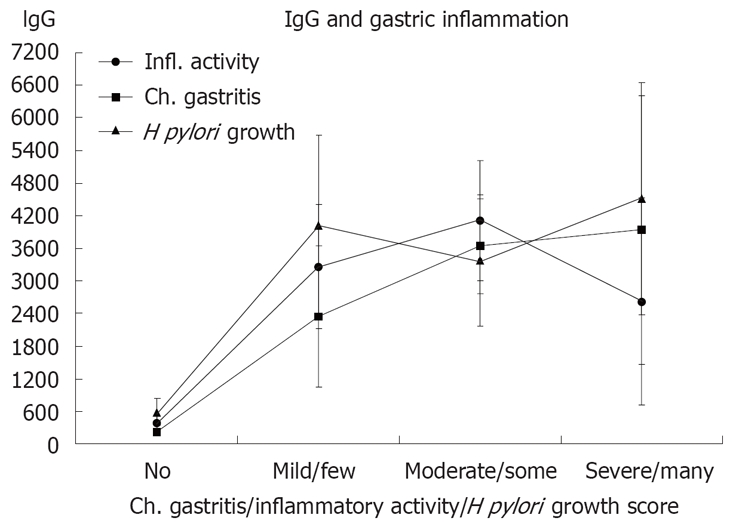
Grade of inflammatory activity (no, mild, moderate, severe), grade of chronic gastritis (no, mild, moderate, severe) and semiquantitative numbers of H pylori (no, few = H pylori like organisms in 1-3 pits, some = H pylori like organisms in more than 3 pits but not all, many = H pylori like organisms in all pits) detected in gastric biopsies from 4 different biopsy sites (prepylorus, angulus, corpus and fundus) related to the mean value with 95% confidence intervals of IgG antibodies against H pylori in each category.
Increasing levels of H pylori IgG antibodies were associated with increasing frequency of subjects with active peptic ulcer (Figure 3). With levels of H pylori IgG antibodies above 1000, there was an increase in PU prevalence (P = 0.03). Still, 80% of the subjects did not have PU.
Figure 3.
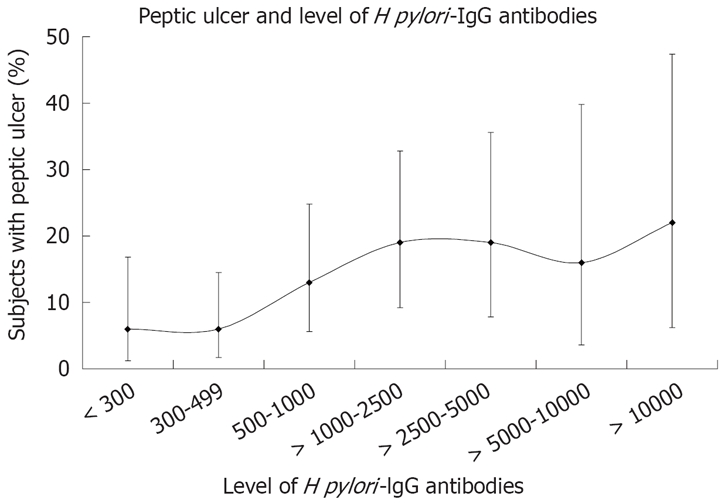
Subjects with peptic ulcer related to level of H pylori IgG antibodies. Frequencies are shown with 95% CI.
DISCUSSION
The prevalence of H pylori in the peptic ulcer patients was 75%-79%. This is somewhat lower than expected in peptic ulcer populations[19,20]. Among the vagotomized or medically treated patients are subjects with gastric ulcers that have a lower H pylori prevalence than duodenal ulcer patients have[19]. In addition, false negative H pylori tests or previous antibiotic treatment of other indications than H pylori infections might also decrease the prevalence of H pylori infection.
In populations with high prevalence of H pylori infection the test characteristics of C14-UBT are very good. While the sensitivity of the serology test is excellent too, the specificity is low. The validity varies with the prevalence of H pylori infection, illustrating the effects of spectrum bias[21]. The test characteristics are, at best, in the control group with the lowest prevalence of H pylori infection. Increasing the threshold of a positive test from 300 to 500 or higher did not improve the validity of the test.
The relative low specificity of the anti H pylori IgG and/or IgA test indicates a high antibody titre despite no actual presence of H pylori in the gastric mucosa. Previous H pylori infection or cross-reacting antibodies from closely related bacteria might explain a positive serology test despite no actual H pylori infection.
Likelihood ratio is a more clinically relevant method of reporting accuracy than only specificity and sensitivity of a test. The probability of having a disease after a positive or negative test can be calculated and reveals more understandable information in the clinical setting and could also be applied to the clinical problem of dyspepsia management[22].
By comparing the true positive rate to the false positive rate, information about the probability of whether a positive test result is likely to be from a truly H pylori positive subject, compared to a similar result to be from a truly H pylori negative subject, can be obtained (positive likelihood ratio)[15]. By this method C14-UBT was about three times better than Hp serology. 13C-UBT is in full concordance with 14C-UBT and should be preferred because of its lack of radioactivity.
The low specificity reduces the clinical utility of a positive test rest result. When, among medically treated peptic ulcer group, applying the test results of a sensitivity of 98%, a specificity of 50%, and a pre-test probability of 75%, the post-test probability of a positive test barely increases to 85%, while the post-test probability of a negative test enlarges from 25% to 90%.
In 21 subjects, there were discrepancies between the results of histological identification of H pylori and growth of the bacteria. As culture growth is highly specific[2], and since none of the 12 subjects with negative histology and positive culture is a false positive, the sensitivity of the histology could not be 100%[16]. If there is a misclassification of the reference standard applied in this study, the direction is towards an overrating of H pylori positive subjects. Consequently, the test characteristics, mainly the sensitivities of serology could be somewhat underestimated.
Others have published sensitivity of 100% and specificity of 79% using the same serology Elisa-kit[23]. However, the population was on average about 10 years younger than in this study and the H pylori prevalence was 82%. The prevalence of H pylori is equivalent to our vagotomy group. While the sensitivity is comparable, the populations differ regarding the specificity of the test. The two populations could differ regarding number of case-mix, or people in North Norway might have more infections that could cross-react with H pylori serology kits.
An objection to this presentation is the lack of validation of the constructed cumulative scores of inflammatory activity, chronic gastritis and H pylori density, based on the histological Sydney System scores at the four different biopsy sites. The chronic gastritis and the subsequent atrophic changes in H pylori infected subjects are commonly described as antrum pre-dominant, corpus-predominant or both (pangastritis)[24]. However, the objective of this study was to detect any association between the global measurement of H pylori serology and the general inflammatory status of the gastric mucosa. Depending on the severity grading, according to the updated Sydney System, the antral predominant chronic gastritis would thus have a relatively high cumulative score, as the condition would be detected in the biopsies from both the pre-pylorous and from the incisura angularis. In addition to the corpus biopsy, the corpus-predominant chronic gastritis should also be reflected in the cumulative scoring system in the transition zone (incisura angularis) as much as the antrum-predominant chronic gastritis. Pangastritis would be reflected by even higher scores by summation from all four biopsy sites.
The percentage of subjects with peptic ulcers was not significantly different at various levels of H pylori IgG antibodies above the recommended cut-off titre value of 300. The same tests could neither differentiate between previous peptic ulcer patients and community controls, nor in the severity score of gastric inflammation measured by inflammatory activity, chronic gastritis or histologically evaluated bacterial growth.
In this study, H pylori IgG antibodies could not be used to differentiate between previous peptic ulcer patients and healthy community controls, nor between patients with or those without active peptic ulcers.
No association was found between the level of positive IgG titres and the cumulative scores of inflammatory activity and H pylori density, according to the updated Sydney System, from the four different biopsy sites in the gastric mucosa. In the clinical setting, this means that the level of positive H pylori titres give no diagnostic information about the degree of inflammation in the gastric mucosa, nor cannot distinguish between subjects with or those without active peptic ulceration, nor between previous peptic ulcer patients and community controls. Others have also reported that H pylori serology is a poor marker of peptic ulcer disease[25-28].
When using H pylori serology tests a negative result is of clinical importance at the recommended cut-off value of IgG titre 300, due to the high sensitivity of the test. A negative serology test result is also reported to almost rule out pre-malignant conditions in the gastric mucosa in screening situations[29]. A low specificity, however, reduces the clinical utility of a positive test result. Independent of previous peptic ulcer status among the tested subjects, H pylori serology and C14-UBT showed comparable sensitivity.
We could not find any association between gastric mucosal morphology and serology, in contrast to what is published by others[30]. However, in that study, a combination of serology tests were used, and a more dichotomous approach to the presence of H pylori infection and its morphological consequences were applied.
Uncritical use of H pylori serology will represent a considerable overestimation of H pylori prevalence in the population tested. H pylori serology is, on the other hand, very reliable to exclude H pylori infection and thereby useful in screening purposes.
COMMENTS
Background
Serology and C14-urea breath test (C14-UBT) are the most commonly used non-invasive tests of Helicobacter pylori (H pylori) infection. Knowledge about the diagnostic validity of particular serological tests is mandatory for inferring its test results. In the diagnostics of H pylori infection, most commercially available serological tests are reported to have both a high sensitivity and a high specificity. The diagnostic characteristics of the tests depend also on the prevalence of H pylori infection in the population tested. Higher prevalences would imply higher sensitivity and lower specificity.
Research frontiers
Application of test characteristics to illustrate limited value of a commonly used blood test (H pylori serology) for detection of H pylori gastric infection or peptic ulcer. However, a negative test result rule out H pylori infection with high certainty.
Applications
General practice and gastroenterological specialist practice.
Terminology
Sensitivity means a test ability to correctly identify a true positive subject with a positive test result (frequency of positive test or true positive rate). Specificity means a test ability to correctly identify a true negative subject with a negative test result (frequency of negative test or true negative rate).
Peer review
This retrospective study has estimated the test characteristics of H pylori serology and of C14-UBT in 83 vagotomized patients, 73 medically treated peptic ulcer patients and 88 gastroscoped community controls in Norway. It is helpful to know the prevalence of the infection in the area of study.
Acknowledgments
The authors want to thank the staff in the Department for Clinical Investigation for their excellent service to the patients and their valuable contribution in the administration of the study. We also want to thank the Department of Microbiology and Department of Pathology for expedited and high quality service of all the biopsy specimens.
Footnotes
Peer reviewer: Amado S Peña, Professor, Department of Pathology, Immunogenetics, VU University Medical Centre, De Boelelaan 1117, PO Box 7057, Amsterdam 1007 MB, Netherlands
S- Editor Li DL L- Editor Rippe RA E- Editor Ma WH
References
- 1.Basset C, Holton J, Ricci C, Gatta L, Tampieri A, Perna F, Miglioli M, Vaira D. Review article: diagnosis and treatment of Helicobacter: a 2002 updated review. Aliment Pharmacol Ther. 2003;17 Suppl 2:89–97. doi: 10.1046/j.1365-2036.17.s2.6.x. [DOI] [PubMed] [Google Scholar]
- 2.de Boer WA. Diagnosis of Helicobacter pylori infection. Review of diagnostic techniques and recommendations for their use in different clinical settings. Scand J Gastroenterol Suppl. 1997;223:35–42. [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirschl AM, Makristathis A. Methods to detect Helicobacter pylori: from culture to molecular biology. Helicobacter. 2007;12 Suppl 2:6–11. doi: 10.1111/j.1523-5378.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 5.Roberts AP, Childs SM, Rubin G, de Wit NJ. Tests for Helicobacter pylori infection: a critical appraisal from primary care. Fam Pract. 2000;17 Suppl 2:S12–S20. doi: 10.1093/fampra/17.suppl_2.s12. [DOI] [PubMed] [Google Scholar]
- 6.Richardson WS, Wilson MC, Keitz SA, Wyer PC. Tips for teachers of evidence-based medicine: making sense of diagnostic test results using likelihood ratios. J Gen Intern Med. 2008;23:87–92. doi: 10.1007/s11606-007-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nurgalieva ZZ, Graham DY. Pearls and pitfalls of assessing Helicobacter pylori status. Dig Liver Dis. 2003;35:375–377. doi: 10.1016/s1590-8658(03)00166-x. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto I, Fukuda Y, Mizuta T, Fukada M, Nishigami T, Shimoyama T. Serum anti-Helicobacter pylori antibodies and gastritis. J Clin Gastroenterol. 1995;21 Suppl 1:S164–S168. [PubMed] [Google Scholar]
- 9.Nakata H, Itoh H, Yokoya Y, Kawai J, Nishioka S, Miyamoto K, Kitamoto N, Miyamoto H, Tanaka T. Serum antibody against Helicobacter pylori assayed by a new capture ELISA. J Gastroenterol. 1995;30:295–300. doi: 10.1007/BF02347502. [DOI] [PubMed] [Google Scholar]
- 10.Kreuning J, Lindeman J, Biemond I, Lamers CB. Relation between IgG and IgA antibody titres against Helicobacter pylori in serum and severity of gastritis in asymptomatic subjects. J Clin Pathol. 1994;47:227–231. doi: 10.1136/jcp.47.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsetmo RO, Johnsen R, Revhaug A. Abdominal and dyspeptic symptoms in patients with peptic ulcer treated medically or surgically. Br J Surg. 1998;85:845–849. doi: 10.1046/j.1365-2168.1998.00711.x. [DOI] [PubMed] [Google Scholar]
- 12.Cutler AF. Diagnostic tests for Helicobacter pylori infection. Gastroenterologist. 1997;5:202–212. [PubMed] [Google Scholar]
- 13.Stolte M, Meining A. The updated Sydney system: classification and grading of gastritis as the basis of diagnosis and treatment. Can J Gastroenterol. 2001;15:591–598. doi: 10.1155/2001/367832. [DOI] [PubMed] [Google Scholar]
- 14.Berstad K, Wilhelmsen I, Berstad A. Biometric evaluation of gastric urease activity in man. Scand J Gastroenterol. 1992;27:977–983. doi: 10.3109/00365529209000174. [DOI] [PubMed] [Google Scholar]
- 15.Feldman RA, Evans SJ. Accuracy of diagnostic methods used for epidemiological studies of Helicobacter pylori. Aliment Pharmacol Ther. 1995;9 Suppl 2:21–31. [PubMed] [Google Scholar]
- 16.Genta RM, Graham DY. Comparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: a topographic study of H. pylori density and distribution. Gastrointest Endosc. 1994;40:342–345. doi: 10.1016/s0016-5107(94)70067-2. [DOI] [PubMed] [Google Scholar]
- 17.Gardner MJ, Gardner SB, Winter PD. Confidence intervals analysis. Vol. 40. BMJ publishing group, London: BMJ Publishing Group; 1998. [Google Scholar]
- 18.Dean AG, Dean FA, Burton AH, Dicker RC Epi Info, Version 6: A word processing, database and statistics system for epidemiology on microcomputers. USD, Stone Mountain, Georgia, 1999 [Google Scholar]
- 19.Marshall BJ. Helicobacter pylori. Am J Gastroenterol. 1994;89:S116–S128. [PubMed] [Google Scholar]
- 20.Bernersen B, Johnsen R, Bostad L, Straume B, Sommer AI, Burhol PG. Is Helicobacter pylori the cause of dyspepsia? BMJ. 1992;304:1276–1279. doi: 10.1136/bmj.304.6837.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lachs MS, Nachamkin I, Edelstein PH, Goldman J, Feinstein AR, Schwartz JS. Spectrum bias in the evaluation of diagnostic tests: lessons from the rapid dipstick test for urinary tract infection. Ann Intern Med. 1992;117:135–140. doi: 10.7326/0003-4819-117-2-135. [DOI] [PubMed] [Google Scholar]
- 22.Moayyedi P, Axon AT. The usefulness of the likelihood ratio in the diagnosis of dyspepsia and gastroesophageal reflux disease. Am J Gastroenterol. 1999;94:3122–3125. doi: 10.1111/j.1572-0241.1999.01502.x. [DOI] [PubMed] [Google Scholar]
- 23.van de Wouw BA, de Boer WA, Jansz AR, Roymans RT, Staals AP. Comparison of three commercially available enzyme-linked immunosorbent assays and biopsy-dependent diagnosis for detecting Helicobacter pylori infection. J Clin Microbiol. 1996;34:94–97. doi: 10.1128/jcm.34.1.94-97.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sipponen P, Kekki M, Seppala K, Siurala M. The relationships between chronic gastritis and gastric acid secretion. Aliment Pharmacol Ther. 1996;10 Suppl 1:103–118. doi: 10.1046/j.1365-2036.1996.22164011.x. [DOI] [PubMed] [Google Scholar]
- 25.Xia HH, Kalantar JS, Mitchell HM, Talley NJ. Can helicobacter pylori serology still be applied as a surrogate marker to identify peptic ulcer disease in dyspepsia? Aliment Pharmacol Ther. 2000;14:615–624. doi: 10.1046/j.1365-2036.2000.00720.x. [DOI] [PubMed] [Google Scholar]
- 26.Quartero AO, Numans ME, de Melker RA, de Wit NJ. In-practice evaluation of whole-blood Helicobacter pylori test: its usefulness in detecting peptic ulcer disease. Br J Gen Pract. 2000;50:13–16. [PMC free article] [PubMed] [Google Scholar]
- 27.Zuniga-Noriega JR, Bosques-Padilla FJ, Perez-Perez GI, Tijerina-Menchaca R, Flores-Gutierrez JP, Maldonado Garza HJ, Garza-Gonzalez E. Diagnostic utility of invasive tests and serology for the diagnosis of Helicobacter pylori infection in different clinical presentations. Arch Med Res. 2006;37:123–128. doi: 10.1016/j.arcmed.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Weijnen CF, Numans ME, de Wit NJ, Smout AJ, Moons KG, Verheij TJ, Hoes AW. Testing for Helicobacter pylori in dyspeptic patients suspected of peptic ulcer disease in primary care: cross sectional study. BMJ. 2001;323:71–75. doi: 10.1136/bmj.323.7304.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storskrubb T, Aro P, Ronkainen J, Vieth M, Stolte M, Wreiber K, Engstrand L, Nyhlin H, Bolling-Sternevald E, Talley NJ, et al. A negative Helicobacter pylori serology test is more reliable for exclusion of premalignant gastric conditions than a negative test for current H. pylori infection: a report on histology and H. pylori detection in the general adult population. Scand J Gastroenterol. 2005;40:302–311. doi: 10.1080/00365520410010625. [DOI] [PubMed] [Google Scholar]
- 30.Mardh E, Mardh S, Mardh B, Borch K. Diagnosis of gastritis by means of a combination of serological analyses. Clin Chim Acta. 2002;320:17–27. doi: 10.1016/s0009-8981(02)00040-2. [DOI] [PubMed] [Google Scholar]