Abstract
AIM: To investigate the effect of Lactobacillus bulgaricus (LBG) on the Toll-like receptor 4 (TLR4) pathway and interleukin-8 (IL-8) production in SGC-7901 cells treated with Helicobacter pyloriSydney strain 1 lipopolysaccharide (H pyloriSS1-LPS).
METHODS: SGC-7901 cells were treated with H pyloriSS1-LPS in the presence or absence of pretreatment for 1 h with viable LBG or supernatant recovered from LBG culture MRS broth (LBG-S). Cellular lysates were prepared for Western blot with anti-TLR4, anti-transforming growth factor β-activated kinase 1 (TAK1), anti-phospho-TAK1, anti-nuclear factor κB (NF-κB), anti-p38 mitogen-activated protein kinase (p38MAPK), and anti-phospho-p38MAPK antibodies. The amount of IL-8 in cell culture medium was measured by ELISA.
RESULTS: H pyloriSS1-LPS up-regulated the expression of TLR4, stimulated the phosphorylation of TAK1, subsequently enhanced the activation of NF-κB and the phosphorylation of p38MAPK in a time-dependent manner, leading to augmentation of IL-8 production in SGC-7901 cells. Viable LBG or LBG-S pretreatment attenuated the expression of TLR4, inhibited the phosphorylation of TAK1 and p38MAPK, prevented the activation of NF-κB, and consequently blocked IL-8 production.
CONCLUSION: H pyloriSS1-LPS induces IL-8 production through activating TLR4 signaling in SGC-7901 cells and viable LBG or LBG-S prevents H pyloriSS1-LPS-mediated IL-8 production via inhibition of the TLR4 pathway.
Keywords: Lactobacillus, Helicobacter pylori, Lipopolysaccharide, Toll-like receptor 4, Interleukin-8
INTRODUCTION
Infection with the human gastric pathogen Helicobacter pylori (H pylori) can develop into chronic gastritis, peptic ulcer and gastric cancer. Some studies[1-5] demonstrated that H pylori can stimulate interleukin-8 (IL-8) production in gastric mucosal epithelia, which induces accumulation of neutrophilic granulocytes in mucosa. Chemotactic response initiates inflammatory damage to gastric mucosa, which plays a crucial role in the pathogenesis of H pylori. However, signal transduction through which H pylori modulates IL-8 production from gastric epithelia is not fully understood.
The product of particular significance for the virulent action of H pylori is its cell wall lipopolysaccharide (LPS). The effects of H pylori lipopolysaccharide (H pylori-LPS) have been manifested by the marked increase of nitric oxide and proinflammatory cytokines including IL-8 in gastric mucosa[6,7], abrogation of proliferation and induction of apoptosis in gastric epithelia[8]. Mammalian Toll-like receptors trigger the signaling pathways involved in innate immune responses to microbial challenge after recognizing pathogen-associated molecular patterns. H pylori-LPS is the natural ligand for Toll-like receptor 4 (TLR4) in gastric epithelia. It has been proposed that H pylori-LPS induces IL-8 production in gastric epithelia through activating the TLR4 signaling pathway[7,9].
Probiotics are living microorganisms with no or low pathogenicity, which exert beneficial effects on the host. Lactobacillus bulgaricus (LBG), a bacterium used in the production of yogurt, is one of the best-studied probiotics. There is increasing evidence[10,11] that LBG has therapeutic effects on H pylori-related diseases, including enhanced eradication of H pylori, amelioration of resistance to antibiotics, down-regulated side effects of antibiotic-based therapy, decreased recurrence of H pylori infection, and inhibition of H pylori-induced apoptosis. The mechanisms underlying these effects include inhibition of H pylori growth and attachment to epithelial cells, inactivation of virulent factors such as urease, and decrease in production of H pylori-induced proinflammatory cytokines[12-16]. However, the signaling pathways which are modulated by LBG in gastric epithelia have not been well elucidated.
In this experiment, we demonstrated that viable LBG inhibited the activation of the TLR4 signaling pathway and IL-8 production induced by H pyloriSydney strain 1 lipopolysaccharide (H pyloriSS1-LPS) in SGC-7901 cells. Furthermore, supernatant recovered from the LBG culture MRS broth (LBG-S) also exerted these effects on SGC-7901 cells treated with H pyloriSS1-LPS. These observations provide the novel insight into the rationale for LBG as a potential treatment for H pylori-related diseases.
MATERIALS AND METHODS
H pyloriSS1 culture and H pyloriSS1-LPS preparation
H pyloriSS1 was kindly offered by Professor Qian Yu (School of Public Health, Sichuan University). H pyloriSS1 was incubated in Brucella broth (bioMérieux Corporate, La Balme-Les Grottes, France) supplemented with 10% fetal calf serum (FCS; Invitrogen GIBCO, Carlsbad, California, USA), 10 mg/L vancomycin, 10 mg/L amphotericin and 2500 U/L polymycin B in a shaking incubator (100 r/min) at 37°C in an atmosphere containing 50 mL/L O2, 100 mL/L CO2 and 850 mL/L N2 for 48 h. H pyloriSS1 was precipitated from Brucella broth by centrifuging at 10 000 r/min for 10 min at 4°C and washed twice with PBS. Then the concentration of H pyloriSS1 in PBS was adjusted to 108 colony forming units (CFU)/mL with optical density determined as 1 at A660. The H pyloriSS1-containing PBS was used to prepare H pyloriSS1-LPS with the LPS extraction kit (bioMérieux) following guidelines from its manufacturer. H pyloriSS1-LPS concentrations were determined with the kinetic Limulus amebocyte lysate assay kit (Cambrex, Walkersville, Maryland, USA) according to the manufacturer’s instructions.
LBG culture and LBG-S preparation
LBG, kindly offered by Professor Qian Yu (School of Public Health, Sichuan University), was incubated in MRS broth (bioMérieux) in a candle jar at 37°C for 24-48 h, precipitated from MRS broth by centrifuging at 5000 r/min for 10 min and washed twice with PBS. Then the concentration of LBG in PBS was adjusted to 107 CFU/mL with optical density determined as 0.5 at A600. LBG was precipitated from PBS by centrifuging at 5000 r/min for 10 min and resuspended with an equivalent volume of RPMI 1640 medium (Invitrogen GIBCO) for pretreatment of SGC-7901 cells.
LBG-S was generated by centrifuging at 1000 × g for 15 min and filtering (0.2 μm) LBG culture MRS broth, then the filtrate was concentrated using Centricon Plus-20 (5-100 kDa; Millipore, Bedford, Massachusetts, USA) by centrifugation at 4000 × g for 1 h following guidelines from the manufacturer. Protein concentrations were determined with the Pierce protein assay kit (Pierce, Rockford, Illinois, USA) using MRS broth as the control.
Cell culture
SGC-7901 cell line was established from human gastric adenocarcinoma cells. Though the characteristics of cell apoptosis and proliferation are different from the cell line derived from normal gastric epithelia, SGC-7901 cells have been widely used as models for investigations on H pylori-induced gastric epithelial inflammatory responses because their inflammatory responsibility is similar to normal gastric epithelia. Therefore, SGC-7901 cells were used in our experiment. They were grown in RPMI 1640 medium supplemented with 10% FCS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in an atmosphere containing 50 mL/L CO2. After 3-4 times of passage, SGC-7901 cells were seeded to generate 1 × 106 cells per 6 cm culture dish and incubated in RPMI 1640 medium containing 10% FCS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in an atmosphere containing 50 mL/L CO2 for 24 h. Then all cells were serum-starved (0.5% FCS) for 24 h before experimentation.
Treatment of cell line
SGC-7901 cells were treated with 25 endotoxin units (EU)/mL H pyloriSS1-LPS for 0, 30, 60 min or 120 min in the absence or presence of pretreatment for 1 h with 107 CFU/mL viable LBG or 10-2 mg/mL LBG-S. At the end of each time point, cells were collected for Western blot, and RPMI 1640 medium was collected for ELISA. Each experiment was in triplicate.
Preparation of cellular lysates and Western blot analysis
Nuclear and cytoplasmic extraction from SGC-7901 cells was performed using the nuclear-cytosol extraction kit (Cell Signaling Technology, Danvers, Massachusetts, USA) following guidelines from the manufacturer. Protein concentrations of all extracts were determined with the Pierce protein assay kit. Each extract was mixed with an equal amount of 2 × loading buffer and heated at 100°C for 5 min. Thirty micrograms of protein was loaded in each lane, resolved in sodium dodecyl sulphate-polyacrylamide gels and electrotransferred to polyvinylidene difluoride membrane (1.2 mA/cm2, 1 h). After blocked with 5% fat-free dried milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h, the membrane was incubated overnight at 4°C with the following primary antibodies: anti-TLR4, anti-transforming growth factor β-activated kinase 1 (TAK1), anti-phospho-TAK1 (p-TAK1), anti-nuclear factor κB (NF-κB), anti-p38 mitogen-activated protein kinase (p38MAPK), anti-phospho-p38MAPK (p-p38MAPK; all at the dilution of 1:1000, Cell Signaling Technology). Anti-β-actin and anti-lamin B1 (both at the dilution of 1:1000, Santa Cruz Biotechnology, Santa Cruz, California, USA) were used for the control of equal protein loading. After washed three times in TBST, the membrane was incubated with horseradish peroxidase-conjugated IgG (1:5000, Santa Cruz Biotechnology) as the secondary antibody at room temperature for 1 h. The photographic film was exposed to bands visualized with the Supersignal West Pico chemiluminescent substrate kit (Pierce). The integrated optical density (IA) of each band was quantified using Quantity One software 4.5.0 (Bio-Rad Laboratories, Hercules, California, USA). Each value for TLR4 band was normalized as the ratio of IA of TLR4 band to that of β-actin band. Each value for p-TAK1 band was normalized as the ratio of IA of p-TAK1 band to that of TAK1 band. Each value for p-p38MAPK band was normalized as the ratio of IA of p-p38MAPK band to that of p38MAPK band. Each value for NF-κB band was normalized as the ratio of IA of NF-κB band to that of lamin B1 band. Each Western blot analysis of extract samples was performed in triplicate.
Detection of IL-8 production
The concentration of IL-8 in RPMI 1640 medium was determined with a commercially available ELISA kit (R&D Systems, Minneapolis, Minnesota, USA) following guidelines from the manufacturer. Each sample was detected thrice.
Statistical analysis
The data were expressed as mean ± SD, and analyzed by SPSS13.0 software (SPSS, Chicago, Illinois, USA) for One-Way ANOVA test. P < 0.05 was considered statistically significant.
RESULTS
Viable LBG or LBG-S inhibited H pyloriSS1-LPS-induced activation of TLR4 signaling pathway
Twenty-five EU/mL H pyloriSS1-LPS up-regulated the expression of TLR4, enhanced the phosphorylation of TAK1 and p38MAPK, and induced the translocation of NF-κB into nuclei in a time-dependent manner (Figure 1A and B, Table 1). These results demonstrate that H pyloriSS1-LPS could activate the TLR4 signaling pathway. Pretreatment for 1 h with 107 CFU/mL viable LBG (Figure 1A) or 10-2 mg/mL LBG-S (Figure 1B) significantly inhibited these effects of H pyloriSS1-LPS on the TLR4 pathway in SGC-7901 cells to a great extent (Table 1).
Figure 1.
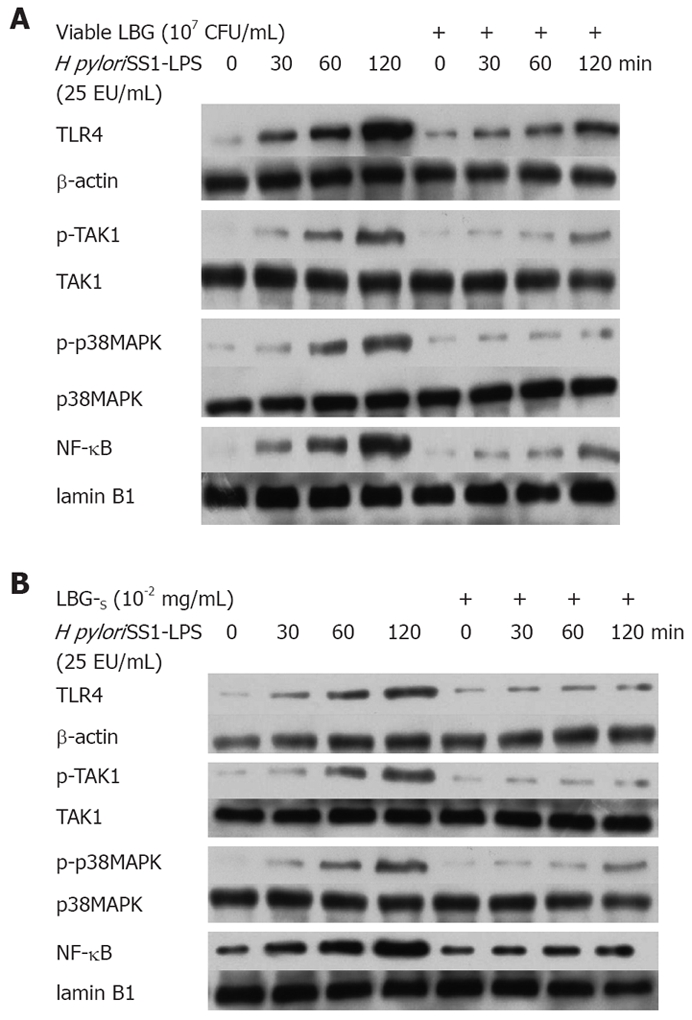
Expression of TLR4 and activation of TAK1, p38MAPK and NF-κB in SGC-7901 cells treated with H pyloriSS1-LPS in the absence or presence of pretreatment with viable LBG (A) and LBG-S (B).
Table 1.
Effects of viable LBG, LBG-S and H pyloriSS1-LPS on the expression of TLR4 and activation of TAK1, p38MAPK and NF-κB in SGC-7901 cells
| LPS 0 min | LPS 30 min | LPS 60 min | LPS 120 min | LBG + LPS 0 min | LBG + LPS 30 min | LBG + LPS 60 min | LBG + LPS 120 min | LBG-S +LPS 0 min | LBG-S + LPS 30 min | LBG-S + LPS 60 min | LBG-S + LPS 120 min | ||
| TLR4/β-actin | 0.014 ± 0.003 | 0.21 ± 0.031 | 0.43 ± 0.051 | 1.21 ± 0.111 | 0.016 ± 0.003 | 0.08 ± 0.012 | 0.15 ± 0.022 | 0.40 ± 0.052 | 0.016 ± 0.003 | 0.088 ± 0.0132 | 0.12 ± 0.022 | 0.18 ± 0.032 | |
| 1t | 2.588 | 3.068 | 3.502 | 2t | 2.328 | 2.425 | 2.302 | 2.182 | 2.733 | 2.901 | |||
| 1P | 0.012 | 0.003 | < 0.001 | 2P | 0.031 | 0.021 | 0.033 | 0.041 | 0.01 | 0.007 | |||
| p-TAK1/TAK1 | 0.008 ± 0.002 | 0.075 ± 0.0091 | 0.17 ± 0.031 | 0.49 ± 0.061 | 0.008 ± 0.001 | 0.025 ± 0.0032 | 0.051 ± 0.0062 | 0.15 ± 0.022 | 0.007 ± 0.002 | 0.031 ± 0.0052 | 0.086 ± 0.0112 | 0.13 ± 0.022 | |
| 1t | 2.445 | 2.871 | 3.421 | 2t | 2.408 | 2.502 | 2.530 | 2.278 | 2.108 | 2.682 | |||
| 1P | 0.018 | 0.005 | < 0.001 | 2P | 0.022 | 0.018 | 0.017 | 0.035 | 0.044 | 0.012 | |||
| p-p38MAPK/p38MAPK | 0.010 ± 0.002 | 0.079 ± 0.0111 | 0.18 ± 0.031 | 0.48 ± 0.081 | 0.009 ± 0.002 | 0.031 ± 0.0042 | 0.058 ± 0.0082 | 0.16 ± 0.032 | 0.013 ± 0.003 | 0.029 ± 0.0042 | 0.079 ± 0.0122 | 0.12 ± 0.022 | |
| 1t | 2.401 | 2.819 | 3.403 | 2t | 2.261 | 2.445 | 2.506 | 2.289 | 2.093 | 2.704 | |||
| 1P | 0.019 | 0.006 | < 0.001 | 2P | 0.036 | 0.02 | 0.018 | 0.034 | 0.046 | 0.011 | |||
| NF-κB/lamin B1 | 0.012 ± 0.002 | 0.18 ± 0.031 | 0.32 ± 0.051 | 1.02 ± 0.131 | 0.012 ± 0.002 | 0.042 ± 0.0052 | 0.084 ± 0.0092 | 0.26 ± 0.042 | 0.014 ± 0.002 | 0.091 ± 0.0122 | 0.17 ± 0.022 | 0.23 ± 0.042 | |
| 1t | 2.523 | 2.968 | 3.458 | 2t | 2.796 | 2.690 | 2.711 | 2.088 | 2.068 | 2.787 | |||
| 1P | 0.014 | 0.004 | < 0.001 | 2P | 0.009 | 0.012 | 0.011 | 0.046 | 0.048 | 0.009 |
t and P value of each group vs corresponding LPS 0 min group;
t and P value of each group vs corresponding LPS group at the same time point.
Viable LBG or LBG-S inhibited H pyloriSS1-LPS-induced IL-8 production
The production of IL-8 in SGC-7901 cells treated with 25 EU/mL H pyloriSS1-LPS for 0, 30 or 60 min in the absence or presence of pretreatment for 1 h with 107 CFU/mL viable LBG or 10-2 mg/mL LBG-S was almost undetectable (Table 2). No significant difference in these data was possibly attributed to their extremely small value. Thirty or 60 min of treatment with H pyloriSS1-LPS might be too short for SGC-7901 cells to produce enough IL-8 for ELISA, because cytokine production usually is posterior to the process of corresponding signal transduction. If we detected IL-8 mRNA in SGC-7901 cells using the retro-transcriptional polymerase chain reaction, results at 30 min or 60 min should have been significantly higher than those at 0 min. Only 120 min of treatment with 25 EU/mL H pyloriSS1-LPS augmented IL-8 production in SGC-7901 cells (Table 2), which was, however, down-regulated by pretreatment for 1 h with 107 CFU/mL viable LBG or 10-2 mg/mL LBG-S (Table 2).
Table 2.
Inhibitory effects of viable LBG or LBG-S on H pyloriSS1-LPS-induced IL-8 production (pg/mL)
| H pyloriSS1-LPS | Viable LBG+LPS | LBG-S+LPS | |||
| 0 min | 2.62 ± 0.43 | 2.56 ± 0.46 | 2.52 ± 0.51 | ||
| 30 min | 2.78 ± 0.38 | 2.67 ± 0.42 | 2.61 ± 0.47 | ||
| 60 min | 3.07 ± 0.35 | 2.93 ± 0.51 | 2.89 ± 0.48 | ||
| 120 min | 40.39 ± 3.01 | 24.12 ± 3.0512 | 18.41 ± 1.8312 | ||
| 1t | 2.832 | 2.601 | 2.506 | ||
| 1P | 0.006 | 0.011 | 0.015 | ||
| 2t | 2.121 | 2.175 | |||
| 2P | 0.047 | 0.044 |
t and P value of each group vs H pyloriSS1-LPS group at 0 min;
t and P value of each group vs H pyloriSS1-LPS group at 120 min.
DISCUSSION
H pylori have recently been considered an indigenous biota of human stomach and a dominant niche in gastric microecology including Lactobacilli and Sacharomyces with the capability of cross-species communication[17-19]. There is evidence that Helicobacter species are ancient inhabitants of human stomachs for at least 60 000 years which have co-evolved with the host and developed their excellent adaption to humans[20,21]. Though the vast majority of people in developing countries carry H pylori, most of them have no clinical manifestations at all[22]. More and more observations are consistent with the hypothesis that H pylori have both pathogenetic and symbiotic features, thus relatively balance their cost and benefit[23,24]. Changes in life style and sanitation conditions cause a probable disturbance of gastric microecology, which plays a more important role in the pathogenetic mechanism of H pylori in the modern era[25,26]. Therefore, eradiation of H pylori does not seem justified for all individuals, especially children. These findings lead to the speculation that we are supposed to domesticate H pylori through restoring the homeostasis of the gastric microecosystem. It was reported that administration of exogenous Lactobacilli has therapeutic effects on H pylori-associated diseases by interfering with the pathogenetic progress of H pylori[27-30]. Inhibition of H pylori-induced proinflammatory factor production by Lactobacilli is a very important aspect. It has been demonstrated that Lactobacilli abrogate H pylori-mediated IL-8 release in vitro and in vivo[31,32]. A large body of evidence has shown that H pylori-LPS-induced inflammation in gastric mucosa has nearly the same pathological characteristics as the mucosal inflammation initiated by H pylori infection[6,7]. Bhattacharyya et al[33] reported that pretreatment with LPS inhibitor greatly attenuated H pylori extract-mediated gastric mucosal inflammation, suggesting that H pylori-LPS may be a major virulent factor for H pylori-associated mucosal inflammation, which urged us to research the effect of Lactobacilli on H pylori-LPS-induced IL-8 production. It has been documented that H pylori-LPS induces mucosal inflammation including IL-8 production via TLR4 signaling. In brief, H pylori-LPS is recognized by TLR4 of the gastric epithelium, and then activates interleukin-1 receptor- associated kinase, tumor necrosis factor receptor-associated factor-6, TAK1 and TAK1-binding protein 1/2, p38MAPK and NF-κB at last in a cascade mechanism[7,9]. However, whether Lactobacilli have the capability of inhibiting H pylori-LPS-activated TLR4 pathway through interacting with gastric epithelia directly has not been extensively researched. Our findings demonstrate that viable LBG and LBG-S can prevent TLR4 signaling activation and IL-8 production mediated by H pyloriSS1-LPS in SGC-7901 cells, which strongly supports the hypothesis that some soluble proteins secreted by LBG and (or) somatic constituents of LBG exert inhibitory effects on the TLR4 pathways in SGC-7901 cells treated with H pyloriSS1-LPS. Yan et al[34] reported that Lactobacillus rhamnosus GG (LGG) or supernatant recovered from LGG culture MRS broth (LGG-S) ameliorated apoptosis of young adult mouse colon cells treated with tumor necrosis factor α (TNF-α), interferon-γ or interleukin-1α through blocking p38MAPK and stress-activated protein kinase/c-Jun amino-terminal kinase pathway. In addition, they have identified two proteins in LGG-S with molecular sizes of 80 and 42 kDa, which may be possible substantial effectors in LGG-S[34]. In a recent study, they purified the two proteins from LGG-S again, ultimately determined their molecular weight as 75 and 40 kDa, and named them p75 and p40 respectively[35]. Their results demonstrate that both p75 and p40 can inhibit TNF-α-induced apoptosis of intestinal epithelia and promote cell growth through activating Akt[35]. LBG-S in our experiment probably contained the similar or even same proteins, which could intervene in H pyloriSS1-LPS-activated TLR4 signaling through modulating other pathways in SGC-7901 cells. Further study is needed to evaluate the hypothesis. In the purification and characterization of the aforementioned potential soluble proteins secreted by LBG, we also detected the effect of heat-killed LBG (hk-LBG) on H pyloriSS1-LPS-activated TLR4 signaling for evaluating the presumption that some somatic constituents of LBG may inhibit the effect of H pyloriSS1-LPS. The incomplete data indicate that hk-LBG could also disrupt the H pyloriSS1-LPS-activated TLR4 pathway. Nevertheless, the effect of hk-LBG was obviously smaller than that of viable LBG at the same concentration.
In conclusion, our evaluation of LBG as a model probiotic organism reveals an important and novel relationship between H pylori-LPS-activated TLR4 signaling and selective microflora, and further our understanding of the signal pathways in gastric epithelia involved in inflammatory responses that are regulated by probiotics and pathogenic bacteria composing the gastric microecosystem.
COMMENTS
Background
Some studies have demonstrated that Helicobacter pylori (H pylori) can stimulate the release of interleukin-8 (IL-8) from gastric epithelia, which initiates inflammatory damage to gastric mucosa and plays a crucial role in the pathogenesis of H pylori infection. H pylori lipopolysaccharide (H pylori-LPS) is a major virulent factor for H pylori-associated mucosal inflammation, which induces IL-8 production via activation of the Toll-like receptor 4 (TLR4) signaling pathway in gastric epithelia. Since H pylori is an indigenous biota in gastric microflora including Lactobacilli and disturbance of the gastric microecosystem plays a more important role in pathogenetic mechanisms of H pylori, eradiation of H pylori in all individuals does not seem justifiable. The gastric microecosystem was restored after treatment with exogenous Lactobacilli. However, whether Lactobacilli inhibit H pylori-LPS-induced IL-8 production through blocking H pylori-LPS-activated TLR4 pathway needs further study.
Research frontiers
Though there is more and more evidence that Lactobacilli have beneficial effects on gastrointestinal diseases, the molecular mechanisms are not well understood, especially in H pylori-associated diseases. Whether certain soluble proteins secreted by Lactobacillus bulgaricus (LBG) and somatic constituents of LBG directly interact with some signaling pathways in gastric epithelia has not been clearly demonstrated. Our study indicated that Lactobacillus rhamnosus GG secreted p75 and p40, two proteins with a molecular size of 75 and 40 kDa respectively, could ameliorate apoptosis of intestinal epithelia treated with tumor necrosis factor α, interferon-γ or interleukin-1α and promote cell growth through activating Akt, blocking p38 mitogen-activated protein kinase and stress-activated protein kinase/c-Jun amino-terminal kinase signaling. The supernatant recovered from LBG culture MRS broth (LBG-S) in our experiment may contain the similar or even same proteins, which could intervene in H pylori Sydney strain 1 lipopolysaccharide (H pyloriSS1-LPS)-activated TLR4 signaling through modulating other pathways in SGC-7901 cells, suggesting that probiotic bacterial components may be useful in preventing H pylori-associated diseases.
Innovations and breakthroughs
Viable LBG or LBG-S blocked the H pyloriSS1-LPS-activated TLR4 pathway in SGC-7901 cells, leading to their inhibitory effects on IL-8 production induced by H pyloriSS1-LPS.
Applications
This study implied that certain soluble proteins secreted by Lactobacilli could directly interact with some signaling pathways in gastric epithelia and partly block the noxious effects of H pylori. This may benefit the exploration of new drugs for the treatment of H pylori-associated diseases.
Peer review
The results of this study indicate that H pyloriSS1-LPS could increase IL-8 production through TLR4 signaling in gastric epithelia and probiotic bacteria attenuate IL-8 production via inhibition of the TLR4 pathway. The manuscript contains some interesting data, viable probiotics and their extracts against H pylori-LPS cytotoxicity.
Acknowledgments
The authors thank Professor Qian Yu (School of Public Health, Sichuan University) for her kind donation of H pylori Sydney strain 1 and Lactobacillus bulgaricus as well as Drs. Xiang Liu, Ming-Jiang Bie and Jian Wang (Laboratory of Medical Laboratory Sciences, School of Public Health, Sichuan University) for their assistance in performing the experiments.
Footnotes
Supported by Basic Research Project of Science and Technology Office of Sichuan Province, No. 04JY029-090-1
Peer reviewer: Tomasz Brzozowski, Professor, Department of Physiology, Jagiellonian University Medical College, 16 Grzegorzecka Str, Cracow 31-531, Poland
S- Editor Zhong XY L- Editor Wang XL E- Editor Lin YP
References
- 1.Felley CP, Pignatelli B, Van Melle GD, Crabtree JE, Stolte M, Diezi J, Corthesy-Theulaz I, Michetti P, Bancel B, Patricot LM, et al. Oxidative stress in gastric mucosa of asymptomatic humans infected with Helicobacter pylori: effect of bacterial eradication. Helicobacter. 2002;7:342–348. doi: 10.1046/j.1523-5378.2002.00107.x. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura N, Suzuki Y, Saito Y. Suppression of Helicobacter pylori-induced interleukin-8 production in gastric cancer cell lines by an anti-ulcer drug, geranylgeranylacetone. J Gastroenterol Hepatol. 2002;17:1153–1160. doi: 10.1046/j.1440-1746.2002.02880.x. [DOI] [PubMed] [Google Scholar]
- 3.Ismail S, Hampton MB, Keenan JI. Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect Immun. 2003;71:5670–5675. doi: 10.1128/IAI.71.10.5670-5675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nozawa Y, Nishihara K, Akizawa Y, Orimoto N, Nakano M, Uji T, Ajioka H, Kanda A, Matsuura N, Kiniwa M. Lafutidine inhibits Helicobacter pylori-induced interleukin-8 production in human gastric epithelial cells. J Gastroenterol Hepatol. 2004;19:506–511. doi: 10.1111/j.1440-1746.2003.03330.x. [DOI] [PubMed] [Google Scholar]
- 5.Lopes AI, Quiding-Jarbrink M, Palha A, Ruivo J, Monteiro L, Oleastro M, Santos A, Fernandes A. Cytokine expression in pediatric Helicobacter pylori infection. Clin Diagn Lab Immunol. 2005;12:994–1002. doi: 10.1128/CDLI.12.8.994-1002.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slomiany BL, Piotrowski J, Slomiany A. Up-regulation of endothelin-converting enzyme-1 in gastric mucosal inflammatory responses to Helicobacter pylori lipopolysaccharide. Biochem Biophys Res Commun. 2000;267:801–805. doi: 10.1006/bbrc.1999.2037. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa T, Asai Y, Sakai Y, Oikawa M, Fukase K, Suda Y, Kusumoto S, Tamura T. Endotoxic and immunobiological activities of a chemically synthesized lipid A of Helicobacter pylori strain 206-1. FEMS Immunol Med Microbiol. 2003;36:1–7. doi: 10.1016/S0928-8244(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 8.Kawahara T, Teshima S, Oka A, Sugiyama T, Kishi K, Rokutan K. Type I Helicobacter pylori lipopolysaccharide stimulates toll-like receptor 4 and activates mitogen oxidase 1 in gastric pit cells. Infect Immun. 2001;69:4382–4389. doi: 10.1128/IAI.69.7.4382-4389.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su B, Ceponis PJ, Lebel S, Huynh H, Sherman PM. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect Immun. 2003;71:3496–3502. doi: 10.1128/IAI.71.6.3496-3502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig Dis Sci. 2004;49:1095–1102. doi: 10.1023/b:ddas.0000037794.02040.c2. [DOI] [PubMed] [Google Scholar]
- 11.Sheu BS, Wu JJ, Lo CY, Wu HW, Chen JH, Lin YS, Lin MD. Impact of supplement with Lactobacillus- and Bifidobacterium-containing yogurt on triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2002;16:1669–1675. doi: 10.1046/j.1365-2036.2002.01335.x. [DOI] [PubMed] [Google Scholar]
- 12.Mukai T, Asasaka T, Sato E, Mori K, Matsumoto M, Ohori H. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med Microbiol. 2002;32:105–110. doi: 10.1111/j.1574-695X.2002.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 13.Oh Y, Osato MS, Han X, Bennett G, Hong WK. Folk yoghurt kills Helicobacter pylori. J Appl Microbiol. 2002;93:1083–1088. doi: 10.1046/j.1365-2672.2002.01779.x. [DOI] [PubMed] [Google Scholar]
- 14.Aiba Y, Suzuki N, Kabir AM, Takagi A, Koga Y. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Am J Gastroenterol. 1998;93:2097–2101. doi: 10.1111/j.1572-0241.1998.00600.x. [DOI] [PubMed] [Google Scholar]
- 15.Michetti P, Dorta G, Wiesel PH, Brassart D, Verdu E, Herranz M, Felley C, Porta N, Rouvet M, Blum AL, et al. Effect of whey-based culture supernatant of Lactobacillus acidophilus (johnsonii) La1 on Helicobacter pylori infection in humans. Digestion. 1999;60:203–209. doi: 10.1159/000007660. [DOI] [PubMed] [Google Scholar]
- 16.Linsalata M, Russo F, Berloco P, Caruso ML, Matteo GD, Cifone MG, Simone CD, Ierardi E, Di Leo A. The influence of Lactobacillus brevis on ornithine decarboxylase activity and polyamine profiles in Helicobacter pylori-infected gastric mucosa. Helicobacter. 2004;9:165–172. doi: 10.1111/j.1083-4389.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 17.Blaser MJ, Parsonnet J. Parasitism by the "slow" bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest. 1994;94:4–8. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sathar MA, Gouws E, Simjee AE, Mayat AM. Seroepidemiological study of Helicobacter pylori infection in South African children. Trans R Soc Trop Med Hyg. 1997;91:393–395. doi: 10.1016/s0035-9203(97)90253-4. [DOI] [PubMed] [Google Scholar]
- 19.Hadley C. The infection connection. Helicobacter pylori is more than just the cause of gastric ulcers--it offers an unprecedented opportunity to study changes in human microecology and the nature of chronic disease. EMBO Rep. 2006;7:470–473. doi: 10.1038/sj.embor.7400699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, Blaser MJ, Graham DY, Vacher S, Perez-Perez GI, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 21.Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, Falush D, Stamer C, Prugnolle F, van der Merwe SW, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915–918. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16 Suppl 1:3–15. doi: 10.1046/j.1365-2036.2002.0160s1003.x. [DOI] [PubMed] [Google Scholar]
- 23.Rajendra S, Ackroyd R, Robertson IK, Ho JJ, Karim N, Kutty KM. Helicobacter pylori, ethnicity, and the gastroesophageal reflux disease spectrum: a study from the East. Helicobacter. 2007;12:177–183. doi: 10.1111/j.1523-5378.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 24.Ackermark P, Kuipers EJ, Wolf C, Breumelhof R, Seldenrijk CA, Timmer R, Segeren KC, Kusters JG, Smout AJ. Colonization with cagA-positive Helicobacter pylori strains in intestinal metaplasia of the esophagus and the esophagogastric junction. Am J Gastroenterol. 2003;98:1719–1724. doi: 10.1111/j.1572-0241.2003.07585.x. [DOI] [PubMed] [Google Scholar]
- 25.Blaser MJ. Hypothesis: the changing relationships of Helicobacter pylori and humans: implications for health and disease. J Infect Dis. 1999;179:1523–1530. doi: 10.1086/314785. [DOI] [PubMed] [Google Scholar]
- 26.Tovey FI, Hobsley M, Kaushik SP, Pandey R, Kurian G, Singh K, Sood A, Jehangir E. Duodenal gastric metaplasia and Helicobacter pylori infection in high and low duodenal ulcer-prevalent areas in India. J Gastroenterol Hepatol. 2004;19:497–505. doi: 10.1111/j.1440-1746.2003.03320.x. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto I, Igarashi M, Kimura K, Takagi A, Miwa T, Koga Y. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J Antimicrob Chemother. 2001;47:709–710. doi: 10.1093/jac/47.5.709. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu T, Haruna H, Hisada K, Yamashiro Y. Effects of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in children. J Antimicrob Chemother. 2002;50:617–618. doi: 10.1093/jac/dkf157. [DOI] [PubMed] [Google Scholar]
- 29.Gotteland M, Cruchet S. Suppressive effect of frequent ingestion of Lactobacillus johnsonii La1 on Helicobacter pylori colonization in asymptomatic volunteers. J Antimicrob Chemother. 2003;51:1317–1319. doi: 10.1093/jac/dkg227. [DOI] [PubMed] [Google Scholar]
- 30.Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, Cammarota G, Anti M, De Lorenzo A, Pola P, et al. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163–169. doi: 10.1046/j.1365-2036.2001.00923.x. [DOI] [PubMed] [Google Scholar]
- 31.Sgouras DN, Panayotopoulou EG, Martinez-Gonzalez B, Petraki K, Michopoulos S, Mentis A. Lactobacillus johnsonii La1 attenuates Helicobacter pylori-associated gastritis and reduces levels of proinflammatory chemokines in C57BL/6 mice. Clin Diagn Lab Immunol. 2005;12:1378–1386. doi: 10.1128/CDLI.12.12.1378-1386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergonzelli GE, Granato D, Pridmore RD, Marvin-Guy LF, Donnicola D, Corthesy-Theulaz IE. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect Immun. 2006;74:425–434. doi: 10.1128/IAI.74.1.425-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacharyya A, Pathak S, Datta S, Chattopadhyay S, Basu J, Kundu M. Mitogen-activated protein kinases and nuclear factor-kappaB regulate Helicobacter pylori-mediated interleukin-8 release from macrophages. Biochem J. 2002;368:121–129. doi: 10.1042/BJ20020555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–50965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


